Abstract
The World Health Organization's Disability Assessment Scale II (WHO-DAS II) is a generic health-status instrument that provides six domain scores and a total, aggregate score. Two of the domain scores, communication and participation, and the total score, have good validity, internal-consistency reliability, and test-retest stability in individuals with adult-onset hearing loss. As such, these two domain scores and the total WHO-DAS II score may be useful as generic outcome measures to assess the effectiveness of hearing aid intervention for this population. Before the use of the WHO-DAS II in hearing aid clinical trials, however, the responsiveness of the instrument and the short- and long-term outcomes to hearing aid intervention had to be determined. Responsiveness and outcomes were assessed in 380 veterans (approximately half received hearing aids and half served as controls) by examining group differences, effect-size estimates, and individual differences as a function of hearing aid intervention. For comparison, data also were obtained on two disease-specific measures, the APHAB and the HHIE. The WHO-DAS II communication domain and total scores were sufficiently responsive to hearing aid intervention for use in future studies in which group differences are to be detected. The WHO-DAS II participation domain was not sufficiently responsive to hearing aid intervention. The APHAB and HHIE, both disease-specific measures, were more sensitive to hearing aid intervention than the generic measure. The short- and long-term outcomes of hearing aid intervention were also examined in the present study. Group outcomes for hearing aid intervention can be expected to be stable for at least 6 months when measured by WHO-DAS II total score and for at least 12 months when measured by the WHO-DAS II communication domain scores. Effect-size estimates and examination of the number of individuals exhibiting change scores exceeding 90% critical differences for true changes in scores indicate that for clinical applications, disease-specific instruments are more useful than the WHO-DAS II. The findings of this study support the use of the WHO-DAS II as a generic measure in hearing aid trials research so as to allow for comparisons of health-status outcomes across different diseases or disorders.
Introduction
According to the National Council on Aging (1999), hearing impairment is one of the most prevalent chronic conditions in the United States, and when left untreated, hearing loss can cause serious emotional and social consequences for older individuals. Other reported adverse effects of adult-onset hearing loss include declines in both cognitive function and subjective well-being (e.g., Bess et al., 1989; Mulrow et al., 1990; Bess et al., 1991; Keller et al., 1999; National Council on Aging, 1999; Strawbridge et al., 2000; Dalton et al., 2003; Pugh, 2004).
Hearing aids are the primary intervention for adult-onset hearing loss, and their use results in beneficial treatment effects that include reductions in psychosocial handicaps associated with hearing loss, reductions of depression, improvements in self-concept, and improvements in interpersonal relationships as documented with disease-specific outcome measures1 (e.g., Harless and McConnell, 1982; Mulrow et al., 1990; Bridges and Bentler, 1998 Kochkin and Rogin, 2000). The broad spectrum of documented declines in functional health status that accompany adult-onset hearing loss, however, has led hearing researchers to supplement disease-specific measures with generic quality-of-life outcome measures (Bess, 2000). The use of generic measures allows for the adoption of a more global approach to measuring treatment efficacy and effectiveness.
Previous studies examining the effects of hearing aid intervention for adult-onset hearing loss have used a variety of generic self-report instruments, such as the Sickness Impact Profile (SIP) (Bergner et al., 1976), the Medical Outcomes Study Short-Form 36 (SF-36) (Ware and Sherbourne, 1992), the Self Evaluation of Life Function (SELF) (Linn and Linn, 1984), the Dartmouth COOP Functional Health Assessment Charts (Nelson et al., 1987) and the EuroQOL (The EuroQOL Group, 1990). In a review of studies that used generic outcomes instruments, Bess (2000) found the instruments failed to show treatment effects for audiologic intervention. In fact, Bess concluded that there was a critical need for the development of a generic instrument that would be sensitive to hearing aid intervention.
Another important occurrence that has highlighted the need for a generic, holistic approach to outcome measurement to assess the effects of hearing aid intervention is the shift in health-care resource allocation from a specific cost for a service to the calculation of the cost for a service as a function of improved patient health status (Beck, 2000). According to Beck, some managed care plans have begun to cover some audiology services and rehabilitative technologies. Increased third-party payment is beneficial to the field of audiology; however, third-party payers will likely demand evidence in the form of quantitative outcome measures that support an improvement in quality of life as a result of hearing aid intervention. Generic instruments are needed to compare improvements in functional health status as a result of hearing aid intervention with improvements in health status that arise from treatment for other chronic diseases or disorders such as high blood pressure, arthritis, and diabetes mellitus.
Recently, a new generic health-status instrument, the World Health Organization's Disability Assessment Schedule (WHO-DAS II) (WHO, 2001) was developed to evaluate dimensions of disability and health status (WHO, 1999). The WHO-DAS II includes items in the domain of communication, with two of the items appearing to be particularly relevant to individuals with hearing loss. The first asks how much difficulty a person has with “generally understanding what people say” and the second asks about difficulty with “starting and maintaining conversations.” The WHO-DAS II also allows for the assessment of functioning in the domains of mobility (getting around), self-care, interpersonal relationships (getting along with others), life activities at home and work, and participation in society. Thus the WHO-DAS II assesses a broad range of specific domains that aggregate to provide a general estimate of health-related quality of life. In examining the psychometric properties of the WHO-DAS II in older individuals with adult-onset hearing loss, Chisolm et al. (2005) found that the communication and participation domains as well as total scores were adequate for use in the adult hearing-impaired population in terms of convergent validity, internal-consistency reliability, and test-retest stability.
Given that the WHO-DAS II is adequate from a psychometric perspective for use in adults with acquired hearing loss, the next step for determining the utility of the WHO-DAS II is an examination of its responsiveness to hearing aid intervention. The responsiveness of any test instrument refers to the extent to which changes in the observed values reflect changes in the “state” of an individual (Hyde, 2000). An instrument's responsiveness can be examined in terms of “individual” or in terms of “group average” effects. In both cases, the state of an individual before the intervention is compared to the state of an individual after the intervention. One reason that an instrument may lack responsiveness to a particular intervention is that the items do not cover adequately the effects of that intervention (Hyde, 2000). As Abrams et al. (2005) point out in their review of the available generic health-status instruments, few contain any items relevant to hearing loss and/or communication.
Thus, one goal of the present study was to determine the responsiveness of the WHO-DAS II communication and participation domains, and total scores, to hearing aid intervention. To assess test responsiveness, treatments or manipulations that are known to have a substantive effect on the attribute of interest are applied and the change that occurs on the test measure becomes an index of the responsiveness of the test measure (Hyde, 2000). To confirm that the hearing aid intervention applied had a substantive effect on the participants in the present study, two disease-specific instruments, the Abbreviated Profile of Hearing Aid Benefit (APHAB) (Cox and Alexander, 1995) and the Hearing Handicap Inventory for the Elderly (HHIE) (Ventry and Weinstein, 1982), were included. Both are known to be responsive to hearing aid intervention.
The present study was also designed to examine the short-term and long-term outcomes of hearing aid intervention using the WHO-DAS II communication and participation domains, and total scores. For comparative purposes, outcomes were measured using summary measures for two disease-specific instruments (i.e., APHAB and HHIE). Outcomes were examined over the course of 1 year.
Examination of both short-term and long-term hearing aid outcomes is important, as the constancy of outcomes measured with disease-specific instruments has been questioned in the literature. Some studies indicate that outcomes are stable over time (e.g., Surr et al., 1998; Malinoff and Weinstein, 1989; Mulrow et al., 1992; Chisolm et al., 2004), whereas other studies show that self-report outcomes decrease over time, with objective measures of benefit remaining stable (e.g., Taylor, 1993; Humes et al., 2002). The utility of the WHO-DAS II as an outcome measure for hearing aid intervention would be strengthened if stability were observed for an extended period of time. In examining the stability of WHO-DAS II outcomes, it is important to determine if the outcomes for the participants in the present study, on commonly used disease-specific self-report instruments, also were stable. Thus, the present study compared outcomes obtained after 2 months of hearing aid use with those obtained at 6 and 12 months postintervention, not only for the WHO-DAS II but also for the APHAB and the HHIE.
Methods
As described in detail in Chisolm et al. (2005), the data presented here were obtained as part of a multisite study designed to examine the effects of hearing aid intervention on quality of life. The study sites were the James H. Quillen Veterans Affairs Medical Clinic (VAMC), Mountain Home, TN; Tennessee Valley Healthcare System, Nashville TN; VA Pittsburgh Healthcare System, Pittsburgh, PA; and James Haley VAMC, Tampa, FL.
Participants
Initially 384 veterans with adult-onset sensorineural hearing loss who were receiving hearing aids for the first time were recruited. Four subjects did not receive a passing score on the Mini Mental State Exam (Folstein et al., 1979) and were withdrawn before the collection of baseline outcome measures, resulting in 380 participants who were eligible to receive hearing aids at no cost to them through the national VA hearing aid program. Additional inclusion criteria consisted of the presence of at least a mild, high-frequency sensorineural hearing loss as evidenced by a pure-tone average of 30 dB HL or more at 2000, 3000, and 4000 Hz in the better ear.
All participants dwelt in the community, had access to a telephone, and had no known neurologic or psychiatric disorders as determined by chart review or known comorbid diseases that would preclude completion of the study. Participants were excluded for conductive or retrocochlear pathology as well as asymmetry of either pure-tone thresholds or speech-recognition scores in quiet. Detailed information about demographic characteristics can be found in Chisolm et al. (2005).
To examine responsiveness of the WHO-DAS II to hearing aid intervention, half of all participants were randomized upon recruitment into an immediate treatment (IT) group and half to a delayed treatment (DT) group. The failure of four participants to pass screening criteria resulted in 189 in the IT group and 191 in the DT group. The IT participants received hearing aids 2 weeks after recruitment into the study. The DT participants, who functioned as the wait-list control group, received hearing aids 10 weeks after recruitment. To examine hearing aid outcomes after the evaluation of initial responsiveness, all participants were followed for 1 year after their hearing aid fittings.
Outcome Measures
The WHO-DAS II is a 36-item instrument that provides 6 domain scores—communication, mobility, self-care; interpersonal; life activities at home and work, and participation—and a total score. In the WHO-DAS II, if respondents do not work, only 32-items are administered and the life activities score is based only on participation in home-related activities. Most of the participants in the present project were retired, so the 32-item version was administered. For each question an individual is asked “In the last 30 days how much difficulty did you have in …?” Responses are given on a 5-point Likert-type scale from 1 (none) to 5 (extreme/cannot do). Raw scores are transformed into standardized scores ranging from 0 to 100, with 0 indicating the best health state and 100 indicating the poorest health state.
Research by Chisolm et al. (2005) demonstrated good convergent validity, internal-consistency reliability, and test-retest stability for the WHO-DAS II communication and participation domains as well as the total score in individuals with adult-onset hearing loss; therefore, these two domain scores and the total score were used to determine responsiveness to hearing aid intervention. Table 1 lists the six items that comprise the communication domain and the eight items that comprise the participation domain.
Table 1.
Items Comprising the Communication and Participation Domains of the World Health Organization's Disability Assessment Schedule II (WHO, 2001)
Communication
|
Participation
|
Two disease-specific instruments, the APHAB and the HHIE, were also administered. The APHAB is a 24-item questionnaire in which individuals report the amount of trouble they have with communication or noises in various everyday situations (Cox and Alexander, 1995). The APHAB produces four subscale scores: ease of communication (EC), listening in background noise (BN), listening in reverberant conditions (RV), and aversiveness of sounds (AV). The APHAB global score, which consists of responses on the EC, BN, and RV subscales, was used as a summary measure in the present study. For the global score, which ranges from 0 to 100, lower scores indicate better performance, and higher scores indicate poorer performance.
The HHIE is a 25-item questionnaire that consists of 13 emotional and 12 social questions (Ventry and Weinstein, 1982). Three scores can be obtained: emotional and social subscale scores, which combine to provide the total score. Total scale scores, which were used in this study, range from 0 to 100, with higher scores indicating greater perceived difficulties and lower scores indicating less difficulty.
Procedures
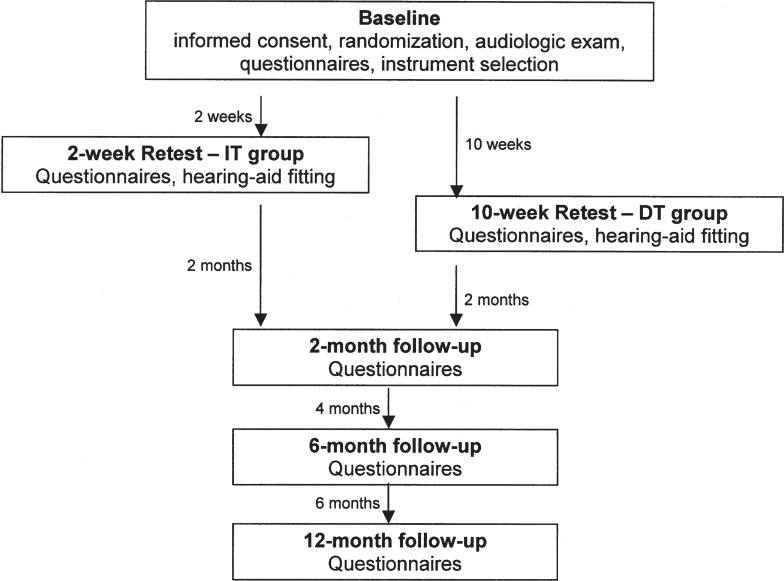
The participants were recruited over an 18-month period at each of the four sites. A timeline of study visits can be seen in Figure 1. In the first research visit, the participants were consented and screened to determine if inclusion criteria were met. A standard clinical audiologic assessment (pure-tone thresholds, speech audiometry, and immittance audiometry) was completed, and participants were counseled regarding the degree and type of hearing loss. Hearing aid options were discussed, and the earmold impressions were made. The participants were required to take a break of at least 30-minutes before questionnaire administration.
Figure 1.
Timeline of study visits.
After the break, baseline administration of the WHO-DAS II, the APHAB, and the HHIE was completed. Order of questionnaires was randomized across participants to control for order effects. Questionnaires were administered in a face-to-face format in which the examiner read aloud each question to the participant who was looking at an easel displaying all possible response alternatives for a specific item. The participant verbally responded and the examiner keyed the response into a customized software program. Participants used a pocket talker during questionnaire administration if difficulty hearing was observed by the examiner.
Immediately before the hearing aid fitting, the second research session occurred. For IT group participants, this was 2 weeks after baseline; for DT group participants, this was 10 weeks after baseline. During the second session, which is referred to as the retest/hearing aid (HA) fitting appointment, participants were administered the WHO-DAS II and the other subjective outcome measures in a different random order than that used in the baseline session. This second administration of outcome measures to the IT group just before hearing aid fitting was done to allow for the examination of short-term (i.e., 2-week interval) and long-term (i.e., 10-week interval) test-retest stability (see Chisolm et al., 2005 for discussion).
After the administration of outcome measures, participants were fitted with their custom in-the-ear digitally-programmable, analog or fully digital hearing aids in accordance with currently accepted clinical practice guidelines (Joint Audiology Committee, 2000). The hearing aids were fit using real-ear measures (Frye, Model 6500). Initially the insertion gain was set to approximate closely an National Acoustic Laboratories-Revised target (Byrne and Dillon, 1986) with a 65-dB SPL input of composite noise and each participant seated 1 meter from the speaker at 45° azimuth. The root-mean-square (rms) difference value (Byrne, 1992) was calculated from 500 to 2000 Hz to measure the accuracy of insertion gain compared with the prescribed target. The fitting parameters were modified, if needed, in accordance with current clinical practice.
After the insertion-gain measurements, real-ear saturation response was completed on each ear using an 85-dB SPL input of composite noise and having the participant look at a loudness scale, similar to that used by Hawkins et al. (1987) to make judgments regarding the volume of the noise. If a participant reported the volume to be uncomfortably loud, then the output was decreased and the task was repeated. If any of the hearing aid parameters were changed, then the rms difference was calculated and documented for the user settings. Hearing aid stability was verified by using 2-cm3 measures throughout the study period.
All participants were seen for a third research visit at 2 months after the retest/HA fitting. For the IT group participants, this third research visit was not only 2 months after the retest/HA fitting but also 10-weeks from when baseline measures were obtained. Thus the responsiveness of the WHO-DAS II to hearing aid intervention could be determined through a comparison of baseline with measures made 10 weeks after baseline for the IT and DT groups.
Both IT and DT participants were also seen 6 and 12 months after their hearing aid fittings to allow for the assessment of long-term outcomes. At each test interval, a different random order of presentation of self-report measures was used. All participants were contacted by telephone monthly to facilitate hearing aid use and to monitor hearing aid performance. The number of participants who returned to complete questionnaires at each test interval is summarized in Table 2.
Table 2.
Number of Participants for Whom Data Were Missing at Each Test Interval and the Reasons for Withdrawal*
| Reason for Withdrawal | Retest/HA-Fitting | 2 Months | 6 Months | 12 Months | ||||
|---|---|---|---|---|---|---|---|---|
| IT | DT | IT | DT | IT | DT | IT | DT | |
| n = 187 | 186 | 176 | 175 | 173 | 167 | 163 | 153 | |
| Death | 1 | 1 | 1 | 0 | 0 | 0 | 3 | 3 |
| Illness | 0 | 2 | 2 | 1 | 3 | 1 | 2 | 5 |
| Withdrew consent | 0 | 0 | 4 | 4 | 0 | 3 | 1 | 1 |
| Protocol deviation | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| Relocation | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Unknown | 0 | 2 | 3 | 2 | 0 | 4 | 4 | 5 |
Withdrawals for the 10-week, post-baseline visit are in the 2 months column for the immediate treatment group (IT) and the retest/hearing aid (HA)-fitting for the delayed treatment group (DT).
Analyses
To examine the group responsiveness of the WHO-DAS II to hearing aid intervention, the data obtained from the IT and DT participants at baseline and 10 weeks later were examined using general linear model repeated-measures analyses of variance (ANOVA). For comparison purposes, separate ANOVAs were also conducted for the two disease-specific measures, APHAB global scores and HHIE total scores. In each analysis, there was one between-group variable (group: IT vs DT) and one within-group variable (test interval: baseline vs 10-week follow-up). Significant interactions were examined further by t test post hoc analyses with Bonferroni corrections made for multiple comparisons. The significance level for each of the ANOVAs and post hoc analyses was set to p < .01. All statistical analyses were performed using SPSS version 13.0 (SPSS, Inc, Chicago, IL) for Windows (Microsoft, Redmond, WA).
As presented in Table 2, at 10 weeks after baseline, data were missing for 13 participants in the IT group and 5 participants in the DT group. Rather than ignore the cases with incomplete data for these and subsequent ANOVAs examining short-term and long-term outcomes of hearing aid intervention, an intention-to-treat analysis (Nich and Caroll, 2002) was conducted with missing data handled by assigning hypothetical outcome scores estimated through the use of the SPSS Missing Values Analysis module. This module uses the iterative expectation maximization approach in which the data are considered missing at random (Overall et al., 1998). That is, the cases with incomplete data are assumed to differ from those with complete data but that the pattern of missing data can be predicted from other variables in the database rather than because of specific outcome variables for which the data are missing (Peduzzi et al., 2002). In estimating the missing data for this study, the variables of age, gender, marital status, audiometric right and left ear air conduction thresholds, baseline, retest, and all available follow-up scores were used.
Effect-size estimates were calculated with the baseline and 10-week follow-up data from the IT participants by using the procedures suggested by Dunlop et al. (1996). The effect sizes were calculated as the difference between baseline and 10-week scores, divided by the pooled standard deviations of the baseline and 10-week scores. The results were interpreted according to Cohen's d (1988) effect-size index in which 0.2 corresponds to a small effect, 0.5 to a moderate, and 0.8 to a large effect.
To address the second goal of this project, the examination of short- and long-term outcomes of hearing aid intervention, the data obtained for all participants regardless of IT or DT treatment group at baseline, retest of baseline, and at 2, 6, and 12 months after the hearing aid fitting were examined by using repeated-measures ANOVAs. For comparison purposes, separate repeated-measures ANOVAs were used to examine the effect of test interval on the APHAB global score and the HHIE total score. Each analysis had one within-group variable (test interval: baseline, retest, 2, 6, and 12 months after the hearing aid fitting).
Significant main effects were examined with post hoc t tests using Bonferroni corrections for multiple comparisons. The significance level for each of the ANOVAs and post hoc analyses was set to p < .01. In addition, the utility of the WHO-DAS II communication and participation domain scores and the total score for detecting individual differences in response to hearing aid intervention was examined by determining the number of participants in each group whose change in scores from retest to 2 months after the hearing aid fitting exceeded 90% critical differences for true changes in scores as reported by Chisolm et al. (2005).
Results and Preliminary Discussion
Responsiveness of the WHO-DAS II
Inferential analyses. Table 3 lists the mean scores and standard deviations obtained at baseline and at the 10-week follow-up for the participants in the IT and DT groups for each of the outcome measures. Also shown are the F values obtained for the interaction between group and test interval in separate ANOVAs for each outcome measure. In each of these analyses, the interaction effect, which was the effect of primary interest, was statistically significant. A post hoc analysis for each outcome measure revealed that the difference between baseline scores for the DT and IT groups was not statistically significant. At the 10-week follow-up, significant differences between groups were found on all outcome measures. More important than these group differences were the differences that occurred as a function of test interval.
Table 3.
Scoresa for the Immediate Treatment (IT) and Delayed Treatment (DT) Groups’ Baseline and 10-Week Follow-Up
| Baseline | IT Group 10-Week Follow-up | Difference | Baseline | DT Group 10-Week Follow-up | Difference | F-value | |
|---|---|---|---|---|---|---|---|
| WHO-DAS II | |||||||
| Communication | 21.58 (16.69) | 13.71 (13.17) | − 7.82* | 23.29 (15.48) | 26.19 (16.47) | 2.89* | 69.16* |
| Participation | 16.37 (17.93) | 14.12 (16.78) | − 2.25* | 16.85 (16.71) | 19.87 (18.65) | 3.02* | 15.66* |
| Total | 15.60 (15.59) | 12.70 (12.90) | − 2.90* | 15.99 (13.24) | 19.16 (15.99) | 3.17* | 38.33* |
| APHAB global | 47.63 (16.38) | 18.11 (−9.81) | − 29.53* | 47.02 (15.82) | 51.21 (15.30) | 4.19* | 538.25* |
| HHIE total | 41.42 (23.43) | 23.98 (23.41) | − 17.54* | 41.30 (21.46) | 43.07 (−22.12) | 1.77 | 86.41* |
WHO-DAS II = World Health Organization, Disability Assessment Schedule II; APHAB = Abbreviated Profile of Hearing Aid Benefit; HHIE = Hearing Handicap Inventory for the Elderly.
Means (standard deviations).
Significant differences in interactions from baseline to the 10-week follow-up at p <.01. Also shown are F-values for each interaction effect of the repeated-measures ANOVAs.
The difference score from baseline to 10-week follow-up for each group is listed in Table 3, with significant differences at p < .01 indicated with an asterisk. As expected, there were significant differences as a function of hearing aid intervention for the IT participants on the two disease-specific measures, the APHAB and the HHIE. Interestingly, the change in scores for the DT participants from baseline to the 10-week follow-up was not statistically significant for the HHIE total score but it was for the APHAB global score. This latter finding was somewhat unexpected and may reflect what is referred to as “response shift” (Schwartz and Sprangers, 1999).
Response shifts may affect the stability of self-report measures and may occur for a variety of reasons, including the individual undergoing changes in (1) internal standards of measurement, (2) values, or (3) conceptualization of a target construct (Schwartz and Sprangers, 1999). Changes in the internal standards of measurement or scale recalibration occur when individuals have new experiences that lead to a change in the perception of functioning (Howard et al., 1979). In examining response shift through administration of a retrospective pretest completed at the time of post-test, Joore et al. (2002) demonstrated response shift on hearing-specific measures in adults being fitted with hearing aids for the first time. In the present study, it is possible that by confirming hearing loss and the need for hearing aids in the DT participants and then requiring them to wait 10 weeks before the hearing aid fitting, the APHAB-assessed perception of communication difficulties in various listening situations was increased for at least some participants.
The results in Table 3 for the WHO-DAS II communication and participation domains and total scores show that similar to the results for the disease-specific measures, significant interactions occurred between group and test interval for each WHO-DAS II measure. Further, both the IT and DT group mean scores significantly changed over time. For both domains, the mean scores for the IT participants decreased significantly and the mean scores for the DT participants increased significantly. Recall that lower scores on the WHO-DAS II indicate better health states and higher scores poorer health states. Thus, the results of these analyses indicate improvements in perceived health status related to the specific domains of communication and participation as a function of hearing aid use.
In addition, when perceived health status in the WHO-DAS II domains of communication, mobility (getting around), self-care, interpersonal relationships (getting along with others), life activities at home and work, and participation in society are aggregated to provide a total estimate of generic health status, improvements as a function of hearing aid intervention also were demonstrated. As with the APHAB global scores, the decrease in mean scores as a function of test interval for the DT group may reflect a “response shift” or it may reflect a true change in health status.
Effect sizes. Effect sizes calculated from the IT group data for the WHO-DAS II communication and participation domains as well as total scale scores are listed in Table 4. For comparison purposes, effect-size measures from the two disease-specific instruments, the APHAB and the HHIE, are also shown. Only one of the effect-size estimates would be interpreted as large, and this is for the APHAB global score. Perhaps this is not surprising, as the primary goal of hearing aid intervention is to improve auditory performance, and the APHAB is specifically designed to assess auditory functioning in daily life and to capture how a hearing aid improves that functioning (Cox and Alexander, 1995).
Table 4.
Simple Effect-Size Measures Calculated from the Baseline and 10-Week Follow-up Data for the Immediate Treatment Group
| Outcome Measure | Effect Size |
|---|---|
| WHO-DASII | |
| Communication | 0.52 |
| Participation | 0.13 |
| Total | 0.20 |
| APHAB global | 2.19 |
| HHIE total | 0.74 |
WHO-DAS II = World Health Organization, Disability Assessment Schedule II; APHAB = Abbreviated Profile of Hearing Aid Benefit; HHIE = Hearing Handicap Inventory for the Elderly.
The effect sizes for the HHIE total score and the WHO-DAS II communication domain score were moderate. The HHIE does not directly assess auditory functioning, but rather, it assesses the emotional and social response to reductions in auditory functioning, which will be influenced by a variety of personality, health, economic, lifestyle, and family variables (Ventry and Weinstein, 1982). Thus, whereas the HHIE is a widely accepted disease-specific outcome measure for hearing aid intervention, factors other than how much the hearing aid improves auditory functioning will likely influence scores. Similarly, an examination of the items that comprise the WHO-DAS II domain score of communication (Table 1) reveals that none of the items specifically refer to auditory functioning, but rather, responses on several items are highly likely to be influenced by auditory status. The finding of a moderate effect size for the WHO-DAS II domain of communication was encouraging as it suggests the use of these domain scores would be appropriate in future studies of hearing aid intervention and possibly for examining individual differences in clinical settings.
The effect-size estimate calculated for the WHO-DAS II participation domain score was negligible, suggesting that this scale is not particularly responsive to hearing aid intervention. The fact that significant group differences were found in the inferential statistical analysis of the IT and DT participants is likely accounted for by the relatively large number of participants. As Cohen (1988) discusses, with large samples, results may indicate statistical significance when, in fact, only a tiny effect is present. Because effect-size indices are independent of sample size, they are often considered a better measure of research outcome when the goal is to determine clinical or practical significance.
The effect size calculated for the WHO-DAS II total score was small. Because the effect size is small, the total score would likely be useful in studies with relatively large numbers of participants but not useful in examining individual differences in clinical settings. As support for the utility of the total score in large group studies, consider the data from the inferential statistical analysis for the IT and DT participants. If an effect size were calculated for the “difference between the change scores for the two groups as a function of intervention,” the result would be an effect-size estimate of 0.62. That is, there would be a mean difference of 0.62 standard deviations between the change scores of the treatment and control groups. As discussed by Hyde (2000), the median effect size recently achieved in studies of treatment effectiveness is about 0.4, or a mean difference of 0.4 standard deviations between treatment and control groups. Thus the finding of an effect size of 0.62 for the “difference” between the IT and control DT group for the WHO-DAS II total change scores in the present study would be reasonable in terms of the responsiveness of many instruments currently utilized in treatment effectiveness research.
Short-Term and Long-Term Outcomes of Hearing Aid Intervention
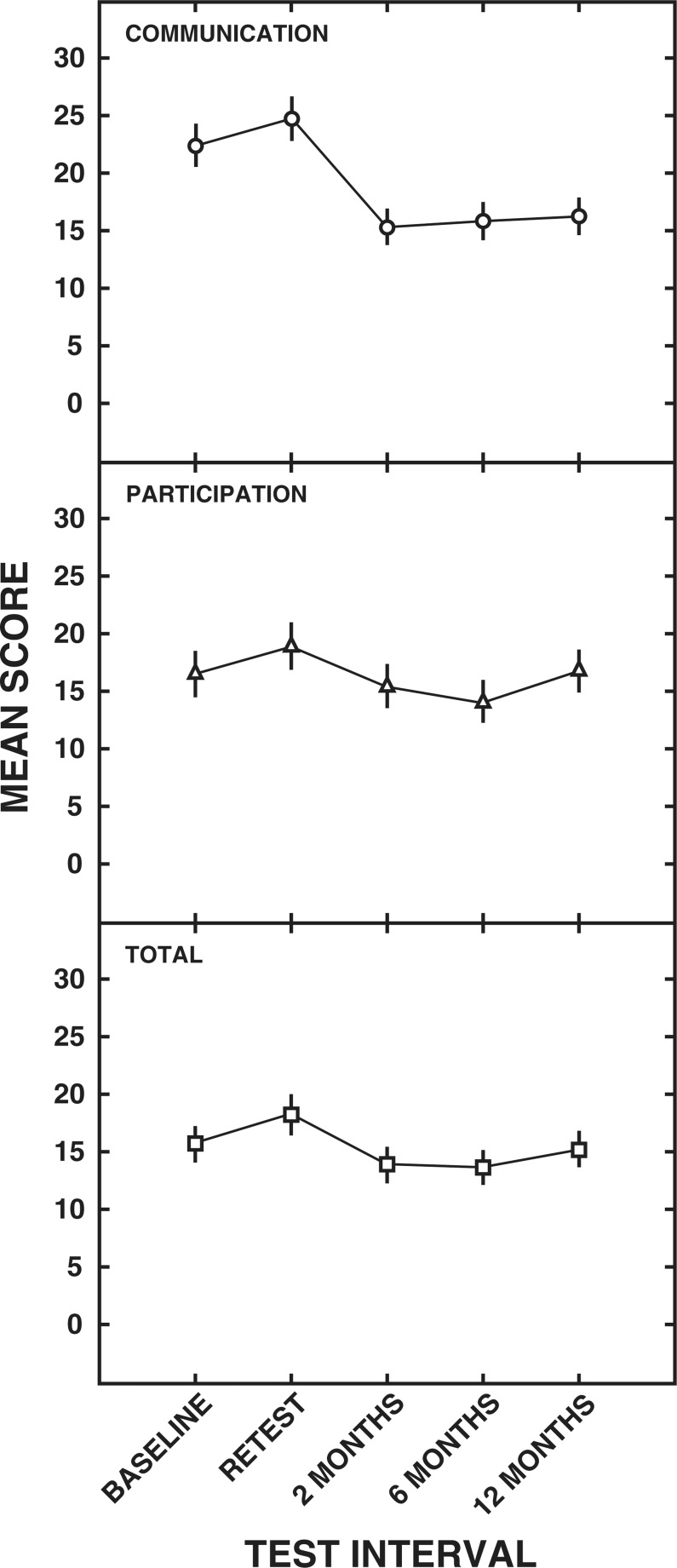
WHO-DAS II. The means and standard errors for the communication domain, participation domain, and total score are depicted in Figure 2. Results of ANOVAs revealed a significant main effect of test interval for the communication domain (F [4,1516] = 99.78), the participation domain (F [4,1516] = 16.45), and the total score (F [4,1516] = 9.92).
Figure 2.
Means and standard errors (± 2) for the World Health Organization, Disability Assessment Schedule II (WHO-DAS II) communication domain (○), participation domain (Δ) and total scores (□) for each test interval.
An examination of the means shown in the top panel of Figure 2 for the communication domain indicates a response shift from baseline to retest such that a significantly higher mean of 24.8 was obtained at retest than at baseline (mean, 22.4). The shift in means suggests a decrease in perceived communication ability during the no-intervention period. Mean differences as a result of hearing aid intervention can also be seen for the communication domain. All postintervention means were significantly lower than both the baseline and retest mean scores. The lowest mean score (15.4) for the communication domain was measured at the 2-month, postintervention visit. Mean scores for the communication domain rose slightly at 6 months after the hearing aid fitting (mean, 15.9) and again at 12 months after the hearing aid fitting (mean, 16.3). However, post hoc testing revealed that there were no significant mean differences among the postintervention scores.
The mean participation domain score of 19.0 (middle panel of Figure 2) obtained at retest also was found to be significantly higher than the mean score of 16.6 obtained at baseline, again indicating a response shift. The mean scores obtained at 6 months (mean, 14.2) postintervention were significantly lower than the scores obtained at baseline and retest. At 2 months (mean, 15.5) and 12 months (mean, 16.9) the scores were significantly less than the retest score but not the baseline score. In addition, the 12 month score was significantly higher than the 6 month score.
For the WHO-DAS II total score, the scores obtained at baseline (mean, 15.8) and retest (mean, 18.3) were significantly different from each other, as shown in the lower panel of Figure 2. At 2 and 6 months postintervention, the mean scores of 14.0 and 13.7, respectively, were found to be significantly less than the mean scores obtained at the baseline and retest sessions. At 12-months postintervention, the mean WHO-DAS II total score increased to 15.3, which was significantly different than the means scores obtained at 2 and 6 months postintervention. Further, the mean 12-month score was not significantly different from that obtained at baseline.
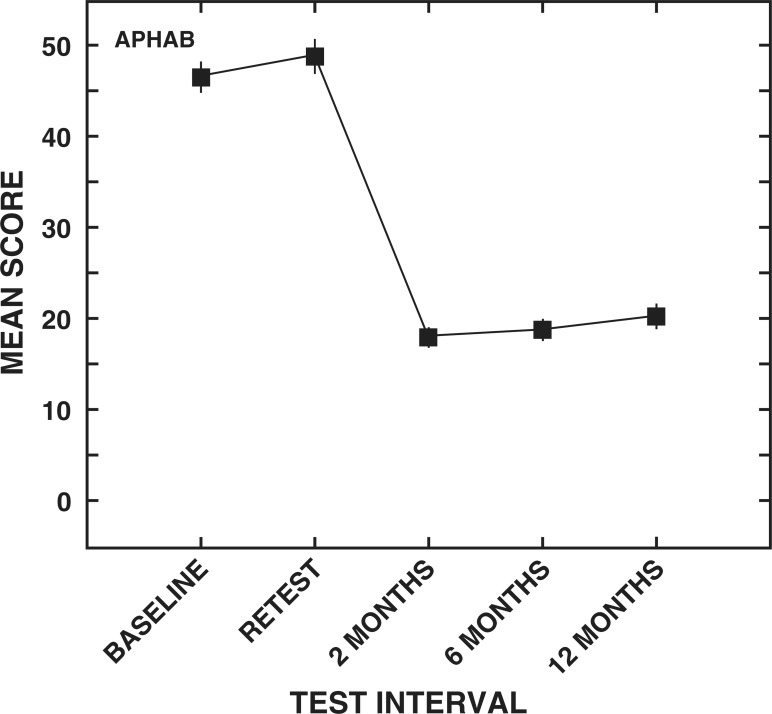
APHAB. The mean scores and standard errors for the APHAB global scores are illustrated in Figure 3. A significant main effect of test interval was observed for the APHAB global scores (F [4,1516] = 895.3). Similar to the results for the WHO-DAS II communication domain scores, the mean baseline global score of 47.3 rose significantly to 49.6 at retest, suggesting a perceived increase in communication difficulties. The mean global score decreased significantly after 2 months of hearing aid use (mean, 18.7). Mean scores at 2 months and 6 months (mean, 19.5) after the hearing aid intervention did not differ significantly, suggesting that the initial improvement in communication ability as measured by the global score of the APHAB was maintained for at least 6 months after the use of hearing aids. The mean global score of 21.1 at 12 months postintervention did show a statistically significant increase in perceived communication difficulty compared with the 2-month and 6-month postintervention scores. On average, however, participants reported significantly less difficulty in self-perceived communication performance after 12 months of hearing aid use compared with the preintervention scores.
Figure 3.
Means and standard errors (± 2) for the Abbreviated Profile of Hearing Aid Benefit (APHAB) global scores for each test interval.
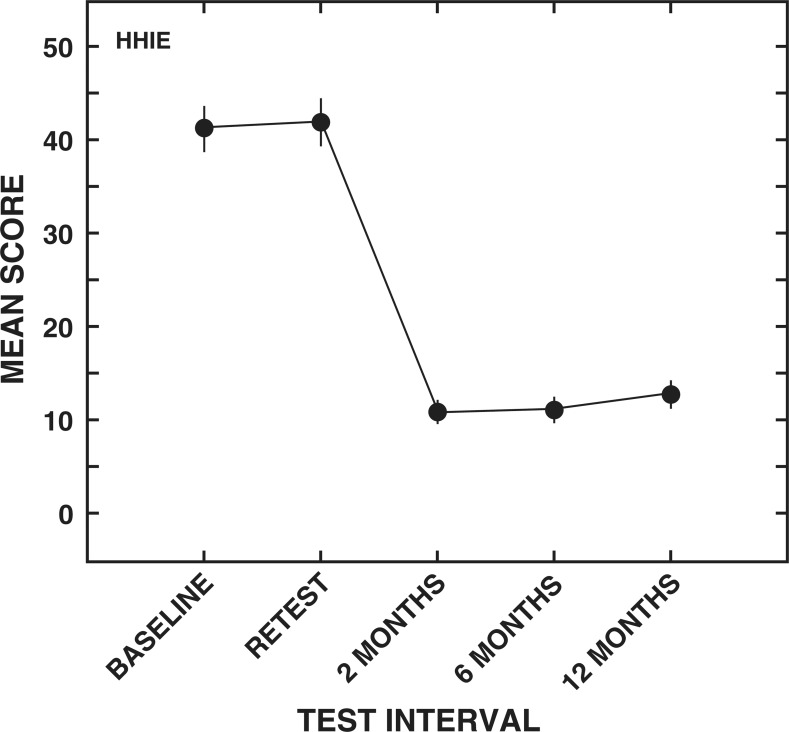
HHIE. The means and standard errors for the HHIE total score can be seen in Figure 4. As expected, a main effect of test interval was obtained for the HHIE total score (F [4,1516] = 605.9). Contrary to the results from the WHO-DAS II communication domain score and the APHAB, but similar to the results of the WHO-DAS II total score, the mean HHIE total score of 41.4 at baseline was not significantly different than retest (mean, 42.0). The finding of similar HHIE total scores at baseline and retest suggests that on average, self-perception of handicap related to hearing loss remained stable during the no-intervention period. A significant decrease in mean HHIE total score to 11.0 was observed after 2 months of hearing aid use and was maintained at the 6-month postintervention follow up (mean, 11.3). Although all of the HHIE mean total scores for the postintervention intervals were significantly less than at baseline or retest, a slight but statistically significant increase to 13.0 was seen at 12 months postintervention compared with the 2-month and 6-month mean data.
Figure 4.
Means and standard errors (± 2) for the Hearing Handicap Inventory for the Elderly (HHIE) total scores for each test interval.
Individual Differences. Table 5 shows the number of participants in the IT and DT groups whose change scores from retest to each of the test sessions after hearing aid intervention exceeded the 90% critical differences for a true change in score for the WHO-DAS II communication and participation domains, total scores, and for the scores from the two disease-specific instruments, the APHAB and the HHIE. Critical difference estimates for all measures were obtained from Chisolm et al. (2005). The decision to use retest rather than baseline data was based on the significant changes in mean scores from baseline to retest for the WHO-DAS II communication and participation domain scores as well as for the APHAB global score. As discussed by Demorest and Erdman (1988), test-retest stability for the determination of critical differences should employ test-retest intervals comparable to the interval over which treatment effects are to be assessed. The long-term critical differences reported by Chisolm et al. (2005) were obtained after 10 weeks of no treatment. Thus, the data obtained at 2 months after the hearing aid intervention are the most reasonable to examine. Although there are no comparable data examining how true scores might vary on the WHO-DAS II, APHAB, or HHIE measures, the change scores for treatment intervals of 6 and 12 months, are still presented for comparative purposes.
Table 5.
Number of Participants Whose Change in Scores from Retest to Each of the Post Hearing-Aid Fitting, Follow-up Visits Exceeded the Long-Term (i.e., 10-week) 90% Critical Differences (CD) for a True Change in Score*
| 90% CD | 2 Months | 6 Months | 12 Months | |
|---|---|---|---|---|
| WHO-DAS II | ||||
| Communication | 12.5 | 115 | 112 | 107 |
| Participation | 9.4 | 71 | 80 | 75 |
| Total | 6.3 | 121 | 117 | 112 |
| APHAB global | 9.9 | 342 | 334 | 319 |
| HHIE total | 14 | 283 | 280 | 265 |
Critical differences for all measures were obtained from Chisolm et al. (2005). WHO-DAS II = World Health Organization, Disability Assessment Schedule II; APHAB = Abbreviated Profile of Hearing Aid Benefit; HHIE = Hearing Handicap Inventory for the Elderly.
An examination of the 2-month data in Table 5 reveals that the APHAB instrument had the largest number of participants who, as a result of hearing aid intervention, exhibited change scores that exceeded critical difference values. This finding is consistent with the results discussed previously for the measurement of effect sizes. That is, the purpose of hearing aid use is to improve auditory performance, and the APHAB directly assesses self-perception of auditory functioning in daily life and how hearing aids improve that functioning. Thus, it is not surprising that the APHAB is the most sensitive measure, among those used in the present study, for detecting clinical effects in individual participants. Approximately two thirds of participants whose change scores exceeded critical differences on the APHAB exhibited change scores that exceeded critical differences for the HHIE. The effect-size estimate for the HHIE (0.74) was much less than that for the APHAB (2.19), so this finding was not surprising.
Because effect sizes on the WHO-DAS II scores were much smaller than for either of the two disease-specific measures, it would be expected that fewer participants would exhibit change scores as a function of hearing aid use that exceeded critical differences. The effect size for the participation domain score was essentially negligible, so it is not surprising that only about one fourth of the participants had change scores that exceeded the critical difference. Caution should be taken in interpreting the data for 6 and 12 months due to a lack of comparable data for nontreated groups at these time intervals; however, the pattern found at 2 months remained the same: the greatest number of individuals exhibiting change scores exceeding critical differences occurred for the APHAB global score and the least number for the WHO-DAS II participation domain scores. Taken as a whole, these findings demonstrate the expected greater sensitivity of the disease-specific measures than generic measures for determining clinically significant individual changes as a function of hearing aid intervention.
Summary and Conclusions
The WHO-DAS II is a new generic health-status instrument that has good potential for use in the assessment of hearing aid intervention in adults with acquired hearing loss. The need for audiology to find a generic instrument for use in assessing intervention strategies for individuals with adult-onset hearing loss stems from a lack of sensitivity to hearing aid intervention for previously examined generic instruments (Bess, 2000; Abrams et al., 2005) and to changes in health-care resource allocation from a specific cost for a service to the calculation of the cost for a service as a function of improved patient health status (Beck, 2000).
Previous work by Chisolm et al. (2005) indicated that the WHO-DAS II domain scores of communication and participation, as well as the WHO-DAS II total score, which is calculated by aggregating across all WHO-DAS II domain scores, met psychometric criteria for consideration as a hearing aid outcome measure. The goal of the present study was twofold. First, the responsiveness of the WHO-DAS II communication domain, participation domain, and total scores to hearing aid intervention was examined. In examining WHO-DAS II responsiveness, the effects of hearing aid intervention on two disease-specific instruments, the APHAB global scale and the HHIE total scale were also examined. The second goal was to examine outcomes of hearing aid intervention over the course of 12 months as measured through the WHO-DAS II scores and also through the disease-specific measures.
Inferential statistical analyses confirmed that all three WHO-DAS II measures resulted in statistically significant changes as a function of hearing aid intervention. As expected, statistical analyses revealed significant hearing aid treatment effects for both the APHAB and the HHIE measures. An unexpected finding for the APHAB global scores and for all of the WHO-DAS II measures was a statistically significant increase in mean scores during the no-intervention period for the wait-list (DT) control group. It was postulated that these differences could either be reflective of response shifts or, possibly, true changes in health status. Future studies in which a retrospective assessment of baseline status is examined would help elucidate this issue.
Examination of effect-size indices revealed, as expected, that disease-specific measures, yield larger effect sizes than do generic measures. In terms of the WHO-DAS II measures, the negligible effect size found for the participation domain scores would argue against the use of these domain scores in future studies of hearing aid intervention. The moderate effect size observed for the communication domain suggests that the scores obtained for this domain might be as useful as the HHIE total score in future studies. Although the effect size for the WHO-DAS II total score was only small, this measure has potential for use in studies that include large numbers of participants, particularly since the administration will also allow for calculation of communication domain scores. It is important to recall that although the WHO-DAS II communication scores assess a particular domain of health status, the items are not specific to hearing loss. Thus the communication domain score as well as the WHO-DAS II total scores would have relevance across a variety of diseases and disorders that might affect the ability of an individual to understand and communicate during activities of daily living.
With regard to the second goal of this study, the examination of outcomes over a 12-month interval, significant effects of hearing aid intervention were found after 2 months of hearing aid use on the WHO-DAS II communication domain, participation domain, and total scores; and, also on the APHAB global and HHIE total scores. The latter finding was expected, as the two disease-specific measures (APHAB and HHIE) are known to be useful in assessing hearing aid efficacy. Similarly, since all WHO-DAS II measures are responsive to hearing aid intervention, the finding of hearing aid benefit at 2 months was not surprising.
More important than the demonstration of short-term benefits of hearing aid intervention, however, was the demonstration of maintained effects of hearing aid intervention up to 12 months, not only for the two disease-specific measures but also as measured by the communication domain scores. In addition, stability of beneficial treatment effects was observed for up to 6 months as measured by the participation domain and total scores.
The finding that the stability of the WHO-DAS II outcomes was most apparent for the WHO-DAS II communication domain seems reasonable, as the primary goal of a hearing aid is to improve speech understanding. The efficacy of hearing aid intervention in achieving the goal of improved speech understanding is also demonstrated in the significant and stable treatment effects demonstrated through the APHAB data.
Although the primary goal of hearing aid intervention is to improve activity limitations related to speech understanding and communication, it is also expected that improvements in understanding and communication will lead to a lessening of activity limitations and participation restrictions that arise secondary to a hearing loss (e.g., Stephans et al., 2003). The efficacy of hearing aids in terms of the lessening of participation restrictions is highlighted by the significant and relatively stable changes in the HHIE over the course of 12 months after the hearing aid fitting. Further, the overall impact of hearing aid intervention for lessening activity limitations and participation restrictions for at least the first 6 months of hearing aid use is supported by the significant changes from baseline that were found at the 2-month and 6-month test intervals for the WHO-DAS II participation domain and total scores.
Over the last decade, an issue in hearing aids outcomes assessment has been the consistency of outcomes over time. As Humes (2001) points out, this issue is important since, if outcomes are stable, then initially obtained results will suffice for either clinical or research purposes. If outcomes change, however, then a longer period of follow-up would be needed. Indeed, Humes (2001) found that for many self-report measures, outcomes obtained at 6 and 12 months after the hearing aid fitting were significantly worse than those obtained at 1 month. Although there appeared to be long-term stability of disease-specific self-report outcomes for the APHAB and the HHIE in the present study, the WHO-DAS II total score outcomes were only stable out to 6 months. This is the case for many self-report outcome measures used to assess hearing aid intervention (e.g., Humes, 2001); however, we concluded that the WHO-DAS II total score shows sufficient stability to be useful in assessing the effects of hearing aid intervention in group studies.
Finally, in the examination of individual differences for the WHO-DAS II measures, the APHAB and the HHIE demonstrate the advantages of using disease-specific questionnaires in clinical settings. The number of participants whose scores exceeded the 90% critical difference, suggesting true change in scores as a result of hearing aid intervention, was much higher for the disease-specific measures than for any of the WHO-DAS II measures. Although disease-specific measures have obvious advantages for use in hearing aid clinical trials, their use alone would negate the possibility of comparison with interventions for other diseases or disorders. To ensure comparability of health status outcomes across different patient groups, it is important to include a generic instrument along with disease-specific instruments in clinical trials research.
Before drawing conclusions from this study, certain limitations must be acknowledged. The primary limitation relates to the participants. Most of the participants were men and all were veterans who received hearing aids at no cost through the VA's National Hearing Aid Program. All participants were new hearing aid users. With these cautions in mind, the following conclusions can be drawn:
An examination of responsiveness through inferential statistical analyses and the calculation of simple effect sizes shows that the WHO-DAS II communication domain scores and total scores are useful generic instruments in future clinical trials of hearing-aid intervention.
Group outcomes for hearing aid intervention can be expected to be stable for at least 6 months when measured by WHO-DAS II total score and for at least 12 months when measured by the WHO-DAS II communication domain scores.
Group outcomes for hearing-aid intervention can be expected to be stable when assessed by using either of the two disease-specific measures used in this study, the APHAB global score and the HHIE total score, for at least 12-months.
Effect-size estimates and examination of the number of individuals exhibiting change scores exceeding 90% critical differences for true changes in scores indicate that for clinical applications, disease-specific instruments are likely to be more useful than the WHO-DAS II.
Acknowledgment
We would like to acknowledge the following individuals for their assistance in data collection and management: Gene Bratt, Paige Harden, Joseph Mikolic, Amanda Pillion Judith Reese, and Maureen Wargo.
Footnotes
Disease-specific measures focus on aspects of health status that are specific to the area of primary interest.
References
- Abrams H, Chisolm TH, McArdle R. (2005). Health-related quality of life and hearing aids. Trends in Amplification. [DOI] [PMC free article] [PubMed]
- Beck LB. (2000). The role of outcomes data in health-care resource allocation Ear Hear 21(suppl): 89–96S [DOI] [PubMed] [Google Scholar]
- Bergner M, Bobbitt RA, Pollard WE, et al. (1976). The Sickness Impact Profile: validation of a health status measure J Med Cr 14: 57–67 [DOI] [PubMed] [Google Scholar]
- Bess FH, Lichtenstein MJ, Logan SA, et al. (1989). Hearing impairment as a determinant of function in the elderly J Am Geriatr Soc 37: 123–128 [DOI] [PubMed] [Google Scholar]
- Bess FH, Lichenstein MJ, Logan SA. (1991). Making hearing impairment functionally relevant: Linkage with hearing disability and handicap Acta Oto 476: 226–231 [DOI] [PubMed] [Google Scholar]
- Bess FH. (2000). The role of generic health-related quality of life measures in establishing audiological rehabilitation outcomes Ear Hear 21(suppl. 4): 74S–99S [DOI] [PubMed] [Google Scholar]
- Bridges J, Bentler RA. (1998). Relating hearing aid use to well-being among older adults Hear J 51: 39–44 [Google Scholar]
- Byrne D. (1992). Key issues in hearing aid selection and evaluation J Am Acad Audiol 3: 67–80 [PubMed] [Google Scholar]
- Byrne D, Dillon H. (1986). The National Acoustics Laboratories' (NAL) new procedure for selecting the gain and frequency response of a hearing aid Ear Hear 7: 257–265 [DOI] [PubMed] [Google Scholar]
- Chisolm T, Abrams H, McArdle R. (2004). Short- and long-term outcomes of adult audiological rehabilitation. Ear Hear 25: 464–477 [DOI] [PubMed] [Google Scholar]
- Chisolm T, Abrams H, McArdle R, et al. (2005). The WHO-DAS II: Psychometric properties in the measurement of functional health status in adults with hearing loss. Trends in Amplification. [DOI] [PMC free article] [PubMed]
- Cohen J. (1988). Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Erlbaum [Google Scholar]
- Cox R, Alexander G. (1995). The Abbreviated Profile of Hearing Aid Benefit Ear Hear 16: 176–186 [DOI] [PubMed] [Google Scholar]
- Dalton DS, Cruickshanks KJ, Klein BE, et al. (2003). The impact of hearing loss on quality of life in older adults Gerontologist 43: 661–668 [DOI] [PubMed] [Google Scholar]
- Demorest ME, Erdman SA. (1988). Retest Stability of the Communication Profile for the Hearing Impaired Ear Hear 9: 237–242 [DOI] [PubMed] [Google Scholar]
- Dunlop WP, Cortina JM, Vaslow JB, Burke MJ. (1996). Meta-analysis of experiments with match groups or repeated measures designs. Psych Methods 1: 179–177 [Google Scholar]
- Folstein MF, Folstein SE, McHugh PJ. (1979). Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiat Res 12: 189–198 [DOI] [PubMed] [Google Scholar]
- Harless E, McConnell F. (1982). Effects of hearing aid use on self concept in older persons J Speech Hear Disord 47: 305–309 [DOI] [PubMed] [Google Scholar]
- Hawkins DB, Walden BE, Montgomery A, et al. (1987). Description and validation of an LDL procedure designed to select SSPL90 Ear Hear 8: 162–169 [DOI] [PubMed] [Google Scholar]
- Howard GS, Ralph KM, Gulanic NA, et al. (1979). Internal validity in pretest-posttest self-report evaluations and a re-evaluation of retrospective pretests Appl Psych Meas 3: 1–23 [Google Scholar]
- Humes LE. (2001). Issues in evaluating the effectiveness of hearing aids in the elderly: What to measure and when Sem Hear 22: 303–315 [Google Scholar]
- Humes LE, Wilson DL, Barlow NN, et al. (2002). Changes in hearing-aid benefit following 1 or years of hearing-aid use by older adults J Speech Hear Res 45: 772–782 [DOI] [PubMed] [Google Scholar]
- Hyde M. (2000). Reasonable psychometric standards for self-report outcome measures in audiological rehabilitiation Ear Hear 21: 24S–36S [DOI] [PubMed] [Google Scholar]
- Joint Audiology Commission , (2000). Statement 3. Joint audiology committee statement on hearing aid selection and fitting (adult). Audiol Today, Special Issue, 42–44
- Joore MA, Potjewijd J, Timmerman AA, et al. (2002). Response shift in the measurement of quality of life in hearing impaired adults after hearing aid fitting Qual Life Res 11: 299–307 [DOI] [PubMed] [Google Scholar]
- Keller BK, Morton JL, Thomas VS, Potter JF. (1999). The effect of visual and hearing impairments on functional status J Am Geriatric Soc 47: 1319–1325 [DOI] [PubMed] [Google Scholar]
- Kochkin S, Rogin CM. (2000). Quantifying the obvious: The impact of hearing instruments on quality of life Hear Rev 7: 31–39 [Google Scholar]
- Linn MW, Linn BS. (1984). Self-evaluation of life function (SELF) scale: A short, comprehensive self-report of health for elderly adults. J Gerontol 39: 603–612 [DOI] [PubMed] [Google Scholar]
- Malinoff R, Weinstein B. (1989). Measurement of hearing aid benefit in the elderly Ear Hear 10: 354–356 [DOI] [PubMed] [Google Scholar]
- Mulrow CD, Tuley MR, Aguilar C. (1992). Correlates of successful hearing aid use in older adults Ear Hear 13: 108–113 [DOI] [PubMed] [Google Scholar]
- Mulrow CD, Aguilar C, Endicott JE, et al. (1990). Quality-of-life changes and hearing impairment: A randomized controlled trial. Ann Intern Med 113: 188–194 [DOI] [PubMed] [Google Scholar]
- The National Council on the Aging (1999). The consequences of untreated hearing loss in older persons. A study conducted by the Seniors Research Group, an alliance between The National Council on the Aging and Market Strategies, Inc. Washington, D.C: The National Council on the Aging [Google Scholar]
- Nelson E, Wasson J, Kirk J, et al. (1987). Assessment of function in routine clinical practice: Description of the COOP chart method and preliminary findings J Chron Dis 40(S1): 55S–63S [DOI] [PubMed] [Google Scholar]
- Nich C, Caroll KM. (2002). Intention-to-treat meets missing data: Implications of alternate strategies for analyzing clinical trials data Drug Alcohol Depend 68: 121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Shobaki G, Shivakumar C, et al. (1998). Adjusting sample size for anticipated dropouts in clinical trials Psychopharmacol Bull 34: 25–33 [PubMed] [Google Scholar]
- Peduzzi P, Henderson W, Hartigan P, et al. (2002), Analysis of randomized controlled trials. Epidemiol Rev 24: 26–38 [DOI] [PubMed] [Google Scholar]
- Pugh KC. (2004). Health status attributes of older African-American adults with hearing loss J Natl Med Assoc 96: 772–779 [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Sprangers MAG. (1999). Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research Soc Sci Med 48: 1531–1548 [DOI] [PubMed] [Google Scholar]
- Stephans D. (2003). The World Health Organization's International Classification of Functioning, Disability and Health (ICF) and its relevance to Audiology AAS Bulletin 28: 40–41 [Google Scholar]
- Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. (2000). Negative consequences of hearing impairment in old age: A longitudinal analysis Gerontologist 40: 320–326 [DOI] [PubMed] [Google Scholar]
- Surr RK, Cord MT, Walden BE. (1998). Long-term versus short-term hearing aid benefit. J Am Acad Audiol 9: 165–171 [PubMed] [Google Scholar]
- Taylor KS. (1993). Self-perceived and audiometric evaluations of hearing benefit in the elderly. Ear Hear 14: 390–394 [DOI] [PubMed] [Google Scholar]
- The EuroQol Group. (1990). EuroQol: A new facility for the measurement of health related quality of life. Health Policy 16: 199–208 [DOI] [PubMed] [Google Scholar]
- The National Council on the Aging (1999). The consequences of untreated hearing loss in older persons. A study conducted by the Seniors Research Group, an alliance between The National Council on the Aging and Market Strategies, Inc. Washington, D.C: The National Council on the Aging [Google Scholar]
- Ventry I, Weinstein B. (1982). The hearing handicap inventory for the elderly: A new tool Ear Hear 3: 128–134 [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. (1992). The MOS 36-item short form health survey (SF-36). Medical Care 30, 473–483 [PubMed]
- World Health Organization (2001). International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization [Google Scholar]
- World Health Organization (1999). The World Health Organization Disability Assessment Schedule Phase II Field Trial Instrument. Geneva: World Health Organization [Google Scholar]
- World Health Organization, Disability Assessment Schedule II (WHO-DAS II) 36-item interviewer administered version. Available from URL: http://www.who.int/icidh/whodas/index.html, updated November 2001