Abstract
Objective
To evaluate demographics, survival, and surgical trends for patients with malignant ovarian germ cell tumors.
Methods
SEER data abstracted from 1988 to 2001 and analyzed using Kaplan–Meier and Cox regression models.
Results
Of 760 patients, the median age was 23 years (range: 1–91 years). Seventy-six percent of patients presented with stage I–II disease, and 24% with stage III–IV. Thirty-two percent were dysgerminomas, 55% immature teratomas, and 13% yolk sac tumors. Fertility-preserving surgery was performed in 41.2% (n =313) of patients. In those <45 years old, the use of fertility-preserving surgery increased from 40.5% to 44.5% to 48.4% over the time periods 1988–1992, 1993–1997, 1998–2001 (P =0.25). The survival of patients who underwent fertility-preserving surgery was not statistically different compared to those who underwent standard surgery (P =0.26). Patients with stage I–II disease had improved survival compared to stage III–IV disease (97.6% vs. 85.5%, P <0.001). The overall survival of women with dysgerminomas, immature teratomas, and yolk sac tumors was 99.5%, 94.3%, and 85.4%, respectively (P <0.001). In multivariate analysis, older age, advanced stage, and yolk sac tumor histology predicted for poorer survival.
Conclusion
Our data suggests that the use of fertility-preserving surgery with concomitant surgical staging for germ cell cancers has increased without compromising survival.
Keywords: germ cell tumors, ovarian cancer, surgical practice
INTRODUCTION
Malignant ovarian germ cell tumors account for approximately 5% of malignant ovarian neoplasms and are usually diagnosed in children and young women from the ages of 10–30 years [1]. The most common presenting symptom is rapidly progressive abdominal pain associated with a palpable abdominal and/or pelvic mass. Fortunately, the vast majority of patients (50–75%) present with International Federation of Gynecology and Obstetrics (FIGO) surgical stage I disease. Treatment with modern systemic chemotherapy can achieve survival in excess of 90% [2–6]. This is in contrast to the age distribution, stage at diagnosis, and survival rates that are observed in the more common epithelial ovarian cancers. Another salient difference between germ cell cancers and the more common epithelial ovarian cancers is that conservative, fertility-preserving surgery can be offered to the majority of girls and young women with malignant germ cell tumors [1].
In a recent analysis of female germ cell tumors from the SEER database, Smith et al., showed that the incidence of germ cell tumors has declined and differs from the rising trends reported for testicular cancers. In addition, these authors found that although the survival rates have improved, they were lower in the older population and non-dysgerminoma subtypes [7]. To expand on these findings, we identified demographic trends and surgical patterns in the treatment of patients with malignant germ cell tumors after FIGO staging. Moreover, we evaluated the efficacy of fertility-preserving surgery.
METHODS
The database used was the Incidence-SEER 9 Regs Public-Use, February 2006 Sub (1973–2002), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch [8]. The years under investigation included 1988–2001. Distribution by ethnicity was calculated for Whites, Blacks, Asians, and others. Stage was stratified into two categories: stage I–II and stage III–IV.
Using the previously reported methodology of Smith et al. [7], malignant female genital germ cell tumors were identified, selecting for females, malignant disease, and morphology codes ((International Classification of Diseases for Oncology [ICD-O] specific for germ cell tumors identified by female genital tract topography codes (ICD 510–519, 529–549, 559, 569, 570–574, 577–579))). The histology codes used were dysgerminoma (9060), yolk sac tumor (9071), and malignant teratoma (9080, 9082–9085, 9090–9091). It should be noted that the ICD-9 codes for malignant teratoma included those teratomas which had undergone malignant degeneration, but were exclusive of teratocarcinoma.
Fertility-sparing surgery was defined as those who underwent a uterine-preserving procedure. The inference was that this should reflect conservation of both the uterus and the contralateral adnexae in most cases. This assumption is based on the fact that: (1) most patients with malignant germ cell tumors of the ovary present with stage I unilateral tumors and (2) since the vast majority are children and young women it is unlikely that the contralateral (i.e., unaffected) ovary would have been previously removed.
Chi-square tests were used to determine differences in three histologic cell types of germ cell tumors with respect to age, race, surgery, and stage. This analysis was also used to detect time trend differences from 1988 to 1992, 1993 to 1997, and 1998 to 2001 with respect to surgical and histologic changes over time. Disease-specific survival is defined as survival in the absence of other causes of death [9]. Kaplan–Meier survival curves were generated using the LIFETEST procedure (SAS 8, SAS Institute Inc., Cary, NC). All prognostic variables found to be significant in univariate analysis were included in multivariate analysis using the Cox proportional hazards model. This study was reviewed and approved by the Institutional Review Board.
RESULTS
Of 760 women with malignant germ cell tumors, the median age was 23 years (range: 1–91 years). 72.1% (n =548) of patients were less than 30 years of age, 18.9% (n =144) were between 30 and 40 years of age, and 8.9% (n =68) were over 40 years old at diagnosis. Seventy-two percent of patients were White or Hispanic (P <0.001), while less than 15% were Black, Asian, and other ethnicities. Approximately 76% (n =581) of patients presented with stage I–II disease, and 24% (n =179) with advanced (stage III–IV) tumors. The tumor subtypes included 55% immature teratomas (n =417, including 74 mixed germ cell tumors), 32% dysgerminomas (n =240), and 13% yolk sac tumors (n =103). Immature teratomas were the most common histologic subtype (P <0.001) (Table I).
TABLE I.
Demographic and Clinical Characteristics
| Characteristics | Total (n =760) | Dysgerminoma (n =240) | Yolk sac tumor (n =103) | Teratoma (n =417) | P-value |
|---|---|---|---|---|---|
| Age of diagnosis (years) | |||||
| Median | 23 | 23 | 23 | 22 | |
| Range | (1–91) | (4–87) | (1–91) | (1–86) | |
| Age ≤30 | 548 (72.1%) | 175 (72.9%) | 78 (75.7%) | 295 (70.7%) | P =0.366 |
| Age 31–40 | 144 (18.9%) | 49 (20.4%) | 14 (13.6%) | 81 (19.4%) | |
| Age >40 | 68 (8.9%) | 16 (6.7%) | 11 (10.7%) | 41 (9.8%) | |
| Race | |||||
| White | 549 (72.2%) | 198 (82.5%) | 71 (68.9%) | 280 (67.1%) | P <0.001 |
| Non-Hispanic | 401 (52.8%) | 151 (76.3%) | 53 (74.6%) | 197 (70.4%) | P =0.339 |
| Hispanic | 148 (19.5%) | 47 (23.7%) | 18 (25.4%) | 83 (29.6%) | |
| Black | 96 (12.6%) | 13 (5.4%) | 16 (15.5%) | 67 (16.1%) | |
| Asian | 77 (10.1%) | 15 (6.3%) | 8 (7.8%) | 54 (12.9%) | |
| Other | 38 (5.0%) | 14 (5.8%) | 8 (7.8%) | 16 (3.8%) | |
| Surgery | |||||
| None | 9 (1.2%) | 1 (0.4%) | 1 (1.0%) | 7 (1.7%) | P =0.307 |
| Yes | 535 (70.4%) | 170 (70.8%) | 66 (64.1%) | 299 (71.7%) | |
| Standarda | 222 (29.2%) | 82 (34.2%) | 33 (32.0%) | 107 (25.7%) | P =0.010 |
| Fertility-preserving | 313 (41.2%) | 88 (36.7%) | 33 (32.0%) | 192 (46.0%) | |
| Unknown | 216 (28.4%) | 69 (28.8%) | 36 (35.0%) | 111 (26.6%) | |
| Stage at diagnosis | |||||
| Stage I | 522 (68.7%) | 158 (65.8%) | 63 (61.2%) | 301 (72.2%) | P =0.058 |
| Stage II | 59 (7.8%) | 23 (9.6%) | 11 (10.7%) | 25 (6.0%) | |
| Stage III | 129 (17.0%) | 47 (19.6%) | 17 (16.5%) | 65 (15.6%) | |
| Stage IV | 50 (6.6%) | 12 (5.0%) | 12 (11.7%) | 26 (6.2%) | |
Hysterectomy and debulking.
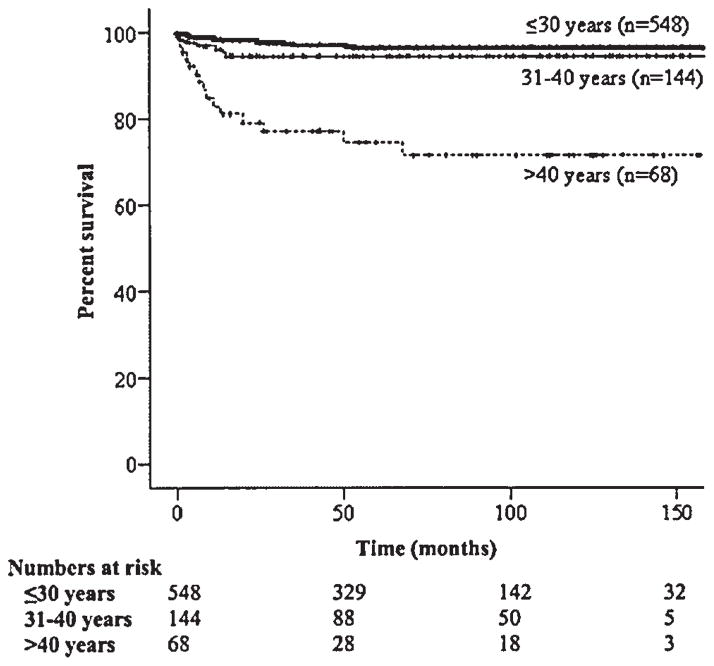
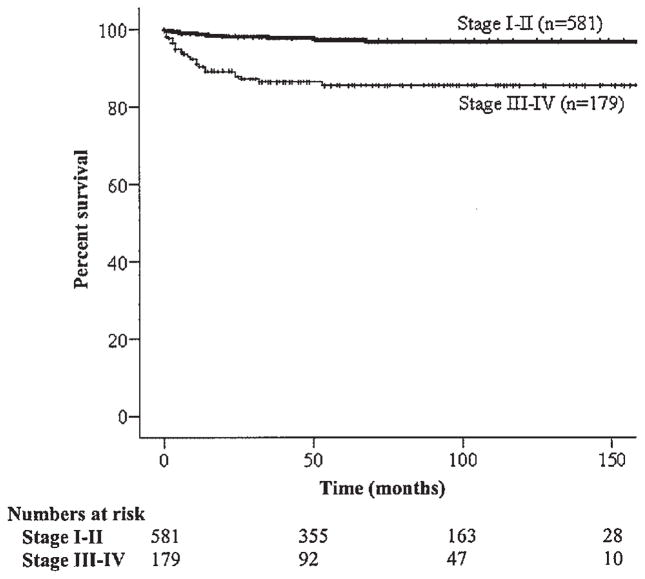
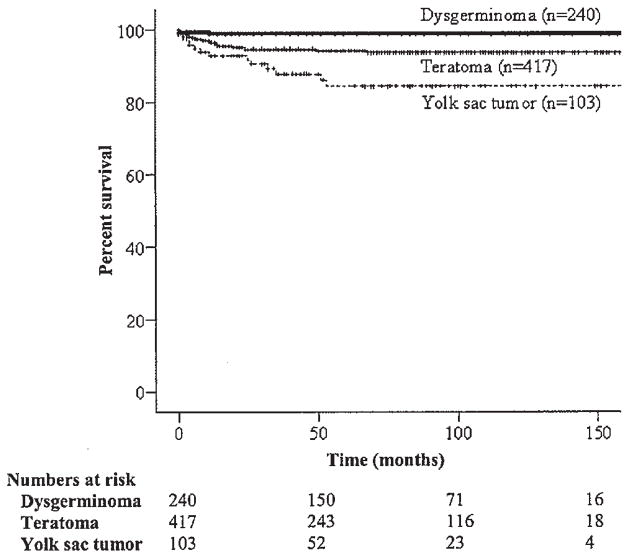
The 5-year relative survival of women less than 30 years of age, 31–40 years of age, and greater than 40 years of age was 96.8%, 95.4%, and 74.5%, respectively (P <0.001) (Table II and Fig. 1). The survival of Whites, Blacks, and Asians were 94.9%, 89.6%, and 98.7%, respectively (P <0.001). Survivorship was significantly higher among patients with stage I–II disease as compared to those with stage III–IV disease, respectively (97.6% vs. 85.5%; P <0.001) (Table II and Fig. 2). The disease-specific survival of women with dysgerminomas, immature teratomas, and yolk sac tumors was 99.5%, 94.3%, and 85.4% respectively (P <0.001) (Table II and Fig. 3). Among patients with stage I–II tumors, those with dysgerminomas, immature teratomas, and yolk sac tumors had survival rates of 100%, 97.1%, and 93.9%, (P =0.049), respectively; in comparison, the survival of stage III–IV patients for these same histologic subtypes were 98.0%, 84.3%, and 64.9% (P <0.001). In multivariate analysis, older age, advanced stage, and yolk sac histology remained as significant independent prognostic factors for poorer survival (Table III).
TABLE II.
Five-Year Disease-Specific Survival
| Subgroup | Total (n =760) | Dysgerminoma (n =240) | Yolk sac tumor (n =103) | Teratoma (n =417) | Log-rank tests |
|---|---|---|---|---|---|
| Malignant ovarian germ cell tumors | 94.8% (±0.9) | 99.5% (±0.5) | 85.4% (±4.0) | 94.3% (±1.2) | P <0.001 |
| Age of diagnosis | P <0.001 | ||||
| Age ≤30 | 96.8% (±0.8) | 100.0% (±0.0) | 85.9% (±4.8) | 97.7% (±0.9) | P <0.001 |
| Age 31–40 | 95.4% (±1.8) | 100.0% (±0.0) | 92.3% (±7.4) | 93.0% (±3.0) | P =0.18 |
| Age >40 | 74.5% (±6.0) | 90.0% (±9.5) | 72.7% (±13.4) | 70.1% (±8.2) | P =0.34 |
| Race | P <0.001 | ||||
| Whites | 94.9% (±1.0) | 99.4% (±0.6) | 88.1% (±4.3) | 93.5% (±1.6) | P =0.002 |
| Non-Hispanic | 94.9% (±1.2) | 100.0% (±0.0) | 89.2% (±4.6) | 92.7% (±2.0) | P =0.003 |
| Hispanic | 94.8% (±2.2) | 97.6% (±2.4) | 83.7% (±11.1) | 95.8% (±2.4) | P =0.27 |
| Blacks | 89.6% (±3.6) | 100.0% (±0.0) | 60.0% (±16.2) | 93.2% (±3.3) | P =0.01 |
| Asians | 98.7% (±1.3) | 100.0% (±0.0) | 87.5% (±11.7) | 100.0% (±0.0) | P =0.02 |
| Surgical treatment | P =0.63 | ||||
| No | 100.0% (±0.0) | 100.0% (±0.0) | 100.0% (±0.0) | 100.0% (±0.0) | — |
| Yes | 96.9% (±0.8) | 99.1% (±0.6) | 84.6% (±4.1) | 94.3% (±1.2) | P <0.001 |
| Type | P =0.26 | ||||
| Standarda | 95.6% (±1.6) | 100.0% (±0.0) | 84.2% (±7.4) | 95.7% (±2.1) | P =0.008 |
| Fertility-preserving | 97.9% (±0.9) | 98.9% (±1.1) | 92.9% (±4.9) | 98.3% (±1.0) | P =0.18 |
| Stage | P <0.001 | ||||
| Stage I | 98.1% (±0.7) | 99.4% (±0.6) | 92.7% (±4.1) | 98.4% (±0.8) | P =0.09 |
| Stage II | 90.8% (±3.9) | 100.0% (±0.0) | 90.9% (±8.7) | 82.9% (±7.8) | P =0.17 |
| Stage III | 88.6% (±3.0) | 97.6% (±2.4) | 63.5% (±13.8) | 88.4% (±4.2) | P =0.007 |
| Stage IV | 77.1% (±6.5) | 100.0% (±0.0) | 66.7% (±13.6) | 73.3% (±9.6) | P =0.19, P <0.001 |
| Stage I–II | 97.6% (±0.7) | 100.0% (±0.0) | 92.6% (±3.6) | 97.1% (±1.0) | P =0.049 |
| Stage III–IV | 85.5% (±2.8) | 98.0% (±1.9) | 64.9% (±9.9) | 84.3% (±4.0) | P <0.001 |
Hysterectomy and debulking.
Fig. 1.
Kaplan–Meier disease-specific survival by age (P <0.001).
Fig. 2.
Kaplan–Meier disease-specific survival by stage (P <0.001).
Fig. 3.
Kaplan–Meier disease-specific survival by histology (P <0.001).
TABLE III.
Multivariate Analysis
| Prognostic factors | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Age of diagnosisa | 1.05 | 1.03–1.07 | P <0.001 |
| Stage at diagnosisb | 2.17 | 1.63–2.90 | P <0.001 |
| Histologyc | 2.31 | 1.32–4.04 | P =0.003 |
Continuous.
I–II versus III–IV.
Dysgerminoma versus yolk sac tumor versus teratoma.
Across the three time intervals under investigation, there was an increase in the performance of surgical staging, as reflected by removal of retroperitoneal lymph nodes. Only 37% of the patients treated from 1988 to 1992 underwent lymph node dissection while over 50% of patients seen between 1998 and 2001 had lymph nodes removed (P =0.011, Table IVA). The increasing performance of lymphadenectomy over time and the 5-year disease specific survival in the study population over time are shown in Tables IVA and IVB.
TABLE IVA.
Patient Characteristics
| Total | 1988–1992 | 1993–1997 | 1998–2001 | P-value | |
|---|---|---|---|---|---|
| FPSa | 313 (58.5%) | 87 (58.4%) | 119 (57.2%) | 107 (60.1%) | P =0.85 |
| Standard surgeryb | 222 (41.5%) | 62 (41.6%) | 89 (42.8%) | 71 (39.9%) | |
| Lymphadenectomy | |||||
| Yes | 301 (43.0%) | 78 (36.6%) | 111 (41.7%) | 112 (50.7%) | P =0.01 |
| No | 399 (57.0%) | 135 (63.4%) | 155 (58.3%) | 109 (49.3%) | |
| Histology | |||||
| Dysgerminoma | 240 (31.6%) | 78 (33.3%) | 86 (30.2%) | 76 (31.5%) | P =0.70 |
| FPSa | 88 (51.8%) | 28 (51.9%) | 27 (45.0%) | 33 (58.9%) | P =0.33 |
| Standard surgeryb | 82 (48.2%) | 26 (48.1%) | 33 (55.0%) | 23 (41.1%) | |
| Immature teratoma | 417 (54.9%) | 128 (54.7%) | 162 (56.8%) | 127 (52.7%) | |
| FPSa | 192 (64.2%) | 48 (61.5%) | 81 (66.9%) | 63 (63.0%) | P =0.71 |
| Standard surgeryb | 107 (35.8%) | 30 (38.5%) | 40 (33.1%) | 37 (37.0%) | |
| Yolk sac | 103 (13.6%) | 28 (12.0%) | 37 (13.0%) | 38 (15.8%) | |
| FPSa | 33 (50.0%) | 11 (64.7%) | 11 (40.7%) | 11 (50.0%) | P =0.30 |
| Standard surgeryb | 33 (50.0%) | 6 (35.3%) | 16 (59.3%) | 11 (50.0%) | |
Fertility-preserving surgery.
Hysterectomy and debulking.
TABLE IVB.
5-Year Disease-Specific Survival Over Time
| Total | 1988–1992 | 1993–1997 | 1998–2001 | P-value | |
|---|---|---|---|---|---|
| Overall survival | 94.8% (±0.9) | 92.1% (±1.8) | 94.6% (±1.4) | 98.5% (±0.9) | P =0.04 |
| Surgery type | P =0.26 | ||||
| FPSa | 97.9% (±0.9) | 96.6% (±1.9) | 97.6% (±1.4) | 100.0% (±0.0) | P =0.28 |
| Standard surgeryb | 95.6% (±1.6) | 93.3% (±3.2) | 95.4% (±2.3) | 100.0% (±0.0) | P =0.29 |
| Lymphadenectomy | P =0.013 | ||||
| Yes | 96.8% (±1.1) | 97.4% (±1.8) | 94.5% (±2.2) | 100.0% (±0.0) | P =0.12 |
| No | 92.3% (±1.4) | 87.8% (±2.9) | 94.7% (±1.8) | 96.6% (±1.9) | P =0.04 |
| Histology | P <0.001 | ||||
| Dysgerminoma | 99.1% (±0.6) | 100.0% (±0.0) | 97.6% (±1.6) | 100.0% (±0.0) | P =0.19, P =0.33 |
| FPSa | 98.9% (±1.1) | 100.0% (±0.0) | 96.3% (±3.6) | 100.0% (±0.0) | P =0.32 |
| Standard surgeryb | 100.0% (±0.0) | 100.0% (±0.0) | 100.0% (±0.0) | 100.0% (±0.0) | — |
| Immature teratoma | 94.3% (±1.2) | 90.3% (±2.7) | 95.0% (±1.7) | 98.8% (±1.2) | P =0.02, P =0.24 |
| FPSa | 98.3% (±1.0) | 97.9% (±2.1) | 97.5% (±1.7) | 100.0% (±0.0) | P =0.56 |
| Standard surgeryb | 95.7% (±2.1) | 89.6% (±5.7) | 97.5% (±2.5) | 100.0% (±0.0) | P =0.13 |
| Yolk sac tumor | 84.6% (±4.1) | 77.8% (±8.0) | 86.1% (±5.8) | 94.5% (±3.8) | P =0.43 |
| FPSa | 92.9% (±4.9) | 81.8% (±11.6) | 100.0% (±0.0) | 100.0% (±0.0) | P =0.19 |
| Standard surgeryb | 84.2% (±7.4) | 80.0% (±17.9) | 81.3% (±9.8) | 100.0% (±0.0) | P =0.61, P =0.45 |
Fertility-preserving surgery.
Hysterectomy and debulking.
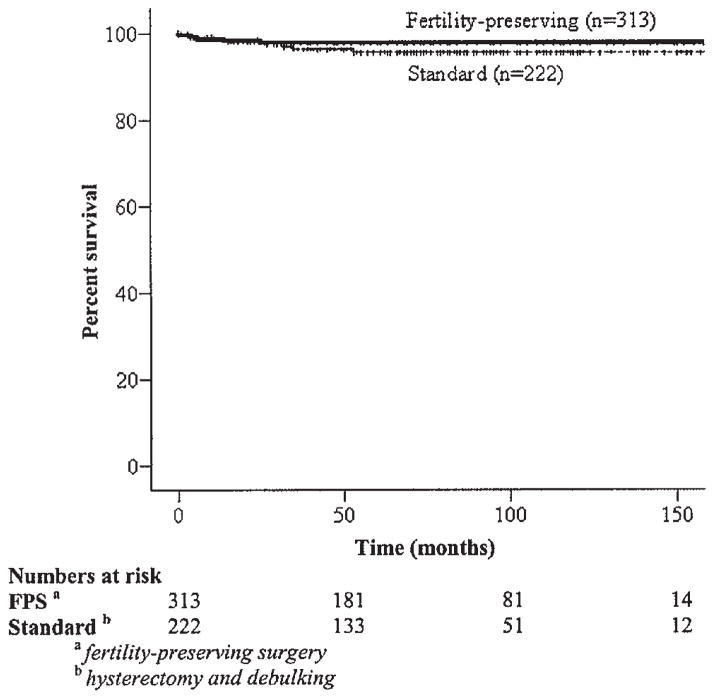
Within each histologic subtype, the majority of patients presented with stage I–II compared with stage III–IV disease (P =0.058). When the study interval from 1988 to 2001 was separated into three periods (1988–1992, 1993–1997, and 1998–2001), there was a trend toward an increase in the use of fertility-preserving surgery over time (40.5% vs. 44.5% vs. 48.4%; P =0.25) in patients <45 years old. Furthermore, among those reproductive-aged (<45 years) patients with stage I–II disease, 62% underwent fertility-preserving surgery. There were no significant survival differences between the overall group of women who underwent fertility-preserving surgery compared to those who had standard surgeries which included hysterectomy (Table IVB and Fig. 4). In addition, over the three time periods, the increased use of fertility-preserving surgeries did not negatively affect the disease-specific survival of these women over time (P =0.276) (Table IVB).
Fig. 4.
Kaplan–Meier disease-specific survival by surgical treatment (P =0.26).
DISCUSSION
In an attempt to document use in the community of the advances made in the management of malignant ovarian germ cell tumors of the ovary, the SEER registry was utilized to document management trends and 5-year disease-specific data from 1988 to 2001. The SEER Program encompasses 13 geographically defined population-based central registries for the United States operated by local nonprofit organizations. It currently includes population-based data from about 14% of the United States population and is reasonable representative of subsets of different racial/ethnic groups residing in the United States.
Smith et al. [7] evaluated the incidence of women with malignant ovarian germ cell tumors over the 30-year period encompassed by the SEER program (1973–2002). Of note, these investigators did not look specifically at yolk sac tumors. These authors also showed that the incidence of germ cell tumors have declined and differ from the rising trends reported for testicular cancers. In addition, these authors found that although the survival rates have improved, they were lower in the older population and non-dysgerminoma subtypes [7]. To expand on these findings, we identified demographic trends and surgical patterns in the treatment of patients with malignant germ cell tumors after FIGO staging. Moreover, we evaluated the efficacy of fertility-preserving surgery.
An observation in our study that is at odds with historical figures is the finding that immature teratomas (inclusive of those with mixed germ cell tumor) were the histologic subtype most frequently represented among the three histological cell types under investigation from 1988 to 2001. Previous reports have often cited the dysgerminoma as being most frequently diagnosed among malignant ovarian germ cell tumors [1]. In Smith’s 30-year SEER study considering 5-year blocks as distinct periods, it is noteworthy that immature teratomas were found to occur more frequently than dysgerminomas in four of six blocks [7]. The age-adjusted incidence rates of immature teratoma and dysgerminoma were similar in one 5-year block (1983–1987) and only in the earliest block under investigation (1973–1977) was the occurrence of dysgerminoma found to be higher than that of immature teratoma [7]. Thus, it is possible that many of the historical series emerging from the 1970s emphasized the relatively high incidence of dysgerminoma.
Fertility sparing procedures should be performed in combination with comprehensive surgical staging surgeries. Prior studies have found that comprehensive surgical staging procedures with lymphadenectomy is associated with an improved outcome [10]. Fertility-preserving surgery for ovarian cancers include an ovarian cystectomy, unilateral salpingo-oophorectomy, unilateral salpingo-oophorectomy plus hysterectomy (with preservation of the contralateral ovary), and bilateral salpingo-oophorectomy (with preservation of the uterus). Clearly, following some of these procedures, assisted reproductive technology would be required to achieve a pregnancy. A significant number of women with germ cell ovarian cancers can be appropriate candidates for fertility-preserving surgeries because these tumors typically affect girls or young women and nearly 75% of these tumors are unilateral and diagnosed at stage I [11].
Many of these patients, other than those with stage I dysgerminoma and stage IA, grade 1 immature teratoma, need further adjuvant chemotherapy. Despite the use of adjuvant chemotherapy, prior studies have reported on the rate of successful pregnancies after fertility-sparing surgeries followed by chemotherapy [11–16]. Because these patients for the most part are in the earlier two-thirds of their reproductive period, we assumed that when fertility-preserving surgery was undertaken, that the contralateral ovary was preserved because: (a) the majority of germ cell tumors are unilateral and (b) young women are unlikely to have had a previous oophorectomy. Indeed, with the great success of adjuvant therapy programs resulting in excellent survival rates for most patients with malignant ovarian germ cell tumors, it has become increasingly apparent that fertility-preserving surgery can be offered to the vast majority of patients with malignant ovarian germ cell tumors, oftentimes regardless of stage [17]. When FPS is embarked upon, the other important principles of comprehensive surgical staging for clinical stage I disease is still warranted [14].
In this study the increased use of fertility-preserving surgery over time is encouraging. This reflects a successful dissemination of knowledge into the community from single institution experiences and collaborative efforts. Low and co-workers performed a retrospective review of 74 patients with malignant ovarian germ cell tumors who were treated by conservative surgery, retaining the uterus and contralateral ovary to preserve ovarian function with or without the use of adjuvant chemotherapy [11]. This group reported survival rates of 98.2% for those with stage I disease, and 94.4% for those with advanced tumors. Importantly, although 61.7% of patients who received adjuvant therapy developed amenorrhea, 91.5% of those women resumed normal menstrual function on completion of chemotherapy, and 14 healthy live births were recorded in this group with no documented birth defects. Similarly Tangir et al. [16] reported the outcomes of 64 patients with malignant ovarian germ cell tumors who were treated with fertility-preserving surgery with or without chemotherapy, all of whom survived and were followed for an evaluation of subsequent menstrual and reproductive function. Thirty-eight patients attempted conception and 29 achieved at least one pregnancy (76%). Importantly, among the patients who conceived, 20 had FIGO surgical stage I tumors, one stage II tumor, and eight stage III disease cancers.
The limitations of the SEER database have been described previously and include their retrospective content. As with other large population-based series, our report was limited by a lack of central pathology review. To determine if there are significant discrepancies between registry and referral pathologists, Tyler et al. [18] performed slide reviews on 477 women diagnosed with ovarian, breast or endometrial cancer and compared the diagnoses of pathologists contributing to tumor registries affiliated with the SEER program to an expert gynecologic pathologists. These authors found an overall agreement of 97% for overall cancers, and the agreement for major cellular subtypes of ovarian cancer was 73% for endometrioid and 100% for clear cell carcinomas. Moreover, there was a 61.7% complete histopathologic agreement with only 1% of cases that were considered as having major differences. There is also limited information on clinically relevant information such as extent and location of residual disease, and type of chemotherapy. In addition, there is no information on the affects of chemotherapy on ovarian function and fertility outcomes. The strengths of the study is that the results from this population-based study can be generalized to the U.S. population since the SEER cancer registries are consistent in representative regions throughout the country [19]. Based on the Northern American Association of Central Cancer Registries, the SEER program’s quality control measures maintain the highest level of certification of data quality and completeness [20]. More specifically, a mechanism involved in the quality assurance program includes an annual review the medical records of sample cases for accuracy. Virnig et al. reported a 98% completeness in each sample case with a >90% rate in the accuracy of reporting adjuvant therapy [21].
In our study, the finding that nearly 25% of young women with malignant ovarian germ cell tumors underwent hysterectomy during the study period is of concern. Moreover, only 50% of women underwent surgical staging with lymphadenectomy for germ cell tumors. Perhaps educational programs are needed to improve the awareness of the appropriate treatment of this curable solid tumor.
References
- 1.Tewari KS, Disaia PJ. On the evolution of a successful treatment program for a solid tumor system. Eur J Gynaecol Oncol. 2000;21:339–347. [PubMed] [Google Scholar]
- 2.Williams SD, Kauderer J, Burnett AF, et al. Adjuvant therapy of completely resected dysgerminoma with carboplatin and etoposide: A trial of the Gynecologic Oncology Group. Gynecol Oncol. 2004;95:496–499. doi: 10.1016/j.ygyno.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Feuer EJ, Frey CM, Brawley OW, et al. After a treatment breakthrough: A comparison of trial and population-based data for advanced testicular cancer. J Clin Oncol. 1994;12:368–377. doi: 10.1200/JCO.1994.12.2.368. [DOI] [PubMed] [Google Scholar]
- 4.Billmire D, Vinocur C, Rescorla F, et al. Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: An intergroup study. J Pediatr Surg. 2004;39:424–429. doi: 10.1016/j.jpedsurg.2003.11.027. discussion 424–9. [DOI] [PubMed] [Google Scholar]
- 5.Cushing B, Giller R, Cullen JW, et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: A pediatric intergroup study–Pediatric Oncology Group 9049 and Children’s Cancer Group 8882. J Clin Oncol. 2004;22:2691–2700. doi: 10.1200/JCO.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Papadimitriou C, Hamilos G, et al. Treatment of ovarian germ cell tumors with a 3-day bleomycin, etoposide, and cisplatin regimen: A prospective multicenter study. Gynecol Oncol. 2004;95:695–700. doi: 10.1016/j.ygyno.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Smith HO, Berwick M, Verschraegen CF, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol. 2006;107:1075–1085. doi: 10.1097/01.AOG.0000216004.22588.ce. [DOI] [PubMed] [Google Scholar]
- 8.Surveillance Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 9 Regs Public-Use, Nov 2004 Sub (1973–2002) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; ( www.seer.cancer.gov) [Google Scholar]
- 9.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: A statistical methodology. Natl Cancer Inst Monogr Sep. 1961;6:101–121. [PubMed] [Google Scholar]
- 10.Chan JK, Munro EG, Cheung MK, et al. Association of lymphadenectomy and survival in stage I ovarian cancer patients. Obstet Gynecol. 2007;109:12–19. doi: 10.1097/01.AOG.0000249610.95885.ef. [DOI] [PubMed] [Google Scholar]
- 11.Gershenson DM. Fertility-sparing surgery for malignancies in women. J Natl Cancer Inst Monogr. 2005:43–47. doi: 10.1093/jncimonographs/lgi011. [DOI] [PubMed] [Google Scholar]
- 12.Gershenson DM. Menstrual and reproductive function after treatment with combination chemotherapy for malignant ovarian germ cell tumors. J Clin Oncol. 1988;6:270–275. doi: 10.1200/JCO.1988.6.2.270. [DOI] [PubMed] [Google Scholar]
- 13.Brewer M, Gershenson DM, Herzog CE, et al. Outcome and reproductive function after chemotherapy for ovarian dysgerminoma. J Clin Oncol. 1999;17:2670–2675. doi: 10.1200/JCO.1999.17.9.2670. [DOI] [PubMed] [Google Scholar]
- 14.Low JJ, Perrin LC, Crandon AJ, et al. Conservative surgery to preserve ovarian function in patients with malignant ovarian germ cell tumors. A review of 74 cases. Cancer. 2000;89:391–398. [PubMed] [Google Scholar]
- 15.Zanetta G, Bonazzi C, Cantu M, et al. Survival and reproductive function after treatment of malignant germ cell ovarian tumors. J Clin Oncol. 2001;19:1015–1020. doi: 10.1200/JCO.2001.19.4.1015. [DOI] [PubMed] [Google Scholar]
- 16.Tangir J, Zelterman D, Ma W, et al. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101:251–257. doi: 10.1016/s0029-7844(02)02508-5. [DOI] [PubMed] [Google Scholar]
- 17.Tewari KS, Di Saia PJ. Ovulatory failure, fertility preservation and reproductive strategies in the setting of gynecologic and non-gynecologic malignancies. Eur J Gynaecol Oncol. 2006;27:449–461. [PubMed] [Google Scholar]
- 18.Tyler CW, Jr, Lee NC, Robboy SJ, et al. The diagnosis of ovarian cancer by pathologists: How often do diagnoses by contributing pathologists agree with a panel of gynecologic pathologists? Am J Obstet Gynecol. 1991;164:65–70. doi: 10.1016/0002-9378(91)90628-5. [DOI] [PubMed] [Google Scholar]
- 19.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: A national resource. Cancer Epidemiol Biomarkers Prev. 1999;8:1117–1121. [PubMed] [Google Scholar]
- 20.North American Association of Central Cancer Registries. Available at: http://www.naaccr.org/
- 21.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40:IV-49-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 22.Synopsis for Table of Contents.