Abstract
Since the advent of cochlear implantation, candidacy criteria have slowly broadened to include increasingly younger patients. Spurred by evidence demonstrating both perioperative safety and significantly increased speech and language benefit with early auditory intervention, children younger than 12 months of age are now being successfully implanted at many centers. This review highlights the unique challenges involved in cochlear implantation in the very young child, specifically diagnosis and certainty of testing, anesthetic risk, surgical technique, intraoperative testing and postoperative programming, long-term safety, development of receptive and expressive language, and outcomes of speech perception. Overall, the current body of literature indicates that cochlear implantation prior to 1 year of age is both safe and efficacious.
Keywords: cochlear implantation, infant, young children, deafness, profound hearing loss
Introduction
Widespread universal newborn hearing screening has led to increased identification of infants with hearing loss worldwide. This increase in early diagnosis has led to greater opportunities for early intervention. Long-term data suggest that auditory rehabilitation (including conventional amplification) commencing prior to 6 months of age leads to significant gains in vocabulary, speech intelligibility, general language abilities, social-emotional development, parental bonding, and parental grief resolution when compared with late-identified peers (Moeller, 2000; Yoshinaga-Itano, 2003). With early diagnosis, increasing numbers of children younger than 1 year with bilateral severe to profound sensorineural hearing loss (SNHL) who do not benefit from a trial of conventional amplification (and who meet anatomic and medical criteria) are now being successfully implanted at many centers.
Since the advent of pediatric cochlear implantation (CI), formal candidacy criteria have slowly broadened to include increasingly younger patients. In 1990, the U.S. Food and Drug Administration approved CIs for children 2 year of age and older with bilateral profound SNHL. Currently, CI is Food and Drug Administration–approved for children 1 year of age and older. Although prior and ongoing research has attempted to address the unique issues of safety, candidacy, programming, and efficacy in this very young age-group, CI in children younger than 12 months of age remains controversial.
The following review will highlight and discuss the following issues specific to the under-1 population, including diagnosis, anesthetic risk, surgical technique, intraoperative testing and postoperative programming, long-term safety, development of receptive and expressive language, and speech perception outcomes.
Diagnosis of Infant Hearing Loss
Inherent in the discussion of CI in children younger than 12 months is the ability to reliably diagnose children of this age-group with bilateral profound deafness. The sensitivity and specificity of both behavioral and objective tests of auditory function are discussed in terms of their application to the under-1 population.
Behavioral Audiometry
Widely accepted as the gold standard for auditometric evaluation, behavioral testing in infants is performed using visual reinforcement audiometry (VRA; Widen et al., 2000). The VRA employs operant conditioning to reinforce an infant's natural inclination to turn toward a sound stimulus (narrow-band noise, pure tones, or speech). A positive response, or head turn toward the stimulus, is visually reinforced until consistent, which is typically a rapid process in infants (Widen et al., 2000). Using VRA, behavioral thresholds for pure-tone stimuli can approximate the reliability obtained in adults and can provide frequency specific information (Olsho, Koch, Carter, Halpin, & Spetner, 1988; Widen et al., 2000). Although VRA can be reliably applied to children who have reached 6 months developmental age, this age may not equal their chronological age in cases of prematurity or neurocognitive delay (Moore, Thompson, & Folsom, 1992). As hearing impairment is not uncommon in premature infants and may accompany other areas of global disability, objective measures of audiometric assessment are required. Objective testing is also appropriate for infants younger than 6 months of age or in the case that VRA conditioning proves ineffective.
Electrophysiologic Tests of Auditory Function
Although behavioral testing provides a global assessment of an infant's auditory function, objective measures target particular areas of the auditory pathway. Otoacoustic emissions (OAEs), whether transient evoked (TEOAE, click stimulus) or distortion product (DPOAE, tone pairs), are generated from the outer hair cells of the cochlea in response to an auditory stimulus. Auditory brainstem response (ABR) testing assesses the inner hair cells, the vestibulocochlear nerve, and their afferent neural connections into the brainstem. Heavily studied and appropriate for individuals of any age, these tests are often used in universal newborn hearing screening programs throughout the United States.
OAEs are rapid, easy to administer, unaffected by test environment or location (sound booth, private room, open nursery, or intensive care unit), and minimally affected by the state of infant arousal. ABR testing produces similarly robust, highly replicable results unaffected by patient state of consciousness (Oates & Stapells, 1998; Stapells 1989). Both therefore satisfy many criteria of an ideal, objective screening test in the newborn population (Norton et al., 2000a, 2000b). In the multicenter “Identification of Newborn Hearing Impairment” study sponsored by the National Institute of Health, TEOAE, DPOAE, and ABR results were compared with behavioral audiometry using VRA as the gold standard. This national study included 7,179 neonates, 4,911 of whom were at high risk for hearing loss as neonatal intensive care unit graduates, for having a family history of congenital hearing loss, or because of other perinatal factors (Norton et al., 2000b). Overall, TEOAE and DPOAE were found to be equivalent in predicting hearing loss greater than 30 dB HL in frequencies higher than 2 Hz. At frequencies 1 Hz and lower, however, ABR was most predictive of auditory function when compared with VRA (N = 2995; Norton et al., 2000a). The sensitivity of each test increased with the magnitude of hearing loss, and all tests (OAE, ABR, and VRA) had difficulty identifying mild functional impairment (Norton et al., 2000a, 2000b). Norton's results have been confirmed by other large-scale, international studies evaluating community-based infant hearing screening programs. Chiong et al. (2007) compared OAEs with ABRs results in 1,133 children younger than 9 months of age and found an OAE sensitivity of 85.4% and specificity of 99.4%.
Although highly specific, ABR testing has some difficulty distinguishing between severe hearing loss and profound hearing loss (Luts, Desloovere, Kumar, Vandermeersch, & Wouters, 2004). The broad-frequency spectral content of the click stimulus leads to wide activation of the basilar membrane and does not allow assessment of frequency-specific hearing loss (Picton, Durieux-Smith, & Moran, 1994; Stapells & Oates, 1997). However, use of tone-burst stimuli, in contrast to a click stimulus, can lead to frequency-specific thresholds and is used reliably in many centers (Oates & Stapells, 1998; Stapells, 1989).
Another objective auditory evoked potential, the auditory steady-state response (ASSR), addresses some of the limitations of ABR. Using longer sinusoidal pure-tone stimuli that are both frequency and amplitude varied, auditory function can be obtained over a larger dynamic range (Picton, John, Dimitrijevic, & Purcell, 2003). Additionally, unlike ABR where the test administrator identifies the presence of various waveforms, ASSR uses computerized statistical software to detect a response (Swanepoel & Ebrahim, 2009). Multiple studies comparing ASSR with ABR and behaviorally obtained thresholds in children have shown increased specificity and specificity of ASSR (Luts et al., 2004; Swanepoel & Ebrahim, 2009).
Following diagnosis of hearing impairment, ABR and ASSR are also important for determining threshold levels for amplification in very young children (Gabbard & Schryer, 2003). As mentioned earlier, the positive impact of intervention prior to 6 months of age has widespread implications, and all children with bilateral hearing loss greater than 20 dB HL should be considered for amplification (Gabbard & Schryer, 2003; Yoshinaga-Itano, 2003). Children younger than 1 year of age with severe to profound bilateral SNHL should undergo a trial of amplification and aural rehabilitation prior to confirmation of CI candidacy (discussed further in upcoming section).
Immittance audiometry, including tympanometry and acoustic reflex testing, is another important component of the objective audiometric evaluation of infants with suspected hearing loss. Using pure tones and variations in pressure in the external auditory canal, tympanometry provides an objective measure of tympanic membrane mobility and middle ear pressure. Middle ear fluid, common in infancy, can diminish or obliterate OAE and significantly compromise ABR testing (El-Rafaie, Parker, & Bamford, 1996; Koivun, Uhari, Laitakari, Alho, & Luotonen, 2000). Studies suggest that conductive hearing loss may be the most common cause of infant hearing screening failures, contributing up to 67% of the false positive findings (Boone, Bower, & Martin, 2005). Unique mechanical properties of the infant ear require the use of a high-frequency 1 kHz probe tone versus a 226 Hz tone typically used in older children and adults. Specifically, the external canal of the newborn is highly compliant because of soft cartilage and an incompletely formed bony canal (Holte, Margolis, & Cavanaugh, 1991). In addition, changes in ossicular bone density and stapedial joint laxity make the infant middle ear a mass-dominated system, versus the stiffness-dominated system found in adults (Holte et al., 1991). Swanepoel et al. (2007) and others have shown high-frequency tympanometry to be reliable and up to 95% specific for accurate characterization of newborn middle ear function (Holte et al., 1991; Kei et al., 2003; Margolis, Bass-Ringdahl, Hanks, Holte, & Zapala, 2003). Thus, although not a test of hearing sensitivity, tympanometry plays a valuable role in the audiologic evaluation of infants (Boone et al., 2005; Harlor & Bower, 2009; Kei et al., 2003; Margolis et al., 2003; Swanepoel et al., 2007).
Using a 1 kHz high-frequency probe tone, acoustic reflexes (AR) have shown similar sensitivity in this very young age-group (Swanepoel et al., 2007). Abnormalities of this bilateral reflex can result from ipsilateral and/or contralateral pathology of the middle ear, ossicles, stapedius muscle, cochlear or facial nerves, or the brainstem. The ipsilateral reflex arc involves the auditory nerve (CN VIII), cochlear nucleus, and the ipsilateral facial nucleus and nerve (CN VII) and ends with contraction of the stapedius muscle, innervated by CN VII. An ipsilateral probe tone can also elicit a stapedial muscle contraction in the contralateral ear because of brainstem crossover at the superior olivary complex. Abnormalities or lack of ARs can be indicative of conductive and/or SNHL, thus these results should be used in combination with other objective audiometric testing, such as tympanometry, ABR, or ASSR, to support a diagnosis of hearing loss in the very young child.
Unlike the above immitance tests, electrocochleography (ECoG) is less commonly employed and has a limited role in the diagnostic assessment of infant hearing loss (Dauman, 1991). ECoG is a method of recording stimulus-related cochlear potentials and typically includes three measurements: the cochlear microphonic (CM), cochlear summating potential, and compound action potential of the auditory nerve. Multiple studies have documented high correlation of thresholds between ECoG, ABR, and behavioral audiometry in children, including those younger than 1 year of age with hearing loss (Aso & Gibson, 1994; Wong, Gibson, & Sanli, 1997). ECoG can be performed trans- or extratympanically. Transtympanic ECoG involves either a needle electrode placed through the tympanic membrane onto the promontory or a round window electrode placed into the region of the round window through a myringotomy (Aso & Gibson, 1994; Wong et al., 1997). Extratympanic recording, a less invasive method, uses a foam ear plug electrode (called a TIPtrode) placed in the medial external auditory canal (P. S. Roland, Yellin, Meyerhoff, & Frank, 1995). Although ECoG can provide information about residual cochlear function, the test requires sedation in children and is typically been performed in conjunction with another procedure for which the child is sedated (Aso & Gibson, 1994; Dauman, 1991; Wong et al., 1997). Currently, ECoG has two primary applications for diagnosis of deafness in the very young child: the multiply handicapped or delayed child and the diagnosis of auditory neuropathy/dys-synchrony (AN/AD, to be discussed in detail in that section). In the former, ECoG has been used as an additional piece of evidence to evaluate cochlear function when prior testing such as VRA, OAE, ABR, or ASSR could not be obtained, or when results obtained were inconclusive or confounded (Aso & Gibson, 1994; Dauman, 1991; Gibson & Sanli, 2007; Wong et al., 1997). In the latter, research suggests that presence of a CM in the absence of ABR recordings may uncover auditory neuropathy or auditory processing disorders in young children (Aso & Gibson, 1994; Gibson & Sanli, 2007; Rance, 2005).
In summary, CI evaluation of a child younger than 1 year of age should include the following: an attempt at behavioral audiometry (i.e., VRA), bilateral OAEs, ear- and frequency-specific ABR or ASSR, bilateral tympanometry, and acoustic reflexes. Present OAEs with abnormal ABR or ASSR and absent reflexes should generate suspicion for AN/AD, and ECoG can be used as an additional test in this setting.
Assessment of Speech Perception and Early Language Development
Speech perception testing in this age-group remains challenging as most tests are language based and not appropriate for children younger than 1 year. Use of the Infant-Toddler Meaningful Auditory Integration Scale (IT-MAIS), a parental survey of early speech development, has been employed reliably in this age-group as a proxy for speech perception and linguistic development (Waltzman & Roland, 2005; Zimmerman-Philips, Osberger, & Robbins, 1997). This 10-question structured interview assesses the three categories of auditory-specific behavior, including vocalization, alerting to sound, and deriving meaning from sound (Zimmerman-Philips et al., 1997). Parents score the frequency of various behaviors on a 4-point scale from never (0) to always (4). Normative is available for normal hearing children throughout the first year of life and older, beginning at 1 month old. As in Waltzman and Roland (2005), the IT-MAIS can be administered to parents of children with suspected hearing loss and compared with age-specific norms in normal hearing infants. In children younger than 1 year of age with suspected hearing loss, the IT-MAIS can be administered again after a trial of hearing aids plus aural rehabilitation to assess progress. Greater certainty regarding CI candidacy is achieved when IT-MAIS scores demonstrate lack of progress following a trial of appropriate amplification.
As with speech perception, traditional tests of early language development used in older children are not appropriate for children younger than 1 year of age. However, using parental report, video-, and audiorecordings, multiple researchers have investigated early vocalization behaviors of normal hearing and early-identified hearing impaired infants. (Davis, Morrison, von Hapsburg, & Warner-Czyz, 2005; Eilers & Oller, 1994; Moeller et al., 2007; Oller & Eilers, 1988; Smith & Oller, 1981) Between 7 and 10 months of age, infants with normal hearing begin babbling, or the consistent production of recognizable consonant–vowel syllables (Oller & Eilers, 1988; Smith & Oller, 1981). Overall, delayed babble onset has been found in children with severe to profound hearing loss (Davis et al., 2005; Eilers & Oller, 1994; Moeller et al., 2007; Oller & Eilers, 1988; Smith & Oller, 1981). However, significant individual variability has been reported, with some profoundly deaf infants beginning to babble at ages comparable with age-matched normal hearing peers (Koopmans-van Beinum, Clement, & van den Dikkenberg-Pot, 2001; Wallace, Menn, & Yoshinaga-Itano, 2000). Although used in a variety of research settings, babble assessment is not currently a standard component of preoperative CI evaluation in children younger than 1. Given the apparent range of vocalization abilities among hearing impaired infants, details of babble onset may not necessarily contribute to a diagnosis of profound hearing loss. Following CI, however, babble assessment and other tests of early vocalization may play an important role in monitoring linguistic progress, especially in children implanted under 12 months of age (Moeller et al., 2007). This will be discussed further in upcoming sections on postimplantation outcomes.
Etiology of Hearing Loss, Genetic Testing, and Radiologic Imaging
In approximately 35% to 50% of children with SNHL, the etiology of hearing impairment can be traced to acquired, environmental factors, including intrauterine infections (commonly referred to as TORCH infections representing the pathogens of toxoplasmosis, rubella, cytolomegalovirus, herpes virus, HIV, syphilis), ototoxic medications, meningitis, maternal or neonatal metabolic disorders, maternal illicit drug use, prematurity, low Apgar scores, neonatal jaundice, and exposure to teratogens (American College Medical Genetics [ACMG], 2002; Mafong, Shin, & Lalwani, 2002; Parving & Stephens, 1997; Preciado et al., 2005). Once an etiology is suspected or identified, knowledge of the specific natural history of auditory insult can be used in combination with hearing testing to increase the certainty of deafness diagnosis. In some instances, such as meningitis where labyrinthitis ossifications leads to rapid ossification of the cochlear lumen and difficulty with electrode insertion, rapid progression to CI following diagnosis is crucial to overall success (J. T. Roland, Coehlo, Pantelides, & Waltzman, 2008). Thus, in children younger than 1 having meningitis, knowledge of the disease process can be a crucial component of CI candidacy evaluation.
In cases of hereditary congenital hearing loss, etiology can be attributed to genetic mutations in approximately 50% (ACMG, 2002) of them. Of these, approximately 30% are associated with clinical features from a known syndrome, whereas 70% are not (i.e., nonsyndromic; ACMG, 2002; Mafong et al., 2002; Parving & Stephens, 1997). Among children with nonsyndromic profound SNHL, approximately half will have mutations in the gene GJB2, which codes for a gap junction protein of the beta 2 until subclass called connexin 26, which is involved in cell-to-cell communication in the inner ear (ACMG, 2002; Steel & Kros, 2001). Preciado et al. (2005) suggest early use of GJB2 testing in the diagnostic algorithm for infants with indications of severe to profound deafness on a hearing screen so as to assist with appropriate, early intervention, including CI. Knowledge of connexin mutation positivity can increase certainty of deafness diagnosis in children younger than 1 year of age and can assist with CI candidacy assessment.
In the evaluation of pediatric SNHL, radiologic imaging, specifically high-resolution computed tomography (HRCT), can be used in conjunction with objective audiologic testing to confer certainty of diagnosis (Antonelli, Varela, & Mancuso, 1999; Preciado et al., 2005). Excluding children with known causes of SNHL such as TORCH infection, prematurity, hyperbilirubinemia, known syndromes, or meningitis, Preciado et al. (2005) found 30% of participants demonstrated radiologic abnormalities on HRCT scan consistent with SNHL. Of these 45 children, only 1 was also positive for GJB2, indicating importance of both genetic testing and imaging in assessing pediatric SNHL (Preciado et al., 2005). Additionally, the diagnostic yield of each HRCT scan was not correlated with hearing loss severity: HRCT abnormalities were equally common in children with mild-moderate hearing loss and those with severe-profound deafness. The abnormalities detected in this study, specifically enlarged vestibular aqueduct (EVA; 53%), cochlear dysplasia (13%), and cochlear hypoplasia (4.4%), can be associated with a range of hearing abilities in young children (Preciado et al., 2005). Thus, information obtained from HRCT should be used in combination with other diagnostic measures, such as audiometric testing described above, to provide insight into a diagnosis of infant hearing loss. Also, because congenital inner ear abnormalities such as EVA or Mondini dysplasia can be associated with syndromic hearing loss, such as Pendred's syndrome, HRCT findings can initiate targeted genetic or serologic testing for a specific syndrome or etiology (Preciado et al., 2005).
Use of other imaging modalities, specifically magnetic resonance imaging (MRI), in the diagnosis of profound hearing loss in children younger than 1 year is controversial. In cases where etiology of deafness is known, such as meningitis, the value of MRI in assessment of cochlear ossification and in preoperative surgical planning is well recognized (J. T. Roland et al., 2008; Trimble, Blaser, James, & Papsin, 2007). Some authors, however, advocate MRI as the primary imaging modality for all children with severe to profound hearing loss (Adunka, Jewells, & Buchman, 2007), whereas others suggest use of a diagnostic algorithm in which patient factors are used to direct imaging (Trimble et al., 2007). Although a complete discussion of this debate is beyond the scope of this article, one controversy surrounds the ability of MRI to diagnosis cochlear nerve (CN) aplasia or absence. Presence of a CN is an absolute requirement for CI at any age; however, diagnosis of CN deficiency can be challenging. Prior reports suggested that structural features seen on HRCT, such as stenosis of the internal auditory canal, were indicative of CN aplasia and, thus, poor performance with a CI (Jackler, Luxford, & House, 1987; Shelton, Luxford, Tonokawa, Lo, & House, 1989). Results of Adunka et al. (2007), however, indicate that neither internal auditory canal nor bony CN canal size can accurately predict CN deficiency. They suggest that MRI is the most reliable imaging modality for diagnosis of CN aplasia and recommend it be used for determining CI candidacy in all children with severe to profound hearing loss (Adunka et al., 2007). Trimble et al. (2007) present an algorithm for preoperative pediatric CI imaging that balances the advantages of HRCT scanning (shorter imaging time requiring less sedation, increased bony detail) with the benefits of MRI (detection of CN aplasia, avoidance of radiation associated with CT, ability to diagnosis intracranial abnormalities). They note that there is concordance between HRCT and MRI and that dual imaging detects abnormalities that would not be identified using either modality alone (Trimble et al., 2007).
Auditory Neuropathy/Dys-Synchrony
The diagnosis and management of children with AN/AD remains controversial, and this debate is heightened in children younger than 1 year of age. AN/AD describes a heterogenous group of auditory-processing abnormalities typically characterized by presence of OAE and or CM potentials with a greatly abnormal or absent ABR and absent acoustic reflexes (Aso & Gibson, 1994; Gibson & Sanli, 2007; Luts et al., 2004; Rance, 2005). In older children and adults, a hallmark of diagnosis involves auditory perceptual deficits out of proportion with behavioral hearing levels (Rance, 2005). As described earlier, both behavioral audiometry and assessment of speech perception prove difficult in very young children, thus illustrating the diagnostic challenge in this age-group. For children (and adults) with AN/AD and minimal auditory capacity, multiple studies have confirmed CI outcomes commensurate with those of peers with other forms of SNHL (Rance, 2005; Rance & Barker, 2009). Recent evidence has suggested, however, that outcomes for a selected group of children with AN/AD treated with hearing aid amplification may equal or exceed that of those managed with CI (Rance & Barker, 2009). Additionally, evidence exists for spontaneous recovery of AN/AD prior to 1 year of age (Attias & Raveh, 2007; Rance, 2005). Clearly a topic of ongoing debate, additional research is necessary to clarify issues of CI candidacy in this population. At this time, children younger than 1 year of age in who stable hearing thresholds in the severe to profound range can be established with both objective testing and reliable conditioned audiometry and who demonstrated no improvement in auditory skill with hearing aids should be considered for CI before their first birthday. Despite known benefits of early implantation (to be discussed in detail in upcoming “Outcomes” section), reliable diagnosis of AN/AD may not be possible in very young children, and therefore, treatment with CI should be delayed until there is a certainty of diagnosis.
Children With Multiple Disabilities
Another group of very young children whose CI candidacy remains controversial are those with multiple disabilities and/or developmental delay. Developmental abnormalities, level of functioning, and behavioral pathology (such as autism) provide additional challenges in the accurate assessment of infant hearing loss. However, evidence suggests that profoundly deaf children in these groups may receive a myriad benefits with CI, including improvement in communication skills, self-sufficiency, and ability to interact with others (Donaldson, Heavner, & Zwolan, 2004; Filipo, Bosco, Mancini, & Ballantyne, 2004). Recent evidence in developmentally delayed children implanted under 36 months of age suggests that early diagnosis and CI is possible and allows improvement in some auditory skills (Wiley, Menizen-Derr, & Choo, 2008). At all ages, a crucial component of the CI evaluation of a child with multiple disabilities is a thorough discussion of parental and care-giver expectations regarding postimplantation outcomes. While presence of multiple disabilities does not preclude CI under 1, expectations for success should be tailored to each child's individual competencies. As more than 40% of children with profound SNHL have other disabilities, later implantation may allow further observation and characterization of suspected deficits, making expectation counseling more accurate (Wiley, Menizen-Derr & Choo, 2008). Based on current evidence, CI candidacy for children younger than 1 year of age with multiple known or suspected disabilities remains controversial and requires consideration of each child's unique constellation of disabilities, level of cognition, and objective audiologic testing.
Summary
With respect to diagnosis of infant hearing loss, a paramount concern is the issue of specificity: the risk of implanting a child without profound deafness. In assessment of test accuracy, specificity refers to the proportion of time patients not having a disease or disorder (i.e., patients with normal hearing) are identified as normal hearing by the test. Its analogous measure, false positive rate, is the proportion of time subjects without the disorder (i.e., normal hearing infants) are failed by the test (i.e., demonstrate hearing loss). Opponents of very early implantation point to misdiagnosis of profound deafness as reason to wait until children are older. In contrast, proponents cite data suggesting an early sensitive period of speech and language development as well as improved outcomes with implantation under 12 months of age (discussed in more detail in the later sections). Ear-specific behavioral audiograms when possible, OAEs, ABR, or ASSR combined with tympanometry can accurately and confidently diagnose bilateral profound hearing loss in most children younger than 1 year of age. When etiology of deafness is known, including GJB2 positivity and congenital cochlear malformations, certainty of diagnosis and confidence in CI candidacy are increased. Progress with appropriate amplification can be evaluated in very young children and, as with older children and adults, poor performance supports CI candidacy. For some groups such as those with AN/AD, multiple disabilities or developmental delay, issues of diagnosis, and candidacy in children younger than 1 year remain controversial.
Anesthetic Risk
Anesthetic risk is an important consideration for children younger than 1 year of age. Epidemiological studies of anesthesia-related complications have found the incidence of morbidity, mortality, and life-threatening adverse events in children younger than 12 months to be significantly higher than children older than 1 year of age (Keenen, Shapiro, & Dawson, 1991; Morray et al., 2000; Tiret, Nivoche, Hatton, Desmonts, & Vourc'h, 1998). Closer evaluation of these population-based studies, however, clarifies the risk specific to children undergoing surgery for CI. Factors that increase the anesthetic-related complications include emergency surgery, inadequate fasting period, and age less than 1 month—none of which typically apply to scheduled CI surgery (M. M. Cohen, Cameron, & Duncan, 1990; Keenen et al., 1991; Moray et al., 2000; Tiret et al., 1998). In a study of all perioperative complications from serious events such as cardiac arrest to minor instances of nausea and vomiting, M. M. Cohen et al. (1990) actually found no difference in incidence between children aged 1 to 12 months and those aged 1 to 5 years. Involvement of a pediatric anesthesiologist has also been shown to significantly decrease perioperative risk (Keenan et al., 1991; Keenan, Shapiro, & Kane, 1994). Keenan et al. (1991) reported incidence of anesthesia-related cardiac arrest of 19.7 per 10,000 procedures in children younger than 12 months, over a period of 7 years, when a nonpediatric anesthesiologist was involved. In contrast, the incidence of cardiac arrest was zero when anesthesia was performed by a pediatric-trained anesthesiologist.
Although anesthetic concerns unique to very young children exist, data in the CI literature support perioperative safety in the under-1 population. Lesinski-Schiedat, Illg, Heermann, Bertram, and Lenarz (2004) reported no higher incidence of surgical complications in 27 children implanted under 1 year of age compared with older toddlers. James and Papsin (2004) analyzed inpatient records of 25 infants implanted between 7 and 12 months of age and found no anesthetic or immediate postoperative complications. Research by Colletti et al. (2005), Miyamoto, Houston, and Bergeson (2005), and Waltzman and Roland (2005), examining 10, 13, and 18 children, respectively, implanted at less than 1 year of age, also found no anesthetic or immediate surgical complications. More recent studies by Dettman, Pinder, Briggs, Dowell, and Leigh (2007), Valencia, Rimell, Friedman, Oblander, and Helmbrecht (2008), and Miyamoto, Hay-McCutcheon, Kirk, Houston, and Bergeson-Dana (2008) report no anesthetic or immediate postoperative complications in 19, 15, and 8 children, respectively, younger than 12 months. Finally, in the largest series to date, J. T. Roland, Cosetti, Wang, Immerman, and Waltzman (2009) report no immediate perioperative adverse events in 50 children implanted under 1 year of age.
Surgical Technique
The facial recess and cochlea are adult sized at birth, so there are no additional risks related to cochleostomy or electrode insertion. However, intraoperative blood loss, facial nerve anatomy, receiver/stimulator fixation, and device migration with skull growth present unique risks to children younger than 1 year. In very young children, a small circulating blood volume (approximately 80 ml/kg in children younger than 12 months) can lead to hypovolemic effects with less than 10% loss of volume (James & Papsin, 2004; Johr, Ho, Wagner, & Linder, 2008). Two sources of blood loss are important in very young children: bone marrow and emissary veins. Pneumatization of the mastoid bone increases with age, approaching 60% at 2 years of age (James & Papsin, 2004). Very young children, therefore, have a greater proportion of bone marrow in their mastoid and greater risk of blood loss during mastoidectomy (Birman, 2009; James & Papsin, 2004; J. T. Roland et al., 2009, Waltzman & Roland, 2005). In addition, blood loss from mastoid emissary veins can have a greater impact on overall circulating blood volume in children younger than 1 year of age (J. T. Roland et al., 2009; Roland & Waltzman, 2005). To minimize blood loss, judicious coagulation of emissary veins and rapid elimination of oozing bone marrow using wax and drilling with diamond burrs are especially important to reduce risks of hypovolemia in this age-group.
Anatomical variables are also of paramount importance in CI surgery under 1 year. Unlike older children, the facial nerve and the semicircular canals are laterally or superficially displaced, especially in the region of the underdeveloped mastoid tip where the facial nerve may lie just deep to the skin (Birman, 2009; J. T. Roland et al., 2009; Roland & Waltzman, 2005). Long accepted as a standard in temporal bone surgery, use of facial nerve monitoring in CI surgery is nearly universal (Silverstein, Smouha, & Jones, 1988). In children younger than 1 year, minimal inferior extension of the postauricular skin incision, careful identification of the facial nerve, and judicious use of intraoperative facial nerve monitoring can assist with these surgical challenges. Attention to receiver/stimulator fixation and flap thickness is also important to the under-1 population. In very young children, the posterior scalp flap is typically thinner and more delicate, necessitating constant care and atraumatic handling of the skin flap and soft tissues (N. L. Cohen & Roland, 2006; J. T. Roland et al., 2009; Roland & Waltzman, 2005). A variety of device fixation techniques have been advocated in children, including creation of a bony well and suture tie-down or use of a tight soft tissue pocket without well or additional fixation (Balkany et al., 2009; N. L. Cohen & Roland, 2006; Davids, Ramsden, Gordon, James, & Papsin, 2009; J. T. Roland et al., 2009). Because of the skull thickness being less than 1mm in young children, some authors advocate circumferential dural exposure for seating of the receiver/stimulator so as to minimize device profile and damage from external trauma (N. L. Cohen & Roland, 2006; J. T. Roland et al., 2009; Waltzman & Roland, 2005). Opponents of this technique indicate that the device stability achieved with a tight soft tissue pocket is equivalent to that accomplished by creation of a bony well while eliminating the risks of intracranial complications (Balkany et al., 2009). Although rare dural disruption has been documented with this technique, a bony well with circumferential dural exposure has been safely drilled without complication in multiple pediatric patients, as documented in Waltzman and Roland (2005), N. L. Cohen and Roland (2006), and J. T. Roland et al. (2009). Even if dural exposure is not performed, evidence suggests bony fixation with ligature tie-down is superior in the pediatric population. In a study of 385 children with 462 implants including those younger than 1 year of age, Davids et al. (2009) found that four out of five major complications involved loss of device fixation. These authors and others advocate use of bony well fixation and ligature tie-down for prevention of traumatic displacement of the device, especially in very young children whose motor development will inevitably allow multiple falls (N. L. Cohen & Roland, 2006; Davids et al., 2009; James & Papsin, 2004; J. T. Roland et al., 1998; J. T. Roland et al., 2009). Ongoing advances in receiver/stimulator design, such as the recently introduced Nucleus 5® (CI512) series by Cochlear, attempt to address these issues by creating a thinner device contoured to the infant skull. The effect of this change in device design is as yet unknown.
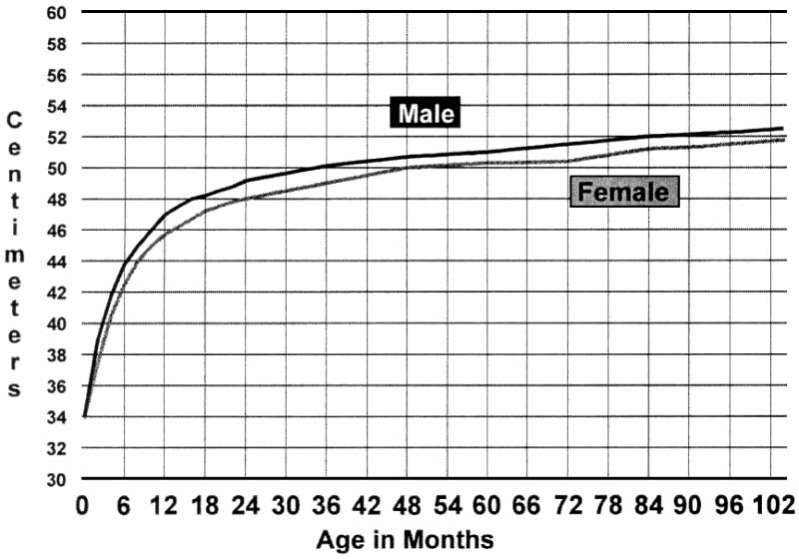
Issues of skull growth and electrode migration are also a consideration in this very young population. As seen in Figure 1, pediatric head circumference undergoes a dramatic change in the first year of life, especially as compared with the rate of growth after 12 months of age. As mentioned previously, the cochlea is adult size at birth and does not enlarge with age; however, with skull growth, the distance between the cochlea and the skull increases and may affect device movement. Although prior studies by J. T. Roland et al. (1998) in older children suggest that device migration because of skull growth is not a concern, the rate of change is much greater in very young children (N. L. Cohen & Roland, 2006; J. T. Roland et al., 2009; Waltzman & Roland, 2005). Long-term follow-up using bony well fixation with ligature tie-down holes in children younger than 1 show no evidence of greater migration in this population, however additional long-term studies using other fixation techniques are necessary (J. T. Roland et al., 2009).
Figure 1.
Head circumference by age in months
Note: The plotted values are the 50th percentile. There is continued gradual growth to approximately 55 cm in girls and 56 cm in boys at age 18. The rate of growth is highest from 0 to 12 months.
Challenges in Programming
As with other areas of CI in children younger than 1, programming presents unique challenges to the CI audiologist. Optimal use of CI technology requires an accurate assessment of threshold and comfort levels, obtained in older children and adults with behavioral testing. As with diagnostic testing, behavioral methods are frequently inappropriate for the very young child.
The introduction of electrophysiologic tools to assess the response of the auditory nerve to peripheral electrical stimulation has had an important impact on the programming of very young CI recipients. Neural response telemetry of Cochlear Corporation (Sydney, Australia), neural response imaging of Advanced Bionics (Valencia, California) and auditory nerve response telemetry of Med-El (Innsbruk, Austria) use an electrode in each array to record a response from the auditory nerve (Abbas, Brown, & Etler, 2006). Responses, termed electrically evoked compound action potentials (ECAPs or EAPs), can be recorded intraoperatively as well as in postoperative programming sessions. Unlike other electrophysiogic tests such as electrically evoked auditory brainstem response (EABR), ECAP is faster and can be performed while children are awake and active (Abbas et al., 2006). Only EABR, however, can provide information about the auditory pathway central to the auditory nerve. Research correlating behavioral with electrophysiologic thresholds has consistently demonstrated high cross-subject variability. However, in very young children, ECAP measurements obtained through NRT/NRI/ART have been used successfully as a basis for threshold (T) and comfort (C) levels in creation of initial speech processor MAPs (reviewed in Abbas et al., 2006; Holstad et al., 2009). Wide agreement exists, however, that the gold standard of postoperative programming involves behavioral responses; electrophysiologic measurements are appropriate only until children reach an age when reliable behavioral assessments are feasible.
Long-Term Safety
In their recent article, J. T. Roland et al. (2009) provide evidence for long-term safety in the under-1 population. A total of 50 patients implanted under 12 months of age were followed for up to 6.8 years postoperatively and demonstrated complication rates comparable with that of older children and adults. Length of device usage was not found to increase long-term complications as all eight complications, three major and five minor, occurred at or before 10 months postoperatively. Major complications included device failure, cerebrospinal fluid leak in a malformed cochlea, and wound infection leading to device exposure and reimplantation. Minor complications included hematoma, cellulitis, skin flap erythema, and two cases of minor wound dehiscence. This long-term complication rate of 16% is comparable with that found in large studies of older children and adults. Specifically, Bhatia, Gibbin, Nikolopoulos, and O'Donoghue (2004) found a 16% overall complication rate in 300 children aged 1 to 5 years followed for 4 years, and Ovesen and Johansen (2008) demonstrated a rate of 15.7% in their combined population of children and adults (N = 313). The predominance of minor complication in very young children highlights issues of surgical technique, such as the importance of delicate tissue handling, careful surgical planning, and meticulous treatment of squamous epithelium (Davids et al., 2009; J. T. Roland et al., 2009). Additionally, more than half of the complications in J. T. Roland et al. (2009) were discovered by either a parent or programming audiologist, emphasizing the role of aggressive, proactive education of family members and audiology staff regarding minor complications. They suggest that this early detection and prompt recognition may allow minor issues to be resolved with minimal intervention before progression to major complications. Whereas no patients in J. T. Roland et al. (2009) developed cholesteatoma, both Lin, Lee, and Peng (2006) and Bhatia et al. (2004) noted cholesteatoma formation among pediatric implant recipients. Unlike scalp health, cholesteatoma diagnosis requires a careful middle ear exam and underscores the need for long-term follow-up by a trained otolaryngologist, routinely seen yearly at our center. In J. T. Roland et al. (2009), long-term follow-up showed no difference in speech perception skills between patients with major or minor complications or in those with early versus delayed complication onset.
Outcomes in Speech Perception and Language Development
Central to the discussion of CI in very young children is the growing body of literature supporting improved auditory and linguistic outcomes in children implanted before 12 months of age (Colletti et al., 2005; Dettman et al., 2007; Holt & Svirsky, 2008; James & Papsin, 2004; Miyamoto et al., 2005; Miyamoto et al., 2008; J. T. Roland et al., 2009; Tait, DeRaeve, & Nikolopoulos, 2007; Waltzman & Roland, 2005).
With respect to auditory outcomes, Lesinski-Schiedat et al. (2004) found children implanted prior to 12 months demonstrated superior speech understanding compared with children who received implants between 1 and 2 years of age. Waltzman and Roland (2005) and J. T. Roland et al. (2009) found that speech understanding results in a select group of early implanted children may allow those children to reach their full hearing potential, which may approach that of normal hearing peers in some cases. Using the Category of Auditory Performance (a global measure of auditory receptive abilities), Colletti et al. (2005) found the outcomes of 10 children younger than 1 were significantly better than children implanted later. In an initial report involving six under-1 CI recipients, Holt & Svirsky (2008) did not find a difference between children implanted prior to 12 months and those implanted between 1 and 2 years old. However, recent data by these authors including CI recipients younger than 1 (N = 35) suggest a significant advantage in areas of speech perception compared with later implanted groups (Tajudeen, Waltzman, Jethanamest, & Svirsky, 2010). These data, however, did not support a sensitive period for word identification before the age of 3 (Tajudeen et al., 2010).
With respect to language development, evidence for a sensitive period within the first 2 years of life is supported by robust outcomes data in the pediatric CI population. Specifically, Waltzman and Cohen (1998), Geers, Brenner, and Davidson (2003), Svirsky, Teoh, and Neuburger (2004), Connor, Craig, Raudenbush, Heavner, and Zwolan (2006), and Miyamoto et al. (2008) provide data for improved speech perception and oral linguistic skills in children implanted before their second birthday compared with children implanted older than 2 year of age. As in diagnostic testing, however, postimplant outcome assessment in children younger than 1 requires unique methodology geared toward age-appropriate abilities. Tests such as the IT-MAIS, described previously, the Rosetti Infant-Toddler Language Scale (RI-TLS), and the Tait Video Analysis are just a few examples of speech and language outcomes assessment tools validated in the under-1 population (Rossetti, 1990; Tait, Nikolopoulos, Wells, & White, 2007; Zimmerman-Philips et al., 1997).
Using the RI-TLS, Dettman et al. (2007) examined communication abilities of 19 children implanted before 12 months of age. These children achieved rates of both receptive and expressive language growth comparable with their normally hearing peers and significantly greater than rates achieved by children implanted between 12 and 24 months of age. Nott, Cowan, Brown, and Wigglesworth (2009) compared lexical acquisition in CI recipients and normal hearing children and found those implanted earlier, prior to 12 months of age, were closest to their hearing peers in time to acquisition of their 1st and 100th word. Unlike Dettman (2007) Holt and Svirsky (2008) found improved receptive language skills in children implanted younger than 1 year of age but negligible differences in expressive ability between under-1 CI recipients and those implanted between 12 and 24 months of age. Taken together, early evidence suggests a higher rate of receptive and language development in children implanted under the age of 1. At present, outcomes data in auditory perception and linguistic development suggest that early-implanted children may be more likely to achieve their full potential and may reduce or eliminate the need for them to “catch up” or learn at a faster than normal rate to achieve age-appropriate norms.
Conclusion
CI in the very young child provides unique challenges in diagnosis and certainty of testing, anesthetic risk, surgical technique, intraoperative testing and postoperative programming, long-term safety, development of receptive and expressive language, and speech perception outcomes. Overall, research to date support minimal anesthetic, surgical, and long-term complications, suggesting that early implantation poses minimal risk to children younger than 1 year of age. Benefit in areas of receptive and expressive language development and speech perception has been suggested by multiple studies. Whether a child implanted under 1 year of age will ultimately develop auditory and/or linguistic skills commensurate with his or her normal hearing peers is difficult to predict; however, data suggest that early implantation can maximize a child's ability to achieve his or her full potential with minimal associated risk. Finally, although difficult to quantify, the impact of early parent–child bonding and interaction should not be ignored. As more than 95% of deaf children are borne to normal hearing parents (National Information Center for Children and Youth With Disabilities, 1998), implantation under 12 months allows normal hearing parents an opportunity to engage in auditory-verbal interaction with their hearing impaired infant at a young age. As a whole, the growing body of evidence suggests that when performed by experienced surgeons and pediatric anesthesiologists and followed by pediatric CI audiologists, the auditory and linguistic benefits of CI prior to 12 months of age outweigh the risks of early implantation.
Declaration of Conflicting Interests
The author(s) declared a potential conflict of interest as follows:
J. Thomas Roland is a consultant for Cochlear and Advanced Bionics, LLC.
Funding
The authors acknowledge Rienzi Foundation for Cochlear Implant Research for their financial support of the research in this article.
References
- Abbas P. J., Crown C. J., Etler C. P. (2006). Electrophysiology and device telemetry. In Waltzman S. B., Roland J. T., Jr. (Eds.), Cochlear implants (pp. 96–109). New York, NY: Thieme [Google Scholar]
- Adunka O. F., Jewells V., Buchman C. A. (2007). Value of computed tomography in the evaluation of children with cochlear nerve deficiency. Otology & Neurotology, 28, 597–604 [DOI] [PubMed] [Google Scholar]
- American College Medical Genetics. (2002). Genetic evaluation guidelines for the etiologic diagnosis of congenital hearing loss, Genetic evaluation of congenital hearing loss expert panel. Genetics in Medicine, 4, 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli P. J., Varela A. E., Mancuso A. A. (1999). Diagnostic yield of high-resolution computer tomography for pediatric sensorineural hearing loss. Laryngoscope, 109, 1642–1647 [DOI] [PubMed] [Google Scholar]
- Aso S., Gibson W. (1994). Electrocochleography in profoundly deaf children: Comparison of promontory and round window techniques. American Journal of Otology, 15, 376–379 [PubMed] [Google Scholar]
- Attias J., Raveh E. (2007). Transient deafness in young candidates for cochlear implants. Audiology & Neuro-Otology, 12, 325–333 [DOI] [PubMed] [Google Scholar]
- Balkany T. J., Whitley M., Shapira Y., Angeli S. I., Brown K., Eter E., Treaba C. (2009). The temporalis pocket technique for cochlear implantation: Anatomic and clinical study. Otology & Neurotology, 30, 903–907 [DOI] [PubMed] [Google Scholar]
- Bhatia K., Gibbin K. P., Nikolopoulos T. P., O'Donoghue G. M. (2004). Surgical complications and their management in a series of 300 consecutive pediatric cochlear implantations. Otology & Neurotology, 25, 730–739 [DOI] [PubMed] [Google Scholar]
- Birman C. (2009). Cochlear implant surgical issues in the very young child. Cochlear Implants International, 10, 19–22 [DOI] [PubMed] [Google Scholar]
- Boone R. T., Bower C. M., Martin P. F. (2005). Failed newborn hearing screens as presentation for otitis media with effusion in the newborn population. International Journal of Pediatric Otorhinolaryngology, 69, 393–397 [DOI] [PubMed] [Google Scholar]
- Chiong C., Ostrea E., Jr., Reyes A., Llanes E. G., Uy M. E., Chan A. (2007). Correlation of hearing screening with developmental outcomes in infants over a 2-year period. Acta Oto-Laryngologica, 127, 384–388 [DOI] [PubMed] [Google Scholar]
- Cohen M. M., Cameron C. B., Duncan P. G. (1990). Pediatric anesthesia morbidity and mortality in the perioperative period. Anesthesia and Analgesia, 70, 160–167 [DOI] [PubMed] [Google Scholar]
- Cohen N. L., Roland J. T., Jr. (2006). Complications of cochlear implant surgery. In Waltzman S. B., Roland J. T., Jr. (Eds.), Cochlear implants (pp. 126–132). New York, NY: Thieme [Google Scholar]
- Colletti V., Carner M., Miorelli V., Guida M., Colletti L., Fiorino F. G. (2005). Cochlear implantation at under 12 months: Report on 10 patients. Laryngoscope, 115, 445–449 [DOI] [PubMed] [Google Scholar]
- Connor C. M., Craig H. K., Raudenbush S. W., Heavner K., Zwolan T. A. (2006). The age at which young deaf children receive cochlear implants and their vocabulary and speech-production growth: Is there an added value for early implantation? Ear and Hearing, 27, 628–644 [DOI] [PubMed] [Google Scholar]
- Dauman R. (1991). Electrocochleography: Applications and limitations in young children. Acta Oto-Laryngologica, Supplementum, 482, 14–26 [DOI] [PubMed] [Google Scholar]
- Davids T., Ramsden J. D., Gordon K. A., James A. L., Papsin B. C. (2009). Soft tissue complications after small incision pediatric cochlear implantation. Laryngoscope, 119, 980–983 [DOI] [PubMed] [Google Scholar]
- Davis B. L., Morrison H. M., von Hapsburg D., Warner-Czyz A. D. (2005). Early vocal patterns in infants with varied hearing levels. Volta Review, 105, 7–27 [Google Scholar]
- Dettman S. J., Pinder D., Briggs R. J., Dowell R. C., Leigh J. R. (2007). Communication development in children who receive the cochlear implant younger than 12 months: Risks versus benefits. Ear and Hearing, 28, 11S–18S [DOI] [PubMed] [Google Scholar]
- Donaldson A. I., Heavner K. S., Zwolan T. A. (2004). Measuring progress in children with autism spectrum disorder who have cochlear implants. Archives of Otolaryngology, Head & Neck Surgery, 130, 666–671 [DOI] [PubMed] [Google Scholar]
- Eilers R. E., Oller D. K. (1994). Infant vocalizations and the early diagnosis of severe hearing impairment. Journal of Pediatrics, 124, 199–203 [DOI] [PubMed] [Google Scholar]
- El-Rafaie A., Parker D. J., Bamford J. M. (1996). Otoacoustic emissions versus ABR screening: The effect of external and middle ear abnormalities in a group of SCBU neonates. British Journal of Audiology, 30, 3–8 [DOI] [PubMed] [Google Scholar]
- Filipo R., Bosco E., Mancini P., Ballantyne D. (2004). Cochlear implants in special cases: Deafness in the presence of disabilities and/or associated problems. Acta Oto-Laryngologica, Supplementum, 552, 74–80 [PubMed] [Google Scholar]
- Gabbard S. A., Schryer J. (2003). Early amplification options. Mental Retardation and Developmental Disabilities Research Reviews, 9, 236–242 [DOI] [PubMed] [Google Scholar]
- Geers A., Brenner C., Davidson L. (2003). Factors associated with development of speech perception skills in children implanted by age five. Ear and Hearing, 24, 245–355 [DOI] [PubMed] [Google Scholar]
- Gibson W. P., Sanli H. (2007). Auditory neuropathy: An update. Ear and Hearing, 28, 102S–106S [DOI] [PubMed] [Google Scholar]
- Harlor A. D., Bower C. (2009) Hearing assessment in infants and children: Recommendations beyond neonatal screening. Pediatrics, 124, 1252–1263 [DOI] [PubMed] [Google Scholar]
- Holstad B. A., Sonneveldt V. G., Fears B. T., Davidson L. S., Aaron R. J., Richter M., Skinner M. W. (2009). Relation of electrically evoked compound action potential thresholds to behavioral T- and C-levels in children with cochlear implants. Ear and Hearing, 20, 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. F., Svirsky M. A. (2008). An exploratory look at pediatric cochlear implantation: Is earliest always best? Ear and Hearing, 29, 492–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holte L., Margolis R. H., Cavanaugh R. (1991). Developmental changes in multifrequency tympanograms. Audiology, 30, 1–24 [DOI] [PubMed] [Google Scholar]
- Jackler R. K., Luxford W. M., House W. F. (1987). Congenital malformations of the inner ear: A classification based on embryogenesis. Laryngoscope, 97, 2–14 [DOI] [PubMed] [Google Scholar]
- James A. L., Papsin B. C. (2004). Cochlear implant surgery at 12 months of age or younger. Laryngoscope, 114, 2191–2195 [DOI] [PubMed] [Google Scholar]
- Johr M., Ho A., Wagner C. S., Linder T. (2008). Ear surgery in infants under one year of age: Its risks and implications for cochlear implant surgery. Otology & Neurotology, 29, 310–313 [DOI] [PubMed] [Google Scholar]
- Keenan R. L., Shapiro J. H., Dawson K. (1991). Frequency of anesthetic cardiac arrests in infants: Effect of pediatric anesthesiologists. Journal of Clinical Anesthesia, 3, 433–437 [DOI] [PubMed] [Google Scholar]
- Keenan R. L., Shapiro J. H., Kane F. R. (1994). Bradycardia during anesthesia in infants: An epidemiological study. Anesthesiology, 80, 976–982 [DOI] [PubMed] [Google Scholar]
- Kei J., Allison-Levick J., Dockray J., Harrys R., Kirkegard C., Wong J., Tudehope D. (2003). High frequency (1000 Hz) tympanometry in normal neonates. Journal of the American Academy of Audiology, 14, 21–28 [DOI] [PubMed] [Google Scholar]
- Koivun P., Uhari M., Laitakari K., Alho O. P., Luotonen J. (2000) Otoacousting emissions and tympanometry in children with otitis media. Ear and Hearing, 21, 212–217 [DOI] [PubMed] [Google Scholar]
- Koopmans-van Beinum F. J., Clement C. J., van den Dikkenberg-Pot I. (2001). Babbling and the lack of auditory speech perception: A matter of coordination? Developmental Science, 4, 61–70 [Google Scholar]
- Lesinski-Schiedat A., Illg A., Heermann R., Bertram B., Lenarz T. (2004). Paediatric cochlear implantation in the first and in the second year of life: A comparative study. Cochlear Implants International, 5, 146–159 [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Lee F. P., Peng S. C. (2006). Complications in children with long-term cochlear implants. ORL, Journal for Oto-Rhino-Laryngology and Its Related Specialties, 68, 237–242 [DOI] [PubMed] [Google Scholar]
- Luts H., Desloovere C., Kumar A., Vandermeersch E., Wouters J. (2004). Objective assessment of frequency-specific hearing thresholds in babies. International Journal of Pediatric Otorhinolaryngology, 68, 915–926 [DOI] [PubMed] [Google Scholar]
- Mafong D. D., Shin E. J., Lalwani A. K. (2002). Use of laboratory evaluation and radiologic imaging in the diagnostic evaluation of children with sensorineural hearing loss. Laryngoscope, 112, 1–7 [DOI] [PubMed] [Google Scholar]
- Margolis R. H., Bass-Ringdahl S., Hanks W. D., Holte K., Zapala D. A. (2003). Tympanometry in newborn infants—1 kHz norms. Journal of the American Academy of Audiology, 14, 383–392 [PubMed] [Google Scholar]
- Miyamoto R. T., Houston D. M., Bergeson T. (2005). Cochlear implantation in deaf infants. Laryngoscope, 115, 1376–1380 [DOI] [PubMed] [Google Scholar]
- Miyamoto R. T., Hay-McCutcheon M. J., Kirk K. I., Houston D. M., Bergeson-Dana T. (2008). Language skills of profoundly deaf children who received cochlear implants under 12 months of age: A preliminary study. Acta Oto-Laryngologica, 128, 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller M. P. (2000). Early intervention and language development in children who are deaf and hard of hearing. Pediatrics, 106, 43–52 [DOI] [PubMed] [Google Scholar]
- Moeller M. P., Hoover B., Putman C., Arbataitis K., Bohnenkamp G., Peterson B., Stelmachowicz P. (2007). Vocalizations of infants with hearing loss compared with infants with normal hearing: Part I—Phonetic development. Ear and Hearing, 28, 605–627 [DOI] [PubMed] [Google Scholar]
- Moore J. M., Thompson G., Folsom F. C. (1992). Auditory responsiveness of premature infants using visual reinforcement audiometry (VRA). Ear and Hearing, 13, 187–194 [DOI] [PubMed] [Google Scholar]
- Morray J. P., Geiduschek J. M., Ramamoorthy C., Haberkern C. M., Hackel A., Caplan R. A., Cheney F. W. (2000). Anesthesia-related cardiac arrest in children: Initial findings of the Pediatric Perioperative Cardiac Arrest (POCA) Registry. Anesthesiology, 93, 6–14 [DOI] [PubMed] [Google Scholar]
- National Information Center for Children and Youth With Disabilities. (1998). Fact sheet: General information about deafness and hearing loss. Washington, DC: Author [Google Scholar]
- Norton S. J., Gorga M. P., Widen J. E., Folsom R. C., Sininger Y. S., Cone-Wesson B., Fletcher K. A. (2000a). Identification of neonatal hearing impairment: Evaluation of TEOAE, DPOAE, and ABR test performance. Ear and Hearing, 21, 508–528 [DOI] [PubMed] [Google Scholar]
- Norton S. J., Gorga M. P., Widen J. E., Folsom R. C., Sininger Y. S., Cone-Wesson B., Fletcher K. A. (2000b). Identification of neonatal hearing impairment: Summary and recommendations. Ear and Hearing, 21, 529–535 [DOI] [PubMed] [Google Scholar]
- Nott P., Cowan R., Brown P. M., Wigglesworth G. (2009). Early language development in children with profound hearing loss fitted with a device at a young age: Part I—The time period taken to acquire first words and first word combinations. Ear and Hearing, 30, 526–40 [DOI] [PubMed] [Google Scholar]
- Oates P., Stapells D. R. (1998). Auditory brainstem response estimates of the pure-tone audiogram: Current status. Seminars in Hearing, 19, 61–85 [Google Scholar]
- Oller D. K., Eilers R. E. (1988). The role of audition in infant babbling. Child Development, 59, 441–449 [PubMed] [Google Scholar]
- Olsho L. W., Koch E. G., Carter E. A., Halpin C. F., Spetner N. B. (1988). Pure-tone sensitivity of human infants. Journal of the Acoustical Society of America, 84, 1316–1324 [DOI] [PubMed] [Google Scholar]
- Ovesen T., Johansen L. V. (2008). Post-operative problems and complications in 313 consecutive cochlear implantations. Journal of Laryngology and Otology, 123, 492–496 [DOI] [PubMed] [Google Scholar]
- Parving A., Stephens D. (1997). Profound permanent hearing impairment in childhood: Causative factors in two European countries. Acta Oto-Laryngologica, 117, 158–160 [DOI] [PubMed] [Google Scholar]
- Picton T. W., Durieux-Smith A., Moran L. M. (1994). Recording auditory brainstem responses from infants. International Journal of Pediatric Otorhinolaryngology, 28, 93–110 [DOI] [PubMed] [Google Scholar]
- Picton T. W., John M. S., Dimitrijevic A., Purcell D. (2003). Human auditory steady-state responses. Journal of the American Academy of Audiology, 15, 541–554 [DOI] [PubMed] [Google Scholar]
- Preciado D. A., Lawson L., Madden C., Myer D., Ngo D., Bradshaw J. K., Grienwald J. H., Jr. (2005). Improved diagnostic effectiveness with a sequential diagnostic paradigm in idiopathic pediatric sensorineural hearing loss. Otology & Neurotology, 26, 610–615 [DOI] [PubMed] [Google Scholar]
- Rance G. (2005). Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends in Amplification, 9, 1–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G., Barker E. J. (2009). Speech and language outcomes in children with auditory neuropathy/dys-synchrony managed with wither cochlear implants or hearing aids. International Journal of Audiology, 48, 313–320 [DOI] [PubMed] [Google Scholar]
- Roland J. T., Jr., Coehlo D. H., Pantelides H., Waltzman S. B. (2008). Partial and double-array implantation of the ossified cochlea. Otology & Neurotology, 29, 1068–1075 [DOI] [PubMed] [Google Scholar]
- Roland J. T., Jr., Cosetti M., Wang K. H., Immerman S., Waltzman S. B. (2009). Cochlear implantation in the very young child: Long-term safety and efficacy. Laryngoscope, 119, 2205–2210 [DOI] [PubMed] [Google Scholar]
- Roland J. T., Jr., Fishman A. J., Waltzman S. B., Alexiades G., Hoffman R. A., Cohen N. L. (1998). Stability of the cochlear implant array in children. Laryngoscope, 108, 1119–1123 [DOI] [PubMed] [Google Scholar]
- Roland P. S., Yellin M. W., Meyerhoff W. L., Frank T. (1995). Simultaneous comparison between transtympanic and extratympanic electrocochleography. American Journal of Otology, 16, 444–450 [PubMed] [Google Scholar]
- Rosetti L. M. (1989). The Rossetti Infant-Toddler Language Scale. East Moline, IL: LinguiSystems [Google Scholar]
- Shelton C., Luxford W. M., Tonokawa L. L., Lo W. W., House W. F. (1989). The narrow internal auditory canal in children: A contraindication to cochlear implants. Otolaryngology—Head and Neck Surgery, 100, 227–231 [DOI] [PubMed] [Google Scholar]
- Silverstein H., Smouha E. E., Jones R. (1988). Routine intraoperative facial nerve monitoring during otologic surgery. American Journal of Otology, 9, 269–275 [PubMed] [Google Scholar]
- Smith B., Oller D. K. (1981). A comparative study of premeaningful vocalizations produced by normally developing and Down's syndrome infants. Journal of Speech and Hearing Disorders, 46, 46–51 [DOI] [PubMed] [Google Scholar]
- Stapells D. R. (1989). Auditory brainstem response assessment of infants and children. Seminars in Hearing, 10, 229–251 [Google Scholar]
- Stapells D. R., Oates P. (1997). Estimation of the pure-tone audiogram by the auditory brainstem response: A review. Audiology & Neuro-Otology, 2, 257–280 [DOI] [PubMed] [Google Scholar]
- Steel K. P., Kros C. J. (2001). A genetic approach to understanding auditory function. Nature Genetics, 27, 143–149 [DOI] [PubMed] [Google Scholar]
- Svirsky M. A., Teoh S.-W., Neuburger H. (2004). Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiology & Neuro-Otology, 9, 224–233 [DOI] [PubMed] [Google Scholar]
- Swanepoel D. W., Ebrahim S. (2009). Auditory steady-state response and auditory brainstem response thresholds in children. European Archives of Oto-Rhino-Laryngology, 266, 213–219 [DOI] [PubMed] [Google Scholar]
- Swanepoel D. W., Werner S., Hugo R., Louw B., Owen R., Swanepoel A. (2007). High frequency immittance for neonates: A normative study. Acta Oto-Laryngologica, 127, 49–56 [DOI] [PubMed] [Google Scholar]
- Tait M., De Raeve L., Nikolopoulos T. P. (2007). Deaf children with cochlear implants before the age of 1 year: Comparison of preverbal communication with normally hearing children. International Journal of Pediatric Otorhinolaryngology, 71, 1605–1611 [DOI] [PubMed] [Google Scholar]
- Tait M. E., Nikolopoulos T. P., Wells P., White A. (2007). The use and reliability of Tait video analysis in assessing preverbal language skills in profoundly deaf and normally hearing children under 12 months of age. International Journal of Pediatric Otorhinolaryngology, 71, 1377–1382 [DOI] [PubMed] [Google Scholar]
- Tajudeen B. A., Waltzman S. B., Jethanamest D., Svirsky M. A. (2010). Speech perception in congenitally deaf children receiving cochlear implants in the first year of life. Unpublished manuscript. [DOI] [PMC free article] [PubMed]
- Tiret L., Nivoche Y., Hatton F., Desmonts J. M., Vourc'h G. (1988). Complications related to anaesthesia in infants and children. A prospective survey of 40240 anaesthetics. British Journal of Anaesthesia, 61, 263–269 [DOI] [PubMed] [Google Scholar]
- Trimble K., Blaser S., James A. L., Papsin B. C. (2007). Computed tomography and/or magnetic resonance imaging before pediatric cochlear implantation? Developing an investigative strategy. Otology & Neurotology, 28, 317–324 [DOI] [PubMed] [Google Scholar]
- Valencia D. M., Rimell F. L., Friedman B. J., Oblander M. R., Helmbrecht J. (2008). Cochlear implantation in infants less than 12 months of age. International Journal of Pediatric Otorhinolaryngology, 72, 767–773 [DOI] [PubMed] [Google Scholar]
- Wallace V., Menn L., Yoshinaga-Itano C. (2000). Is babble the gateway to speech for all children? A longitudinal study of children who are deaf or hard of hearing. Volta Review, 100, 121–148 [Google Scholar]
- Waltzman S. B., Cohen N. L. (1998). Cochlear implantation in children younger than 2 years old. American Journal of Otology, 18, 158–62 [PubMed] [Google Scholar]
- Waltzman S. B., Roland J. T., Jr. (2005). Cochlear implantation in children younger than 12 months. Pediatrics, 116, 487–493 [DOI] [PubMed] [Google Scholar]
- Widen J. E., Folsom R. C., Cone-Wesson B., Carty L., Dunnell J. J., Koebsell K., Norton S. J. (2000). Identification of neonatal hearing impairment: Hearing status at 8 to 12 months corrected age using a visual reinforcement audiometry protocol. Ear and Hearing, 21, 471–487 [DOI] [PubMed] [Google Scholar]
- Wiley S., Meinsen-Derr J., Choo D. (2008). Auditory skills development among children with developmental delays and cochlear implants. Annals of Otology, Rhinology & Laryngology, 117, 711–718 [DOI] [PubMed] [Google Scholar]
- Wong S. H., Gibson W. P., Sanli H. (1997). Use of transtympanic round window electrocochleography for threshold estimations in children. American Journal of Otology, 18, 632–636 [PubMed] [Google Scholar]
- Yoshinaga-Itano C. (2003). Early intervention after universal neonatal hearing screening: Impact on outcomes. Mental Retardation and Developmental Disabilities Research Reviews, 9, 252–266 [DOI] [PubMed] [Google Scholar]
- Zimmerman-Philips S., Osberger M. J., Robbins A. M. (1997). Infant-Toddler Meaningful Auditory Integration Scale. Sylmar, CA: Advanced Bionics Corporation [Google Scholar]