Abstract
Episodic memory decline is a hallmark of normal cognitive aging. Here, we report the first event-related fMRI study to directly investigate age differences in the neural reactivation of qualitatively rich perceptual details during recollection. Younger and older adults studied pictures of complex scenes at different presentation durations along with descriptive verbal labels, and these labels subsequently were used during fMRI scanning to cue picture recollections of varying perceptual detail. As expected from prior behavioral work, the two groups subjectively rated their recollections as containing similar amounts of perceptual detail, despite objectively measured recollection impairment in older adults. In both age groups, comparisons of retrieval trials that varied in recollected detail revealed robust activity in brain regions previously linked to recollection, including hippocampus and both medial and lateral regions of the prefrontal and posterior parietal cortex. Critically, this analysis also revealed recollection-related activity in visual processing regions that were active in an independent picture-perception task, and these regions showed age-related reductions in activity during recollection that cannot be attributed to age differences in response criteria. These fMRI findings provide new evidence that aging reduces the absolute quantity of perceptual details that are reactivated from memory, and they help to explain why aging reduces the reliability of subjective memory judgments.
Keywords: aging, episodic memory, fMRI, recollection, reactivation
1. Introduction
Neuroimaging studies have provided key insights into the mechanisms of age-related episodic memory decline, or the finding that older adults are less likely than younger adults to accurately recollect specific aspects of past experiences (for overviews, see Dennis et al., 2013). Many studies have focused on brain activity during encoding, and a common finding is that older adults show different patterns of activity than younger adults in regions of the medial temporal lobes (MTL, including hippocampus) and prefrontal cortex (PFC) (e.g., Daselaar et al., 2003; Gutchess et al., 2005), as well as differential patterns of functional connectivity between these regions (e.g., Grady et al., 2003). These activity differences in older adults often are attributed to suboptimal binding of event details into a coherent memory (a process that depends on MTL), as well as suboptimal use of elaborative encoding strategies (a process that depends on PFC), consistent with age-related structural decline in these regions (for discussion see Craik & Rose, 2012; Mitchell & Johnson, 2009). Older adults also show reduced encoding-related activity in regions associated with visual perception including fusiform gyrus, parahippocampal gyrus, and occipital cortex (e.g., Burgmans et al., 2010; Grady et al. 1999; Gutchess et al., 2005; Persson et al., 2011; but see Dulas & Duarte, 2011), and reduced functional connectivity between these regions and hippocampus (e.g., Leshikar et al., 2010; Dennis et al., 2008b), implicating reduced encoding of visual details.
Fewer neuroimaging studies have investigated brain activity during memory retrieval, and early findings suggested that age-related activity differences at retrieval are less pronounced than at encoding (for review see Persson & Nyberg, 2006). However, more recent fMRI studies have revealed important age differences at retrieval. For example, several studies have found age-related differences in brain activity in lateral PFC regions that are linked to strategic or controlled aspects of memory retrieval (e.g., McDonough et al., 2013; Mitchell et al., 2013; Velanova et al., 2007), as well as age differences in the functional connectivity between these PFC regions and hippocampus during retrieval (Dew et al., 2012). These findings are consistent with behavioral research indicating that older adults are less likely than younger adults to engage in strategic retrieval processes that allow them to successfully retrieve and evaluate encoded information (e.g., Jacoby et al., 2005; Koutstaal, 2003; Thomas & Bulevich, 2006; McDonough & Gallo, 2013).
Neuroimaging studies of memory retrieval also have revealed age-related differences in MTL activity, potentially reflecting the disproportionate age impairment of recollection (i.e., the conscious experience of retrieving specific contextual details) compared with familiarity (i.e., a decontextualized feeling of oldness towards a retrieval cue, see Diana et al., 2007). Age-related reductions have been found in hippocampal activity associated with recollection (see Daselaar et al., 2006; Giovanello et al., 2010), although this effect is not always found (e.g., Angel et al., 2013; Duverne et al., 2008; Dulas & Duarte, 2012; Persson et al., 2011). In contrast, some studies have found age-related increases in parahippocampal gyrus at retrieval (e.g. Daselaar et al., 2006; Giovanello et al., 2010), potentially implicating a greater reliance on familiarity. These findings underscore that – depending on the nature of the memory task – age differences in brain activity at retrieval can have multiple interpretations, including differences in the integrity of recollection or familiarity processes, or differences in the strategic reliance on these processes.
1.1 Recollection and Perception-Specific Reactivation
Although MTL and PFC regions are classically associated with episodic memory (e.g., Rugg & Vilberg, 2012), a key discovery from neuroimaging studies is that recollection involves activity in some of the same brain regions that process perceptual features experienced in the original event. This research relies on the use of a reactivation task, in which perceptually detailed stimuli (such as pictures) are studied, and on a later memory test, participants are instructed to retrieve as much perceptual detail from the study stimuli as possible, using only words as retrieval cues. In one of the first uses of this kind of reactivation task, Wheeler et al. (2000; see also Nyberg et al., 2000) presented participants with common object labels (e.g., the word “dog”) and asked them to recollect whether the label had, in an earlier encoding session, been associated with the presentation of a picture or a sound (e.g., whether they recollected earlier seeing a picture of a dog or hearing the sound of barking). Using event-related fMRI at retrieval, they found greater brain activity in secondary visual processing regions (e.g., inferior temporal and occipital regions) when recollecting pictures compared with sounds, but greater activity in auditory processing regions (e.g., superior temporal regions) when recollecting sounds compared with pictures. Critically, because recollection was cued only with words, differential activity in these perceptual regions could be attributed to reactivation of perceptual features from memory – a phenomenon dubbed “memory’s echo.” Other fMRI studies in younger adults have found similar reactivation effects for visually-detailed memories (e.g., Johnson & Rugg, 2007; Kahn et al., 2004; Khader et al., 2005; Wheeler & Buckner, 2003; Woodruff et al., 2005), and activity in these regions also can be related to the encoding and retrieval of false memories (Gonsalves et al., 2004; Kahn et al., 2004; Stark et al., 2010).
To our knowledge, no prior fMRI study has used a reactivation task to investigate age differences in the recollection of qualitatively rich perceptual details of studied stimuli, and the most relevant literature has provided mixed results. A handful of aging studies have re-presented studied pictures at test to compare brain activity associated with subjective reports of recollection versus familiarity (Angel et al., 2013; Dennis et al., 2013; Duarte et al., 2010). Each of these studies found age-related reductions in activity in posterior cortical regions (e.g., fusiform, lingual, and occipital gyri), potentially reflecting decreased perception-specific reactivation. However, because these studies re-presented studied pictures at test, activity in posterior regions could reflect differential processing of the test pictures themselves, as opposed to reactivation from memory. For example, younger participants might attend to more distinctive perceptual features of test pictures in an attempt to trigger recollection, thereby increasing activity in visual processing regions for recognized items. Because aging reduces activity in visual regions during the perceptual processing of pictures at study (e.g., Burgmans et al., 2010; Grady et al. 1999; Gutchess et al., 2005; Persson et al., 2011), one would expect similar reductions associated with the perceptual processing of pictures at test. Another interpretative difficulty of these studies is that the most commonly used subjective judgments of recollection do not specify the kinds of recollected information (cf. Tulving, 1985), and different age groups might choose to rely on different types of information when making these judgments (e.g., semantic vs. perceptual, see Dennis et al., 2008a; Norman & Schacter, 1997).
Source memory tasks provide another popular way to assess the recollection of specific information (for review see Mitchell & Johnson, 2009). In one line of research relevant to reactivation effects, studied pictures were re-presented at test and participants had to recollect specific aspects of their previous encoding context (e.g., whether they were earlier studied on the top or bottom of the computer screen; Duarte et al. 2008; Dulas & Duarte, 2012; Duvrne et al., 2008; Morcom et al., 2007). These studies tended to find overall similar patterns of brain activity in younger and older adults, or (sometimes) age-related increases in activity in regions associated with recollection, such as PFC, MTL, and posterior regions. These age-related increases in activity were attributed to neural inefficiency in some studies (see Duverne et al., 2008; Dulas & Duarte, 2012; Morcom et al., 2007), but at least for the activity in posterior regions, the re-presentation of pictures at test again makes a reactivation interpretation difficult. Moreover, the age-related increases in more posterior activity clearly are at odds with the reductions observed in the studies using subjective judgments.
Two more recent studies avoided the difficulties associated with re-presenting pictures at test, using words as retrieval cues for the recollection of previously studied pictures or words. As such, these studies were more similar to the reactivation tasks described earlier, but unlike reactivation tasks these studies did not require participants to retrieve as much perceptual detail as possible. In the most relevant conditions of one study, Mitchell et al. (2013) found age-related reductions in posterior activity that may have reflected reactivation of perceptual details from memories (i.e., middle and inferior temporal gyri, extending into middle occipital gyrus). However, this study used very short delays (i.e., a few seconds), so the extent that these results apply to perceptual reactivation at longer delays is unclear. A study by McDonough et al. (2013) used a longer delay that is more typical of episodic memory studies. This study was designed to investigate fMRI of retrieval monitoring processes, but an unpublished analysis of retrieval success effects (i.e., picture target hits > nonstudied lure rejections) revealed activity in posterior precuneus and inferior temporal gyrus that might have reflected perceptual reactivation. This activity did not differ between the age groups, contrasting with the aforementioned result of Mitchell et al. (2013), but note that overall activity was not very widespread in posterior visual processing regions in either of these studies, likely because the relevant tasks only required participants to recollect whether (or not) a picture had been studied compared with other sources of information.
In sum, no prior aging study has used verbal retrieval cues to investigate the reactivation of perceptual regions when participants are explicitly attempting to recollect as much perceptual detail from study stimuli as possible (i.e., a reactivation task). Moreover, although several studies have used subjective judgments or source memory tasks to investigate age differences in the recollection of pictures, some of these studies have shown age-related decreases in brain activity in perceptual regions while others have shown age-related increases. In the current study, we used a cued-recollection task for visually complex scenes, and we directly investigated age differences in perceptual reactivation under conditions where participants were explicitly attempting to retrieve as much perceptual detail as possible.
1.2 The Current Study
Using fMRI to measure perceptual reactivation during recollection can address a key theoretical question about aging that has been difficult to resolve using behavioral data alone (cf. Levy & Wagner, 2013). Older adults often report subjectively vivid or detailed recollections to the same extent as younger adults, while objective measures of recollection accuracy reveal significant age-related declines (e.g., Dodson et al., 2007; Gallo et al. 2011; Jacoby et al., 2005; McCabe et al., 2009; McDonough & Gallo, 2013; Wong et al., 2012). What accounts for this dissociation between subjective and objective measures of memory?
This behavioral dissociation can be interpreted in two different ways. According to a recollection quantity interpretation, older adults retrieve less information overall than younger adults, but they recalibrate their subjective judgments to this reduced level of output, thereby yielding the same distribution of strong and weak recollection judgments as younger adults. Such recalibration could be a natural consequence of differences in recollection quantity, and it also could be affected by age differences in metamemory (see Wong et al., 2012). According to a recollection quality interpretation, older adults sometimes retrieve the same overall amount of subjective detail as younger adults (as reflected in their subjective judgments), but a larger proportion of this retrieved information is irrelevant or distorted, thereby leading to reduced accuracy on objective measures (see Dodson et al., 2007). Note that the key question here is not whether aging affects recollection quantity or quality (it likely affects both), but rather it is how to interpret age-invariance in subjective judgments in the face of age differences in objective accuracy.
The two alternative interpretations are difficult to disentangle with only behavioral measures, but neuroimaging of perception-specific reactivation can potentially inform them. If older adults give similar subjective judgments as younger adults primarily because they recalibrate their subjective judgments after retrieving fewer perceptual details, then reactivation-related activity should be reduced in older compared with younger adults. But if older adults give similar subjective judgments as younger adults primarily because they retrieve more false or distorted details, then reactivation-related activity should be similar or even greater in older compared with younger adults.
To measure perception-related reactivation, as well as the neural correlates of perceptually detailed recollections more generally, the current study used a cued recollection task that capitalized on both subjective and objective components. During the study phase, participants were presented with visually complex pictures at various presentation durations, along with descriptive verbal labels that would later be presented as retrieval cues. We used complex pictures and varied presentation duration to elicit a wide range of recollection details. Importantly, in addition to studying labels associated with pictures (targets), participants also studied labels without accompanying pictures (lures) that served as catch trials on the subsequent cued recollection test. Familiarizing these lures during the study phase encouraged the use of picture recollection (instead of familiarity) to discriminate between targets and lures on the cued recollection test, and provided a means of assessing recollection judgment accuracy.
Event-related fMRI scanning was conducted during the cued-recollection test. On this test, the verbal labels from the study phase were presented and participants attempted to recollect the picture (if any) associated with the label and rate the amount of visual details that they could recollect. Only verbal labels were used as retrieval cues, allowing us to assess recollection-related brain activity in perceptual regions without having to compete with brain activity associated with picture viewing at test. After the cued-recollection test, we also collected fMRI data during a separate picture-perception task, allowing us to identify regions involved in the visual processing of pictures. Finally, following fMRI scanning, participants took a two-alternative-forced-choice (2AFC) test to objectively assess the quality of detail associated with their earlier subjective judgments of recollected detail.
Our primary analysis of brain activity contrasted targets associated with varying levels of subjectively reported recollection detail (strong or weak), using correct rejections of lures as a baseline. This analysis allowed us to directly compare brain activity associated with retrieval success across the two age groups, using a within-subject task-related baseline to avoid interpretive issues when comparing raw BOLD activity between age groups (e.g., unknown age-related physiological effects). Memory reactivation could occur during weak or strong recollection, so that age differences in brain activity relevant to reactivation could be found either in the main effect of age (i.e., different hit > correct rejection effects across the groups) or in the age x recollection detail interaction (i.e., different strong > weak effects across the groups; see section 2.5 for fMRI analysis details). To the extent that older adults retrieve fewer perceptual details than younger adults when making the same subjective recollection judgments, we expected decreased perceptual-reactivation effects (in either contrast) in older compared with younger adults (i.e., reduced recollection quantity). On the other hand, to the extent that older adults retrieve more irrelevant or distorted details when making the same subjective recollection judgments as younger adults (i.e., reduced recollection quality), we expected similar or greater perceptual-reactivation effects in older compared with younger adults (in either contrast). We also conducted a secondary fMRI analysis comparing brain activity to targets of varying durations regardless of participants’ responses, to ensure that any age-related reactivation effects observed in our primary analysis were not due to age differences in response criteria (see section 3.2.3).
2. Materials and Methods
2.1 Participants
Twenty-two younger and 27 older adults participated in the experiment for monetary compensation. Data from nine older adults were excluded from analysis due to movement artifact (5), difficulties with the task in the MRI environment (3), or scanner malfunction (1), yielding a final sample of 22 younger adults aged 19–29 years (M = 21.23; 13 female) and 18 older adults aged 65–82 (M = 73.72; 16 female). All participants were recruited from Chicago and the surrounding area, were right-handed, and none reported neuropsychological conditions associated with cognitive decline (e.g., Alzheimer’s disease, Parkinson’s disease, etc.), taking excessive alcohol or narcotics, having a history of psychiatric diagnoses, or having recent head trauma. All participants gave informed consent using methods approved by the appropriate human participants committees at the University of Chicago. Vision was normal or corrected to normal using MR-compatible glasses or contact lenses. The older adults were also screened for high mental functioning with the Mini-Mental State Examination (M = 28.29; Folstein, et al., 1975).
2.2 Materials
The experimental stimuli consisted of 128 colored photographs of objects and people in naturalistic contexts from the International Affective Picture System (IAPS; Lang et al., 2005). Each picture was given a unique 2 to 3 word descriptive label (e.g., “city skyline”, “messed up linens”, “golfer in sand”) that could be used to subsequently cue recollection of the picture at test (cf. Gallo et al., 2009). For each participant, 32 pictures and their associated labels were assigned to each of the study conditions (short study duration, medium study duration, and long study duration) with an additional 32 labels assigned to the no picture condition. All labels and associated pictures were counterbalanced across participants. Care was taken to select unique pictures that avoided graphic content, and to match the counterbalancing lists on arousal and valence based on the IAPS norms.
2.3 Design and Procedure
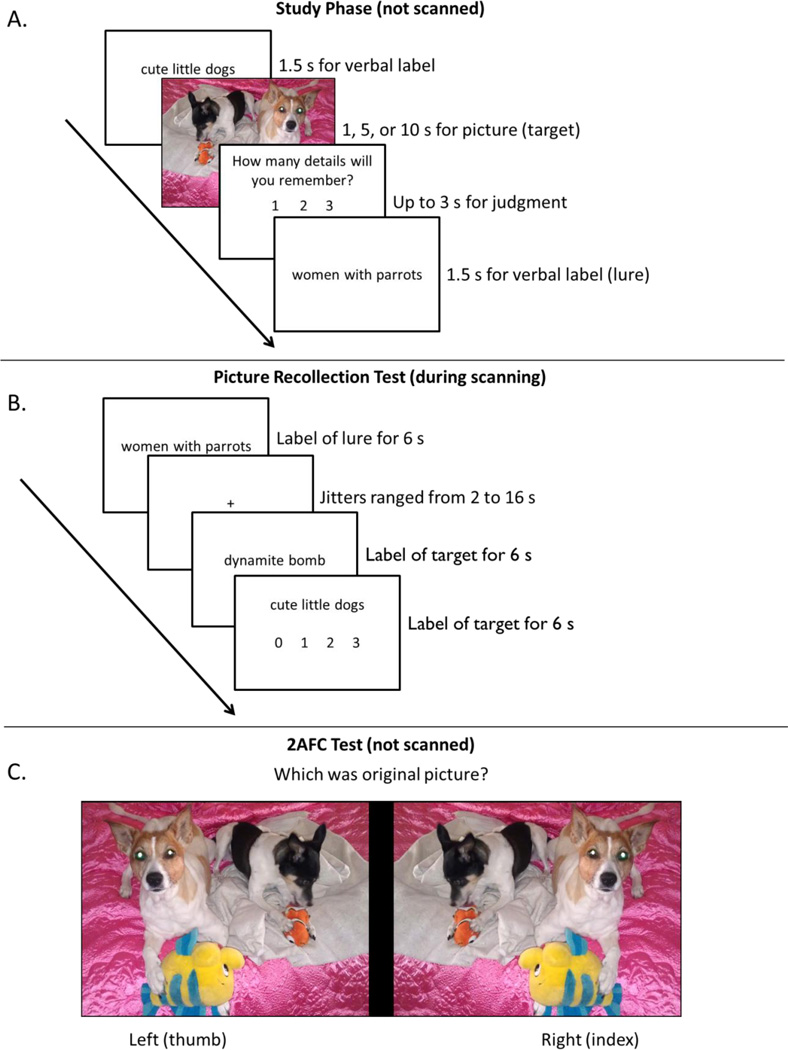
Figure 1 presents a schematic of the procedure. In the study phase (outside the scanner), participants viewed 128 labels in black font for 1.5 seconds each. Some of the labels were presented without their corresponding picture, and others were immediately followed by their corresponding picture for a varying duration (1 s, 5 s, or 10 s), all randomly ordered. Participants were told to remember the pictures and labels because their memory would later be tested with the labels as retrieval cues. After viewing each picture, participants were given 3 seconds to rate the amount of detail they believed that they would remember on the subsequent memory test (i.e., a judgment of learning), on a scale of 1 (low), 2 (medium), or 3 (high). Each event was separated by 500 ms.
Figure 1.
Panel A shows a schematic of the study phase. Verbal labels were presented for 1.5 s followed by a picture for either 1 s, 5 s, or 10 s. Participants then had up to 3 s to judge how many details from the picture they might remember. Panel B shows a schematic for the cued-recollection test during the scan period. Verbal labels were presented for 6 s and participants judged the amount of recollected details. Jittered fixations were interspersed among verbal labels (ranging from 2 s to 16 s). Panel C shows a schematic for the 2AFC Test. Participants were presented with the original picture and its mirror image and asked to judge which one was the originally presented image (self-paced).
Following the study phase, memory was tested in the MRI scanner. The scanning session included two functional runs for the cued-recollection test (approx. 9 min each), two functional runs for a perception localizer (approx. 5.5 min each), and a two-alternative-forced choice (2AFC) recognition test administered during an anatomical scan (approx. 2–7 min). Each functional run was buffered by 16 seconds of fixation. In the scanner, all stimuli were presented using mirrored projection, and responses were recorded using an MRI-compatible button box with the participants’ right hand.
During the cued-recollection test, all 128 labels from the study phase were presented as retrieval cues for the studied pictures. Test labels were presented in black font for 6 seconds each, and were randomly ordered and separated by a fixation cross of varying duration (jittered 2–12 s). Participants were told that all of the labels had been studied, but some had been studied with an associated picture (targets) and others had been studied without pictures (lures). For each test label, participants were instructed to attempt to recollect an associated picture from the study phase and form a mental image of the picture as best as possible, and then to rate the amount of detail that they were able to recollect on a scale of 0 (no detail), 1 (low detail), 2 (medium detail), or 3 (high detail). Participants were told that test labels that had been studied without a picture should receive a rating of 0 (no picture details). Presentation of the stimuli was time-locked to the MR signal, and their order was determined using Optseq (part of the FS-FAST analysis tools written by D. Greve, Charlestown, MA) to maximize the MR signal (e.g., Dale, 1999).
After the cued-recollection test, participants passively viewed 30 new IAPS pictures and 30 new word labels (unrelated to the pictures) during a picture-perception scan. We used IAPS pictures with similar valence and levels of arousal that were not in the primary experiment to assess picture perception independently from memory effects. Participants were told that this was a perception task, and that they should simply view each picture or label, without forming additional mental images or associations. These items were presented in alternating blocks of 15 pictures and 15 labels (4 s for each item), with each block separated by 16 seconds of fixation. This procedure was repeated in each of 2 functional runs, so that each picture or label was seen twice across runs. The order of the picture and label blocks was counterbalanced across participants.
After the picture-perception task, participants took the 2AFC test to objectively measure the detail associated with previous subjective recollection judgments. Participants viewed each of the 96 originally studied pictures and their mirror images on a split screen, randomly ordered. Care was taken to blur any details that would indicate a mirror image of the picture (e.g., words that appeared on street signs). They were asked to identify the originally studied picture, which was presented equally often on the left or the right side of the screen. This test was self-paced, and no functional data were acquired during this test.
2.4 MRI Data Acquisition and Preprocessing
A 3T Philips scanner was used to collect MR data at The University of Chicago Brain Research Imaging Center. Structural scans were acquired using high resolution T1-weighted structural Turbo Field Echo (TR = 7.4 ms, TE = 3.4 ms, flip angle = 8 degrees, FOV = 250 mm, matrix = 240 × 240 mm2, in-plane resolution = 1.04 × 1.04 mm2). For functional scans, T2*-weighted images are used to estimate neural activity via the BOLD response (30 interleaved axial slices, 4 mm thickness, .5 mm skip) were acquired for whole-brain coverage, using an EPI sequence (TR = 2000 ms, TE = 25 ms, flip angle = 80 degrees, FOV = 230 mm, matrix = 76 × 77 mm, in-plane resolution = 2.88 × 2.88 mm2). Four dummy scans were collected prior to the experiment, resulting in 618 total volumes acquired across the two functional runs for the primary functional scans and 336 total volumes acquired across the two secondary (picture perception) functional scans for each participant.
Data were processed using SPM8 (Wellcome Dept. of Imaging Neuroscience). Preprocessing steps included calculating rigid body motion correction parameters, manually reorienting the functional images to the ACPC including the mean image, coregistering structural to functional images, segmenting the structural intro gray matter, white matter, and CSF, normalizing to the MNI template (2 mm cubic voxels), and spatial smoothing (10 mm FWHM kernel). All functional data were visually inspected for quality control, and one run from an older adult was removed from subsequent analysis due to artifact.
2.5 fMRI Data Analysis
Functional MRI data for the cued-recollection test were analyzed under the assumptions of the general linear model (GLM). We estimated brain activity for each participant on a voxel-by-voxel basis for each of the event types of interest (see below). Brain activity was estimated for each event using a canonical hemodynamic response function (HRF) that was convolved with a mini-epoch of variable duration, starting at the onset of the test label and ending when participants pressed a response button (responses occurring after the stimulus left the display or when multiple responses were recorded were given an average response time value across all trials for that participant). This method is preferable to a stick function of zero duration because the retrieval processes of interest are thought to occur throughout these mini-epochs (cf. Grinband et al., 2008), and we found this method was more sensitive to memory effects in a previous aging study (McDonough et al., 2013). Temporal derivatives were also included in the model for each trial type for each individual to capture variable onset times across age groups (cf. Rombouts, 2005). The two runs were modeled as two separate sessions, using rigid body motion parameters, outliers due to movement/signal spikes (Mazaika et al., 2005), and session effects as regressors. A high-pass filter of 128 seconds was used.
In the first level of functional analysis, contrasts characterized activity associated with each event type of interest in each individual. To match the number of observations at each level of recollection detail in each age group, our primary fMRI analyses collapsed the two lower ratings of recollection detail into a single “weak recollection” response bin. Thus, four event types were modeled from the cued-recollection test, including ratings of strong recollected detail for targets (a response of “3”, or strong-hits), ratings of weak recollected detail for targets (a response of “1” or “2”, weak-hits), ratings of no recollected detail for targets (i.e. a response of “0”, misses), and ratings of no recollected detail for lures (i.e., a response of “0”, correct rejections). There were too few false alarms to separately model (i.e. ratings of 1, 2, or 3 to lures), so these responses were modeled as a junk variable along with trials that had missing responses.
To investigate effects associated with picture recollection, contrasts for each participant were generated for strong and weak hits using correct rejections as a baseline, which were then entered into a second-level analysis treating participants as a random effect, using a 2 (Age: young, old) × 2 (Recollection Detail: strong, weak) full factorial ANOVA. Using the correct rejections of lures as a baseline is theoretically important, because in our study these test labels had been studied along with those associated with the targets (and hence both should have been familiar), but unlike the target labels the lure labels had not been associated with a study picture. For this factorial analysis, we only included those voxels within a gray matter mask that also showed positive memory effects using an inclusive mask (hits > correct rejections) at a liberal threshold of p < .05. For the main effects of recollection detail and age, we further ensured that none of these regions showed a trend for an interaction, so all main effects were exclusively masked with the interaction F-test at p < .1 (to give a one-sided threshold of p < .05). Note that effects of age in this analysis could reflect differences in target picture recollection (i.e., retrieval success), as well as other cognitive processes that may vary with retrieval success, while controlling for possible age differences in brain activity that might have affected all of the test items (i.e., age-related physiological differences). All effects were considered significant if they exceeded a threshold of p < .05, corrected for multiple comparisons using Monte Carlo simulations (AlphaSim) at 10,000 iterations (equivalent to p < .0006 and 40 contiguous voxels).
Because our primary analysis involved subject-specific responses, it may have been affected by unintended group differences in response criteria (see Results section). To avoid these interpretative issues, a secondary analysis compared brain activity at retrieval as a function of the duration that pictures were presented during the study phase, using lures as a baseline. All targets and lures were included regardless of participants’ test responses. In this analysis, four event types were modeled: items previously studied with no picture (lures), with a picture for 1 s (short), with a picture for 5 s (medium), and with a picture for 10 s (long). Contrasts at the individual level using lures as a baseline were then entered in to a 2 (Age: younger, older) x 2 (Duration: short, medium, long) full factorial ANOVA. As with the recollection detail analysis, the results were inclusively masked to include only gray matter voxels with positive memory effects (targets > lures at p <.05), and main effects were exclusively masked with the interaction F-test (p < .1). Voxels were considered significant if they exceeded a threshold of p < .05, corrected for multiple comparisons using Monte Carlo simulations at 10,000 iterations (equivalent to p < .0006 and 33 contiguous voxels).
For the picture-perception task, the two conditions of interest (picture and labels) were modeled as a block design leaving fixation trials unmodeled. Brain activity was estimated on a voxel-by-voxel basis for each condition, modeled via convolution with a canonical hemodynamic response function (HRF) over a duration of 61.5 seconds for each condition. Two runs were modeled as two separate sessions, using rigid body motion parameters and session effects as regressors. A high-pass filter of 155 seconds was used due to the length of each block. To obtain our picture-perception effects, contrasts for each subject were generated for picture and label blocks against the implicit baseline. These contrasts were then entered into a second-level analysis treating participants as a random effect, using a 2 (Age: young, old) × 2 (Item: picture, label) full factorial ANOVA. From this full factorial analysis, a picture > label contrast was created collapsing across age group (p < .05 using Monte-Carlo correction and 92 contiguous voxels within a gray matter mask) to identify regions associated with picture perception. That is, both picture and label trials required basic visual and semantic processing, but viewing pictures required engagement of more detailed and complex visual processing than that required by the labels.
3. Results
3.1 Behavioral Data
3.1.1 Cued-recollection test
As expected, picture recollection was superior in younger than in older adults. Table 1 presents the overall rate of target hits (i.e., a non-zero recollection detail rating on the 0–3 scale) and lure correct rejections (i.e., a rating of 0) that were made as a proportion of the total number of targets (or lures) for which a response was registered during the fMRI session (97% of items for older adults, 99% for younger adults). As expected, younger adults had greater hits than older adults (.85 vs. .78), t(38) = 2.12, SEM = .032, p = .04, and fewer false alarms (i.e., 1 - correct rejections, or .10 vs .29), t(38) = 3.90, SEM = .048, p < .001, although both age groups clearly were able to successfully discriminate between targets and lures (both p’s < .001). Critically, even though older adults were less accurate than younger adults, the average recollection-detail rating for target hits was matched across age groups (both means = 1.83, t (38) < 1). This pattern replicates the typical behavioral finding described in the Introduction (section 1.2), showing similar subjective judgments of recollection detail for targets across the age groups, despite differences in objective measures of accuracy (also see below).
Table 1.
Cued-recollection test: Correct Responses and Latencies to Targets and Lures
| Mean Proportion | Response Latencies (s) | |||
|---|---|---|---|---|
| Younger | Older | Younger | Older | |
| Target Hits (total) | .85 (80.9) | .78 (73.6) | 2.37 | 2.65 |
| “Strong” hits | .39 (37.6) | .37 (35.7) | 2.20 | 2.45 |
| “Weak” hits | .45 (43.3) | .40 (37.9) | 2.52 | 2.91 |
| Lure Correct Rejections | .90 (28.6) | .71 (22.2) | 2.28 | 3.07 |
Note. Targets were verbal descriptions that had been studied with pictures of varying durations, whereas lures were descriptions that had been studied without a picture. Recollection detail ratings of “3” were coded as strong recollections and ratings of “2” or “1” were coded as weak recollections (i.e., target hits or lure false alarms); ratings of “0” were coded as no recollection (i.e., target misses or lure correct rejections). The average number of observations is included in parentheses.
To parallel the response bins used in our primary fMRI analysis, we divided target hits into those given strong recollection responses (a rating of “3”) and those given weak recollection responses (a rating of “1” or “2”). A 2 (Age: Younger, Older) × 2 (Recollection Detail: Strong, Weak) ANOVA on these data revealed only a main effect of age, F(1,38) = 4.46, MSE = .006, p = .041, ηp2 = .11, indicating that younger adults had greater overall hits than older adults but the proportion of weak and strong hits did not differ from each other or across age groups (all p’s > .40). Thus, there were a similar number of strong hits and weak hits across the two age groups for our fMRI analyses.1 A similar ANOVA on latencies for target hits revealed a main effect of age, F (1,38) = 5.81, MSE = .34, p = .02, ηp2 = .13, as older adults were slower than younger adults. There also was a main effect of recollected details, F (1,38) = 51.76, MSE = .058, p < .001, ηp2 = .18, and no interaction, F (1,38) = 1.50, MSE = .058, p = .23, ηp2 = .04, indicating that participants were faster to report strong hits than weak hits. This effect suggests that strong recollections were relatively easier to retrieve, while weaker recollections potentially required more retrieval-monitoring effort (i.e., search and evaluation). This effect is important for our fMRI analysis, because it implies that greater differences in brain activity for strong than weak recollection responses cannot be attributed to more time on task or retrieval-monitoring effort for stronger recollections.
As an alternative way to analyze data from the cued-recollection test, we separately calculated the average rating of recollected details (from 0 to 3) for the test items in each study duration condition (Table 2). To analyze these ratings, we subtracted the mean rating for lures from the mean rating for the targets in each study duration condition. This correction adjusts for the relatively higher recollection rating for lures in older adults than in younger adults, t(38) = 4.68, SEM = .076, p < .001 (i.e., the age difference in false alarms). A 2 (Age: younger, older) x 3 (Duration: short, medium, long) ANOVA on these adjusted ratings revealed a main effect of age, F(1,38) = 11.04, MSE = .64, p = .002, ηp2 = .23, as younger adults were more effective than older adults at using these ratings to differentiate between targets and lures. There also was a main effect of duration, F(2,76) = 128.40, MSE = .030, p < .001, ηp2 = .77, with higher ratings for longer durations. Finally, there was an age x duration interaction, F(2,76) = 4.42, MSE = .030, p = .015, ηp2 = .10, indicating that younger adults benefitted more than older adults from additional study time. Follow-up t-tests within each age group showed that recollection ratings differed as a function of study duration (long > medium > short) for younger (all p’s < .001) and older adults (all p’s < .001). Thus, recollection detail ratings were sensitive to the experimental manipulation of picture study duration in both age groups, demonstrating the validity of these subjective ratings.
Table 2.
Cued-recollection test: Recollection Detail Ratings and Latencies Across Picture Study Durations
| Mean Detail Ratings | Mean Latencies (s) | |||
|---|---|---|---|---|
| Study Duration | Younger | Older | Younger | Older |
| No Picture | 0.13 (.05) | 0.49 (.06) | 2.30 (.38) | 3.00 (.68) |
| Picture 1 s | 1.54 (.10) | 1.53 (.11) | 2.38 (.32) | 2.61 (.51) |
| Picture 5 s | 1.96 (.09) | 1.81 (.10) | 2.34 (.35) | 2.66 (.56) |
| Picture 10 s | 2.28 (.09) | 2.04 (.09) | 2.38 (.44) | 2.58 (.53) |
Note. Recollection ratings were on a scale of 0 (no detail) to 3 (high detail). Standard error of each mean is in parenthesis.
3.1.2 2AFC Test
The 2AFC test was administered after the fMRI session, providing an objective measure of memory accuracy for the items that were given subjective recollection ratings during fMRI. We analyzed 2AFC accuracy as a function of the recollection-detail bins used in our primary fMRI analysis (see Table 3). A 2 (Age: younger, older) × 2 (Recollection Detail: strong, weak) ANOVA on these 2AFC data revealed a main effect of age (younger > older), F (1,38) = 43.19, MSE = .014, p < .001, ηp2 = .53, a main effect of detail (strong > weak), F (1,38) = 25.83, MSE = .007, p < .001, ηp2 = .41, and no interaction (p = .69). It is worth underscoring that these age differences in 2AFC accuracy were found even when comparing items that were initially given the same recollection rating in each age group (i.e., greater accuracy in younger than older adults for strong recollection ratings, t(38) = 5.54, SEM = .03, p < .001, and for weak recollection ratings, t(38) = 5.34, SEM = .03, p < .001). These findings indicate that the initial subjective recollection judgments overestimated the amount of accurate details that older adults could retrieve compared with younger adults, again demonstrating the typical age dissociation between subjective and objective measures described in the Introduction (section 1.2). Nevertheless, these subjective recollection ratings tracked subsequent 2AFC accuracy in both younger adults, t(21) = 5.28, SEM = .02, p < .001, and older adults, t(17) = 2.84, SEM = .04, p = .01, and the lack of an interaction reveals that the 2AFC accuracy increase from weak to strong recollections was similar in magnitude in younger adults (.08) and older adults (.10). Because there was no age x detail interaction in the behavioral analysis, any brain activity showing an age x detail interaction cannot be attributed to a differential recollection detail effect between the two age groups, conceptually analogous to behavioral procedures that are sometimes used to match age groups on recollection accuracy (Angel et al., 2013; Duverne et al., 2008; Morcom et al., 2007).
Table 3.
2AFC Test Accuracy as a Function of Cued-recollection Test Response to Targets
| Younger | Older | |
|---|---|---|
| Weak Recollection | 0.80 (.02) | .61 (.03) |
| Strong Recollection | 0.88 (.02) | .71 (.02) |
Note. Standard error of the mean in parenthesis. Chance responding = 50%.
3.2 fMRI Data
3.2.1 Picture-Perception Localizer Task
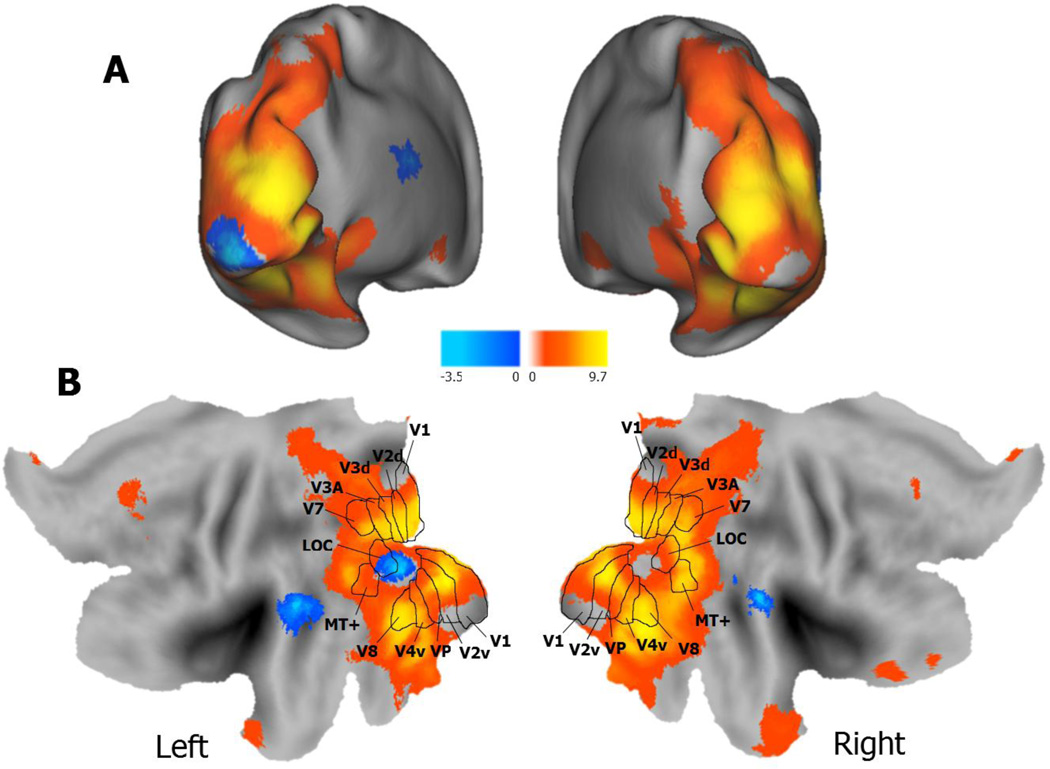
To identify regions associated with the visual processing of pictures more than the descriptive verbal labels, we compared blocked trials of passively viewing either pictures or descriptive verbal labels (p < .05, Monte-Carlo corrected). As seen in Table 4 and Figure 2, a pictures > labels contrast (collapsing across age group) revealed significant voxels with extensive and continuous activity in visual processing regions from occipital and extending dorsally to midline parietal regions, and ventrally to inferior temporal regions including the fusiform gyri and the parahippocampal gyri, all bilaterally. Additional clusters of activity were found in anterior cingulate cortex, left superior temporal gyrus, and left cerebellum. The activity in some of these latter regions may have reflected nonperceptual processes that were more likely to be initiated by visually processing pictures than verbal labels (e.g., generating semantic associations, working to encode perceptual details, etc.), but several of the more posterior regions likely were involved in greater perceptual processing of pictures. The reverse contrast (labels > pictures) revealed several significant clusters including left bilateral superior temporal gyri, middle temporal gyrus, left inferior occipital gyrus, left thalamus, left caudate, and right superior temporal gyrus. Only two clusters revealed an Age x Item interaction including the right lingual gyrus and the right fusiform gyrus. The interaction was due to a greater difference in brain activity for pictures than verbal labels for younger than older adults, possibly due to dedifferentiation of visual ventral activity in older adults (e.g., Grady et al., 1994; Park et al., 2012).
Table 4.
Peak Coordinates for the Picture-Perception Localizer Task Collapsed Across Age Groups
| MNI Coordinates (x, y, z) |
Region | BA | T | Cluster size (voxels) |
||
|---|---|---|---|---|---|---|
| Picture > Labels | ||||||
| −8 | 44 | 56 | L Superior Frontal Gyrus | 8/9 | 4.01 | 221 |
| −4 | 52 | 50 | L Medial Frontal Gyrus | 8 | 3.80 | - |
| 14 | 46 | 54 | R Superior Frontal Gyrus | 8 | 3.77 | - |
| 30 | 18 | −32 | R Superior Temporal Gyrus | 38/11 | 5.63 | 456 |
| 30 | 32 | −26 | R Inferior Frontal Gyrus | 11 | 3.66 | - |
| 0 | 14 | −10 | Bi Anterior Cingulate | 25 | 5.06 | 190 |
| 6 | −84 | −8 | R Lingual Gyrus | 18 | 13.64 | 32994 |
| 30 | −48 | −14 | R Fusiform Gyrus | 37 | 12.33 | - |
| −28 | −62 | −14 | L Cerebellum | - | 9.14 | - |
| 30 | −66 | −12 | R Fusiform Gyrus | 19 | 11.14 | - |
| 46 | −74 | −4 | R Inferior Temporal Gyrus | - | 9.80 | - |
| 36 | −78 | 14 | R Middle Occipital Gyrus | 18/19 | 11.93 | - |
| 30 | −84 | 32 | R Precuneus | 19 | 9.67 | - |
| −10 | −96 | 8 | L Middle Occipital Gyrus | 18 | 11.12 | - |
| 2 | −52 | 6 | R Posterior Cingulate | 29 | 3.99 | 108 |
| Labels > Pictures | ||||||
| −12 | −24 | 22 | L Thalamus | - | 4.04 | 99 |
| −12 | −24 | 28 | L Caudate Body | - | 3.89 | - |
| −64 | −28 | 2 | L Superior Temporal Gyrus | 22 | 4.33 | 352 |
| −54 | −40 | 6 | L Middle Temporal Gyrus | 22 | 3.90 | - |
| 68 | −36 | 14 | R Superior Temporal Gyrus | 22/41 | 4.17 | 151 |
| −28 | −96 | −10 | L Inferior Occipital Gyrus | 18 | 5.41 | 191 |
Notes. Coordinates are sorted front to back, top to bottom, left to right. L = Left, R = Right, Bi = Bilateral, BA = Approximate Brodmann Area. Regions with no cluster sizes represent sub-peaks within a cluster.
Figure 2.
Brain activity from the perceptual localizer task across age groups. Activity associated with picture perception (pictures > verbal labels) is depicted in yellow/orange and word perception (verbal labels > pictures) in blue/aqua. Panel A depicts brain activity on inflated brains in Panel A and on flattened brains in Panel B. The overlaid visual area boundaries on the flatted map (Panel B) are from van Essen (2003).
3.2.2 Cued-recollection Test: Retrieved Detail Analysis
Our primary analysis identified retrieval success effects (i.e., target hits > lure correct rejections) for two different levels of target recollection detail (strong and weak), and entered these effects into a 2 (Age: younger, older) × 2 (Recollection Detail: strong, weak) full factorial ANOVA. For all of these analyses, target hits > lure correct rejections was used as an inclusive mask to ensure all effects only showed positive memory effects. For the main effects, we exclusively masked the interaction effects to eliminate main effects that were qualified by the interaction. Rather than further masking the results by the picture-perception localizer contrast, we indicate which regions overlapped with the localizer in all figures and tables.
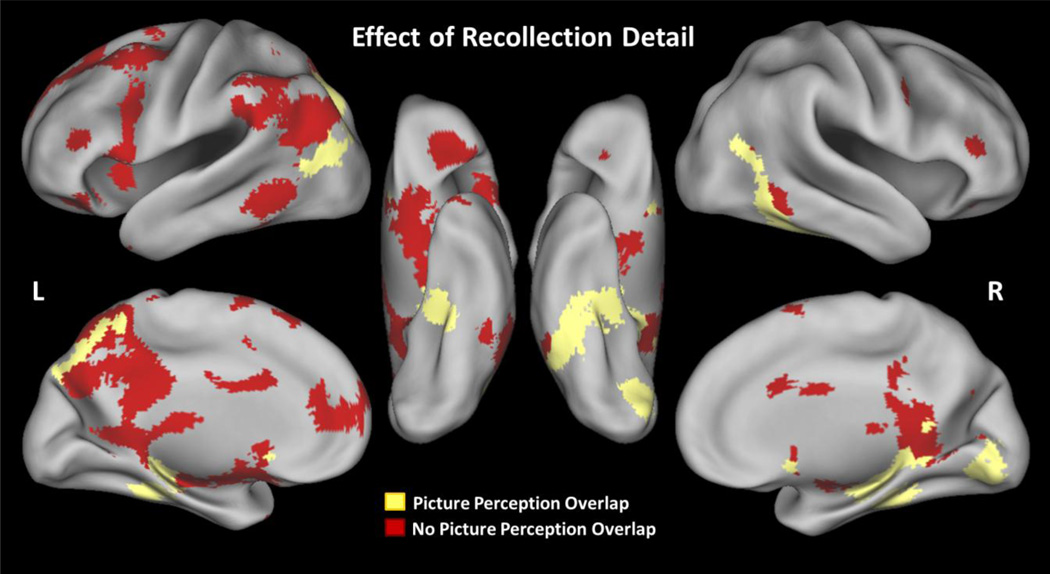
As seen in Figure 3, the main effect of recollection detail (strong > weak) yielded activity common to both younger and older adults in the core recollection network (Rugg & Vilberg, 2012), as well as activity that potentially reflected perceptual reactivation (e.g., Nyberg et al., 2000; Wheeler et al., 2000; see Table 5 for a full list of regions). No regions showed the opposite pattern (weak > strong). One large posterior cluster spanned left posterior parahippocampal gyrus (BA 36), right posterior cingulate gyrus (31), bilateral middle temporal gyrus (BA 37/39), right precuneus (BA 7), right lingual gyrus (BA 18), and the right cerebellum. Critically, many of these regions overlapped with the picture-perception contrast (shown in yellow in Figure 3). We also found activity differences in bilateral middle/inferior frontal gyrus (BA 9/45/46/47), left medial frontal gyrus (BA 10), right putamen, and left caudate. Overall, these results are consistent with younger adult research showing activation in core recollection regions and perception-related regions, and critically, we found similar activity in these regions for both younger and older adults.2
Figure 3.
Brain activity associated with recollection detail (strong > weak) in both younger and older adults. Red = active regions that did not overlap with the picture-perception contrast (pictures > words), Yellow = active regions that overlapped with the picture-perception contrast.
Table 5.
Peak Coordinates for the Recollection Detail Analysis of the Cued-recollection test.
| MNI Coordinates (x, y z) |
Region | BA | Z | Cluster size (voxels) |
||
|---|---|---|---|---|---|---|
| Main Effect of Recollection Detail (Strong > Weak) | ||||||
| −6 | 68 | 18 | L Medial Frontal Gyrus | 10 | 6.73 | 26072 |
| 4 | 10 | −8 | R Anterior Cingulate | 25 | 5.93 | - |
| −28 | −32 | −14 | L Parahippocampal Gyrus* | 36 | 6.09 | - |
| −12 | −46 | 38 | L Cingulate Gyrus | 31 | 5.97 | - |
| −54 | −62 | −4 | L Middle Temporal Gyrus | 37 | 6.20 | - |
| 8 | −70 | −18 | R Cerebellum | - | 6.34 | - |
| 18 | −78 | 4 | R Lingual Gyrus | 18 | 5.93 | - |
| 32 | −78 | −38 | R Cerebellum* | - | 5.98 | - |
| 50 | 38 | 6 | R Inferior Frontal Gyrus | 46 | 4.82 | 151 |
| 28 | 38 | −8 | R Middle Frontal Gyrus | 11 | 4.10 | 55 |
| −28 | 38 | −12 | L Middle Frontal Gyrus | 11 | 6.14 | 697 |
| −40 | 28 | −22 | L Inferior Frontal Gyrus | 47 | 5.16 | - |
| −36 | 18 | −40 | L Superior Temporal Gyrus | 38 | 3.92 | 45 |
| −44 | 6 | −36 | L Middle Temporal Gyrus | 21 | 3.53 | - |
| 50 | 4 | 38 | R Middle Frontal Gyrus | 9 | 3.89 | 101 |
| 48 | −2 | 36 | R Precentral Gyrus | 6 | 3.58 | - |
| 16 | 4 | 16 | R Caudate | - | 3.73 | - |
| 18 | −2 | 16 | R Putamen | - | 3.77 | 107 |
| 24 | −62 | 30 | R Precuneus* | 7 | 4.09 | - |
| 32 | −64 | 22 | R Middle Temporal Gyrus* | 39 | 4.80 | 350 |
| 28 | −68 | 18 | R Posterior Cingulate* | 31 | 4.29 | - |
| Main Effect of Age (Younger > Older) | ||||||
| 2 | 12 | 26 | R Anterior Cingulate | 33 | 4.21 | 85 |
| R Anterior Parahippocampal | ||||||
| 26 | −20 | −22 | Gyrus* | 35 | 3.60 | 43 |
| 24 | −36 | −18 | R Fusiform Gyrus* | 36 | 3.94 | 62 |
| R Posterior Parahippocampal | ||||||
| 32 | −40 | −16 | Gyrus* | 37 | 3.72 | - |
| −16 | −80 | 52 | L Precuneus* | 7 | 4.62 | 124 |
Notes. Coordinates are sorted front to back, top to bottom, left to right. L = Left, R = Right, Bi = Bilateral, BA = Approximate Brodmann Area. Regions with no cluster sizes represent sub-peaks within a cluster.
denotes regions overlapping with the picture-perception contrast.
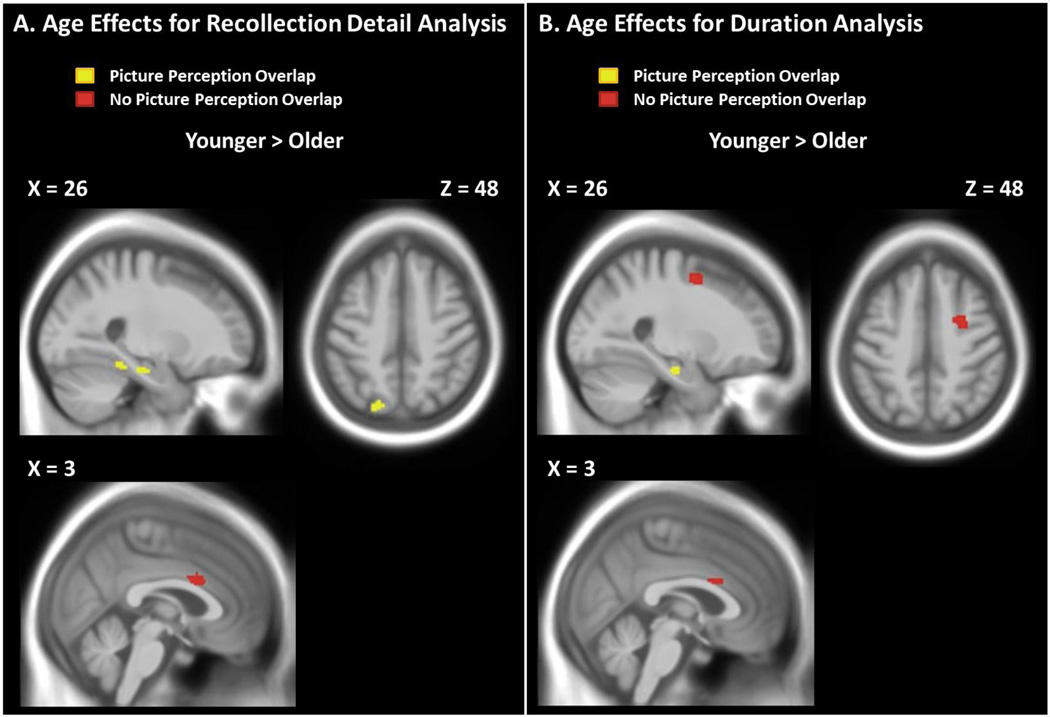
The main effect of age on these fMRI data revealed regions showing differential reactivation effects across all levels of detail (i.e., hits > correct rejections) between the two age groups.3 Note that this comparison matches the two age groups on subjective recollection detail for targets (mean ratings for hits = 1.83 in both age groups). All of the regions from this comparison showed greater retrieval success effects in younger adults relative to older adults (see Table 5 and Figure 4A). Regions showing more activity in younger adults included right anterior cingulate cortex (BA 24/33), right parahippocampal gyrus (BA 35/37), right fusiform gyrus (BA 36/37, and left precuneus (BA 7). Most of these posterior regions overlapped with the picture-perception contrast (shown in yellow in Figure 4A). The age-related reductions in more posterior regions provide unique support for the idea that aging reduces the amount of perceptual detail that is reactivated during the subjective experience of recollection (i.e., age reductions in recollection quantity).
Figure 4.
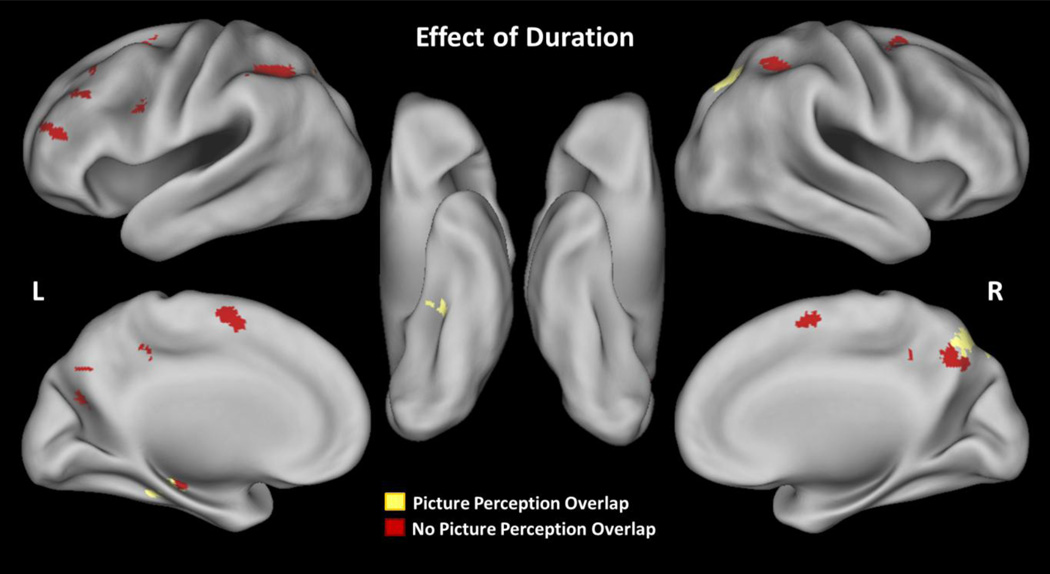
Panel A shows brain activity (hits > correct rejections) associated with age that is greater in younger than older adults in the recollection detail analysis. Panel shows brain activity (targets > lures) associated with age that is greater in younger than older adults in the duration analysis. Neither analysis revealed significant clusters that showed greater effects for older than younger adults. Red = active regions that did not overlap with the picture-perception contrast (pictures > words), Yellow = active regions that overlapped with the picture-perception contrast.
Lastly, no regions revealed a significant Age × Detail interaction. At a more liberal threshold (p < .001, uncorrected), three clusters showed greater effects of recollection detail (strong > weak) in older than younger adults. These significant clusters included the left thalamus, right lentiform nucleus extending into the globus pallidus, and the right inferior parietal lobule (BA 40), but none of these clusters overlapped with the picture-perception contrast, and none showed a main effect of age when collapsing across strong and weak recollections.
In sum, our comparison of trials that varied in recollection detail (strong > weak) showed robust activity in regions associated with episodic memory retrieval and perceptual reactivation. Most of this brain activity did not significantly differ between younger and older adults (Figure 3), but out of those regions that did show age effects, most regions—particularly regions that overlapped with the picture-perception contrast— showed larger retrieval success effects in younger than in older adults (Figure 4). Together these findings suggest that while the neural substrates of recollection are largely similar between younger and older adults, age-related declines are present in a subset of core recollection and perception-related regions.
3.2.3 Cued-recollection test: Study Duration Analysis
Although our recollection-detail analysis used a relatively standard contrast to identify retrieval success effects for targets (i.e., hits > correct rejections), this reliance on subject-specific responses may have introduced unanticipated age differences in response bias and associated item-selection effects into the fMRI analysis. For example, if younger adults were more conservative than older adults in making their recollection responses (i.e., claiming to recollect only the most vividly experienced details at retrieval), then this difference in response bias would change the average amount of perception-specific details associated with target hits and lure rejections in the two age groups, even if the two groups retrieved a similar amount of details (accurate or not) across all items. Depending on the variability of the target and lure memory distributions, these item-selection effects could increase or decrease age differences in fMRI activity. To avoid these interpretative issues we conducted a secondary fMRI analysis that analyzed all test items, regardless of subjects’ responses (i.e., all targets > all lures). Although this analysis obviously would be less sensitive to retrieval success effects than our primary analysis, if this analysis revealed similar age effects on perceptual reactivation as in our primary fMRI analysis, it would bolster an interpretation based on age differences in the amount of recollected detail as opposed to response criteria.
For this analysis we compared all test items (i.e., targets > lures) as a function of age and study duration, using a 2 (Age: younger, older) x 3 (Study Duration: short, medium, long) full factorial ANOVA (p < .05, Monte-Carlo corrected). As with the previous analysis, we inclusively masked all results by the target > lure contrast and exclusively masked the main effects by the interaction contrast. The results can be found in Table 6. As expected based on the link between study duration and recollection detail in the behavioral data, the main effect of duration showed activity common to both younger and older adults in regions that overlapped with those observed in the analysis of recollection detail (see Figure 5). All significant clusters showed greater activity as a function of study duration (long > medium > short) and included left superior frontal gyrus (BA 6,10), bilateral middle frontal gyri (8/9/10/44), left inferior frontal gyrus (BA 9), left hippocampus/parahippocampal gyrus (BA 36), left fusiform gyrus (BA 37), bilateral parietal cortex (BA 39/40), and bilateral precuneus (BA 7). Many of these posterior regions overlapped with the picture-perception contrast (see Figure 5 in yellow). Compared with our primary analysis, there were fewer temporal regions with this duration analysis, but this is to be expected given that the current analysis was less sensitive to retrieval success.
Table 6.
Peak Coordinates for Study Duration Analysis of the Cued-recollection test.
| MNI Coordinates (x, y, z) |
Region | BA | Z | Cluster size (voxels) |
||
|---|---|---|---|---|---|---|
| Main Effect of Duration (Long > Medium > Short) | ||||||
| −40 | 52 | 24 | L Middle Frontal Gyrus | 9/10 | 5.40 | 234 |
| −26 | 50 | 20 | L Superior Frontal Gyrus | 10 | 3.32 | - |
| −36 | 32 | 50 | L Middle Frontal Gyrus | 8 | 4.15 | 78 |
| 28 | 0 | 56 | R Medial Frontal Gyrus | 6 | 4.57 | 187 |
| −6 | −2 | 60 | L Medial Frontal Gyrus | 6 | 4.21 | 372 |
| 6 | 2 | 62 | R Medial Frontal Gyrus | 6 | 4.01 | - |
| −8 | −8 | 66 | L Superior Frontal Gyrus | 6 | 3.71 | - |
| −48 | 6 | 28 | L Inferior Frontal Gyrus | 9 | 3.86 | 55 |
| −30 | −26 | −14 | L Hippocampus* | - | 4.18 | 147 |
| −32 | −32 | −16 | L Parahippocampal Gyrus* | 36 | 3.91 | - |
| −32 | −38 | −16 | L Fusiform Gyrus* | 37 | 3.49 | - |
| −8 | −44 | 48 | L Paracentral Lobule | 5 | 4.17 | 46 |
| 8 | −44 | 46 | R Cingulate Gyrus | 31 | 3.42 | - |
| −14 | −62 | 48 | L Precuneus | 7 | 4.95 | 2185 |
| −46 | −46 | 44 | L Inferior Parietal Lobule | 40 | 4.48 | - |
| 22 | −58 | 46 | R Precuneus* | 7 | 4.89 | - |
| 28 | −64 | 44 | R Superior Parietal Lobule* | 7 | 4.76 | - |
| 32 | −66 | 36 | R Angular Gyrus* | 39 | 4.40 | - |
| Main Effect of Age (Younger > Older) | ||||||
| 2 | 12 | 26 | Bi Anterior Cingulate | 33 | 4.12 | 33 |
| 4 | 6 | 28 | R Cingulate Gyrus | 24 | 3.59 | - |
| 26 | 0 | 52 | R Middle Frontal Gyrus | 6 | 4.10 | 113 |
| 32 | −26 | −24 | R Parahippocampal Gyrus* | 35 | 4.11 | 177 |
| 28 | −18 | −22 | R Hippocampus* | - | 3.85 | - |
| 32 | −38 | −18 | R Fusiform Gyrus* | 37 | 3.66 | - |
Notes. Coordinates are sorted front to back, top to bottom, left to right. L = Left, R = Right, Bi = Bilateral, BA = Approximate Brodmann Area. Regions with no cluster sizes represent sub-peaks within a cluster.
denotes regions overlapping with the picture-perception contrast.
Figure 5.
Brain activity associated with study duration (long > medium > short) in both younger and older adults. Red = active regions that did not overlap with the picture-perception contrast (pictures > words), Yellow = active regions that overlapped with the picture-perception contrast.
More critically, the main effect of age showed largely similar regions of activity as in our primary analysis, all of which showed stronger effects in younger than older adults (see Figure 6A). Significant clusters of activity included bilateral anterior cingulate (BA 24/32), right fusiform gyrus (BA 37), and right parahippocampal gyrus (BA 35). Several of these again overlapped with the picture-perception contrast. Two additional regions also were found in this contrast including right middle frontal gyrus (BA 6), and right hippocampus. The recollection detail analysis (section 3.2.2) also revealed these latter regions at a more liberal threshold (p < .001, uncorrected).
No regions showed an Age x Duration interaction, and at a more liberal threshold (p < .001, uncorrected) only one cluster in the left inferior parietal lobule was significant (brain activity greater for the long condition than the short condition in younger adults than older adults). Even at this more liberal threshold, no regions overlapped with the picture-perception contrast nor did the significant cluster overlap with the recollection detail analysis. In sum, this analysis revealed many of the same age effects on perceptual reactivation that were observed in our primary analysis (younger > older), bolstering the idea that these age differences were due to differences in recollection quantity and not response criteria.
4. Discussion
We used a cued recollection task to identify brain activity associated with the reactivation of perceptually-detailed memories in younger and older adults. Our behavioral results confirmed that that subjective judgments of recollection detail tracked objective accuracy differences within each age group. They also replicated a common finding in the behavioral literature, showing age differences in objectively measured recollection accuracy despite similar subjective ratings of recollection detail for targets across the age groups. This behavioral dissociation is open to multiple theoretical interpretations that our fMRI analysis of perceptual reactivation can inform. We discuss our key fMRI results below, starting with those results that are relevant to age differences in perceptual reactivation.
4.1 Age differences in reactivation
Our analysis of retrieval success effects revealed age-related reductions in brain activity in posterior regions that overlapped with our picture-perception contrast (viewing pictures > labels), implicating less perceptual reactivation from memory in older than in younger adults. These regions included left precuneus and right posterior parahippocampal gyrus implicated in processing visual scene context (e.g., Ranganath & Ritchey, 2012), and right fusiform gyrus implicated in representing visually presented objects and faces (e.g., Haxby et al., 2001; Kanwisher et al., 1997). These age effects were found in our primary analysis of recollected targets, and also in our secondary analysis of all items regardless of response, showing that they cannot be attributed to age differences in response bias (i.e., differential use of the response scale as a function of recollection detail, which could introduce item-selection effects into the fMRI analysis). Instead, these reactivation results implicate a reduced retrieval of perceptual details associated with recollection in older adults compared with younger adults. These results are consistent with previous findings of age-related reductions in posterior regions associated with recollection (Angel et al., 2013; Dennis et al., 2013; Duarte et al., 2010; Mitchell et al., 2013), and they more directly show that activity specifically linked to perceptual reactivation is reduced in older adults.
These age effects on perceptual reactivation provide new evidence that aging reduces the overall amount of retrieved details from memory, consistent with the recollection quantity account discussed in the Introduction (section 1.2). According to this account, subjective recollection judgments do not always track age differences in memory accuracy because older adults recalibrate their use of subjective judgments relative to the overall amount of information that they can actually retrieve. As a result, although older adults recollect fewer details than younger adults overall (i.e., reduced quantity), their subjective judgments of recollection overestimate the actual amount of recollected detail they retrieve relative to younger adults. Our fMRI results are consistent with this idea, to the extent that the age-related reduction in perceptual reactivation that we observed reflects a reduced amount of recollected details in older adults. Note that older adults also might have retrieved more irrelevant or false information relative to younger adults (see section 4.2), but our fMRI data do not directly speak to this issue. Instead, the age-related reductions in perceptual reactivation that we observed provide new evidence for an overall reduction in the amount of retrieved information in older compared to younger adults, regardless of the accuracy of this retrieved information.
It is important to note that the use of fMRI activity to draw theoretical conclusions about cognitive processes relies on the logic of reverse inference, or the assumption that brain activity in a given set of regions (e.g., visual processing areas) reflects the presence of a given cognitive process (e.g., perception-related reactivation). This logic needs to be exercised judiciously given the correlational nature of fMRI (see Poldrack, 2006), as well as studies of cognitive aging (see Salthouse, 2011). We believe that this logic is justified for interpretation of our reactivation activity in the current study for three main reasons. First, we used a cued recollection task that explicitly targeted the perceptual reactivation of picture details from memory, and our objective measures confirmed that the subjective recollection ratings tracked actual differences in the retrieval of perceptual details. Second, we used a picture-perception localizer task to identify regions that were more active during picture than verbal label processing, and similar to prior work in younger adults, we found considerable overlap between these perceptual regions and those active during picture recollection (cf. Johnson & Rugg, 2007; Kahn et al., 2004; Khader et al., 2005; Nyberg et al., 2000; Wheeler et al., 2000; Wheeler & Buckner, 2003; Woodruff et al., 2005). Finally, our response latency data speak against the major alternative to this perceptual reactivation interpretation of our fMRI results, which is that subjects were more likely to search memory for strong than weak recollections using a mental imagery strategy, and hence more likely to activate perception-related regions on this basis alone. If this had been the case, we should have found slower latencies for strong rather than weak recollection trials, but we found the opposite. Considered together, our findings indicate that the subjective experience of recollected details was associated with perception-specific brain activity, consistent with the more general idea that retrieval recapitulates some of the same processes involved during initial perception and encoding (e.g., Damasio, 1989; Kosslyn, 1994; Roediger et al., 2002).
4.2 Other Retrieval Effects
In addition to these perceptual-reactivation effects, we also found robust activity in the core recollection network in both younger and older adults, similar to prior work (e.g., Duarte et al., 2008; Dulas & Duarte, 2012; Duverne et al., 2008). These core recollection regions included medial and lateral prefrontal cortex, as well as medial and lateral posterior parietal cortex. Importantly, in our study this activity was observed when comparing two conditions (strong > weak) that had a similar increment in objective accuracy on the 2AFC test in both age groups. That is, even though older adults were less accurate than younger adults overall, we found mostly similar patterns of recollection-related brain activity when comparing trials that showed the same magnitude of recollection differences in each age group. These results are consistent with prior work showing similar recollection-related activity when comparing conditions that matched younger and older adults on recollection accuracy (e.g., Duverne et al., 2008; Morcom et al., 2007).
Notably, this analysis also revealed age-invariant activity in lateral temporal, parahippocampal, and precuneus regions that overlapped with our picture-perception localizer task. Thus, in addition to the age-related reductions in perceptual reactivation described in the prior section, these other posterior regions showed qualitatively similar reactivation effects across the two age groups. The relative proportion of this reactivation activity compared with that showing age-related reductions might speak to the overall ease of the current task (i.e., despite retrieving fewer details than younger adults, older adults still retrieved many accurate picture details). It also is possible that some of this reactivation activity reflected relatively more inaccurate or distorted information in older adults compared with younger adults, as older adults did make more inaccurate recollection ratings (for lures) compared with younger adults. However, this conclusion would be premature, because our study was designed to target retrieval success effects and we had too few lure false alarms for a meaningful fMRI analysis. Future work aimed at creating false recollection effects with a reactivation task could address this issue.
Although we found similar activity in many recollection regions across the age groups, we also found age-related decline in other regions that are often associated with episodic recollection. Most notable was age-related decline in the hippocampus—a region more anterior than the posterior hippocampal activity that showed age-invariant effects. Age-related decline in hippocampal activity is sometimes (e.g., Daselaar et al., 2006; Dennis et al., 2008c; Giovanello et al., 2010), but not always associated with reduced recollection in aging (e.g., Angel et al., 2013; Duverne et al., 2008; Dulas & Duarte, 2012; Persson et al., 2011). In the present study, the different age effects found in the more anterior hippocampal region might be related to an anterior-to-posterior volume loss that has recently been associated with advanced age (e.g., Gordon et al., 2013), as well as the finding that anterior hippocampus might be more sensitive to age-related functional declines, although the literature is somewhat mixed in this regard (see Ta et al., 2012).
We also found age-related reductions in activity in a region in right middle frontal gyrus, consistent with other studies indicating that aging can reduce the effectiveness with which older adults are able to recruit PFC regions during retrieval (e.g., McDonough et al., 2013; Mitchell et al., 2013; Velanova et al., 2007). Interestingly, we found no evidence for age-related increases in PFC activity that has been observed across a variety of tasks (Cabeza et al., 2004; for review see Dennis et al., 2013), an effect that is often attributed to the attempted recruitment of compensatory processes in older adults. It could be argued that we did not see this pattern in older adults because our cued-recollection test was perceived to be relative easy and straightforward in both age groups, so that older adults saw little need to strategically engage in more effortful or compensatory retrieval monitoring processes. However, in a study that was designed to engage retrieval monitoring processes, we also failed to find greater PFC recruitment in older adults compared with younger adults (McDonough et al., 2013). One common feature to both of our tasks was that we looked for age differences using within-task contrasts (i.e., age x task interactions), as opposed to directly comparing task-related BOLD activity between age groups (relative to an implicit baseline). When BOLD activity was directly compared between age groups, we did find evidence for more PFC activity in older adults than younger adults in McDonough et al. (2013), but a similar analysis here (not shown) revealed no reliable age effect.
Finally, it should be explicitly noted that while our fMRI analysis in the current study focused on retrieval, many of the age differences in brain activity that we observed are likely due to a combination of encoding and retrieval processes (see Craik & Rose, 2012; Mitchell & Johnson, 2009). For instance, older adults may have failed to properly encode or bind together as many perceptual details as younger adults, and they also may have had difficulties searching for encoded details at retrieval. Our study was aimed at investigating the reactivation of perceptual details from memory, and our conclusions relevant to reactivation apply regardless of whether the locus of these age-related differences was at encoding or retrieval (or both). That said, it is worthwhile to note that when contrasting novel pictures and verbal descriptions in our perceptual localizer task, which likely recruited encoding processes, we found no interactions with age in the same regions that showed age-related reactivation differences in our recollection task. Thus, the age-related reductions we observed in perceptual reactivation do not seem to be due to age differences in perceptual processing in these regions, although other encoding-related processes may have differed between younger and older adults.
4.3 Broader Implications
In conclusion, we found robust activity in both younger and older adults in the core recollection network, with the majority of age-related declines occurring in regions involved in perception-related reactivation as well as other key regions (such as hippocampus, prefrontal, and parietal cortices). These age differences in brain activity indicate that older adults retrieved less detailed information than younger adults, even when they subjectively reported similarly detailed recollections, and they highlight the difficulty of using subjective report as an index of the amount of actual detail retrieved from memory in different groups. Our results suggest that older adults retrieve fewer perceptual details overall compared with younger adults, but they then recalibrate their subjective memory judgments across this reduced level of output.
These conclusions also may extend to tasks that test memory for more complex autobiographical memories created outside the laboratory. Recent fMRI work has shown that older adults are less likely than younger adults to activate more posterior brain regions when retrieving autobiographical memories from verbal cues, potentially implicating reduced recollection of perception-related detail (Addis et al., 2011; St-Laurent et al., 2011). Although it is difficult to disentangle brain activity associated with recollection as opposed to mental imagery in autobiographical memory, these results are consistent with the current findings, as is behavioral work with autobiographical tasks indicating that older adults report similarly vivid subjective details despite lower objectively measured accuracy compared with younger adults (see Gallo et al., 2011; McDonough & Gallo, 2013).
More generally, the current study found brain activity in regions that are typically associated with episodic memories created in the lab as well as regions associated with more distant autobiographical memories (for meta-analysis, see McDermott et al., 2009). This aspect of our findings argues against the proposal that the retrieval of memories created in the laboratory taps a fundamentally different memory system than autobiographical memory retrieval, although as argued by Roediger and McDermott (2013), this proposal may be true of old/new recognition memory tasks that are commonly used in the laboratory. In this light, it is worth noting that a common feature of the current reactivation task and autobiographical memory tasks is that both required the recollection of rich perceptual details from verbal retrieval cues. Unlike laboratory tasks that require recognition or source memory decisions, the use of retrieval cues to trigger the elaborative recollection of more detailed information may be more representative of the way episodic memory is used outside the laboratory.
Highlights.
First aging study targeting neural reactivation of recollected perceptual details.
Robust activity was found in the core recollection network in both age groups.
Reactivation of perceptual regions during recollection was reduced with age.
New evidence that aging reduces the absolute quantity of recollected details.
Abbreviations
- PFC
Prefrontal cortex
- MTL
medial temporal lobe
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There were too few false alarms to lures to conduct fMRI analyses, but a similar breakdown of lure responses revealed more weak false alarms than strong false alarms for both younger adults (.09 vs. .01; t(21) = 3.75, SEM = .023, p = .001) and older adults (.23 vs. .05; t(17) = 5.40, SEM = .035, p < .001). Thus, although older adults made more false alarms, in both age groups these errors were associated with a relatively weak sense of recollection (e.g., misremembering vague or imagined picture details for items that were not, in fact, studied with a picture).
The regions showing common strong > weak effects across the two age groups in the factorial analysis also were found if we separately analyzed each age group for hit > CR effects (p < .05, Monte-Carlo corrected). This latter analysis also revealed common regions that were not found in the strong > weak factorial analysis, including dorsomedial PFC and right lateral frontal/parietal cortex.
Because the main effect of age is a contrast between young (hit vs. correct rejections) and old (hit vs. correct rejections), this effect can be interpreted as an interaction with age. However, we do not refer to this effect as an interaction so as not to confuse this effect with the age x recollection detail interaction, which contrasted young (strong vs. weak, correct rejection baseline) and old (strong vs. weak, correct rejection baseline).
References
- Addis DR, Roberts RP, Schacter DL. Age-related neural changes in autobiographical remembering and imagining. Neuropsychologia. 2011;49:3656–3669. doi: 10.1016/j.neuropsychologia.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel L, Bastin C, Genon S, Balteau E, Phillips C, Luxen A, Maquet P, Salmon E, Collette F. Differential effects of aging on the neural correlates of recollection and familiarity. Cortex. 2013;49:1585–1597. doi: 10.1016/j.cortex.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Burgmans S, van Boxtel MPJ, Vuurman EFPM, Evers EAT, Jolles J. Increased neural activation during picture encoding and retrieval in 60-year-olds compared with 20-year-olds. Neuropsychologia. 2010;48:2188–2197. doi: 10.1016/j.neuropsychologia.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Rose NS. Memory encoding and aging: a neurocognitive perspective. Neurosci. Biobehav. Rev. 2012;36:1729–1739. doi: 10.1016/j.neubiorev.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum. Brain. Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: a system-level proposal for the neuronal substrates of recall and recognition. Cognit. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb. Cortex. 2006;16:1771–82. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonker C. Deep processing activates the medial temporal lobe in young but not in old adults. Neurobiol. Aging. 2003;24:1005–1011. doi: 10.1016/s0197-4580(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. Third edition. Mahwah, NJ: Erlbaum; 2008. pp. 1–54. [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J. Exp. Psychol. Learn. Mem. Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Kim H, Cabeza R. Age-related differences in brain activity during true and false memory retrieval. J. Cogn. Neurosci. 2008;20:1390–1402. doi: 10.1162/jocn.2008.20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Bowman CR, Peterson KM. Age-related differences in the neural correlates mediating false recollection. Neurobiol. Aging. 2013;35:395–407. doi: 10.1016/j.neurobiolaging.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Dew ITZ, Buchler N, Dobbins IG, Cabeza R. Where is ELSA? The early to late shift in aging. Cereb. Cortex. 2012;22:2542–2553. doi: 10.1093/cercor/bhr334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson CS, Bawa S, Krueger LE. Aging, metamemory, and high-confidence errors: A misrecollection account. Psychol. Aging. 2007;22:122–133. doi: 10.1037/0882-7974.22.1.122. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effect of aging on the neural correlates of subjective and objective recollection. Cereb. Cortex. 2008;18:2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN. Age-related differences in neural activity associated with familiarity, recollection and false recognition. Neurobiol. Aging. 2010;31:1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage. 2011;57:1192–1204. doi: 10.1016/j.neuroimage.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. The effects of aging on material-independent and material-dependent neural correlates of source memory retrieval. Cereb. Cortex. 2012;22:37–50. doi: 10.1093/cercor/bhr056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol. Aging. 2008;29:1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the mental state of patients for the clinician. J. Psychiat. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Foster KT, Johnson EL. Elevated false recollection of emotional pictures in young and older adults. Psychol. Aging. 2009;24:981–988. doi: 10.1037/a0017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo DA, Korthauer LE, McDonough IM, Teshale S, Johnson EL. Age-related positivity effects and autobiographical memory detail: Evidence from a past/future source memory task. Memory. 2011;19:641–652. doi: 10.1080/09658211.2011.595723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Kensinger EA, Wong AT, Schacter DL. Age-related neural changes during memory conjunction errors. J. Cogn. Neurosci. 2010;22:1348–1361. doi: 10.1162/jocn.2009.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves B, Reber PJ, Gitelman DR, Parrish TB, Mesulam M-M, Paller KA. Neural evidence that vivid imagining can lead to false remembering. Psychol. Sci. A. J. Am. Psychol. Soc. 2004;15:655–660. doi: 10.1111/j.0956-7976.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Blazey T, Benzinger TL, Head D. Effects of aging and Alzheimer’s disease along the longitudinal axis of the hippocampus. J. Alzheimers. Dis. 2012;37:41–50. doi: 10.3233/JAD-130011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J. Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FIM. The effects of age on the neural correlates of episodic encoding. Cereb. Cortex. 1999;9:805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Grinband J, Wager TD, Lindquist M, Ferrera VP, Hirsch J. Detection of time-varying signals in event-related fMRI designs. Neuroimage. 2008;43:509–520. doi: 10.1016/j.neuroimage.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. J. Cogn. Neurosci. 2005;17:54–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Bishara AJ, Hessels S, Toth JP. Aging, subjective experience, and cognitive control: dramatic false remembering by older adults. J. Exp. Psychol. General. 2005;134:131–148. doi: 10.1037/0096-3445.134.2.131. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cereb. Cortex. 2007;17:2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J. Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader P, Burke M, Bien S, Ranganath C, Rösler F. Content-specific activation during associative long-term memory retrieval. Neuroimage. 2005;27:805–816. doi: 10.1016/j.neuroimage.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Cambridge, MA: MIT Press; 1994. Image and Brain: The Resolution of the Imagery Debate. [Google Scholar]
- Koutstaal W. Older adults encode--but do not always use--perceptual details: intentional versus unintentional effects of detail on memory judgments. Psychol. Sci. 2003;14:189–193. doi: 10.1111/1467-9280.01441. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Gainesville: University of Florida; 2005. International affective picture system (IAPS): Affective ratings of pictures instruction manual (Technical Report No. A-6) [Google Scholar]
- Leshikar ED, Gutchess AH, Hebrank AC, Sutton BP, Park DC. The impact of increased relational encoding demands on frontal and hippocampal function in older adults. Cortex. 2010;46:507–521. doi: 10.1016/j.cortex.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Measuring Memory Reactivation With Functional MRI: Implications for Psychological Theory. Perspect. Psychol. Sci. 2013;8:72–78. doi: 10.1177/1745691612469031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika P, Whitfield S, Cooper JC. Detection and repair of transient artifacts in fMRI data. Neuroimage. 2005;26(Supplement 1) [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA. Aging reduces veridical remembering but increases false remembering: neuropsychological test correlates of remember-know judgments. Neuropsychologia. 2009;47:2164–2173. doi: 10.1016/j.neuropsychologia.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Szpunar KK, Christ SE. Laboratory-based and autobiographical retrieval tasks differ substantially in their neural substrates. Neuropsychologia. 2009;47:2290–2298. doi: 10.1016/j.neuropsychologia.2008.12.025. [DOI] [PubMed] [Google Scholar]
- McDonough IM, Gallo DA. Impaired retrieval monitoring for past and future autobiographical events in older adults. Psychol. Aging. 2013;28:457–466. doi: 10.1037/a0032732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough IM, Wong JT, Gallo DA. Age-related differences in prefrontal cortex activity during retrieval monitoring: testing the compensation and dysfunction accounts. Cereb. Cortex. 2013;23:1049–1060. doi: 10.1093/cercor/bhs064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Ankudowich E, Durbin KA, Greene EJ, Johnson MK. Age-related differences in agenda-driven monitoring of format and task information. Neuropsychologia. 2013;51:2427–2441. doi: 10.1016/j.neuropsychologia.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychol. Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cereb. Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Norman KA, Schacter DL. False recognition in younger and older adults: exploring the characteristics of illusory memories. Mem. Cognit. 1997;25:838–848. doi: 10.3758/bf03211328. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. Reactivation of encoding-related brain activity during memory retrieval. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Carp J, Kennedy KM, Rodrigue KM, Bischof GN, Huang C-M, Rieck JR, Polk TA, Park DC. Neural broadening or neural attenuation? Investigating age-related dedifferentiation in the face network in a large lifespan sample. J. Neurosci. 2012;32:2154–2158. doi: 10.1523/JNEUROSCI.4494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Kalpouzos G, Nilsson L-G, Ryberg M, Nyberg L. Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus. 2011;21:753–766. doi: 10.1002/hipo.20794. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L. Altered brain activity in healthy seniors: what does it mean? Prog. Brain Res. 2006;157:45–56. doi: 10.1016/s0079-6123(06)57004-9. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Gallo DA, Geraci L. Processing approaches to cognition: The impetus from the levels-of-processing framework. Memory. 2002;10:319–332. doi: 10.1080/09658210224000144. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Two types of event memory. Proc. Natl. Acad. Sci. U. S. A. 2013:2–3. doi: 10.1073/pnas.1321373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SARB, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. Neuroimage. 2005;26:1078–1085. doi: 10.1016/j.neuroimage.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. Brain networks underlying episodic memory retrieval. Curr. Opin. Neurobiol. 2012;23:255–260. doi: 10.1016/j.conb.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol. Bull. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent M, Abdi H, Burianová H, Grady CL. Influence of aging on the neural correlates of autobiographical, episodic, and semantic memory retrieval. J. Cogn. Neurosci. 2011;23:4150–4163. doi: 10.1162/jocn_a_00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Okado Y, Loftus EF. Imaging the reconstruction of true and false memories using sensory reactivation and the misinformation paradigms. Learn. Mem. 2010;17:485–488. doi: 10.1101/lm.1845710. [DOI] [PubMed] [Google Scholar]
- Thomas AK, Bulevich JB. Effective cue utilization reduces memory errors in older adults. Psychol. Aging. 2006;21:379–389. doi: 10.1037/0882-7974.21.2.379. [DOI] [PubMed] [Google Scholar]
- Tulving E. How many memory systems are there? Am. Psychol. 1985;40:385–398. [Google Scholar]
- Van Essen DC. Organization of Visual Areas in Macaque and Human Cerebral Cortex. In: Chalupa L, Werners J, editors. In Visual Neurosciences. Cambridge, MA: The MIT Press; 2003. pp. 507–521. [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cereb. Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J. Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: Vivid remembering reactivates sensory-specific cortex. Proc. Natl. Acad. Sci. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JT, Cramer SJ, Gallo DA. Age-related reduction of the confidence accuracy relationship in episodic memory: Effects of recollection quality and retrieval monitoring. Psychol. Aging. 2012;27:1053–1065. doi: 10.1037/a0027686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]