Abstract
Recent advances in cochlear implant technology have focused renewed attention on the preservation of residual hearing. The focus on preservation of residual hearing is driven by the concept of electroacoustic stimulation. This option depends on the insertion of a short cochlear implant electrode into the basal region of the cochlea while preserving native function in the apical region. The desire to preserve residual hearing has led to the development of the soft-surgery cochlear implantation technique. Here, the authors evaluate its various components. Avoiding entry of blood into the cochlea and the use of hyaluronate seem to be reasonably supported, whereas the use of topical steroids is unlikely to be beneficial. The site of entry into the cochlea, the use of contoured or straight devices, and the depth of insertion are also evaluated. The authors highlight the importance of systematic recording of outcomes and surgical events.
Keywords: cochlear implant, soft surgery, electroacoustic
Introduction
Recent advances in cochlear implant technology have focused renewed attention on the preservation of residual hearing. Initially, the preservation of residual hearing was sought as a backup to traditional implantation so that implantation candidacy criteria could be expanded to include those without total deafness (Lehnhardt, 1993). Subsequently, it was thought that residual hearing would improve implant performance. Although this has not proven true with regard to standard word and sentence recognition testing in quiet, there may be a benefit when listening in noise or when listening to music (Gantz, Turner, Gfeller, & Lowder, 2005; Gfeller, Olszewski, Turner, Gantz, & Oleson, 2006; Turner, Reiss, & Gantz, 2008). Currently, the focus on preservation of residual hearing is driven by the concept of electroacoustic stimulation. This option depends on the insertion of a short cochlear implant electrode into the basal region of the cochlea (high frequencies) while preserving native function in the apical region (low frequencies). Poor traditional hearing aid users with steeply sloping audiograms could thus benefit from acoustic stimulation of their low frequencies and electric stimulation of their high frequencies. This bimodal rehabilitation would expand the criteria for cochlear implantation and could provide the added benefits of improved hearing in noise and music appreciation. The desire to preserve residual hearing has led to the development of the soft-surgery cochlear implantation technique.
The soft-surgery cochlear implantation technique was first proposed in 1993 by Lehnhardt (Lehnhardt, 1993). Many of the tenets of the soft-surgery technique arise from this initial report and are based on a commonsense approach to the opening and manipulation of the cochlea. Some of the principles of the soft-surgery technique are also based on early experience with stapedectomy and subsequent experience with cochlear implantation. Additional principles have their grounding in clinical case series and outcome measures. There is further support for surgical protocols from human and animal temporal bone studies examining intracochlear trauma, which is indirectly correlated with hearing outcomes.
Despite these experiences, there is little direct evidence supporting the soft-surgery protocol or defining which steps may be crucial to a favorable outcome. There are no randomized controlled clinical trials investigating each of these components. Much of the evidence is anecdotal, whereas stronger evidence comes from anatomical studies in animal models and human temporal bones. This article examines the individual components of the soft-surgery methodology and reviews current evidence for their efficacy and justification for their continued use in this form of cochlear implant surgery.
Method
The goal of the soft-surgery technique is to avoid mechanical trauma to the cochlea and to reduce the introduction of factors that may cause adverse intracochlear reactions. The latter includes gross responses such as fibrosis and new bone formation as well as molecular responses such as hair cell apoptosis or alterations in the endocochlear potential. Surgical modifications to achieve these goals include the location of the cochleostomy, the protocol to enter the cochlea, the use of adjunct materials and drugs, and the maintenance of a meticulous surgical field (Cohen, 1997; Eshraghi, 2006).
The surgical procedure, albeit with some minor variation depending on the instrumentation available, begins with the mastoidectomy and preparation of the device well. This is done prior to opening of the cochleostomy to minimize the time of exposure of the scala tympani to the external environment. Care is taken during the approach to the cochlea to reduce the potential for acoustic trauma such as from contact of the drill with the ossicular chain. Bone dust and pate are thoroughly irrigated away to prevent entry into the cochlea at the cochleostomy or by contact with the electrode array.
Blood entry into the cochlea is also avoided. The promontory is prepared to minimize bleeding and reduce the potential for blood to enter the cochlea. This is accomplished by either raising a mucosal flap, microcautery, or the use of vasoconstrictors such as topical epinephrine. A cotton pledget can be placed in the antrum to prevent blood from entering the middle ear from the mastoid or postauricular wound. Silastic, or similar material, can be used to cover the mastoid and medial facial recess when inserting the electrode to prevent contact of the electrode array with raw tissues.
Electrode insertion is performed either through a cochleostomy or via the round window membrane. Each has advantages and disadvantages, which are discussed below. If performing a cochleostomy, it is best placed anterior and inferior to the round window membrane (Figure 1). Exposing the round window membrane and taking down the tectulum, or “lip” of the anterior round window niche, will guide the positioning of the cochleostomy. The cochleostomy can be positioned as an anterior extension of the round window, the so-called extended round window approach. Drilling is performed with a 1 mm diamond burr using ample irrigation and low drill speeds until the endosteum of the scala tympani is revealed. The endosteum is entered using a microlancet or small hook, and care is taken to avoid suctioning of the perilymph. Steroid solution, sodium hyaluronate (i.e., Healon®), or both can be placed over the endosteum prior to opening the inner ear. The cochleostomy should be opened just to the size necessary to accommodate the array. For most implants this is about 0.8 to 1.0 mm.
Figure 1.
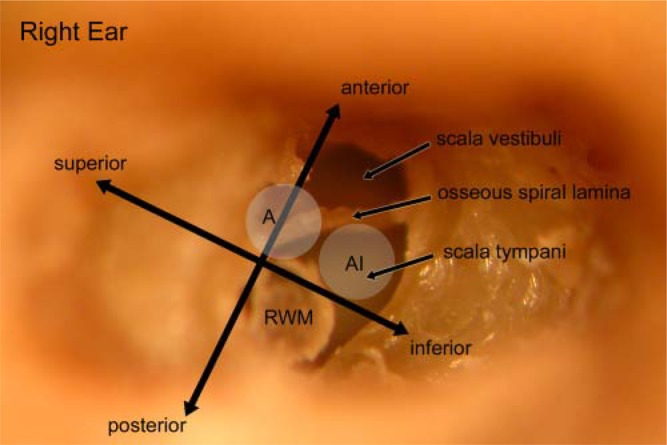
Photograph of right temporal bone with the basal cochlea turn drilled out. The round window membrane (RWM) has been rotated inferiorly and outward for visualization but normally lies beneath and in the plane of the anterior-posterior directional. To safely enter the scala tympani, the cochleostomy needs to be performed anterior and inferior to the round window membrane (AI). The commonly used anterior only approach (A) can lead to scala vestibuli insertions and osseous spiral lamina fractures.
The round window insertion is performed through an incision placed in the anterior border of the round window membrane. To provide access and ample room for the insertion, the posterior tectulum of the niche needs to be removed. The insertion is performed at a slight superior-to-inferior angle to avoid penetration of the basilar membrane. Steroid solution and/or Healon® may also be placed over the membrane prior to opening and insertion.
The insertion is performed with as little pressure as possible. Resistance may indicate contact of the electrode tip with the basilar membrane, osseous spiral lamina, or vasculature along the lateral cochlea wall. The electrode array can be coated in steroid or Healon® to provide lubrication and easier insertion. Differences in electrode flexibility, size, and inherent curvature may also influence the ease of insertion. In hearing preservation surgery, when acoustic hearing will also be used postoperatively, a partial insertion is often desired to avoid interference with natural low-frequency cochlea function. The length of electrode to be inserted should be determined preoperatively. It is best to visually identify the electrode contact that should be at the cochleostomy site and advance to that point. Some surgeons will use a small collar of fascia positioned around the electrode array at the desired limit of insertion.
Once the electrode is inserted to the desired depth, the cochleostomy should be sealed to avoid perilymph leakage or postoperative pneumolabyrinth. Typically, a small piece of temporalis muscle or fascia is plugged around the array at the cochleostomy. If a fascia collar is used around the array this is seated down on the surrounding promontory and otic capsule. An appropriately sized cochleostomy will be essentially sealed by the array, but tissue supplementation is recommended.
Many surgeons will use systemic steroids during and following surgery. This may entail a single dose of decadron intraoperatively followed by a prednisone taper in the immediate postoperative period. Additional local and systemic medications, such as antioxidants, are not routinely used, but ongoing studies may inform such additions (see discussion). Postoperative antibiotics are given at surgeon preference. Typical postoperative instructions include short-term avoidance of increased intracranial and middle-ear pressure from heavy lifting, nose blowing, valsalva, or airplane flights.
Discussion
A literature search (PubMed) was undertaken to identify reports supporting the basic principles of soft-surgery cochlear implantation. This was not a formal evidence-based medicine analysis but rather an investigation to put into historical context the reports, studies, and experiments that have helped develop this surgical approach. This information may place greater emphasis on some aspects of the surgical protocol and prompt better reporting and documentation correlating residual hearing outcomes with intraoperative events.
Reaction to Implantation or Opening of the Cochlea
Despite meticulous surgical technique, opening of the cochlea and placement of a foreign body within the scala tympani will elicit cellular and molecular responses. These may be inherently unavoidable or may require modifications of the approach, surgical methodology, or implant composition. Furthermore, they may require the addition of molecular inhibitors and adjuncts in the perioperative period. Many of the steps in soft surgery are already directed at reducing such responses, but their efficacy remains to be determined. Also, it is not clear which intracochlear responses to implantation are, in fact, detrimental to the preservation of residual hearing. This section briefly reviews the cochlear response to implantation and exposure of perilymph to put into context the subsequent discussions on specific surgical protocols and guidelines.
New bone formation within the cochlea is an expected sequela of cochlear implantation. It typically occurs at the cochleostomy site but can track along the path of implantation and even extend to the nonimplanted apex of the cochlea (Nadol & Eddington, 2006). Neo-osteogenesis appears to be more pronounced in placements using a cochleostomy compared with those through the round window membrane (Shepherd, Clark, Xu, & Pyman, 1995). Despite the frequent occurrence of new bone formation, there is no information regarding its effect on residual hearing.
Fibrosis within the cochlea and around the electrode array is an almost universal finding following implantation. Fibrosis occurs as a reaction to disruption of inner ear anatomy even without electrode placement (Canalis, Gussen, Abemayor, & Andrews, 1987; Smouha, 2003). With cochlear implantation, there is often fibrosis at the cochleostomy site and along the array (Marsh, Jenkins, & Coker, 1992; Nadol et al., 2001; Schindler & Bjorkroth, 1979). Fibrosis appears to be worse along the basal turn and can interfere with cochlear reimplantation (Alexiades et al., 2001). Fibrosis along the basal turn is predicted to alter vibration of the apical basilar membrane and thus interfere with residual low-frequency acoustic hearing (Choi & Oghalai, 2005). Surgical modifications to reduce postoperative fibrosis within the cochlea, as reviewed in this article, are therefore important in hearing preservation cochlear implantation.
Nonspecific inflammatory reactions within the cochlea may also cause loss of residual hearing post-implantation (Rizer, Arkis, Lippy, & Schuring, 1988). An autoimmune-like response may be evoked simply from opening the cochlea as can be seen after stapedectomy (Paparella, Lim, & Sugiura, 1967). Most reactions, however, are likely to be in response to the implant itself or to damage within the cochlea (Cervera-Paz & Linthicum, 2005). Excess tissue manipulation around the cochleostomy, as typically occurs in reimplantation, can cause a robust inflammatory response (Jackler, Leake, & McKerrow, 1989). Changes in fluid pressure within the cochlea may cause hearing loss or cellular changes away from the cochleostomy (Carvalho & Lalwani, 1999; Kelly & Khanna, 1984). Such pressure waves may incite the molecular response that leads to delayed hearing loss after implantation (Eshraghi et al., 2005).
It is the goal of the soft-surgery technique to reduce these reactions to implantation. Until we fully understand their causes, we must proceed with a protocol that minimizes intrascalar trauma, avoids the elicitation of foreign substance reactions, and attenuates the reparative response. These surgical steps are discussed below along with available evidence as to their efficacy and indications in hearing preservation cochlear implantation.
Avoidance of Blood Entry Into the Scala Tympani
Experienced stapes surgeons will argue that the avoidance of blood entry into the vestibule is an important component in preventing sensorineural hearing loss (Causse, Causse, Wiet, & Yoo, 1983). Despite this, a blood clot is often used to help seal the stapedectomy site (House, 1993). Also, the introduction of blood into the vestibule to retrieve a footplate during stapedectomy has been reported (Wayoff, Moeller, & Roche, 1971). We found no publications in the past 40 years presenting a case or experiment demonstrating a correlation between blood contamination of the vestibule and poor stapedectomy outcomes. There are case reports for other disorders, however, that suggest that blood is an inner-ear toxin. A report by Franco-Vidal, Songu, Blanchet, Barreau, and Darrouzet (2007) showed hemorrhage in the cochlea coincident with sudden hearing loss after gamma knife surgery. Other coincidental findings are the demonstration of intracochlear high signal on 3D-FLAIR MRI images in cases of sudden hearing loss, which may indicate hemorrhage or high protein levels (Otake, Sugiura, Naganawa, & Nakashima, 2006; Yoshida et al., 2008). A direct association between the presence of intrascalar blood and etiology of hearing loss is difficult to establish.
Only one study has specifically examined the effect of blood in the inner ear on hearing thresholds. Radeloff and colleagues (2007) introduced 3 μl of blood into the guinea pig cochlea via a cochleostomy and tested thresholds at various postoperative times. There was evidence in both control and study animals of immediate and persistent high-frequency hearing loss that was likely related to surgical trauma and possibly the introduction of additional fluid into the cochlea. The study animals, however, showed a more pronounced low-frequency permanent threshold shift that was considered to be secondary to intrascalar blood. Postulated mechanisms for the hearing loss included oxidative damage induced by hemoglobin or inflammatory reaction to blood and its breakdown products. The threshold shift was significant but relatively small and would not, if of the same magnitude in humans, necessarily preclude the use of acoustic stimulation for hearing rehabilitation.
Avoidance of Bone Dust in the Cochlea
Avoiding bone dust entering the cochlea is also a principle of soft surgery. Similar to blood, this “foreign” material may elicit an inflammatory response within cells of the scala media and negatively affect residual hearing. Furthermore, it may provide a nidus for intracochlear bone formation that can mechanically alter acoustic hearing. McElveen, Wolford, and Miyamoto (1995) clearly showed the potential for bone pâte to promote osteogenesis and scar formation when used to seal the cochleostomy. The implication of particles of bone dust entering the cochlea is not as clear.
Kiefer and coinvestigators (2004a) adhered to a strict soft-surgery protocol including the flushing away of bone dust prior to insertion. They preserved hearing in 12 of 14 patients, but there were no surgical deviations noted in the two patients losing hearing. Thus, the implantation steps that were critical to success could not be determined. Garcia-Ibanez and colleagues (2008) prospectively looked at 26 hearing preservation surgeries and had 1 case in which bone dust entered the cochlea, although this patient also had perilymph suctioning. This was 1 of 8 cases with a significant postoperative threshold shift and therefore provided a weak correlation with outcomes. Overall, there is little direct evidence to support or refute the principle of flushing away bone dust prior to implantation. Given the potential for larger amounts of bone pâte to promote osteogenesis, it seems reasonable to continue this simple step during implantation.
Application of Steroid at the Cochleostomy Site
Systemic steroids are widely used in otology for treating and preventing hearing loss in a variety of conditions. Intratympanic steroids are increasingly being used in similar situations. As such, there is the concept that applying steroids directly on the round window, cochleostomy site, and/or the electrode array can inhibit inflammatory and molecular responses to implantation and avoid the loss of residual hearing. This is supported by animal experiments. For example, the drop in guinea pig CAP (cochlear action potential) thresholds caused by performing a cochleostomy was reversed by the addition of triamcinolone, as compared with saline controls (Kiefer et al., 2007; Ye, Tillein, Hartmann, Gstoettner, & Kiefer, 2007). Furthermore, there was no toxic effect of triamcinolone on the cochlea. In other animal experiments, ABR (auditory brainstem response) threshold changes in a model of electrode insertion trauma were significantly better at 1 month when dexamethasone was infused into the cochlea as compared with only artificial perilymph (Eshraghi et al., 2007; Vivero et al., 2008).
These studies provide strong evidence that steroids can protect the cochlea from adverse reaction to cochleostomy and electrode insertion. The protective effect, however, was only seen with intrascalar administration of the steroid. Steroids administered topically, such as by intratympanic injection, diffuse into the scala tympani but exhibit a strong concentration gradient, with the apex receiving relatively little steroid (Plontke, Biegner, Kammerer, Delabar, & Salt, 2008). Furthermore, when a steroid is applied topically, it reaches peak concentration within an hour and lasts less than 24 hours (Hargunani, Kempton, DeGagne, & Trune, 2006). Thus, if steroids are protective and useful for preserving residual low-frequency hearing, it is doubtful that simple placement of solution on the cochleostomy site is providing a high enough, prolonged enough, or apically directed dosage. Systemic steroid use, however, may provide the necessary dosage and duration of treatment to protect the cochlea. Further studies would be needed to address whether coating the electrode array with steroid solution is a feasible delivery system. Additionally, there may be a role for steroid-eluting electrodes specifically for hearing preservation surgeries.
Application of Healon® to the Cochleostomy and Electrode Array
In his initial description of the soft-surgery technique Lehnhardt (1993) specifically noted the use of Healon® at the cochleostomy site and on the electrode array. Healon® (sodium hyaluronate, aka hyaluronic acid or hyaluronan) is a normal constituent of the extracellular matrix. It is commonly used in implant surgery as a lubricant for electrode insertion (Laszig, Ridder, & Fradis, 2002). Its clarity and viscoelastic properties also make it useful for covering the cochleostomy to prevent entry of blood and bone dust while allowing visualization for incising the endosteum or inserting the electrode array. It may also reduce scar tissue formation within the middle and inner ears (Huang, Tykocinski, Stathopoulos, & Cowan, 2007; Laurent, Hellstrom, & Stenfors, 1986).
The safety of hyaluronic acid in the middle ear is well established. Animal studies have shown that tran-stympanic application does not cause hair cell death or measurable decline in hearing thresholds (Anniko & Arnold, 1995; Anniko, Hellstrom, & Laurent, 1987; Bjurstrom, Slepecky, & Angelborg, 1987; Engstrom, Bjurstrom, Jansson, Engstrom, & Angelborg, 1987; Martini et al., 1992). In contrast, intracochlear injection of hyaluronic acid resulted in permanent sensorineural hearing loss in rats (Roland, Magardino, Go, & Hillman, 1995). This study showed no loss of spiral ganglion cells or changes in their axodendritic morphology, so it was concluded that hyaluronate is safe for general cochlear implant surgery. If the goal is hearing preservation, however, this result is troubling. It is not clear whether the observed hearing loss was from toxicity or from mechanical changes induced by injecting additional fluid into the cochlea.
In a study more representative of soft-surgery use, Laurent, Hellstrom, and Anniko (1992) placed hyaluronan solution over the rat round window membrane prior to incising the membrane. At 2 months postsurgery, hair cell morphology and ABR thresholds were normal despite an immediate drop in thresholds at the time of round window membrane incision. In humans, Angeli (2006) used hyaluronate during stapedotomy and therefore established contact between the gel and the perilymph. In 27 patients, there were no instances of sensorineural hearing loss, and postoperative dizziness was, in fact, less in patients in whom he used hyaluronate.
There are several reports of outcomes in animals and humans with the soft-surgery technique and the use of hyaluronic acid. Rogowski, Reiss, and Lehnhardt (1995) implanted guinea pigs using Healon® as a lubricant. Histological examination showed minimal sensory cell damage within the cochlea, suggesting that the technique, including the use of hyaluronate, was minimally traumatic to the organ of Corti. In humans, Skarzynski was able to preserve some hearing in 21 of 26 adults and children with regular use of Healon® (Skarzynski et al., 2002). Kiefer and coworkers (2004a) used Healon® to seal the steroid solution within the cochleostomy site and preserved residual hearing to within 20 dB of preoperative thresholds in 12 of 14 patients. Garcia-Ibanez, in the prospective study described earlier, had 8 patients in whom Healon® was not used, of whom 4 (50%) showed a >40 dB threshold shift in some frequency range. This contrasts with 17 patients in whom Healon® was used of whom 4 (24%) had a similar decline (Garcia-Ibanez et al., 2008). The use of Healon® in this study showed a weak correlation with hearing preservation.
These data show that topical application of Healon® over the open cochlea and contact of the electrode array during insertion with Healon® does not appear to be ototoxic. The use of a hyaluronate-based lubricant in fact appears to be beneficial to promoting hearing preservation when opening the inner ear. This may be secondary to cytostatic properties of the hyaluronate, reduced friction and trauma during electrode placement, prevention of perilymph leakage, and/or prevention of cochlea contamination with blood and bone dust. Despite these favorable outcomes, there is some indication that gross introduction of hyaluronic acid into the cochlea may have cytotoxic effects and should be avoided in hearing preservation surgery. Judicious topical use of hyaluronic acid therefore appears to be reasonable and probably beneficial in soft cochlear implantation.
Avoidance of Perilymph Leakage and Suctioning
Experience with stapes surgery, perilymphatic fistulae, and labyrinthine fistulae from cholesteatoma has strongly suggested that loss of perilymph from the inner ear can have detrimental effects on coch-leovestibular function. However, the amount of leakage and location of the leak may influence the site and degree of loss of function. For example, Gjuric, Wigand, and Hosemann (1992) have shown that simple fenestration of the rabbit lateral canal does not result in any hearing loss. In contrast, removal of the lateral canal after fibrin glue plugging caused total hearing loss in one third of animals, yet only a mild loss in the two thirds that retained measurable ABRs. Thus, gentle perilymph exposure may be quite safe, and even more radical manipulation of the inner ear can still have some good outcomes.
Actual removal of the perilymph was studied by Hara, Nomura, and Saito (1990) who suctioned 4 μl of perilymph through the round window membrane and examined morphological changes in the guinea pig cochlea. In two of nine specimens studied 1 to 3 months after surgery, there was outer hair cell loss in the apical turns. No electrophysiological testing was performed, but such an apical loss would correlate with elevated low-frequency thresholds. In several other specimens, there were membranous labyrinth and organ of Corti abnormalities, suggesting that hearing loss is likely with suctioning at the round window.
Clinically, suctioning at the cochleostomy site weakly correlated with loss of residual hearing in the prospective Garcia-Ibanez study cited earlier (Garcia-Ibanez et al., 2008). Yet Skarzynski and colleagues Skarzynski et al., 2002) found a 19% loss of residual hearing while adhering to the soft-surgery method but no intraoperative factors to correlate with the losses. Cohen cautions against the suctioning of perilymph and raises the additional concerns that the suction tip can cause mechanical damage to the basilar membrane and osseous spiral lamina (Cohen, 1997). Although direct evidence against suctioning in cochlear implantation is scarce, it suggests that suctioning increases the risk of harm, and as it is not necessary during implantation, it should probably be avoided.
Route of Electrode Insertion: Cochleostomy Versus Round Window
There are two principal means of accessing the scala tympani for placement of the electrode array: via a basal turn cochleostomy or via the round window membrane. Lehnhardt initially rejected the round window approach because of its surgical anatomy, effect on intracochlear fluid dynamics, and potential to disrupt the cochlear aqueduct (Lehnhardt, 1993). Based on anatomical studies and clinical outcomes, the round window approach has emerged as a rational alternative to a cochleostomy. This section examines current data supporting each approach.
The round window insertion has been studied as a less traumatic and more direct approach to the scala tympani than the traditional cochleostomy. When using a custom short array, this approach produces little intracochlear damage (Briggs et al., 2006; Lenarz et al., 2006). Using clinically available electrodes, minimal damage to basal turn structures was also observed (Adunka et al., 2004c). Direct comparison between approaches showed the round window approach to be significantly less traumatic than the cochleostomy insertion (Adunka et al., 2004a). This may be electrode specific, however, because a follow-up study using a perimodiolar electrode showed significant damage to basal turn structures using the round window approach (Adunka, Pillsbury, & Kiefer, 2006). Similarly, early generation electrodes inserted via the round window produced significant damage to the osseous spiral lamina and/or membranous partitions in 50% of the cases (O'Leary, Fayad, House, & Linthicum, 1991).
The cochleostomy is the traditional and more commonly used approach to the scala tympani. Correct placement of the cochleostomy, however, appears critical for avoiding damage to inner ear structures. A cochleostomy located anterior-inferior to the round window membrane avoids damage to the osseous spiral lamina as well as inadvertent entry into the scala media or scala vestibuli (Briggs et al., 2001; Briggs, Tykocinski, Stidham, & Roberson, 2005). Modeling of the hook region of the basal turn also confirms the anterior-inferior margin of the round window membrane as the least likely to disrupt intracochlear anatomy (Li, Wang, Northrop, Merchant, & Nadol, 2007). In a systematic temporal bone study, Adunka, Radeloff, Gstoettner, Pillsbury, and Buchman (2007) showed that starting inferior to the level of the annulus of the round window and proceeding anteroinferiorly along the basal turn produced the least trauma to the osseous spiral lamina. This was in contrast to starting the cochleostomy directly anterior to the round window membrane, which disrupts the osseous spiral lamina (see figure 1).
Clinically, few series have used the round window approach for hearing preservation surgery. In adults, the round window insertion preserved useful levels of low-frequency hearing in the majority of patients (9 of 10 patients; (Skarzynski et al., 2007b). Similar results were found in children in which 8 of 9 patients preserved useful low-frequency hearing (Skarzynski et al., 2007a). In contrast, a separate report showed significant loss of residual hearing via the round window approach in 6 of 8 patients, although all aspects of the soft-surgery technique may not have been followed (Berrettini, Forli, & Passetti, 2008).
The cochleostomy approach has been more extensively studied regarding postoperative residual hearing outcomes. A comparison of location of the cochleostomy found the anterior-inferior site to correlate with better residual hearing outcomes than a strictly inferior or anterior entry point (Garcia-Ibanez et al., 2008). In another study, the anterior-inferior cochleostomy, in comparison to the anterior cochleostomy, also showed a higher rate of hearing preservation (80% vs. 45%; Berrettini et al., 2008). Using the anterior-inferior cochleostomy, Gantz and colleagues (2005) preserved hearing at 1 month postoperatively in all 24 patients receiving a short array implant. A cochleostomy 1 mm caudal to the round window also preserved useful low-frequency hearing in the majority of patients (Kiefer et al., 2004a). A small cochleostomy similarly enabled the preservation of some hearing in 21 of 26 patients using the soft-surgery technique (Skarzynski et al., 2002).
There is no definitive evidence defining the best route for hearing preservation implantation. There are mixed results with round window insertion and an indication that this approach may not be suitable for all electrode configurations. The cochleostomy approach is familiar to nearly all implant surgeons and would seem a reasonable approach if the soft-surgery technique was widely used beyond specific implant centers. The cochleostomy, however, needs to be placed appropriately, and a recent study suggests that there is still wide variability in surgeon approaches to the basal turn (Adunka & Buchman, 2007). The extended round window insertion, a hybrid of both approaches, may provide a specific list of surgical steps that could ensure a uniform protocol and correct placement of the entry site into the cochlea.
Electrode Parameters
Among the principal factors that may contribute to intracochlear trauma during implantation are the inherent properties of the electrode array. These variables include whether the electrode is straight or contoured, stiff or flexible, or long or short. The latter affect depth of insertion, which is addressed in a separate section below. The electrode properties can be selected by the surgeon (by selecting specific companies and devices), and it is critical to understand their potential relationship to the preservation of residual hearing.
Most information regarding electrode properties and intracochlear trauma come from temporal bone insertion studies. These studies, however, vary widely in their selection of types of electrodes and the approach to the cochlea. Lenarz and coworkers (2006), for example, showed that a custom short and straight electrode implanted via the round window membrane caused no basal damage in 95% of bones. In contrast, Adunka and colleagues (2006) found Grade 4 damage (i.e., fracture of the osseous spiral lamina; scale defined by Eshraghi, Yang, & Balkany, 2003) in 75% of bones using the same approach but with a contoured electrode. Similarly, using a contoured electrode with an anterior cochleostomy also caused substantial intracochlear damage (Aschendorff et al., 2003).
It may be premature to conclude from these data that contoured electrodes cause more damage than straight electrodes. When performed through an anterior-inferior cochleostomy the contoured electrodes do, in fact, appear to provide for relatively atraumatic insertions. This is particularly true when using perimodiolar electrodes inserted with the off-stylet technique (Roland, 2005; Stover et al., 2005). Atraumatic insertion through an appropriately placed cochleostomy has also been consistently observed with precurved electrodes placed with insertion tools (Wright, Roland, & Kuzma, 2005).
Direct comparison of straight and precurved electrodes inserted through an anterior-inferior cochleostomy showed little difference in resultant intrascalar trauma (Wardrop et al., 2005b). Both electrodes caused trauma along the insertion path, but this study used full-length electrodes with deep insertions. In three cases with a short insertion (i.e., <15 mm) of the straight electrode, which is more akin to hearing preservation surgery, no intracochlear trauma was observed. In a companion study, a different precurved electrode and electrode with positioner were similarly studied (Wardrop et al., 2005a). Again, shorter insertions via the anterior-inferior cochleostomy showed minimal trauma of basal turn structures.
Similar to the contoured devices, straight electrode arrays inserted via an anterior-inferior cochleostomy have also shown minimal intracochlear trauma. Examination of an early generation straight array inserted only until resistance was met was least likely to tear the membranous labyrinth (Welling, Hinojosa, Gantz, & Lee, 1993). Similarly, a modern thin and flexible straight array caused no intracochlear trauma and was able to be inserted to an average of 360° of cochlear coverage (Adunka et al., 2004b). Some damage in this latter study was attributed to performance of the cochleostomy and not to the array. A comparison of straight and precurved arrays showed that the straight flexible arrays were the least traumatic and that contoured arrays had a higher incidence of basilar membrane perforation (Briggs et al., 2001).
Stiffness of the electrode array may also correlate with the incidence of intracochlear trauma (Briggs et al., 2001). Modeling of insertion forces and the propensity for electrodes to penetrate the basilar membrane show that graded stiffness and soft-tip electrodes are less likely to cause damage than uniformly stiff electrodes (Chen, Clark, & Jones, 2003). Similar modeling shows that both straight and curved electrodes can cause basilar membrane perforations, although the tip of the curved electrodes produced greater contact stress on the lateral wall of the scala tympani (Kha & Chen, 2006). Interestingly, slightly withdrawing the stylet from a precurved electrode was predicted to significantly reduce intrascalar damage. This may account for the favorable anatomical findings described above with the off-stylet technique.
Clinical outcomes may provide more compelling evidence than anatomical studies regarding the selection of electrode configuration and properties for hearing preservation surgery. Hearing preservation rates among 48 patients undergoing either a contoured or straight electrode implantation were compared (Soda-Merhy, Gonzalez-Valenzuela, & Tirado-Gutierrez, 2008). There was no statistically significant difference in residual hearing changes between electrode styles or among individual devices. Using only a straight electrode, Gstoettner and colleagues (2008) found that 67% of patients were able to benefit from acoustic stimulation, and hearing was preserved at any level in 83%. These results are similar to those from other investigations also using a straight electrode (Gstoettner et al., 2004; Skarzynski et al., 2002; Skarzynski et al., 2007a, 2007b). The use of contoured electrodes with the off-stylet technique compares favorably with the soft-surgery protocol, and approximately 80% of patients maintain a measurable level of residual hearing (Berrettini et al., 2008; Garcia-Ibanez et al., 2008; James et al., 2005). These studies represent cochleostomy insertions with either configuration and round window insertions with only the straight configuration. Clinical studies of contoured electrodes and the round window insertion were not found.
There are far more anatomical studies comparing electrode configuration with intracochlear trauma than clinical studies comparing the configuration with hearing preservation outcomes. Despite this, hearing outcomes represent the best endpoint for assessing efficacy and safety. These data suggest that when using an anterior-inferior cochleostomy for implantation, either a straight or contoured electrode may be of benefit. They also suggest that straight electrodes have similar outcomes when using the round window approach. Based on the lack of clinical data and the evidence of trauma in anatomical studies, contoured electrodes may not be suitable for the round window approach. Extending the round window opening, however, may provide an alternative for using the contoured arrays (Todt et al., 2008).
Depth of Insertion
Optimal insertion depth of the electrode array is a balance between sufficient depth to provide the necessary high-frequency electrical stimulation and avoiding interference with functioning cochlear structures and subsequent loss of residual hearing. Although intracochlear damage with electrode insertion is not universally seen, the risk of trauma increases with deeper insertions (Adunka & Kiefer, 2006; Baumgartner et al., 2007; James et al., 2005; Wardrop et al., 2005a).
Several histological and clinical studies have concluded that damage occurs when the electrode is inserted past the point where resistance is first detected. Insertion past the point of first resistance forces the array into the intracochlear structures and may result in electrode buckling and subsequent further trauma; damage from forced electrode insertion ranges from basilar membrane rupture to fracture of the modiolus (Adunka & Kiefer, 2006; Gstoettner et al., 1997; Gstoettner et al., 1999; Neumann, Aschendorff, Schipper, Laszig, & Klenzner, 2005). When inserted without feeling resistance, however, full electrode insertions of standard-length arrays can be accomplished without intracochlear damage or with minimal interference, such as slight displacement of the basilar membrane (Gstoettner et al., 1997; Lenarz et al., 2006). In general, the depth at which first resistance occurs varies considerably and is dependent on the electrode properties, surgical approach, and normal individual variations in cochlear anatomy (Erixon, Hogstorp, Wadin, & Rask-Andersen, 2009; Gstoettner et al., 1997; Gstoettner et al., 1999; James et al., 2005).
Soft, thin, and flexible electrode arrays are being developed as one approach for minimizing traumatic effects of the array on cochlear structures, thereby increasing potential insertion depth (Adunka, Pillsbury & Kiefer, 2006; Lenarz et al., 2006). A direct comparison of insertion forces between standard and atraumatic electrode arrays was performed in temporal bone acrylic models by Adunka and Kiefer (2006). For both arrays, insertion forces increased steeply as insertion depths progressed beyond 18 mm; however, the average forces were reduced by nearly 30% for the atraumatic array.
Although hearing preservation surgery is typically associated with short electrodes, Baumgartner and colleagues (2007) investigated hearing outcomes in 23 patients with deep insertion of a full-length (31 mm) atraumatic electrode array using standard cochlear implant surgical procedures and found hearing preservation in 50% and 25% of patients at the 1-month and 12-month postoperative intervals, respectively. According to the surgical reports, no “serious” resistance was found in 18 surgeries, but this was not related to the hearing preservation outcomes. In another report of 26 patients, using soft surgical techniques and full insertion of a standard 31 mm array, hearing preservation within 5 dB of preoperative thresholds was found in 62% of patients at the 1-month postoperative interval (Skarzynski et al., 2002). Thus, hearing preservation with full electrode insertion is feasible and may be related to the avoidance of resistance and the physical properties of the electrode array (i.e., thinness, flexibility).
Soft-surgical procedures combined with shorter insertions (17–20 mm) of standard arrays has resulted in hearing preservation in which the average low-frequency threshold remained within 35 dB of preoperative measures (James et al., 2005; Skarzynski et al., 2007b). Similarly, full insertion of short arrays (15 mm) have maintained postoperative low-frequency thresholds within 10 dB (Lenarz et al., 2006). Among these investigations, Skarzynski and colleagues (2007b) presented hearing preservation data from as far out as 12 months postimplant. Insertion depths were determined preoperatively based on the patients' audiograms, and long-term hearing preservation was observed in 9 of 10 patients.
Clinical outcomes of insertion depth on postoperative hearing thresholds vary widely among investigations in terms of the type of electrode array, surgical technique, and definition of hearing preservation. In general, however, these data suggest that deeper insertions carry a greater risk of loss of residual hearing, not necessarily by interfering with normal cochlear physiology but from mechanical damage at points of resistance during implantation. Therefore, the surgeon must achieve a balance between the cochlear coverage needed to provide high-frequency electrical stimulation and intraoperative tactile feedback of when resistance is encountered. It is prudent to determine the ideal electrode insertion depth for each individual patient based on their preoperative audiogram. Intraoperatively, the surgeon may decide to modify the final insertion depth depending on whether or not resistance is encountered during implantation.
Other Considerations During Soft-Surgery Cochlear Implantation
The protocol for soft-surgery cochlear implantation as originally described by Lehnhardt has changed little over the past 15 years. The original protocol was developed based on surgical experience, common sense, and knowledge of cochlear anatomy and physiology. Many of the tenets of that surgery have been supported by further surgical experience, direct animal testing, and prospective studies. During this time, however, additional studies have identified factors that can also influence hearing outcomes and the intracochlear reaction to surgical trauma. In light of this, there are potential modifications to the soft-surgery technique that may merit consideration in order to improve the residual hearing preservation rate even further.
Noise and vibration. Cochlea-induced trauma from surgical drilling is of concern in the individual with substantial residual hearing. Such trauma may be secondary to noise or to vibration. Pau, Just, Bornitz, Lasurashvilli, and Zahnert (2007) in a temporal bone study showed that drilling on the promontory exceeds 100 dB SPL, and touching the cochlea endosteum with a rotating burr can exceed 130 dB SPL as measured at the round window membrane (Pau et al., 2007). Similarly, Hickey and O'Connor (1991), using an indirect method of intraoperative ECoG masking in humans, determined that drill noise exceeds 90 dB HL while performing a superficial mastoidectomy and 100 dB HL in the deeper dissections.
Vibration has also been identified as an independent factor in causing cochlea damage and sensorineural hearing loss during drilling (Zou, Bretlau, Pyykko, Starck, & Toppila, 2001). One potential site of damage may be within the stria vascularis. Specifically, vibration of the ossicular chain in a guinea pig model showed disruption of vascular integrity and degradation of intermediate cells in this region (Seki, Miyasaka, Edamatsu, & Watanabe, 2001). Hair cells also seem to be affected by drilling vibration. Leonard and Khanna (1984) showed damage to these structures in both the drilled and non-drilled ears of cats, suggesting cranial vibration to be the culprit. Thus, performance of the mastoidectomy and cochleostomy, or inadvertent contact with the ossicular chain, during implant surgery can potentially cause sensorineural hearing loss.
These studies support the tenet of avoiding contact with the ossicular chain while drilling and support removing the last layer of bone over the cochlea endosteum without drilling. Further investigation may provide alternatives to the mastoidectomy—for example, the suprameatal approach (Kronenberg & Migirov, 2005). Although this entails drilling, it may represent less cumulative vibration and noise to the inner ear. Alternatives to the cochleostomy include the round window approach, whose benefits and concerns are discussed above, and the use of the laser in place of drilling. Some otologic lasers, however, appear to be more damaging to residual hearing than mechanical drilling (Kiefer, Tillein, Ye, Klinke, & Gstoettner, 2004b). At present, drilling will continue to be a mainstay of implant surgery. Therefore, prevention of damage to residual hearing may require adjuncts and additives to prevent noise- and vibration-induced trauma.
Hypothermia. A relatively easy addition to the soft-surgery protocol would be the induction of mild hypothermia during the procedure. Balkany and colleagues (2005) showed that mild hypothermia (i.e., 34°C vs. 37°C) in rats significantly reduced immediate and delayed hearing loss caused by implantation trauma (Balkany et al., 2005). Cooling blankets, and possibly cooled irrigation, may help preserve residual hearing, and these options could be easily studied.
Antioxidants/Neurotrophins. Many substances have cochlear protective effects against noise-induced and toxin-induced hearing loss. However, few substances have studied in specific relation to cochlear implantation. The Van de Water group has shown in vitro that the JNK/c-Jun pathway can be inhibited by several peptides and oligonucleotides. These substances rescue auditory neurons from apoptosis caused by oxidative stressors (Scarpidis et al., 2003). Subsequently, the group demonstrated in vivo that inhibition of this pathway by D-JNKI-1 could prevent ABR and DPOAE (distortion product otoacoustic emission) changes after electrode insertion trauma (Eshraghi et al., 2006). Other neurotrophins and antioxidants have not proved to be as effective. N-acetylcysteine, brain derived neurotrophic factor, and ciliary neurotrophic factor failed to provide significant protective effects against vibration-induced cochlear toxicity (Zou et al., 2003).
Additional steps. Other protocols to help preserve cochlea function during electrode insertion are under investigation. These steps include better sealing of the cochleostomy site to prevent perilymph leakage, as with the use of a fascia washer around the array (Gantz et al., 2005). Performance of a less traumatic cochleostomy with the use of a micromanipulator coupled to the drill is also being investigated (Manrique et al., 2007). The use of intraoperative electrophysiological monitoring of residual hearing may prove to be a critical addition to the soft-surgery protocol and allow the surgeon real-time feedback regarding the effects of insertion on cochlea function (Oghalai et al., 2008).
Conclusions
The surgical methodology that provides the highest probability of preserving usable low-frequency hearing during partial cochlear implantation has not been fully defined. Evidence thus far suggests several critical and important guidelines that the surgeon should adhere to during soft surgery. Avoiding blood entry into the cochlea, using hyaluronate as a protectant and lubricant, and avoiding drill contact with the ossicular chain and cochlear endosteum are all reasonably supported. Bone dust contamination of the cochlea and suctioning of perilymph are probably harmful and are easy to avoid. Topical steroid at the cochleostomy site is not supported but systemic use is likely to be protective. Either round window or cochleostomy approaches can be safely used, but the former may not be suitable for contoured devices. The cochleostomy approach appears safe for straight and contoured devices but must be placed anterior-inferior to the round window membrane to avoid intrascalar trauma. During either insertion route, the surgeon must be mindful of any resistance so as to avoid mechanical trauma to the cochlea. This article highlights the importance of systematic recording of outcomes and surgical events, so that a safe protocol for hearing preservation cochlear implantation can ultimately be defined.
Acknowledgment
The authors would like to acknowledge the contribution of Richard M. Runge, Ph.D., for his translation of the manuscripts written in German.
References
- Adunka O. F., Buchman C. A. (2007). Scala tympani cochleostomy I: Results of a survey. Laryngoscope, 117, 2187–2194 [DOI] [PubMed] [Google Scholar]
- Adunka O., Gstoettner W., Hambek M., Unkelbach M. H., Radeloff A., Kiefer J. (2004a). Preservation of basal inner ear structures in cochlear implantation. ORL: Journal for Oto-Rhino-Laryngology and Its Related Specialties, 66, 306–312 [DOI] [PubMed] [Google Scholar]
- Adunka O., Kiefer J., Unkelbach M. H., Lehnert T., Gstoettner W. (2004b). Development and evaluation of an improved cochlear implant electrode design for electric acoustic stimulation. Laryngoscope, 114, 1237–1241 [DOI] [PubMed] [Google Scholar]
- Adunka O., Unkelbach M. H., Mack M., Hambek M., Gstoettner W., Kiefer J. (2004c). Cochlear implantation via the round window membrane minimizes trauma to cochlear structures: A histologically controlled insertion study. Acta Oto-laryngologica, 124, 807–812 [DOI] [PubMed] [Google Scholar]
- Adunka O., Kiefer J. (2006). Impact of electrode insertion depth on intracochlear trauma. Otolaryngology—Head and Neck Surgery, 135, 374–382 [DOI] [PubMed] [Google Scholar]
- Adunka O. F., Pillsbury H. C., Kiefer J. (2006). Combining perimodiolar electrode placement and atraumatic insertion properties in cochlear implantation: Fact or fantasy? Acta Oto-laryngologica, 126, 475–482 [DOI] [PubMed] [Google Scholar]
- Adunka O. F., Radeloff A., Gstoettner W. K., Pillsbury H. C., Buchman C. A. (2007). Scala tympani cochleostomy II: Topography and histology. Laryngoscope, 117, 2195–2200 [DOI] [PubMed] [Google Scholar]
- Alexiades G., Roland J. T., Jr, Fishman A. J., Shapiro W., Waltzman S. B., Cohen N. L. (2001). Cochlear reimplantation: Surgical techniques and functional results. Laryngoscope, 111, 1608–1613 [DOI] [PubMed] [Google Scholar]
- Angeli S. I. (2006). Hyaluronate gel stapedotomy. Otolaryngology—Head and Neck Surgery, 134, 225–231 [DOI] [PubMed] [Google Scholar]
- Anniko M., Arnold W. (1995). Hyaluronic acid as a molecular filter and friction-reducing lubricant in the human inner ear. ORL: Journal for Oto-rhino-laryngology and Its Related Specialties, 57, 82–86 [DOI] [PubMed] [Google Scholar]
- Anniko M., Hellstrom S., Laurent C. (1987). Reversible changes in inner ear function following hyaluronan application in the middle ear. Acta Oto-laryngologica. Supplementum, 442, 72–75 [DOI] [PubMed] [Google Scholar]
- Aschendorff A., Klenzner T., Richter B., Kubalek R., Nagursky H., Laszig R. (2003). Evaluation of the HiFocus electrode array with positioner in human temporal bones. Journal of Laryngology and Otology, 117, 527–531 [DOI] [PubMed] [Google Scholar]
- Balkany T. J., Eshraghi A. A., Jiao H., Polak M., Mou C., Dietrich D. W., et al. (2005). Mild hypothermia protects auditory function during cochlear implant surgery. Laryngoscope, 115, 1543–1547 [DOI] [PubMed] [Google Scholar]
- Baumgartner W. D., Jappel A., Morera C., Gstoettner W., Muller J., Kiefer J., et al. (2007). Outcomes in adults implanted with the FLEXsoft electrode. Acta Oto-laryngologica, 127, 579–586 [DOI] [PubMed] [Google Scholar]
- Berrettini S., Forli F., Passetti S. (2008). Preservation of residual hearing following cochlear implantation: Comparison between three surgical techniques. Journal of Laryngology and Otology, 122, 246–252 [DOI] [PubMed] [Google Scholar]
- Bjurstrom S., Slepecky N., Angelborg C. (1987). A histopathological study of the inner ear after the administration of hyaluronan into the middle ear of the guinea pig. Acta Oto-laryngologica. Supplementum, 442, 62–65 [DOI] [PubMed] [Google Scholar]
- Briggs R., Tykocinski M., Saunders E., Hellier W., Dahm M., Pyman B., et al. (2001). Surgical implications of perimodiolar cochlear implant electrode design: Avoiding intracochlear damage and scala vestibuli insertion. Cochlear Implants International, 2, 135–149 [DOI] [PubMed] [Google Scholar]
- Briggs R. J., Tykocinski M., Stidham K., Roberson J. B. (2005). Cochleostomy site: Implications for electrode placement and hearing preservation. Acta Oto-laryngologica, 125, 870–876 [DOI] [PubMed] [Google Scholar]
- Briggs R. J., Tykocinski M., Xu J., Risi F., Svehla M., Cowan R., et al. (2006). Comparison of round window and cochleostomy approaches with a prototype hearing preservation electrode. Audiology and Neuro-otology, 11 (Suppl. 1), 42–48 [DOI] [PubMed] [Google Scholar]
- Canalis R. F., Gussen R., Abemayor E., Andrews J. (1987). Surgical trauma to the lateral semicircular canal with preservation of hearing. Laryngoscope, 97, 575–581 [DOI] [PubMed] [Google Scholar]
- Carvalho G. J., Lalwani A. K. (1999). The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. American Journal of Otology, 20, 87–90 [PubMed] [Google Scholar]
- Causse J. B., Causse J. R., Wiet R. J., Yoo T. J. (1983). Complications of stapedectomies. American Journal of Otology, 4, 275–280 [PubMed] [Google Scholar]
- Cervera-Paz F. J., Linthicum F. H., Jr. (2005). Cochlear wall erosion after cochlear implantation. Annals of Otology, Rhinology, and Laryngology, 114, 543–546 [DOI] [PubMed] [Google Scholar]
- Chen B. K., Clark G. M., Jones R. (2003). Evaluation of trajectories and contact pressures for the straight nucleus cochlear implant electrode array: A two-dimensional application of finite element analysis. Medical Engineering & Physics, 25, 141–147 [DOI] [PubMed] [Google Scholar]
- Choi C. H., Oghalai J. S. (2005). Predicting the effect of post-implant cochlear fibrosis on residual hearing. Hearing Research, 205, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. L. (1997). Cochlear implant soft surgery: Fact or fantasy? Otolaryngology—Head and Neck Surgery, 117, 214–216 [DOI] [PubMed] [Google Scholar]
- Engstrom B., Bjurstrom S., Jansson B., Engstrom H., Angelborg C. (1987). An ultrastructural and functional study of the inner ear after administration of hyaluronan into the middle ear of the guinea pig. Acta Oto-laryngologica. Supplementum, 442, 66–71 [DOI] [PubMed] [Google Scholar]
- Erixon E., Hogstorp H., Wadin K., Rask-Andersen H. (2009). Variational anatomy of the human cochlea: Implications for cochlear implantation. Otology & Neurotology, 30, 14–22 [DOI] [PubMed] [Google Scholar]
- Eshraghi A. A. (2006). Prevention of cochlear implant electrode damage. Current Opinion in Otolaryngology & Head and Neck Surgery, 14, 323–328 [DOI] [PubMed] [Google Scholar]
- Eshraghi A. A., Adil E., He J., Graves R., Balkany T. J., Van De Water T. R. (2007). Local dexamethasone therapy conserves hearing in an animal model of electrode insertion trauma-induced hearing loss. Otology & Neurotology, 28, 842–849 [DOI] [PubMed] [Google Scholar]
- Eshraghi A. A., He J., Mou C. H., Polak M., Zine A., Bonny C., et al. (2006). D-JNKI-1 treatment prevents the progression of hearing loss in a model of cochlear implantation trauma. Otology & Neurotology, 27, 504–511 [DOI] [PubMed] [Google Scholar]
- Eshraghi A. A., Polak M., He J., Telischi F. F., Balkany T. J., Van De Water T. R. (2005). Pattern of hearing loss in a rat model of cochlear implantation trauma. Otology & Neurotology, 26, 442–447, discussion 447. [DOI] [PubMed] [Google Scholar]
- Eshraghi A. A., Yang N. W., Balkany T. J. (2003). Comparative study of cochlear damage with three perimodiolar electrode designs. Laryngoscope, 113, 415–419 [DOI] [PubMed] [Google Scholar]
- Franco-Vidal V., Songu M., Blanchet H., Barreau X., Darrouzet V. (2007). Intracochlear hemorrhage after gamma knife radiosurgery. Otology & Neurotology, 28, 240–244 [DOI] [PubMed] [Google Scholar]
- Gantz B. J., Turner C., Gfeller K. E., Lowder M. W. (2005). Preservation of hearing in cochlear implant surgery: Advantages of combined electrical and acoustical speech processing. Laryngoscope, 115, 796–802 [DOI] [PubMed] [Google Scholar]
- Garcia-Ibanez L., Macias A. R., Morera C., Rodriguez M. M., Szyfter W., Skarszynski H., et al. (2008, August 27). An evaluation of the preservation of residual hearing with the Nucleus(R) Contour Advancetrade mark electrode. Acta Oto-laryngologica, 1–14 [DOI] [PubMed] [Google Scholar]
- Gfeller K. E., Olszewski C., Turner C., Gantz B., Oleson J. (2006). Music perception with cochlear implants and residual hearing. Audiology and Neuro-otology, 11 (Suppl. 1), 12–15 [DOI] [PubMed] [Google Scholar]
- Gjuric M., Wigand M. E., Hosemann W. (1992). Selective resection of the semicircular canals of rabbits with preservation of hearing. Acta Oto-laryngologica, 112, 907–915 [DOI] [PubMed] [Google Scholar]
- Gstoettner W., Franz P., Hamzavi J., Plenk H., Jr., Baumgartner W., Czerny C. (1999). Intracochlear position of cochlear implant electrodes. Acta Oto-laryngologica, 119, 229–233 [DOI] [PubMed] [Google Scholar]
- Gstoettner W., Kiefer J., Baumgartner W. D., Pok S., Peters S., Adunka O. (2004). Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Oto-laryngologica, 124, 348–352 [DOI] [PubMed] [Google Scholar]
- Gstoettner W., Plenk H., Jr., Franz P., Hamzavi J., Baumgartner W., Czerny C., et al. (1997). Cochlear implant deep electrode insertion: Extent of insertional trauma. Acta Oto-laryngologica, 117, 274–277 [DOI] [PubMed] [Google Scholar]
- Gstoettner W. K., van de Heyning P., O'Connor A. F., Morera C., Sainz M., Vermeire K., et al. (2008). Electric acoustic stimulation of the auditory system: Results of a multi-centre investigation. Acta Oto-laryngologica, 128, 968–975 [DOI] [PubMed] [Google Scholar]
- Hara M., Nomura Y., Saito K. (1990). Histopathologic study of the perilymph-suctioned labyrinth. Annals of Otology, Rhinology, and Laryngology, 99, 316–320 [DOI] [PubMed] [Google Scholar]
- Hargunani C. A., Kempton J. B., DeGagne J. M., Trune D. R. (2006). Intratympanic injection of dexamethasone: Time course of inner ear distribution and conversion to its active form. Otology & Neurotology, 27, 564–569 [DOI] [PubMed] [Google Scholar]
- Hickey S. A., O'Connor A. F. (1991). Measurement of drill-generated noise levels during ear surgery. Journal of Laryngology and Otology, 105, 732–735 [DOI] [PubMed] [Google Scholar]
- House J. W. (1993). Stapedectomy technique. Otolaryngologic Clinics of North America, 26, 389–393 [PubMed] [Google Scholar]
- Huang C. Q., Tykocinski M., Stathopoulos D., Cowan R. (2007). Effects of steroids and lubricants on electrical impedance and tissue response following cochlear implantation. Cochlear Implants International, 8, 123–147 [DOI] [PubMed] [Google Scholar]
- Jackler R. K., Leake P. A., McKerrow W. S. (1989). Cochlear implant revision: Effects of reimplantation on the cochlea. Annals of Otology, Rhinology, and Laryngology, 98, 813–820 [DOI] [PubMed] [Google Scholar]
- James C., Albegger K., Battmer R., Burdo S., Deggouj N., Deguine O., et al. (2005). Preservation of residual hearing with cochlear implantation: How and why. Acta Oto-laryngologica, 125, 481–491 [DOI] [PubMed] [Google Scholar]
- Kelly J. P., Khanna S. M. (1984). Distribution of cochlear damage caused by the removal of the round window membrane. Hearing Research, 16, 109–126 [DOI] [PubMed] [Google Scholar]
- Kha H. N., Chen B. K. (2006). Determination of frictional conditions between electrode array and endosteum lining for use in cochlear implant models. Journal of Biomechanics, 39, 1752–1756 [DOI] [PubMed] [Google Scholar]
- Kiefer J., Gstoettner W., Baumgartner W., Pok S. M., Tillein J., Ye Q., et al. (2004a). Conservation of low-frequency hearing in cochlear implantation. Acta Oto-laryngologica, 124, 272–280 [DOI] [PubMed] [Google Scholar]
- Kiefer J., Tillein J., Ye Q., Klinke R., Gstoettner W. (2004b). Application of carbon dioxide and erbium:yttriumaluminum-garnet lasers in inner ear surgery: An experimental study. Otology & Neurotology, 25, 400–409 [DOI] [PubMed] [Google Scholar]
- Kiefer J., Ye Q., Tillein J., Adunka O., Arnold W., Gstoettner W. (2007). Protecting the cochlea during stapes surgery: Is there a role for corticosteroids? Advances in Oto-rhino-laryngol, 65, 300–307 [DOI] [PubMed] [Google Scholar]
- Kronenberg J., Migirov L. (2005). Is mastoidectomy indispensable in cochlear implant surgery? Journal of Otolaryngology, 34, 29–31 [DOI] [PubMed] [Google Scholar]
- Laszig R., Ridder G. J., Fradis M. (2002). Intracochlear insertion of electrodes using hyaluronic acid in cochlear implant surgery. Journal of Laryngology and Otology, 116, 371–372 [DOI] [PubMed] [Google Scholar]
- Laurent C., Hellstrom S., Anniko M. (1992). Cochlear effects of hyaluronan applied on ruptured round window membrane. Acta Oto-laryngologica. Supplementum, 493, 63–67 [PubMed] [Google Scholar]
- Laurent C., Hellstrom S., Stenfors L. E. (1986). Hyaluronic acid reduces connective tissue formation in middle ears filled with absorbable gelatin sponge: An experimental study. American Journal of Otolaryngology, 7, 181–186 [DOI] [PubMed] [Google Scholar]
- Lehnhardt E. (1993). Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO, 41, 356–359 [PubMed] [Google Scholar]
- Lenarz T., Stover T., Buechner A., Paasche G., Briggs R., Risi F., et al. (2006). Temporal bone results and hearing preservation with a new straight electrode. Audiology and Neuro-otology, 11 (Suppl. 1), 34–41 [DOI] [PubMed] [Google Scholar]
- Leonard D. G., Khanna S. M. (1984). Histological evaluation of damage in cat cochleas used for measurement of basilar membrane mechanics. Journal of the Acoustic Society of America, 75, 515–527 [DOI] [PubMed] [Google Scholar]
- Li P. M., Wang H., Northrop C., Merchant S. N., Nadol J. B., Jr. (2007). Anatomy of the round window and hook region of the cochlea with implications for cochlear implantation and other endocochlear surgical procedures. Otology & Neurotology, 28, 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique M. J., Savall J., Cervera-Paz F. J., Rey J., Der C., Echeverria M., et al. (2007). Atraumatic surgical approach to the cochlea with a micromanipulator. Acta Oto-laryngologica, 127, 122–131 [DOI] [PubMed] [Google Scholar]
- Marsh M. A., Jenkins H. A., Coker N. J. (1992). Histopathology of the temporal bone following multichannel cochlear implantation. Archives of Otolaryngol—Head and Neck Surgery, 118, 1257–1265 [DOI] [PubMed] [Google Scholar]
- Martini A., Rubini R., Ferretti R. G., Govoni E., Schiavinato A., Magnavita V., et al. (1992). Comparative ototoxic potential of hyaluronic acid and methylcellulose. Acta Oto-laryngologica, 112, 278–283 [DOI] [PubMed] [Google Scholar]
- McElveen J. T., Jr., Wolford R. D., Jr., Miyamoto R. T. (1995). Implications of bone pate in cochlear implant surgery. Otolaryngology—Head and Neck Surgery, 112, 457–460 [DOI] [PubMed] [Google Scholar]
- Nadol J. B., Jr., Eddington D. K. (2006). Histopathology of the inner ear relevant to cochlear implantation. Advances in Oto-rhino-laryngology, 64, 31–49 [DOI] [PubMed] [Google Scholar]
- Nadol J. B., Jr., Shiao J. Y., Burgess B. J., Ketten D. R., Eddington D. K., Gantz B. J., et al. (2001). Histopathology of cochlear implants in humans. Annals of Otology, Rhinology, and Laryngology, 110, 883–891 [DOI] [PubMed] [Google Scholar]
- Neumann M., Aschendorff A., Schipper J., Laszig R., Klenzner T. (2005). The influence of insertion depth on the preservation of residual hearing after cochlear implantation. Laryngorhinootologie, 84, 113–116 [DOI] [PubMed] [Google Scholar]
- Oghalai J. S., Tonini R., Rasmus J., Emery C., Manolidis S., Vrabec J. T., et al. (2008). Intra-operative monitoring of cochlear function during cochlear implantation. Cochlear Implants International, 10, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary M. J., Fayad J., House W. F., Linthicum F. H., Jr. (1991). Electrode insertion trauma in cochlear implantation. Annals of Otology, Rhinology, and Laryngology, 100, 695–699 [DOI] [PubMed] [Google Scholar]
- Otake H., Sugiura M., Naganawa S., Nakashima T. (2006). 3D-FLAIR magnetic resonance imaging in the evaluation of mumps deafness. International Journal of Pediatric Otorhinolaryngology, 70, 2115–2117 [DOI] [PubMed] [Google Scholar]
- Paparella M. M., Lim D. J., Sugiura S. (1967). Inner ear pathology following stapedectomy in animals. Transactions—American Academy of Ophthalmology and Otolaryngology, 71, 408–415 [PubMed] [Google Scholar]
- Pau H. W., Just T., Bornitz M., Lasurashvilli N., Zahnert T. (2007). Noise exposure of the inner ear during drilling a cochleostomy for cochlear implantation. Laryngoscope, 117, 535–540 [DOI] [PubMed] [Google Scholar]
- Plontke S. K., Biegner T., Kammerer B., Delabar U., Salt A. N. (2008). Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otology & Neurotology, 29, 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeloff A., Unkelbach M. H., Tillein J., Braun S., Helbig S., Gstoettner W., et al. (2007). Impact of intrascalar blood on hearing. Laryngoscope, 117, 58–62 [DOI] [PubMed] [Google Scholar]
- Rizer F. M., Arkis P. N., Lippy W. H., Schuring A. G. (1988). A postoperative audiometric evaluation of cochlear implant patients. Otolaryngology—Head and Neck Surgery, 98, 203–206 [DOI] [PubMed] [Google Scholar]
- Rogowski M., Reiss G., Lehnhardt E. (1995). Morphologic study of the guinea pig cochlea after cochlear implantation using the “soft surgery” technique. Annals of Otology, Rhinology, & Laryngology Supplement, 166, 434–436 [PubMed] [Google Scholar]
- Roland J. T., Jr. (2005). A model for cochlear implant electrode insertion and force evaluation: Results with a new electrode design and insertion technique. Laryngoscope, 115, 1325–1339 [DOI] [PubMed] [Google Scholar]
- Roland J. T., Jr., Magardino T. M., Go J. T., Hillman D. E. (1995). Effects of glycerin, hyaluronic acid, and hydroxypropyl methylcellulose on the spiral ganglion of the guinea pig cochlea. Annals of Otology, Rhinology, & Laryngology Supplement, 166, 64–68 [PubMed] [Google Scholar]
- Scarpidis U., Madnani D., Shoemaker C., Fletcher C. H., Kojima K., Eshraghi A. A., et al. (2003). Arrest of apoptosis in auditory neurons: implications for sensorineural preservation in cochlear implantation. Otology & Neurotology, 24, 409–417 [DOI] [PubMed] [Google Scholar]
- Schindler R. A., Bjorkroth B. (1979). Traumatic intracochlear electrode implantation. Laryngoscope, 89, 752–758 [DOI] [PubMed] [Google Scholar]
- Seki M., Miyasaka H., Edamatsu H., Watanabe K. (2001). Changes in permeability of strial vessels following vibration given to auditory ossicle by drill. Annals of Otology, Rhinology, and Laryngology, 110, 122–126 [DOI] [PubMed] [Google Scholar]
- Shepherd R. K., Clark G. M., Xu S. A., Pyman B. C. (1995). Cochlear pathology following reimplantation of a multichannel scala tympani electrode array in the macaque. American Journal of Otolaryngology, 16, 186–199 [PubMed] [Google Scholar]
- Skarzynski H., Lorens A., D'Haese P., Walkowiak A., Piotrowska A., Sliwa L., et al. (2002). Preservation of residual hearing in children and post-lingually deafened adults after cochlear implantation: An initial study. ORL: Journal for Oto-Rhino-Laryngology and Its Related Specialties, 64, 247–253 [DOI] [PubMed] [Google Scholar]
- Skarzynski H., Lorens A., Piotrowska A., Anderson I. (2007a). Partial deafness cochlear implantation in children. InternationalJournal of Pediatric Otorhinolaryngology, 71, 1407–1413 [DOI] [PubMed] [Google Scholar]
- Skarzynski H., Lorens A., Piotrowska A., Anderson I. (2007b). Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach. Acta Oto-laryngologica, 127, 41–48 [DOI] [PubMed] [Google Scholar]
- Smouha E. E. (2003). Surgery of the inner ear with hearing preservation: Serial histological changes. Laryngoscope, 113, 1439–1449 [DOI] [PubMed] [Google Scholar]
- Soda-Merhy A., Gonzalez-Valenzuela L., Tirado-Gutierrez C. (2008). Residual hearing preservation after cochlear implantation: Comparison between straight and perimodiolar implants. Otolaryngology—Head and Neck Surgery, 139, 399–404 [DOI] [PubMed] [Google Scholar]
- Stover T., Issing P., Graurock G., Erfurt P., ElBeltagy Y., Paasche G., et al. (2005). Evaluation of the advance off-stylet insertion technique and the cochlear insertion tool in temporal bones. Otology & Neurotology, 26, 1161–1170 [DOI] [PubMed] [Google Scholar]
- Todt I., Rademacher G., Wagner J., Gopel F., Basta D., Haider E., et al. (2008). Evaluation of cochlear implant electrode position after a modified round window insertion by means of a 64-multislice CT. Acta Oto-laryngologica, 1–5 [DOI] [PubMed] [Google Scholar]
- Turner C. W., Reiss L. A., Gantz B. J. (2008). Combined acoustic and electric hearing: Preserving residual acoustic hearing. Hearing Research, 242, 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivero R. J., Joseph D. E., Angeli S., He J., Chen S., Eshraghi A. A., et al. (2008). Dexamethasone base conserves hearing from electrode trauma-induced hearing loss. Laryngoscope, 118, 2028–2035 [DOI] [PubMed] [Google Scholar]
- Wardrop P., Whinney D., Rebscher S. J., Luxford W., Leake P. (2005a). A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. II: Comparison of Spiral Clarion and HiFocus II electrodes. Hearing Research, 203, 68–79 [DOI] [PubMed] [Google Scholar]
- Wardrop P., Whinney D., Rebscher S. J., Roland J. T., Jr., Luxford W., Leake P. A. (2005b). A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I: Comparison of Nucleus banded and Nucleus Contour electrodes. Hearing Research, 203, 54–67 [DOI] [PubMed] [Google Scholar]
- Wayoff M. R., Moeller R. B., Roche R. (1971). A new and safe technique for removal of a dropped footplate during stapedectomy. Eye, Ear, Nose & Throat Monthly, 50, 246–248 [PubMed] [Google Scholar]
- Welling D. B., Hinojosa R., Gantz B. J., Lee J. T. (1993). Insertional trauma of multichannel cochlear implants. Laryngoscope, 103, 995–1001 [DOI] [PubMed] [Google Scholar]
- Wright C. G., Roland P. S., Kuzma J. (2005). Advanced bionics thin lateral and Helix II electrodes: A temporal bone study. Laryngoscope, 115, 2041–2045 [DOI] [PubMed] [Google Scholar]
- Ye Q., Tillein J., Hartmann R., Gstoettner W., Kiefer J. (2007). Application of a corticosteroid (Triamcinolon) protects inner ear function after surgical intervention. Ear and Hearing, 28, 361–369 [DOI] [PubMed] [Google Scholar]
- Yoshida T., Sugiura M., Naganawa S., Teranishi M., Nakata S., Nakashima T. (2008). Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings and prognosis in sudden sensorineural hearing loss. Laryngoscope, 118, 1433–1437 [DOI] [PubMed] [Google Scholar]
- Zou J., Bretlau P., Pyykko I., Starck J., Toppila E. (2001). Sensorineural hearing loss after vibration: An animal model for evaluating prevention and treatment of inner ear hearing loss. Acta Oto-laryngologica, 121, 143–148 [DOI] [PubMed] [Google Scholar]
- Zou J., Bretlau P., Pyykko I., Toppila E., Olovius N. P., Stephanson N., et al. (2003). Comparison of the protective efficacy of neurotrophins and antioxidants for vibration-induced trauma. ORL: Journal for Oto-Rhino-Laryngology and Its Related Specialties, 65, 155–161 [DOI] [PubMed] [Google Scholar]


