Abstract
This consensus guideline was prepared on behalf of the International Society for Holter and Noninvasive Electrocardiology and is cosponsored by the Japanese Circulation Society, the Computers in Cardiology Working Group on e-Cardiology of the European Society of Cardiology, and the European Cardiac Arrhythmia Society. It discusses the electrocardiographic phenomenon of T-wave alternans (TWA) (i.e., a beat-to-beat alternation in the morphology and amplitude of the ST- segment or T-wave). This statement focuses on its physiological basis and measurement technologies and its clinical utility in stratifying risk for life-threatening ventricular arrhythmias. Signal processing techniques including the frequency-domain Spectral Method and the time-domain Modified Moving Average method have demonstrated the utility of TWA in arrhythmia risk stratification in prospective studies in >12,000 patients. The majority of exercise-based studies using both methods have reported high relative risks for cardiovascular mortality and for sudden cardiac death in patients with preserved as well as depressed left ventricular ejection fraction. Studies with ambulatory electrocardiogram-based TWA analysis with Modified Moving Average method have yielded significant predictive capacity. However, negative studies with the Spectral Method have also appeared, including 2 interventional studies in patients with implantable defibrillators. Meta-analyses have been performed to gain insights into this issue. Frontiers of TWA research include use in arrhythmia risk stratification of individuals with preserved ejection fraction, improvements in predictivity with quantitative analysis, and utility in guiding medical as well as device-based therapy. Overall, although TWA appears to be a useful marker of risk for arrhythmic and cardiovascular death, there is as yet no definitive evidence that it can guide therapy.
Keywords: ambulatory ECG monitoring, cardiovascular mortality, exercise testing, risk stratification, sudden cardiac death, T-wave alternans
The International Society for Holter and Noninvasive Electrocardiology together with the Japanese Circulation Society, the Working Group on e-Cardiology of the European Society of Cardiology, and the European Cardiac Arrhythmia Society charged the authors with reviewing the topic of microvolt T-wave alternans (TWA) to provide a consensus guideline with regard to its physiological basis, measurement methods, and clinical utility.
Physiology of TWA
The TWA phenomenon, a repeating ABABAB pattern in the morphology and amplitude of the ST-segment or T-wave, has long been recognized and linked to arrhythmogenesis (1). This pattern distinguishes TWA from other types of ST-segment and T-wave variability (Fig. 1). TWA reflects spatiotemporal heterogeneity of repolarization, is sensitive to perturbations in intracellular calcium handling, and serves as a mechanism of arrhythmogenesis by amplifying repolarization heterogeneity. It arises from beat-to-beat alternation of action potential duration at the level of cardiac myocytes. TWA can be either spatially concordant, when action potentials in neighboring cell regions alternate in phase, or discordant, when they are out of phase (Figs. 2 and 3) (2,3). The physiological basis of TWA has recently been reviewed in detail (2–7).
Figure 1. TWA and Nonalternating Fluctuations.
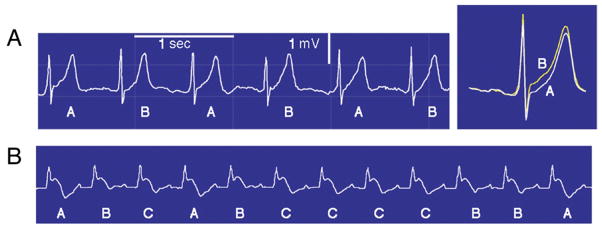
(A) Precordial (V4) electrocardiogram rhythm strip (left) and high-resolution template of QRS-aligned complexes (right) during routine exercise testing from a patient with coronary artery disease. The template illustrates T-wave alternans (TWA) as a separation between ST-T segments in A and B beats. TWA magnitude = 106 μV. Sec = second. (B) Nonalternating fluctuations in T-wave amplitude after ajmaline administration in a Brugada syndrome patient.
Figure 2. Discordant Alternans Leading to VF.

Action potential propagation between 2 ventricular sites (A to B) is shown with transition from concordant to discordant alternans and development of ventricular fibrillation (VF). Shaded areas indicate dispersion of repolarization between sites. *Premature beats. L = long action potential duration; S = short action potential duration. Adapted, with permission, from Oshodi et al. (2).
Figure 3. Interlead Discordant Alternans.

Mid-myocardial (Mid)/epicardial (Epi) recordings after an abrupt decrease in pacing cycle length from 1,000 to 500 ms. Ventricular tachycardia was initiated by a premature focal discharge from a left ventricular endocardial site, which induced areas of functional conduction block and re-entrant excitation. Numbers above the tracing are calculated activation–recovery intervals (ARI). Numbers below the tracing are dispersion of ARI between mid-myocardial and epicardial sites. *Marked discordant alternans is associated with the onset of ventricular tachyarrhythmia after a premature beat. Reprinted, with permission, from Chinushi et al. (3).
The association of TWA with spatiotemporal heterogeneity of repolarization (i.e., transient or lasting repolarization differences among neighboring myocardial regions) is supported by a number of investigations, including in vitro optical mapping (2,3,5,7–9), intact large animal experimental laboratory studies (10–13), computer simulations, and clinical studies involving programmed electrical stimulation (14,15). Higher levels of TWA indicate greater risk for arrhythmias (13,16), which becomes especially elevated when alternation in neighboring areas is discordant (i.e., out of phase) (3,10). Changes in action potential duration and conduction velocity restitution, premature beats, and functional and/or anatomic gradients in action potential duration facilitate development of steep, heterogeneous repolarization gradients conducive to re-entry and wavebreak.
TWA magnitude coincides with vulnerability to lethal ventricular tachyarrhythmias. It is amplified by increased heart rates (16), ventricular premature beats (17), coronary artery occlusion and reperfusion (5,12,14,18–20), adrenergic stimulation (18,21), and mental stress (22). Blockade of beta-adrenergic receptors (23,24), sympathetic denervation (18), and vagus nerve and spinal cord stimulation (25), which reduce susceptibility to ventricular tachyarrhythmias, decrease TWA magnitude. Clinical studies with ambulatory electrocardiogram (ECG) monitoring (26) and recordings from intracardiac leads of implanted devices (27) reveal a progressive increase in TWA preceding the onset of spontaneous ventricular arrhythmias.
Heart rate influences TWA, likely by impacting on intracellular calcium cycling (4) and engaging the steepest portions of the electrical restitution curve (7,28). Even in the normal heart, excessive heart rates of >170 beats/min in guinea pigs and >200 beats/min in canines are capable of inducing TWA (29). During myocardial ischemia or heart failure, the onset heart rate for TWA is considerably lower (9,12) due to impaired capacity of the sarcoplasmic reticulum to reuptake calcium. Heart rate is not the sole determinant of TWA, because autonomic neurotransmitters and changes in myocardial substrate can lead to elevated levels of TWA during fixed rate pacing (16,18). Furthermore, pacing alone does not replicate the TWA enhancement at comparable heart rates due to adrenergic stimulation or myocardial ischemia (21–24).
Myocardial ischemia can increase TWA magnitude, as was evidenced in animals during coronary artery occlusion (16,18,19) and in humans during angioplasty (16,20). Experimental studies with fixed heart rates indicated that myocardial ischemia- and reperfusion-induced increases in TWA magnitude paralleled (r2 = 0.98) incidence of ventricular tachycardia and fibrillation (16). Marked changes in T-wave complexity and heterogeneity (i.e., nonuniformity in T-wave morphology) (12,13) occurred simultaneously with TWA magnitude surges, with transition from concordant to discordant alternans. Loss of intercellular coupling by ischemia was implicated when rotigaptide decreased connexin43 dephosphorylation in parallel with ischemia-induced TWA and dispersion of repolarization (30).
Derangements in calcium cycling and conduction constitute ionic bases for TWA during myocardial ischemia and heart failure (8,9). With luminescent dyes, Clusin (8) demonstrated ischemia-induced concordant and discordant alternation in calcium transients. Moreover, calcium-channel blockade reversed ischemia-induced TWA in parallel with arrhythmia suppression in anesthetized canines (31). Experimental models of heart failure demonstrated associations of TWA with disturbances in calcium handling and development of discordant alternans, which set up pro-arrhythmic preconditions for conduction block and re-entry. In particular, heart failure reduced sarcoplasmic reticulum calcium ion (Ca2+)-adenosine triphosphatase expression and inhibited ryanodine receptor function, resulting in impaired reuptake and release of calcium in the sarcoplasmic reticulum, both contributing to marked derangements in intracellular Ca2+ handling (9). Recent studies in cardiomyopathy patients showed that TWA may be attributable to oscillations in the action potential plateau that, in computational models, were best explained by reduced calcium uptake into the sarcoplasmic reticulum (15,28).
Sympathetic nerve activity and abnormalities in calcium handling may serve as arrhythmogenic factors in nonischemic disease patients, in whom enhanced adrenergic activity compensates for reduced myocardial contractility. The vulnerable myocardial substrate is susceptible to transient alterations in neural activity and electrolyte imbalance, which may initiate disturbances in cardiac repolarization and ventricular arrhythmogenesis. Imaging studies (32) demonstrated the important role of increased sympathetic nerve activity in provoking TWA in idiopathic dilated cardiomyopathy. During exercise, patients with dilated or hypertrophic cardiomyopathy also experience repolarization abnormalities including TWA that are associated with ventricular tachycardia. Histopathological changes, particularly fiber disarray and/or fibrosis, are correlated with TWA occurrence and ventricular tachyarrhythmias in hypertrophic cardiomyopathy (33).
Methodology for TWA Assessment
Two contemporary techniques employed in sizeable clinical studies for arrhythmia risk stratification by microvolt-level TWA are the Spectral and the Modified Moving Average (MMA) Methods (Tables 1 and 2) (34–74). Both allow detection of subtle levels of TWA in the nonvisible micro-volt range as well as visible, macroscopic TWA. Experience with the Spectral Method is more extensive. Other analytical methods employed in experimental and clinical studies (18,20,26,75,76) have been reviewed (75).
Table 1.
Clinical Studies With the Spectral Method Enrolling >100 Patients
| First Author (Ref. #) | Patient Population (Enrollment, Disease) | Mean LVEF | Hazard Ratios (95% CI) for TWA |
|---|---|---|---|
| Predictive studies | |||
|
| |||
| Cardiomyopathy | |||
| Kitamura et al. (34) | 104 patients with DCM | 37% | 11.9 (1.53–92.59) for SCD, VF, or sustained VT at 21 ± 14 months |
| Hohnloser et al. (35) | 137 patients with DCM, LVEF ≤35% | 29 ± 11% | 3.44 for ventricular tachyarrhythmic events at 14 ± 6 months |
| Chow et al. (36) | 514 patients with ischemic cardiomyopathy, LVEF ≤35%, no previous sustained ventricular arrhythmia and positive or indeterminate TWA test results (patients included in Chow et al. [38]) | 26%–29% | 2.24 (1.34–3.75) for all-cause mortality; 2.29 (1.00–5.24) for arrhythmic mortality; NS for nonarrhythmic mortality |
| Salerno-Uriarte et al. (ALPHA) (37) | 446 patients with DCM, LVEF ≤40% | 29.5% | 4.0 (1.4–11.4) for cardiac death and life-threatening arrhythmias at 18 months |
| Chow et al. (38,39) | 768 patients with ischemic cardiomyopathy, LVEF ≤35%, no previous sustained ventricular arrhythmia | 26%–29% | 2.27 (1.22–4.24) for all-cause mortality in patients without an ICD; 2.42 (1.07–5.41) for all-cause mortality or appropriate ICD discharge in patients with an ICD. ICD use was associated with lower all-cause mortality in MTWA non-negative patients (0.45, 0.27–0.76) but not in MTWA negative patients (0.85, 0.33–2.20), with mortality benefit in MTWA-non-negative patients largely mediated through arrhythmic mortality reduction (0.30, 0.13–0.68). |
| Chan et al. (40) | 768 patients with ischemic cardiomyopathy, LVEF ≤35%, no previous sustained ventricular arrhythmia (same patients as Chow et al. [38]) | 26%–29% | 2.19 (1.1–4.34) for all-cause mortality and appropriate ICD shocks at 1 yr; 3.36 (1.28–8.83) at 2 yrs |
| Costantini et al. (ABCD) (41) | 566 patients with ischemic cardiomyopathy, LVEF ≤40%, and NSVT | 28 ± 8% | 2.1 for SCD or appropriate ICD discharge at 1 yr |
| Congenital heart disease | |||
| Alexander et al. (42) | 304 consecutive pediatric patients with congenital heart disease, myopathy, syncope, or history of cardiac arrest | Not stated | 7.9 (2.2–28.1) for ventricular arrhythmia; 6.7 (1.6–28.1) for cardiac arrest at <3 yrs |
| Depressed LVEF | |||
| Rashba et al. (43) | 108 consecutive patients with CAD and LVEF ≤40% | 28 ± 7% | 2.2 (1.1–4.7) for death, sustained ventricular arrhythmias, appropriate ICD discharge at 18 ± 13 months in patients with normal QRS segment; NS in patients with prolonged QRS segment |
| Rashba et al. (44) | 144 patients with CAD and LVEF ≤40% | 28 ± 7% | 2.2 (1.1–4.7) for death, sustained ventricular arrhythmia, or appropriate ICD discharge at 17 ± 13 months; NS in patients with LVEF <30%; ∞ in patients with LVEF >30% |
| Bloomfield et al. (45) | 549 patients with LVEF ≤40%, no history of sustained ventricular arrhythmias | 25% | 6.5 (2.4–18.1) for all-cause mortality or nonfatal sustained ventricular arrhythmia at 2 yrs |
| Cantillon et al. (46) | 286 patients with LVEF ≤35%, NSVT or syncope | 26 ± 7% | 2.33 (1.44–3.67) for arrhythmia-free survival at 38 ± 11 months |
| Morin et al. (47) | 386 patients with CAD, NSVT, LVEF ≤40% | 26%–30% | 1.64 for ventricular tachyarrhythmia or death in patients with narrow QRS segment at 40 ± 19 months; NS in patients with wide QRS segment |
| Heart failure | |||
| Klingenheben et al. (48) | 107 consecutive patients with congestive heart failure, LVEF ≤45%, no history of arrhythmia, and no recent MI | 28 ± 7% | ∞ for SCD, arrhythmias, sustained VT at 14.6 months |
| Gorodeski et al. (49) | 303 consecutive patients with heart failure and LVEF ≤40% | 24% | 1.89 (1.05–3.39) for total mortality or cardiac transplantation; NS after adjustment for metabolic measures at 2.8 yrs. Concordance index for time- to-event outcomes = 0.75. C statistic for propensity score = 0.79. |
| Post-MI | |||
| Ikeda et al. (50) | 102 post-MI patients | 20%–40% | 16.8 (2.2–127.8) for arrhythmic events |
| Ikeda et al. (51) | 850 post-MI patients | 51 ± 13% | 5.9 (1.6–21.4) for SCD or resuscitated VF at 25 ± 13 months; 82% were monitored at 2–10 weeks after MI |
| Bloomfield et al. (52) | 177 MADIT-II like post-MI patients with LVEF ≤30 | 23 ± 6% | 4.8 for all-cause mortality at 2 yrs |
| Ikeda et al. (53) | 1,041 post-MI patients with LVEF >40% | 55 ± 10% | 23.5 monitored at 48 ± 66 days for SCD or life- threatening arrhythmia at 32 ± 14 months |
| Exner et al. (REFINE) (54) | 322 post-MI patients with LVEF <50% | 40% within 1 week and 47% at 8 weeks after MI | 2.75 (1.08–7.02) monitored at 10–14 weeks after MI for cardiovascular death or resuscitated cardiac arrest (primary endpoint) at 47 months; NS if monitored at 2–4 weeks after MI. AUC for primary endpoint = 0.62; for combination of TWA + HRT, AUC = 0.70. |
| Referred for electrophysiological study | |||
| Gold et al. (55) | 313 patients | 44 ± 18% | 10.9 for SCD, sustained VT, VF, or appropriate ICD discharge at 400 days |
| Rashba et al. (56) | 251 patients with CAD and LVEF | 27 ± 8% | 2.2 (1.1–4.7) for arrhythmic events (arrhythmic death, VT, aborted VF) at 499 ± 395 days; NS for TWA during atrial pacing at 100–120 beats/min |
|
| |||
| Nonpredictive studies | |||
|
| |||
| Schwab et al. (57) | 140 post-MI patients | 56 ± 14% | NS for TWA monitored at 15 ± 6 days after MI for SCD or sustained VT at 451 ± 210 days |
| Tapanainen et al. (58) | 379 consecutive post-MI patients | 45 ± 10% | NS for TWA monitored at approximately 8 days after MI for all-cause mortality or cardiac death at 14.8 months |
| Grimm et al. (59) | 343 patients with DCM, LVEF ≤45% | 31 ± 10% | NS for SCD, VF, or sustained VT at 52 months |
| Ikeda et al. (60) | 124 consecutive subjects with Brugada-type ECG | (not stated) | NS for SCD or VT at 40 ± 19 months |
| Gold et al. (SCD-HeFT TWA substudy) (61) | 490 patients with congestive heart failure | 24 ± 7% | NS for SCD, sustained VT/VF, or appropriate ICD discharge at 2.5 yrs |
| Chow et al. (MASTER) (62) | 575 post-MI patients with LVEF ≤30% | 24 ± 5% | 2.04 (1.10–3.78) for total mortality at 2.1 ± 0.9 yrs; NS for SCD or appropriate ICD discharge |
| Huikuri et al. (CARISMA) (63) | 312 post-MI patients | 31 ± 6% | NS for TWA monitored at 6 weeks after MI for VF or symptomatic, sustained VT at 2 yrs |
See text for discussion of nonsignificant prediction among studies with high or low implantable cardioverter-defibrillator (ICD) use, continuance or withdrawal of beta-adrenergic blockade for test, or test time after myocardial infarction (MI). This table was developed from searches of the published medical data on the terms alternans and alternation in PubMed (National Library of Medicine, Bethesda, Maryland) and Paperchase (Bedford, Massachusetts) databases. Reference lists from these studies, from meta-analyses (88,112), and from recent reviews (4–7) were also scanned. Only clinical studies that enrolled over 100 patients and reported hazard ratios were included.
ABCD = Alternans Before Cardioverter Defibrillator; ALPHA = T-Wave Alternans in Patients With Heart Failure; AUC = area under the receiver-operator characteristic curve; CAD = coronary artery disease; CARISMA = Cardiac Arrhythmias and Risk Stratification after Acute Myocardial Infarction; CI = confidence interval; DCM = dilated cardiomyopathy; ECG = electrocardiogram; EP = electrophysiological; HRT = heart rate turbulence; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; MADIT = Multicenter Automatic Defibrillator Trial; MASTER = Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients; NSVT = nonsustained ventricular tachycardia; REFINE = Risk Estimation Following Infarction, Noninvasive Evaluation; ROC = receiver-operator characteristic curve; SCD = sudden cardiac death; SCD-HeFT = Sudden Cardiac Death in Heart Failure Trial; TWA = T-wave alternans; VF = ventricular fibrillation; VT = ventricular tachycardia.
Table 2.
Clinical Studies With the Modified Moving Average Method
| Test Setting First Author (Trial) (Ref. #) | Patient Population (Enrollment, Disease, Mean Age) | Mean LVEF | Hazard Ratios (95% CI) for TWA; NPV and PPV |
|---|---|---|---|
| Routine exercise testing | |||
| Nieminen et al. (FINCAVAS) (64) | 1,037 consecutive patients referred for routine exercise testing; 58 ± 13 yrs (patients included in Leino et al. [66]) | Mostly preserved | 6.0 (2.8–12.8) for CV death, 7.4 (2.8–19.4) for SCD at 44 ± 7 months for 65-μV TWA cutpoint; NPV for CV death = 97.6; PPV = 12.6; NPV for SCD = 98.6, PPV = 8.0 |
| Minkkinen et al. (FINCAVAS) (65) | 2,119 consecutive patients referred for routine exercise testing; 57 ± 13 yrs (patients included in Leino et al. [66]) | Mostly preserved | 4.6 (2.2–9.9) for CV death, 4.4 (1.5–12.7) for SCD at 47 months for 65-μV cutpoint; NPV for CV death = 97.4; PPV = 10.2 |
| Leino et al. (FINCAVAS) (66) | 3,598 consecutive patients referred for routine exercise testing; 56 ± 13 yrs | Mostly preserved | 1.55 (1.150–2.108, p < 0.004) for CV death; 1.58 (1.041–2.412; p < 0.033) for SCD at 55 months per 20 μV TWA in lead V5. |
|
| |||
| Exercise recovery | |||
| Exner et al. (REFINE) (54) | 322 post-MI patients; 62 (interquartile range: 53–70) yrs | Moderately depressed (38%–48%) | 2.94 (1.10–7.87) monitored at 10–14 weeks after event for CV death or resuscitated cardiac arrest (primary endpoint) at 47 months; NS when monitored at 2–4 weeks after MI. For primary endpoint for TWA, AUC = 0.62; for combination of TWA + HRT, AUC = 0.71. |
| Slawnych et al. (REFINE/FINCAVAS) (67) | 322 post-MI patients (from REFINE) and 681 CAD patients (from FINCAVAS); 69 (interquartile range: 57–76) yrs | Moderately depressed (38%–48%) and preserved (56%–63%) groups | 2.5 (1.1–6.0) for CV death at 48 months for 60-μV cutpoint; NPV = 96%; PPV= 13%. AUC for CV mortality = 0.69. |
| Leino et al. (FINCAVAS) (68) | 1,972 consecutive patients referred for routine exercise testing; 57 ± 13 yrs (patients included in Leino et al. [66]) | Mostly preserved | 3.5 (1.6–7.9) for CV death at 48 months for 60-μV cutpoint. For CV death for TWA alone, C-statistic = 0.550–0.606; for combination of TWA + HRR, C-statistic = 0.671–0.691. |
|
| |||
| Ambulatory ECG monitoring | |||
| Verrier et al. (ATRAMI) (69) | Acute post-MI; case: control analysis (15 cases: 29 control subjects) from 1,284 ATRAMI patients, monitored at 15 ± 10 days post-MI; 60–62 yrs | Moderately depressed (42 ± 3%) | 7.9 (1.9–33.1) for cardiac arrest or arrhythmic death at 21 months for a priori 75th percentile cutpoint (47 μV); patients were monitored at 15 ± 10 days post-MI |
| Stein et al. (EPHESUS) (70) | Acute post-MI, LVEF ≤40%, and heart failure; case: control analysis (46 cases: 92 control subjects) from 6,632 EPHESUS patients, monitored at 2–10 days post-MI; 68 ± 11 yrs | Depressed (34 ± 5%) | 5.5 (2.2–13.8) for SCD at 16.4 months for 47-μV cutpoint; patients were monitored at 2–10 days post- MI. For SCD, AUC = 0.73 for TWA in lead V1 and = 0.70 in lead V3 (p < 0.001). |
| Sakaki et al. (71) | 295 consecutive cardiomyopathy patients with ischemic or nonischemic left ventricular dysfunction; 66 ± 16 yrs | Depressed (34 ± 6%) | 17.1 (6.3–46.6) for CV death, 22.6 (2.6–193.7) for witnessed SCD at 1 yr for 65-μV cutpoint; NPV for CV death = 97%; PPV = 37% |
| Maeda et al. (72) | 63 consecutive patients including 21 control subjects, 21 post-MI patients without VT, and 21 post-MI patients with VT; 65 ± 11 yrs | Depressed (36%–43%) for post-MI group | 6.1 (1.1–34.0) for sustained VT or VF at 6 yrs for 65-μV cutpoint |
| Stein et al. (CHS) (73) | General population patients age ≥65 yrs; case: control analysis (49 cases: 98 control subjects) from 1,649 CHS patients | Not tested, assumed preserved | 4.8 (1.48–15.81) for SCD at 14 yrs |
| Hou et al. (74) | 219 consecutive acute post-MI patients; 55 yrs | >35% in 201; ≤35% in 18 | 17.78 (3.75–84.31) for SCD within 16 months for 47-μV cutpoint; patients were monitored at 1–15 days post-MI; NPV = 99%; PPV = 17% |
This table is based on searches of the published medical data on the terms alternans and alternation for all clinical studies that reported hazard ratios in PubMed (National Library of Medicine, National Institutes of Health, Bethesda Maryland) and Paperchase (Bedford Massachusetts) databases. Reference lists from these studies and from recent reviews (4–7) were also scanned.
ATRAMI = Autonomic Tone and Reflexes after Myocardial Infarction; CHS = Cardiovascular Health Study; CV = cardiovascular; EPHESUS = Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; FINCAVAS = Finnish Cardiovascular Study; HRR = heart rate recovery; NPV = negative predictive value; PPV = positive predictive value; other abbreviations as in Table 1.
ANALYSIS APPROACH
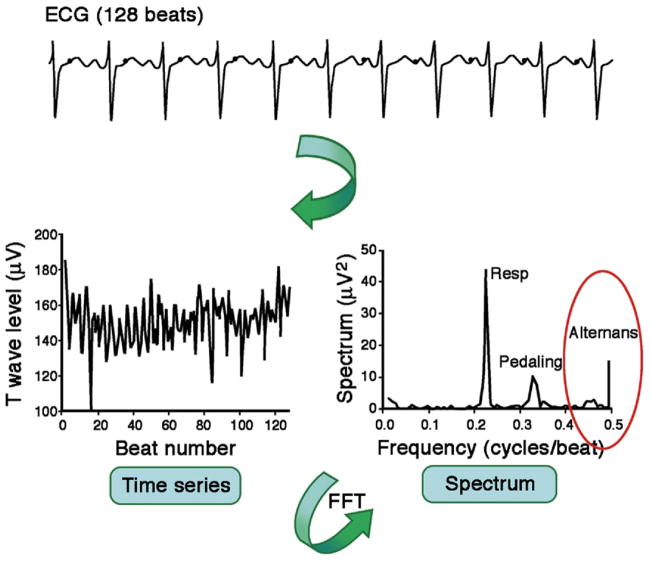
The concept of analyzing morphology fluctuations in the T-wave by computerized spectral techniques dates from experimental studies (29) employing the Fast Fourier Transform (FFT) (Fig. 4) (77). In detail, sequential ECG cycles are aligned to their QRS complex, and the amplitude of the ST segments and T waves at 128 pre-defined points t (relative to the JT interval) is measured. Subsequently, each beat-to-beat series of amplitude fluctuations is processed with FFT to generate a separate spectrum for each point t. The spectra corresponding to different time points along the JT interval are averaged, producing a composite spectrum. The voltage difference between the overall mean beat and the even- or odd-numbered mean beats, or the alternans voltage in microvolts, represents the square root of the alternans power at 0.5 cycle/beat. The significance of TWA is expressed by the alternans ratio (K score), calculated as the ratio of alternans power at 0.5 cycle/beat divided by the standard deviation of spectral noise (78). The greater the power, the higher is the alternans voltage. The ECG fluctuations occurring at other frequencies are not included in TWA assessment. Use of this technique in clinical practice is evidenced by substantial sales of commercial equipment and consumables as publicly reported by Cambridge Heart, Inc.
Figure 4. Spectral TWA Method.
Schematic representation of T-wave alternans (TWA) assessment of the electrocardiogram (ECG) with the Spectral Method (77). See text for details. FFT = Fast Fourier Transform.
TEST METHODOLOGY
Because the Spectral Method involves a graded increase in heart rate, the TWA test is usually conducted during bicycle or treadmill exercise to an optimum heart rate (79). Some investigators infused chronotropic agents (21,24) or employed atrial pacing to elevate and stabilize heart rate (23,56,80–82). TWA can occur in normal individuals at heart rates >120 beats/min. Consequently, a target heart rate range of 105 to 110 beats/min was determined for pathologic alternans in adults (78,82). Practice has varied with regard to withholding beta-adrenergic blockade to allow patients to reach this heart rate (83). The current recommendation is to maintain chronic medications during the test (23).
TWA is determined from standard precordial and orthogonal X,Y,Z leads. Special high-resolution electrodes can minimize noise. TWA is not measured in ECG portions in which ectopic or premature beats constitute >10% of beats (78), because the readings can be influenced by excessive numbers of premature beats or by attendant phase changes of alternans (e.g., from “ABAB” to “BABA”).
CLASSIFICATION OF TEST RESULTS
TWA level >1.9-μV cutpoint with alternans signal-to-noise ratio K >3 sustained for >2 min is defined as a positive test result on the basis of outcome data of clinical studies (55,78). Test results below this level are considered negative.
Because of a relatively high incidence (20% to 40% of all cases) of indeterminate test results, a test classification of “abnormal due to patient factors” was introduced (84). This classification is employed when the test is associated with excessive ectopy (approximately 32%), lack of capacity to reach a target rate of 105 to 110 beats/min (approximately 51%), or nonsustained TWA (approximately 10%). Abnormal test results due to patient factors carry greater risk than positive test results. In contrast, the occurrence of muscle, respiration, or other movement artifacts or electrode noise accounts for 6.4% of indeterminate test results in experienced centers and is referred to as “technically indeterminate.” Those tests have no prognostic value per se.
ANALYSIS APPROACH
The MMA method employs the noise-rejection principle of recursive averaging (75). The algorithm continuously streams odd and even beats into separate bins and creates median complexes for each bin (Fig. 5) (19). These complexes are then superimposed, and the maximum difference between the odd and even median complexes at any point within the JT segment is averaged for every 10 to 15 s and reported as the TWA value. The moving average allows control of the influence of new incoming beats on the median templates with an adjustable update factor (i.e., the fraction of morphology change that an incoming beat can contribute). The recommended rapid update factor of one-eighth provides greater sensitivity and capacity to detect transient but clinically important surges in TWA than one-sixteenth or one-thirty-second (64). Noise measurements are in part derived from mismatch of the even or odd median complexes outside the JT segment. The algorithm excludes extrasystoles, noisy beats, and the beats preceding them and filters effects of noise, movement, and respiration.
Figure 5. MMA TWA Method.
Flow chart of the major components of the Modified Moving Average (MMA) method of T-wave alternans (TWA) analysis. See text for details.
Adapted, with permission, from Nearing et al. (19).
TEST METHODOLOGY
The MMA allows microvolt TWA analysis during routine, symptom-limited exercise stress testing (64 – 66,68) and during post-exercise recovery (54,67,68) as well as during ambulatory ECG monitoring (69–74) in the flow of clinical evaluation (Table 2). Risk stratification is based on the peak TWA value throughout the 24-h ambulatory ECG recording or the symptom-limited exercise test.
MMA-based TWA is calculated from standard precordial ECG leads with standard electrodes (64–74). Because limb leads are prone to motion artifact (65), TWA in these leads should be interpreted with caution. The MMA method generates high-resolution templates of superimposed QRS-aligned complexes showing the alternation pattern, which permit visual examination to verify the presence and magnitude of TWA (Fig. 1) (65,70). TWA values should be over-read for verification down to 20 μV.
CLASSIFICATION OF TEST RESULTS
MMA-based TWA studies with ambulatory ECG recordings and exercise support the concept that TWA represents a continuum of risk with higher TWA levels indicating greater risk (64–68). With the recommended update factor of one-eighth, TWA ≥60 μV during routine exercise testing (64–68) and ambulatory ECG monitoring (69–74) indicates severely elevated risk for sudden cardiac death and/or cardiovascular mortality. In patients during the early post-MI phase with or without heart failure, a cutpoint of ≥47 μV also predicted sudden cardiac death (69,70,74). Leino et al. (66) demonstrated a 55% and 58% increase in risk of cardiovascular and sudden cardiac death, respectively, per 20 μV of TWA.
Immediate repeatability of the Spectral Method test results was verified with bicycle ergometry with 15 min and 4 h between the tests (85) as well as during atrial pacing (24). Data on long-term stability of TWA results with the Spectral Method are sparse (86). Repeatability has not been reported with the MMA method.
The methods are analytically comparable, although they differ in noise processing. The 4- to 10-fold differences in TWA voltages reported by MMA and Spectral Method tests can be accounted for by the update factor (87). Also, the Spectral Method reports one-half of the average TWA magnitude across the entire JT interval for 128 beats, whereas the MMA method reports the peak TWA level at any point within the JT interval for each 10-to 15-s interval.
Clinical Utility
Significant predictivity of TWA analysis by the Spectral Method has been prospectively demonstrated in >7,200 patients with various types of cardiovascular disease, including myocardial infarction, congestive heart failure, ischemic cardiomyopathy, and nonischemic dilated cardiomyopathy (Table 1) (Figs. 6A and 6B) (37,54). Lack of significant predictivity in a few observational studies has been attributed to short periods after myocardial infarction during ongoing remodeling (57,58), washout of beta-blocking agents before the test (23,59,83), and use of implantable cardioverter-defibrillator (ICD) firing as a surrogate endpoint for arrhythmic death (61,62,88).
Figure 6. Survival Curves for Prospective TWA Studies.
(A) The ALPHA (T-Wave Alternans in Patients with Heart Failure) T-wave alternans (TWA) study: Kaplan-Meier cumulative event-free (cardiac death/life-threatening arrhythmias) survival according to TWA Spectral Method testing (37). Patients at risk are shown at selected time points. (B) The REFINE (Risk Estimation Following Infarction, Noninvasive Evaluation) TWA study (54). Risk of cardiac death or resuscitated cardiac arrest (primary outcome) among patients with impaired autonomic tone, measured with heart rate turbulence + abnormal repolarization alternans + ejection fraction <50% versus remaining patients. Left panel indicates TWA results with the Spectral Method during exercise; right panel indicates results of MMA analysis during recovery from exercise. Patients at risk are shown at selected time points. (C) TWA sub-study of SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) (61). Comparison of primary event rates for microvolt T-wave alternans (MTWA) in non-negative (dashed line) and negative (solid line) patients (n = 490). No significant difference between event rates was found for the 2 groups. Reprinted, with permission, from Gold et al. (61). (D) Ambulatory electrocardiogram (ECG) TWA study (71). Event-free survival from cardiac mortality in ischemic (left) and nonischemic (right) subgroups on the basis of maximum TWA voltage by the Modified Moving Average (MMA) method. CI = confidence interval.
A focus of most TWA studies with the Spectral Method has been on identifying patients who would not benefit from ICD implantation, particularly those fulfilling the MADIT II (Multicenter Automatic Defibrillator Trial II) criteria, namely, prior myocardial infarction and left ventricular ejection fraction (LVEF) ≤30% (52,62). The need for accurate identification of patients who would not benefit from ICD implantation is underscored by the facts that one-third of ICD patients receives an inappropriate shock within 1 to 3 years of implantation (89,90), and ICD shock is associated with a 2- to 5-fold increase in mortality, most commonly due to progressive heart failure (90). Event-free survival from all-cause or cardiac mortality and/or ventricular tachyarrhythmias averaged 97% to 98% in patients with negative TWA test results, pointing to a potential to improve exclusion of patients who may not benefit from primary prophylactic ICD implantation, despite meeting evidence-based criterion of LVEF <35% (52,91).
TWA IN INTERVENTIONAL ICD TRIALS
TWA stratified total mortality in the MASTER (Microvolt T Wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial of MADIT II-type patients but did not predict sudden cardiac death or appropriate ICD discharge in the MASTER trial (62) or sudden cardiac death, sustained ventricular tachycardia or fibrillation, or appropriate ICD discharge in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) TWA substudy (Fig. 6C) (61). A recent meta-analysis addressing lack of predictivity of ICD discharge in these trials indicated that predictive accuracy was strong in the TWA studies enrolling relatively few patients with ICDs, with a composite hazard ratio for prediction by abnormal versus negative TWA of 13.6 (95% confidence interval [CI]: 8.5 to 30.4) and that predictive accuracy in studies with high ICD use was low at 1.6 (95% CI: 1.2 to 2.1) (88). Thus, ICD firing, employed as a surrogate endpoint for sudden cardiac death, may have underrepresented the utility of the test. An alternative explanation implicates withholding beta-adrenergic blocking agents to explain the inconsistent results (Fig. 7) (83).
Figure 7. Beta-Blocker TWA Meta-Analysis.
Association between microvolt TWA analyzed by the Spectral Method and ventricular arrhythmic events, stratified by screening protocol discontinuation of beta-blocker therapy. Washout of beta-adrenergic blockade impaired predictivity. Risk ratio (RR): 12.44 reflects 0.5 correction factor; therefore, no 95% CI is depicted. *Study estimates for meta-analysis were hazard or risk ratios. **No events in microvolt TWA negative group (p = 0.04). ***Test for heterogeneity: p = 0.025. Abbreviations as in Table 1. Reprinted, with permission, from Chan et al. (83).
GUIDING ICD IMPLANTATION
Data to support use of TWA to withhold or delay ICD implantation are insufficient at present. To date, only the ABCD (Alternans Before Cardioverter Defibrillator) trial (41) has tested the capacity of TWA to guide prophylactic ICD implantation. Event rates were higher in patients with either a positive TWA (hazard ratio: 2.1, p = 0.03) or a positive electrophysiological study (hazard ratio: 2.4, p = 0.007) than in those whose TWA and electrophysiological tests were either negative or indeterminate at the pre-specified primary time point of 1 year. Moreover, the event rate in patients with both negative TWA and electrophysiological study test results was lower than in patients with 2 positive tests (2% vs. 12%; p = 0.017), suggesting synergy between the tests. But, TWA did not predict endpoint events at 2 years, suggesting potential time dependence.
In their meta-analysis, Hohnloser et al. (88) proposed a clinical algorithm to identify patients who would not benefit from ICD implantation for primary prevention. (See their Fig. 2.) Their analysis revealed that the mortality rate of TWA negative patients with LVEF ≤35% but no history of ventricular arrhythmias and no prior ICD implantation was 4-fold lower than that of MADIT II or SCD-HeFT trial patients randomized to ICD therapy. Accordingly, the negative predictive value derived for this group is >99%. This algorithm will require testing in a prospective trial.
TWA IN HEREDITARY CHANNELOPATHIES
Although intra-cardiac repolarization alternans plays a pivotal pathogenetic role in the genesis of malignant arrhythmias, particularly torsades de pointes, in channelopathies (92), prospective studies have not demonstrated that TWA analysis by the Spectral Method identifies patients with long QT (93,94) or Brugada syndromes (60,95) who will experience episodes of torsades de pointes. The disappearance of TWA during atrial pacing at 110 beats/min provides clues with regard to difficulties in sensing TWA during target heart-rate exercise in Brugada patients (96).
Predictivity of TWA analysis by the MMA method has been demonstrated in >4,800 patients, including those with coronary artery disease, recent or old myocardial infarction, congestive heart failure, or cardiomyopathy (Table 2). In FINCAVAS (Finnish Cardiovascular Study), the largest investigation of TWA to date, TWA predicted sudden cardiac death and cardiovascular and total mortality in a general population of >3,500 low-risk patients referred for routine, symptom-limited exercise testing (64 – 66,68). MMA-based TWA is also predictive when monitored during immediate post-exercise recovery (54,67,68) or from ambulatory ECG records (69 –74) (Fig. 6D). Sudden cardiac death was witnessed (71) or judged by physician review (68,73,97) or an events committee (98,99). TWA detected with continuous 24-h ECG monitoring reflects the influences of daily activity, mental stress (22), sleep states (100), and sleep apnea (101). Results of these investigations support MMA analysis of TWA during the flow of routine clinical assessment.
Hazard ratios for prediction by the Spectral and MMA methods are similar, whether in the same population (54) or in studies overall (Tables 1 and 2). Exner et al. (54) determined that TWA assessed by the Spectral Method during exercise and by the MMA method during the post-exercise recovery phase yielded significant odds ratios of 2.75 and 2.94, respectively, in 322 post-myocardial infarction patients with better-preserved LVEF (mean 47% measured at 8 weeks after myocardial infarction) but without an ICD who were enrolled in the REFINE (Risk Estimation Following Infarction, Noninvasive Evaluation) study (Fig. 6B). The concordance of the 2 methods measured simultaneously during exercise has not been systematically investigated. In a study using atrial pacing (n = 41), Cox et al. (80) found that the Spectral Method was predictive (p < 0.02) and that, with their customized software and with one-sixteenth rather than the recommended one-eighth update factor, MMA was nearly significant (p = 0.06) in the same patients. TWA analyzed by both the Spectral Method and MMA was significantly elevated at 8 to 15 min before the onset of spontaneous ventricular tachyarrhythmias in ambulatory ECGs in the ESVEM (Electrophysiologic Study Versus Electrocardiographic Monitoring) trial (26).
An important frontier in arrhythmia risk stratification concerns patients with preserved LVEF, in whom the majority of sudden cardiac deaths occur albeit with low incidence (102). Ikeda et al. (53) with the Spectral Method and the FINCAVAS investigators (64–68) with the MMA method showed that TWA can identify individuals with preserved LVEF but heightened risk for sudden cardiac death. Positive predictivity in this population is relatively low, in the 8% to 10% range, typical for prognostic markers applied to low-risk groups.
Improved risk stratification in patients with preserved LVEF can be attained by a combination of noninvasive markers, because the capacity to detect risk increases markedly when more than 1 factor is analyzed (103). Hazard ratios increased by 30% to 212% (54,68) and positive predictive value by 78% (50) when cardiac electrical instability quantified by TWA was combined with signal-averaged ECG, heart rate turbulence, or heart rate recovery, which are independent markers of risk of cardiovascular death and may disclose the mechanistic basis for TWA. Use of combinations of noninvasive parameters, specifically including TWA, was recently recommended in a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop report on sudden cardiac death prediction and prevention (104), citing complexities of myocardial substrates underlying sudden cardiac death risk. However, high levels of TWA even in the absence of additional risk markers should be noted.
GRADED ASSESSMENT OF RISK
To date, most TWA studies have employed a binary approach to classify test results as either negative or abnormal on the basis of a cutpoint. However, because TWA reflects a continuum of cardiac electrical instability, its quantification offers the potential for diagnosing levels of risk and for improving prognostics. Treating a continuous variable as binary reduces predictive power by one-third to one-half (105).
Clinical studies using either the Spectral (26,106) or MMA methods (26,64–68) reveal that higher TWA magnitudes indicate increased risk for ventricular tachyarrhythmias. Klingenheben et al. (106) found in patients with infarct-related or nonischemic cardiomyopathy that TWA magnitude and not merely its presence was associated with tachyarrhythmic complications. In the FINCAVAS database, Minkkinen et al. (65), Slawnych et al. (67), and Leino et al. (66) reported that sudden cardiac death incidence and cardiovascular and total mortality rose with elevated TWA values (Fig. 8) (67). Leino et al. (66) determined that risk for cardiovascular death and sudden cardiac death increased by 55% and 58%, respectively, for each 20 μV of TWA. A crescendo in TWA magnitude consistently occurred before onset of life-threatening ventricular tachyarrhythmias in ambulatory subjects with ischemic heart disease enrolled in the ESVEM (Electrophysiologic Study Versus Electrocardiographic Monitoring) trial (26). During the arrhythmia-free period, TWA was significantly lower than during the 15 min immediately preceding ventricular tachycardia onset.
Figure 8. The REFINE/FINCAVAS TWA Study.
Rates of cardiovascular death (orange bars) and total mortality (blue bars) at 4 years, by quintile of TWA magnitude with the MMA method (67). FINCAVAS = Finnish Cardiovascular Study; other abbreviations as in Figure 6.
Quantitative TWA analysis may allow tracking changes in risk over time, as patients recover and the myocardium remodels, as cardiac disease or heart failure status are altered, or in response to medical therapy. TWA quantification also permits comparison of risk levels across different populations and disease states. For example, it was recently shown that TWA is markedly elevated (≥60 μV) in dialysis patients in the absence of an acute cardiovascular event, consistent with their established heightened risk for sudden cardiac death (107).
GUIDING MEDICAL THERAPY
Clinical studies and reports suggest that the magnitude of TWA reflects the effects of pharmacologic therapy without reducing the predictive capacity of the phenomenon (108). Beta-adrenergic (23,24,83) and sodium channel blocking agents (109) diminish TWA magnitude, reflecting the capacity of these agents to reduce sudden cardiac death and cardiovascular mortality (110). In the Brugada syndrome, sodium channel blockade provokes the diagnostic ECG changes as well as macroscopic TWA and arrhythmias (111). The proarrhythmic effects associated with cardiovascular and noncardiovascular agents are also disclosed by elevated levels of TWA.
Because antiadrenergic and antiarrhythmic drugs influence TWA, the decision to withhold these agents at the time of TWA testing is a matter of debate. Some investigators withheld beta-blocker therapy to avoid drug-induced chronotropic incompetence (36,38,39,41,61,62), whereas others performed the test with full cardioprotective medication (35,37,45,48). In a meta-analysis, Chan et al. (83) determined that the predictive capacity of TWA for ventricular arrhythmic events was significantly weaker in studies withholding beta-blockade therapy (relative risk: 1.40; 95% CI: 1.06 to 1.84; p = 0.02) than in studies performed with discharge medications (relative risk: 5.39; 95% CI: 2.68 to 10.84; p <0.001) (Fig. 7). An important corollary of these findings is that the effects of medications on TWA represents not a disruption in measurement but an indication of therapeutic efficacy (108).
Summary and Recommendations for Clinical Practice
An extensive body of evidence from prospective studies in >12,000 patients with depressed or preserved LVEF supports the use of TWA analysis in assessing risk for cardiovascular mortality and sudden cardiac death (Tables 1 and 2) (112). Hazard ratios generated by the Spectral and MMA methods are similar, whether in the same population (54) or in studies overall. Multivariate analyses confirm that TWA provides information on risk beyond standard clinical variables for cardiovascular disease, including demographic factors (e.g., age, sex, and race) and traditional cardiovascular risk markers (e.g., smoking, blood pressure, history, and medications). Currently, the only established risk marker for sudden cardiac death and the only parameter approved to identify high-risk patients for ICD implantation is depressed LVEF (113). This parameter has significant limitations, as underscored by the fact that the majority of individuals who die suddenly have preserved LVEF (102). TWA is being assessed along with candidate genes as arrhythmia markers in a large cohort of patients with ICDs for ICD shock risk and mortality in EUTrigTreat (EU TrigTreat Clinical Study: An Arrhythmia Risk Stratification and Genetic Trial) (Clinical trials identifier: NCT01209494) and in the GAME (Medtronic Genetic Arrhythmia Markers for Early Detection) study (Clinical trials identifier: NCT00664807).
Accordingly, we concur with the recommendations of guidelines committees of the American Heart Association, American College of Cardiology, European Society of Cardiology, and/or Heart Rhythm Society chaired by Zipes et al. (114), who assigned Class I Level of Evidence: A and Class IIa Level of Evidence: A indications to TWA testing for arrhythmia risk, and led by Goldberger et al. (115), who determined that TWA provides valuable information with regard to risk for cardiovascular mortality and sudden cardiac death. Additional applications recommended by task force or consensus statement authors are in neonates, in whom the appearance of TWA signals the highest level of risk (116), and in hospital settings to warn of the development of torsade de pointes (117).
Interventional trials, which have been performed to date only with the Spectral Method, have not demonstrated that a negative TWA test result can sufficiently guide decision-making with regard to ICD implantation (41,61,62). Thus, TWA should not be used as a sole parameter either to rule in or to rule out ICD use. A clinical algorithm that incorporates LVEF in the decision-making process appears promising on the basis of a meta-analysis (88). However, this proposal will require prospective testing. Also, use of more advanced statistical approaches and criteria for evaluation of novel markers of cardiovascular risk is recommended (118).
Acknowledging the complexities of myocardial substrates underlying sudden cardiac death risk, a recent National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop report on prediction and prevention proposed combinations of risk markers specifically including TWA (104). The utility of ambulatory TWA combined with heart rate turbulence, an indicator of baroreceptor sensitivity (119), to identify post-myocardial infarction patients with preserved LVEF who would benefit from ICD implantation is the subject of an ongoing clinical trial, REFINE-ICD (Risk Estimation Following Infarction, Noninvasive Evaluation of ICD Therapy) (120) (Clinical trials identifier: NCT00673842).
The time period for optimum predictive accuracy of TWA for arrhythmic events, cardiovascular mortality, and total mortality in patients with acute myocardial infarction is under investigation in a clinical trial, T-Wave Alternans in Acute Myocardial Infarction: An Evaluation of the Time of Testing on Its Prognostic Accuracy (Clinical trials identifier: NCT00589849). Exner et al. (54) determined that, for long-term prediction, TWA testing should be performed at 10 to 14 weeks after myocardial infarction, while others (69,70,74) found significant risk stratification with TWA monitoring at ≤15 days post-MI. There is a rationale for considering earlier TWA testing, because 1.4% of patients (approximately 17,500 in the United States) experience sudden cardiac death in the first month after myocardial infarction during the acute remodeling period (121).
Evidence supports the value of quantitative TWA analysis for both the Spectral and MMA methods. Quantification offers inherent advantages with respect to gauging level of risk across time after cardiovascular events and in evaluation of the potential anti- and pro-arrhythmic effects of medical therapy by conventional and nonconventional agents (108). These applications merit exploration in interventional trials.
It is recommended that TWA testing be performed on discharge medications, particularly with respect to beta-blockade therapy (23,83), to ensure that the test results reflect the effects of chronic drug therapy.
Conclusions
Overall, our assessment is that it is reasonable to consider TWA evaluation whenever there is suspicion of vulnerability to lethal cardiac arrhythmias. However, there is as yet no definitive evidence from interventional trials that it can guide therapy.
Acknowledgments
No financial support was received for preparation of this consensus guideline. Dr. Verrier receives royalty income from Georgetown University and Beth Israel Deaconess Medical Center for intellectual property licensed to GE Healthcare and to Medtronic. Dr. Exner received equipment donations from Cambridge Heart and GE Healthcare; and research grants from GE Healthcare, Cambridge Heart Inc., Medtronic, and St. Jude Medical. Dr. Hohnloser is on the Speakers’ Bureau of Cambridge Heart; and is a consultant for St. Jude Medical. Dr. Narayan receives lecture honoraria from St. Jude Medical, Medtronic, Biotronik, and previously from Cambridge Heart; and has ownership interest in Topera Inc. Dr. Rosenbaum is a consultant to Cambridge Heart. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Verrier, Klingenheben, and Malik comprise the International Society for Holter and Noninvasive Electrocardiology Study Group Writing Committee for this consensus guideline. Daniel J. Cantillon, MD, served as Guest Editor for this paper.
Abbreviations and Acronyms
- CI
confidence interval
- ECG
electrocardiogram
- FFT
Fast Fourier Transform
- ICD
implantable cardioverter-defibrillator
- LVEF
left ventricular ejection fraction
- MMA
Modified Moving Average
- TWA
T-wave alternans
References
- 1.Hering HE. Experimentelle Studien an Säugethieren über das Elektrocardiogram. Zschr Exper Path Therapie. 1909;7:363–78. [Google Scholar]
- 2.Oshodi GO, Wilson LD, Costantini O, Rosenbaum DS. Microvolt T wave alternans: mechanisms and implications for prediction of sudden cardiac death. In: Gussak I, Antzelevitch C, Wilde AAM, Friedman P, Ackerman M, Shen WK, editors. Electrical Diseases of the Heart: Genetics, Mechanisms, Treatment, Prevention. New York, NY: Springer-Verlag; 2007. pp. 394–408. [Google Scholar]
- 3.Chinushi M, Kozhevnikov D, Caref EB, Restivo M, El-Sherif N. Mechanism of discordant T wave alternans in the in vivo heart. J Cardiovasc Electrophysiol. 2003;14:632–8. doi: 10.1046/j.1540-8167.2003.03028.x. [DOI] [PubMed] [Google Scholar]
- 4.Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–81. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 5.Cutler MJ, Rosenbaum DS. Explaining the clinical manifestations of T wave alternans in patients at risk for sudden cardiac death. Heart Rhythm. 2009;6:S22–8. doi: 10.1016/j.hrthm.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verrier RL, Kumar K, Nearing BD. Basis for sudden cardiac death prediction by T-wave alternans from an integrative physiology perspective. Heart Rhythm. 2009;6:416–22. doi: 10.1016/j.hrthm.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res. 2011;108:98–112. doi: 10.1161/CIRCRESAHA.110.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol. 2008;294:H1–10. doi: 10.1152/ajpheart.00802.2007. [DOI] [PubMed] [Google Scholar]
- 9.Wilson LD, Jeyaraj D, Wan X, et al. Heart failure enhances susceptibility to arrhythmogenic cardiac alternans. Heart Rhythm. 2009;6:251–9. doi: 10.1016/j.hrthm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konta T, Ikeda K, Yamaki M, et al. Significance of discordant ST alternans in ventricular fibrillation. Circulation. 1990;82:2185–9. doi: 10.1161/01.cir.82.6.2185. [DOI] [PubMed] [Google Scholar]
- 11.Chinushi M, Restivo M, Caref EB, El-Sherif N. Electrophysiological basis of arrhythmogenicity of QT/T alternans in the long-QT syndrome: tridimensional analysis of the kinetics of cardiac repolarization. Circ Res. 1998;83:614–28. doi: 10.1161/01.res.83.6.614. [DOI] [PubMed] [Google Scholar]
- 12.Nearing BD, Verrier RL. Progressive increases in complexity of T-wave oscillations herald ischemia-induced ventricular fibrillation. Circ Res. 2002;91:727–32. doi: 10.1161/01.res.0000038887.17976.33. [DOI] [PubMed] [Google Scholar]
- 13.Nearing BD, Verrier RL. Tracking cardiac electrical instability by computing interlead heterogeneity of T-wave morphology. J Appl Physiol. 2003;95:2265–72. doi: 10.1152/japplphysiol.00623.2003. [DOI] [PubMed] [Google Scholar]
- 14.Selvaraj RJ, Picton P, Nanthakumar K, Mak S, Chauhan VS. Endocardial and epicardial repolarization alternans in human cardiomyopathy: evidence for spatiotemporal heterogeneity and correlation with body surface T-wave alternans. J Am Coll Cardiol. 2007;49:338–46. doi: 10.1016/j.jacc.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 15.Narayan SM, Bayer JD, Lalani G, Trayanova NA. Action potential dynamics explain arrhythmic vulnerability in human heart failure: a clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol. 2008;52:1782–92. doi: 10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia induced vulnerability by precordial T wave alternans analysis in dog and human. Cardiovasc Res. 1994;28:1440–9. doi: 10.1093/cvr/28.9.1440. [DOI] [PubMed] [Google Scholar]
- 17.Narayan SM, Lindsay BD, Smith JM. Demonstrating the pro-arrhythmic preconditioning of single premature extrastimuli using the magnitude, phase, and temporal distribution of repolarization alternans. Circulation. 1999;100:1887–93. doi: 10.1161/01.cir.100.18.1887. [DOI] [PubMed] [Google Scholar]
- 18.Nearing BD, Huang AH, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T wave. Science. 1991;252:437–40. doi: 10.1126/science.2017682. [DOI] [PubMed] [Google Scholar]
- 19.Nearing BD, Verrier RL. Modified moving average analysis of T-wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol. 2002;92:541–9. doi: 10.1152/japplphysiol.00592.2001. [DOI] [PubMed] [Google Scholar]
- 20.Martinez JP, Olmos S, Wagner G, Laguna P. Characterization of repolarization alternans during ischemia: time-course and spatial analysis. IEEE Trans Biomed Eng. 2006;53:701–11. doi: 10.1109/TBME.2006.870233. [DOI] [PubMed] [Google Scholar]
- 21.Kaufman ES, Mackall JA, Julka B, Drabek C, Rosenbaum DS. Influence of heart rate and sympathetic stimulation on arrhythmogenic T wave alternans. Am J Physiol Heart Circ Physiol. 2000;279:H1248–55. doi: 10.1152/ajpheart.2000.279.3.H1248. [DOI] [PubMed] [Google Scholar]
- 22.Lampert R, Shusterman V, Burg M, et al. Anger-induced T-wave alternans predicts future ventricular arrhythmias in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 2009;53:774–8. doi: 10.1016/j.jacc.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klingenheben T, Gronefeld G, Li YG, Hohnloser SH. Effect of metoprolol and d,l-sotalol on microvolt-level T-wave alternans. Results of a prospective, double-blind, randomized study. J Am Coll Cardiol. 2001;38:2013–9. doi: 10.1016/s0735-1097(01)01661-8. [DOI] [PubMed] [Google Scholar]
- 24.Rashba EJ, Cooklin M, MacMurdy K, et al. Effects of selective autonomic blockade on T-wave alternans in humans. Circulation. 2002;105:837–42. doi: 10.1161/hc0702.104127. [DOI] [PubMed] [Google Scholar]
- 25.Ferrero P, Castagno D, Massa R, et al. Spinal cord stimulation affects T-wave alternans in patients with ischaemic cardiomyopathy: a pilot study. Europace. 2008;10:506–8. doi: 10.1093/europace/eun052. [DOI] [PubMed] [Google Scholar]
- 26.Shusterman V, Goldberg A, London B. Upsurge in T-wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation. 2006;113:2880–7. doi: 10.1161/CIRCULATIONAHA.105.607895. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu RK, Costantini O, Cummings JE, Poelzing S, Rosenbaum DS, Quan KJ. Intracardiac alternans compared to surface T-wave alternans as a predictor of ventricular arrhythmias in humans. Heart Rhythm. 2008;5:1003–8. doi: 10.1016/j.hrthm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Bayer JD, Narayan SM, Lalani GG, Trayanova NA. Rate-dependent action potential alternans in human heart failure implicates abnormal intracellular calcium handling. Heart Rhythm. 2010;7:1093–101. doi: 10.1016/j.hrthm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JM, Clancy EA, Valeri CR, Ruskin JM, Cohen RJ. Electrical alternans and cardiac electrical instability. Circulation. 1988;77:110–21. doi: 10.1161/01.cir.77.1.110. [DOI] [PubMed] [Google Scholar]
- 30.Kjolbye AL, Dikshteyn M, Eloff BC, Deschenes I, Rosenbaum DS. Maintenance of intercellular coupling by the antiarrhythmic peptide rotigaptide suppresses arrhythmogenic discordant alternans. Am J Physiol Heart Circ Physiol. 2008;294:H41–9. doi: 10.1152/ajpheart.01089.2006. [DOI] [PubMed] [Google Scholar]
- 31.Nearing BD, Hutter JJ, Verrier RL. Potent antifibrillatory effect of combined blockade of calcium channels and 5-HT2 receptors with nexopamil during myocardial ischemia and reperfusion in dogs: Comparison to diltiazem. J Cardiovasc Pharmacol. 1996;27:777–87. doi: 10.1097/00005344-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Harada M, Shimizu A, Murata M, et al. Relation between microvolt-level T-wave alternans and cardiac sympathetic nervous system abnormality using iodine-123 metaiodobenzylguanidine imaging in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2003;92:998–1001. doi: 10.1016/s0002-9149(03)00988-3. [DOI] [PubMed] [Google Scholar]
- 33.Kon-No Y, Watanabe J, Koseki Y, et al. Microvolt T wave alternans in human cardiac hypertrophy: electrical instability and abnormal myocardial arrangement. J Cardiovasc Electrophysiol. 2001;12:759– 63. doi: 10.1046/j.1540-8167.2001.00759.x. [DOI] [PubMed] [Google Scholar]
- 34.Kitamura H, Ohnishi Y, Okajima K, et al. Onset heart rate of microvolt-level T-wave alternans provides clinical and prognostic value in nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2002;39:295–300. doi: 10.1016/s0735-1097(01)01718-1. [DOI] [PubMed] [Google Scholar]
- 35.Hohnloser SH, Klingenheben T, Bloomfield D, Dabbous O, Cohen RJ. Usefulness of microvolt T-wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: results from a prospective observational study. J Am Coll Cardiol. 2003;41:2220–4. doi: 10.1016/s0735-1097(03)00467-4. [DOI] [PubMed] [Google Scholar]
- 36.Chow T, Kereiakes DJ, Bartone C, et al. Prognostic utility of microvolt T-wave alternans in risk stratification of patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2006;47:1820–7. doi: 10.1016/j.jacc.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 37.Salerno-Uriarte JA, De Ferrari GM, Klersy C, et al. Prognostic value of T-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: results of the ALPHA study. J Am Coll Cardiol. 2007;50:1896–904. doi: 10.1016/j.jacc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Chow T, Saghir S, Bartone C, et al. Usefulness of microvolt T-wave alternans in predicting outcome in patients with ischemic cardiomyopathy with and without defibrillators. Am J Cardiol. 2007;100:598–604. doi: 10.1016/j.amjcard.2007.03.069. [DOI] [PubMed] [Google Scholar]
- 39.Chow T, Kereiakes DJ, Bartone C, et al. Microvolt T-wave alternans identifies patients with ischemic cardiomyopathy who benefit from implantable cardioverter-defibrillator therapy. J Am Coll Cardiol. 2007;49:50–8. doi: 10.1016/j.jacc.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 40.Chan PS, Kereiakes DJ, Bartone C, Chow T. Usefulness of microvolt T-wave alternans to predict outcomes in patients with ischemic cardiomyopathy beyond one year. Am J Cardiol. 2008;102:280–4. doi: 10.1016/j.amjcard.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 41.Costantini O, Hohnloser SH, Kirk MM, et al. The ABCD (Alternans Before Cardioverter Defibrillator) trial: strategies using T-wave alternans to improve efficiency of sudden cardiac death prevention. J Am Coll Cardiol. 2009;53:471–9. doi: 10.1016/j.jacc.2008.08.077. [DOI] [PubMed] [Google Scholar]
- 42.Alexander ME, Cecchin F, Huang KP, Berul CI. Microvolt T-wave alternans with exercise in pediatrics and congenital heart disease: limitations and predictive value. Pacing Clin Electrophysiol. 2006;29:733–41. doi: 10.1111/j.1540-8159.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 43.Rashba EJ, Osman AF, MacMurdy K, et al. Influence of QRS duration on the prognostic value of T wave alternans. J Cardiovasc Electrophysiol. 2002;13:770–5. doi: 10.1046/j.1540-8167.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- 44.Rashba EJ, Osman AF, Macmurdy K, et al. Enhanced detection of arrhythmia vulnerability using T wave alternans, left ventricular ejection fraction, and programmed ventricular stimulation: a prospective study in subjects with chronic ischemic heart disease. J Cardio-vasc Electrophysiol. 2004;15:170–6. doi: 10.1046/j.1540-8167.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- 45.Bloomfield DM, Bigger JT, Steinman RC, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–63. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 46.Cantillon DJ, Stein KM, Markowitz SM, et al. Predictive value of microvolt T-wave alternans in patients with left ventricular dysfunction. J Am Coll Cardiol. 2007;50:166–73. doi: 10.1016/j.jacc.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 47.Morin DP, Zacks ES, Mauer AC, et al. Effect of bundle branch block on microvolt T-wave alternans and electrophysiologic testing in patients with ischemic cardiomyopathy. Heart Rhythm. 2007;4:904–12. doi: 10.1016/j.hrthm.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 48.Klingenheben T, Zabel M, D’Agostino RB, Cohen RJ, Hohnloser SH. Predictive value of T-wave alternans for arrhythmic events in patients with congestive heart failure [letter] Lancet. 2000;356:651–2. doi: 10.1016/s0140-6736(00)02609-x. [DOI] [PubMed] [Google Scholar]
- 49.Gorodeski EZ, Cantillon DJ, Goel SS, et al. Microvolt T-wave alternans, peak oxygen consumption, and outcome in patients with severely impaired left ventricular systolic function. J Heart Lung Transplant. 2009;28:689–96. doi: 10.1016/j.healun.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikeda T, Sakata T, Takami M, et al. Combined assessment of T-wave alternans and late potentials used to predict arrhythmic events after myocardial infarction. A prospective study. J Am Coll Cardiol. 2000;35:722–30. doi: 10.1016/s0735-1097(99)00590-2. [DOI] [PubMed] [Google Scholar]
- 51.Ikeda T, Saito H, Tanno K, et al. T-wave alternans as a predictor for sudden cardiac death after myocardial infarction. Am J Cardiol. 2002;89:79–82. doi: 10.1016/s0002-9149(01)02171-3. [DOI] [PubMed] [Google Scholar]
- 52.Bloomfield DM, Steinman RC, Namerow PB, et al. Microvolt T-wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: a solution to the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II conundrum. Circulation. 2004;110:1885–9. doi: 10.1161/01.CIR.0000143160.14610.53. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda T, Yoshino H, Sugi K, et al. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: results of a collaborative cohort study. J Am Coll Cardiol. 2006;48:2268–74. doi: 10.1016/j.jacc.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 54.Exner DV, Kavanagh KM, Slawnych MP, et al. Noninvasive risk assessment early after a myocardial infarction the REFINE study. J Am Coll Cardiol. 2007;50:2275–84. doi: 10.1016/j.jacc.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 55.Gold MR, Bloomfield DM, Anderson KP, et al. A comparison of T-wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol. 2000;36:2247–53. doi: 10.1016/s0735-1097(00)01017-2. [DOI] [PubMed] [Google Scholar]
- 56.Rashba EJ, Osman AF, MacMurdy K, et al. Exercise is superior to pacing for T wave alternans measurement in subjects with chronic coronary artery disease and left ventricular dysfunction. J Cardiovasc Electrophysiol. 2002;13:845–50. doi: 10.1046/j.1540-8167.2002.00845.x. [DOI] [PubMed] [Google Scholar]
- 57.Schwab JO, Weber S, Schmitt H, et al. Incidence of T wave alternation after acute myocardial infarction and correlation with other prognostic parameters: results of a prospective study. Pacing Clin Electrophysiol. 2001;24:957–61. doi: 10.1046/j.1460-9592.2001.00957.x. [DOI] [PubMed] [Google Scholar]
- 58.Tapanainen JM, Still AM, Airaksinen KE, Huikuri HV. Prognostic significance of risk stratifiers of mortality, including T wave alternans, after acute myocardial infarction: results of a prospective follow-up study. J Cardiovasc Electrophysiol. 2001;12:645–52. doi: 10.1046/j.1540-8167.2001.00645.x. [DOI] [PubMed] [Google Scholar]
- 59.Grimm W, Christ M, Bach J, Muller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg cardiomyopathy study. Circulation. 2003;108:2883–91. doi: 10.1161/01.CIR.0000100721.52503.85. [DOI] [PubMed] [Google Scholar]
- 60.Ikeda T, Takami M, Sugi K, Mizusawa Y, Sakurada H, Yoshino H. Noninvasive risk stratification of subjects with a Brugada-type electrocardiogram and no history of cardiac arrest. Ann Noninvasive Electrocardiol. 2005;10:396–403. doi: 10.1111/j.1542-474X.2005.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gold MR, Ip JH, Costantini O, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: Primary results from the T-wave Alternans Sudden Cardiac Death in Heart Failure Trial substudy. Circulation. 2008;118:2022–8. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chow T, Kereiakes DJ, Onufer J, et al. Does microvolt T-wave alternans testing predict ventricular tachyarrhythmias in patients with ischemic cardiomyopathy and prophylactic defibrillators? The MASTER (Microvolt T-wave Alternans Testing for Risk Stratification of Post-Myocardial Infarction Patients) trial. J Am Coll Cardiol. 2008;52:1607–15. doi: 10.1016/j.jacc.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 63.Huikuri HV, Raatikainen MJ, Moerch-Joergensen R, et al. Prediction of fatal or near-fatal cardiac arrhythmia events in patients with depressed left ventricular function after an acute myocardial infarction. Eur Heart J. 2009;30:689–98. doi: 10.1093/eurheartj/ehn537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nieminen T, Lehtimaki T, Viik J, et al. T-wave alternans predicts mortality in a population undergoing a clinically indicated exercise test. Eur Heart J. 2007;28:2332–7. doi: 10.1093/eurheartj/ehm271. [DOI] [PubMed] [Google Scholar]
- 65.Minkkinen M, Kahonen M, Viik J, et al. Enhanced predictive power of quantitative TWA during routine exercise testing in the Finnish Cardiovascular Study. J Cardiovasc Electrophysiol. 2009;20:408–15. doi: 10.1111/j.1540-8167.2008.01325.x. [DOI] [PubMed] [Google Scholar]
- 66.Leino J, Verrier RL, Minkkinen M, et al. Importance of regional specificity of T-wave alternans in assessing risk for cardiovascular mortality and sudden cardiac death during routine exercise testing. Heart Rhythm. 2011;8:385–90. doi: 10.1016/j.hrthm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Slawnych MP, Nieminen T, Kahonen M, et al. Post-exercise assessment of cardiac repolarization alternans in patients with coronary artery disease using the modified moving average method. J Am Coll Cardiol. 2009;53:1130–7. doi: 10.1016/j.jacc.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 68.Leino J, Minkkinen M, Nieminen T, et al. Combined assessment of heart rate recovery and T-wave alternans during routine exercise testing improves prediction of total and cardiovascular mortality: The Finnish Cardiovascular Study. Heart Rhythm. 2009;6:1765–71. doi: 10.1016/j.hrthm.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 69.Verrier RL, Nearing BD, La Rovere MT, et al. Ambulatory electrocardiogram-based tracking of T wave alternans in post-myocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol. 2003;14:705–11. doi: 10.1046/j.1540-8167.2003.03118.x. [DOI] [PubMed] [Google Scholar]
- 70.Stein PK, Sanghavi D, Domitrovich PP, Mackey RA, Deedwania P. Ambulatory ECG-based T-wave alternans predicts sudden cardiac death in high-risk post-MI patients with left ventricular dysfunction in the EPHESUS study. J Cardiovasc Electrophysiol. 2008;19:1037–42. doi: 10.1111/j.1540-8167.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 71.Sakaki K, Ikeda T, Miwa Y, et al. Time-domain T-wave alternans measured from Holter electrocardiograms predicts cardiac mortality in patients with left ventricular dysfunction: a prospective study. Heart Rhythm. 2009;6:332–7. doi: 10.1016/j.hrthm.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 72.Maeda S, Nishizaki M, Yamawake N, et al. Ambulatory ECG-based T-wave alternans and heart rate turbulence predict high risk of arrhythmic events in patients with old myocardial infarction. Circ J. 2009;73:2223–8. doi: 10.1253/circj.cj-09-0420. [DOI] [PubMed] [Google Scholar]
- 73.Stein PK, Sanghavi D, Sotoodehnia N, Siscovick DS, Gottdiener J. Association of Holter-based measures including T-wave alternans with risk of sudden cardiac death in the community-dwelling elderly: the cardiovascular health study. J Electrocardiol. 2010;43:251–9. doi: 10.1016/j.jelectrocard.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hou Y, Fang PH, Wu Y, et al. Prediction of sudden cardiac death in patients after acute myocardial infarction using T-wave alternans: a prospective study. J Electrocardiol. 2011 doi: 10.1016/j.jelectrocard.2011.07.015. In press. [DOI] [PubMed] [Google Scholar]
- 75.Martinez JP, Olmos S. Methodological principles of T wave alternans analysis: a unified framework. IEEE Trans Biomed Eng. 2005;52:599–613. doi: 10.1109/TBME.2005.844025. [DOI] [PubMed] [Google Scholar]
- 76.Monasterio V, Clifford GD, Laguna P, Martinez JP. A multilead scheme based on periodic component analysis for T-wave alternans analysis in the ECG. Ann Biomed Eng. 2010;38:2523–41. doi: 10.1007/s10439-010-0029-z. [DOI] [PubMed] [Google Scholar]
- 77.Cohen RJ. TWA and Laplacian imaging. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 5. Philadelphia: Saunders; 2009. p. 889. [Google Scholar]
- 78.Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol. 2002;13:502–12. doi: 10.1046/j.1540-8167.2002.00502.x. [DOI] [PubMed] [Google Scholar]
- 79.Turitto G, Caref EB, El-Attar G, et al. Optimal target heart rate for exercise-induced T-wave alternans. Ann Noninvasive Electrocardiol. 2001;6:123–8. doi: 10.1111/j.1542-474X.2001.tb00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cox V, Patel M, Kim J, Liu T, Sivaraman G, Narayan SM. Predicting arrhythmia-free survival using spectral and modified-moving average analyses of T-wave alternans. Pacing Clin Electrophysiol. 2007;30:352–8. doi: 10.1111/j.1540-8159.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- 81.Tanno K, Ryu S, Watanabe N, et al. Microvolt T-wave alternans as a predictor of ventricular tachyarrhythmias: a prospective study using atrial pacing. Circulation. 2004;109:1854–8. doi: 10.1161/01.CIR.0000124717.77777.EC. [DOI] [PubMed] [Google Scholar]
- 82.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–41. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 83.Chan PS, Gold MR, Nallamothu BK. Do beta-blockers impact microvolt T-wave alternans testing in patients at risk for ventricular arrhythmias? A meta-analysis. J Cardiovasc Electrophysiol. 2010;21:1009–14. doi: 10.1111/j.1540-8167.2010.01757.x. [DOI] [PubMed] [Google Scholar]
- 84.Kaufman ES, Bloomfield DM, Steinman RC, et al. “Indeterminate” microvolt T-wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;48:1399–404. doi: 10.1016/j.jacc.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 85.Turitto G, Mirandi AP, Pedalino RP, Uretsky S, El-Sherif N. Short-term reproducibility of T wave alternans measurement. J Cardiovasc Electrophysiol. 2002;13:641–4. doi: 10.1046/j.1540-8167.2002.00641.x. [DOI] [PubMed] [Google Scholar]
- 86.Oliveira MM, Fiarresga A, Pelicano N, et al. Temporal variations in microvolt T-wave alternans testing after acute myocardial infarction. Ann Noninvasive Electrocardiol. 2007;12:98–103. doi: 10.1111/j.1542-474X.2007.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hostetler B, Xue J, Young B, et al. Detect short run of TWA event with time-domain algorithm. Comput Cardiol. 2005:483–6. [Google Scholar]
- 88.Hohnloser SH, Ikeda T, Cohen RJ. Evidence regarding clinical use of microvolt T-wave alternans. Heart Rhythm. 2009;6:S36–44. doi: 10.1016/j.hrthm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 89.Ellenbogen KA, Levine JH, Berger RD, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–82. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 90.Mishkin JD, Saxonhouse SJ, Woo GW, et al. Appropriate evaluation and treatment of heart failure patients after implantable cardioverter-defibrillator discharge: time to go beyond the initial shock. J Am Coll Cardiol. 2009;54:1993–2000. doi: 10.1016/j.jacc.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 91.Hohnloser SH, Ikeda T, Bloomfield DM, Dabbous OH, Cohen RJ. T-wave alternans negative coronary patients with low ejection and benefit from defibrillator implantation. Lancet. 2003;362:125–6. doi: 10.1016/s0140-6736(03)13865-2. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz PJ, Malliani A. Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q-T syndrome. Am Heart J. 1975;89:45–50. doi: 10.1016/0002-8703(75)90008-3. [DOI] [PubMed] [Google Scholar]
- 93.Kaufman ES, Priori SG, Napolitano C, et al. Electrocardiographic prediction of abnormal genotype in congenital long QT syndrome: experience in 101 related family members. J Cardiovasc Electrophysiol. 2001;12:455–61. doi: 10.1046/j.1540-8167.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 94.Schmitt J, Baumann S, Klingenheben T, et al. Assessment of microvolt T-wave alternans in high-risk patients with the congenital long-QT syndrome. Ann Noninvasive Electrocardiol. 2009;14:340–5. doi: 10.1111/j.1542-474X.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirchhof P, Eckardt L, Rolf S, et al. T wave alternans does not assess arrhythmic risk in patients with Brugada syndrome. Ann Noninvasive Electrocardiol. 2004;9:162–5. doi: 10.1111/j.1542-474X.2004.92541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishizaki M, Fujii H, Sakurada H, Kimura A, Hiraoka M. Spontaneous T wave alternans in a patient with Brugada syndrome—responses to intravenous administration of class I antiarrhythmic drug, glucose tolerance test, and atrial pacing. J Cardiovasc Electrophysiol. 2005;16:217–20. doi: 10.1046/j.1540-8167.2004.40411.x. [DOI] [PubMed] [Google Scholar]
- 97.Pajunen P, Koukkunen H, Ketonen M, et al. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12:132–7. doi: 10.1097/00149831-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 98.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ for the ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 99.Pitt B, Remme W, Zannad F, et al. for the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 100.Verrier RL, Josephson ME. Impact of sleep on arrhythmogenesis. Circ Arrhythm Electrophysiol. 2009;2:450–9. doi: 10.1161/CIRCEP.109.867028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takasugi N, Nishigaki K, Kubota T, et al. Sleep apnoea induces cardiac electrical instability assessed by T-wave alternans in patients with congestive heart failure. Eur J Heart Fail. 2009;11:1063–70. doi: 10.1093/eurjhf/hfp138. [DOI] [PubMed] [Google Scholar]
- 102.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–82. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 103.Buxton AE. Risk stratification for sudden death in patients with coronary artery disease. Heart Rhythm. 2009;6:836–47. doi: 10.1016/j.hrthm.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 104.Fishman GI, Chugh SS, DiMarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society workshop. Circulation. 2010;122:2335–48. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–41. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 106.Klingenheben T, Ptaszynski P, Hohnloser SH. Quantitative assessment of microvolt T-wave alternans in patients with congestive heart failure. J Cardiovasc Electrophysiol. 2005;16:620–4. doi: 10.1111/j.1540-8167.2005.40708.x. [DOI] [PubMed] [Google Scholar]
- 107.Secemsky EA, Verrier RL, Cooke G, et al. High prevalence of cardiac autonomic dysfunction and T-wave alternans in dialysis patients. Heart Rhythm. 2011;8:592–8. doi: 10.1016/j.hrthm.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 108.Verrier RL, Nieminen T. T-wave alternans as a therapeutic marker for antiarrhythmic agents. J Cardiovasc Pharmacol. 2010;55:544–54. doi: 10.1097/FJC.0b013e3181d6b781. [DOI] [PubMed] [Google Scholar]
- 109.Nieminen T, Nanbu DY, Datti IP, et al. Antifibrillatory effect of ranolazine during severe coronary stenosis in the intact porcine model. Heart Rhythm. 2011;8:608–14. doi: 10.1016/j.hrthm.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 110.Olsson G, Wikstrand J, Warnold I, et al. Metoprolol-induced reduction in postinfarction mortality: pooled results from five double-blind randomized trials. Eur Heart J. 1992;13:28–32. doi: 10.1093/oxfordjournals.eurheartj.a060043. [DOI] [PubMed] [Google Scholar]
- 111.Tada T, Kusano KF, Nagase S, et al. Clinical significance of macroscopic T-wave alternans after sodium channel blocker administration in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2008;19:56–61. doi: 10.1111/j.1540-8167.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- 112.Gehi AK, Stein RH, Metz LD, Gomes JA. Microvolt T-wave alternans for the risk stratification of ventricular tachyarrhythmic events: a meta-analysis. J Am Coll Cardiol. 2005;46:75–82. doi: 10.1016/j.jacc.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 113.Goldenberg I, Gillespie J, Moss AJ, et al. the Executive Committee of the Multicenter Automatic Defibrillator Implantation Trial II. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122:1265–71. doi: 10.1161/CIRCULATIONAHA.110.940148. [DOI] [PubMed] [Google Scholar]
- 114.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a Report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J Am Coll Cardiol. 2006;48:e247–346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 115.Goldberger JJ, Cain ME, Hohnloser SH, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society Scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death. A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. J Am Coll Cardiol. 2008;52:1179–99. doi: 10.1016/j.jacc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 116.Schwartz PJ, Garson A, Paul T, et al. Guidelines for the interpretation of the neonatal electrocardiogram. A task force of the European Society of Cardiology. Eur Heart J. 2002;23:1329–44. doi: 10.1053/euhj.2002.3274. [DOI] [PubMed] [Google Scholar]
- 117.Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–47. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bauer A, Malik M, Schmidt G, et al. Heart rate turbulence: standards of measurement, physiological interpretation, and clinical use. International Society for Holter and Noninvasive Electrocardiology Consensus. J Am Coll Cardiol. 2008;52:1353–65. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 120.Exner D. Noninvasive risk stratification after myocardial infarction: rationale, current evidence and the need for definitive trials. Can J Cardiol. 2009;25 (Suppl A):21A–7A. doi: 10.1016/s0828-282x(09)71050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Solomon SD, Zelenkofske S, McMurray JJ, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. 2005;352:2581–8. doi: 10.1056/NEJMoa043938. [DOI] [PubMed] [Google Scholar]