Abstract
Circulating 45 and 62 kDa antibodies targeting the cerebellum were previously associated with Autism Spectrum Disorder (ASD), lower adaptive/cognitive function and aberrant behaviors. Moreover, 37, 39 and 73 kDa maternal antibodies (mAb) targeting the fetal brain were previously correlated with broad autism spectrum, irritability, abnormal brain enlargement and impaired expressive language. The present study aims towards clinically characterizing individuals with brain-targeted IgG and/or exposed to maternal antibrain antibodies in a large sample of Italian autistic children (N = 355), their unaffected siblings (N = 142) and mothers (N = 333). The presence of patient- and mother-produced anti-brain antibodies does not confer increased risk of autism within the same sibship. However, the 45 and 62 kDa antibodies are correlated with autism severity: the 45 kDa Ab is associated with cognitive impairment and lower scores at the Vineland Adaptive Behavior Scales, the 62 kDa Ab with motor stereotypies, while both correlate with larger head circumference (all P < 0.05). On the other hand, maternal 37, 39 and 73 kDa antibrain antibodies, either alone or in combination, are correlated with impaired verbal and non-verbal language development, neurodevelopmental delay and sleep/wake cycle disturbances in their autistic children (P < 0.05). Presence of the 62 kDa autoAb in the child is significantly associated with presence of the 39 and/or 73 kDa antibodies in his/her mother. Our results confirm and extend previous observations in an ethnically distinct sample, providing further evidence of a pathomorphic role for antibrain antibodies in autism while demonstrating their familial clustering.
Keywords: Autism, Autism Spectrum Disorder, Autoantibodies, Autoimmunity, Cerebellum, Cognitive impairment, Language development, Macrocephaly, Sleep/wake cycle
1. Introduction
In recent years, several studies reported the presence of circulating IgG autoantibodies that react with components of the central nervous system (CNS) in individuals with Autism Spectrum Disorder (ASD) (Enstrom et al., 2009), a neurodevelopmental disease characterized by deficits in social reciprocity and communication, as well as by unusually restricted interests and repetitive behaviors (American Psychiatric Association, 2013). These autoantibodies, displaying a significantly lower prevalence in population controls compared to autistic individuals (Wills et al., 2009; Goines et al., 2011), seemingly target different brain regions, including thalamus, hypothalamus, caudate nucleus, cerebral cortex, and putamen, but most consistently the cerebellum (Cabanlit et al., 2007; Wills et al., 2007, 2011). Cerebellar specific IgG autoantibodies, initially estimated approximately 52 kDa in size and directed mainly against calretinin-positive GABAergic Golgi cells, were identified by Western Blot analysis in plasma from children with ASD (Cabanlit et al., 2007; Wills et al., 2011). Histopathological and brain imaging studies have unveiled multiple abnormalities in the cerebellum of many autistic individuals, including a significant reduction in Purkinje cell number, cytoarchitectonic abnormalities and cytoplasmic inclusions in the deep cerebellar nuclei, and vermal hypoplasia (Fatemi et al., 2012). At the clinical level, Goines et al. (2011) later correlated the presence of anti-cerebellar autoantibodies defined as recognizing bands at 45 and 62 kDa by western blot, with an autism diagnosis, lower adaptive and cognitive function, as well as aberrant behaviors.
Meanwhile maternal antibodies have also been reported, specifically recognizing proteins highly expressed in fetal brain tissue and exerting a negative impact on neural development during gestation; these maternal antibrain antibodies display greater prevalence in mothers of autistic children than in mothers of typically developing children (Dalton et al., 2003; Zimmerman et al., 2007; Braunschweig et al., 2008; Brimberg et al., 2013; Nordahl et al., 2013; Rossi et al., 2013; for review see Braunschweig and Van de Water, 2012). In fact, early in prenatal life, the fetus relies on maternal IgG passing across the placenta into the fetal circulation for protective immunity. This is passive immunity is triggered by the environmental exposures of the mother and remains until the child is capable of mounting an adaptive immune response. However, IgG that recognizes self-antigens can also cross the placenta and reach the CNS before blood brain barrier formation, potentially interfering with fetal neurodevelopment.
A thorough understanding of the implication of autoimmunity in autism thus requires that the relative role of child- and mother-produced antibrain antibodies be distinguished. The aim of the present study was to tease out the complex interplay of these antibodies through the characterization of the clinical and behavioral features associated with the presence of either the patient-produced 45 and 62 kDa autoantibodies to adult brain, or the maternal 37, 39, and 73 kDa anti-fetal brain antibodies. This was accomplished by assessing a large sample of Italian children diagnosed with ASD together with their unaffected siblings and mothers. In addition to clinical signs and symptoms, behavioral features and family history, we also looked at macrocephaly/macrosomy, a well-established autism endophenotype consistently found in 18% of all ASD individuals assessed to date (Sacco R and Persico AM, submitted), as well as principal components of the autism phenotype and defined patient clusters previously identified by our group (Sacco et al., 2010, 2012). Finally, we determined whether the presence of autoantibodies in ASD children is part of a broader autoimmune liability expressed as either the presence of antibrain antibodies in their mothers or as a positive history of autoimmune disorders in family members. To our knowledge, this is the first study (a) exploring in parallel the presence of both maternal and children autoantibodies, as well as their relationship with clinical and behavioral variables; (b) using an ethnically distinct sample (Italian) as compared with previous studies, and (c) characterizing unaffected siblings as controls to investigate the risk of autism conferred by autoantibodies within the same sibship.
2. Methods
2.1. Subjects
A sample of 355 Italian autistic patients, 142 unaffected siblings and 333 mothers were included in the investigation. Demographic and clinical characteristics of the sample are summarized in Table 1. A total of 353 of these autistic individuals were born to mothers characterized for the presence/absence of anti-fetal brain antibodies (M = 300; F = 53; age = 8.67 ± 5.63). Autistic behaviors were assessed using the Autism Diagnostic Interview-Revised (ADI-R) (Rutter et al., 2003), and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2002). Adaptive functioning was characterized using the Vineland Adaptive Behavior Scales (VABS) (Sparrow et al., 1984). Developmental, clinical, and family history variables were characterized using a previously-described questionnaire (see Supplementary methods in Sacco et al., 2010). All parents gave written informed consent for themselves and for their children. The consent form was approved by the Institutional Review Board of University “Campus Bio-Medico” (Rome, Italy). Head circumference was measured in 222 ASD patients aged <16 years old, and transformed into percentiles using the sex- and age-specific standard tables currently adopted in the vast majority of European countries and by the Italian Pediatric Association, as described (Sacco et al., 2010). In addition, we investigated the association of child- and mother-produced antibrain antibodies with the four Principal Components of the ASD phenotype described by Sacco et al. (2010), namely “circadian & sensory dysfunction”, “immune dysfunction”, “neurodevelopmental delay”, and “stereotypic behaviors”. The association of autoantibodies with one specific component could pathophysiologically link dysimmune processes with neurobiological mechanisms specifically relevant to a limited subset of autistic signs and symptoms.
Table 1.
Demographic and clinical characteristics of the sample. Sample size (N) as stated at the top, unless otherwise specified.
| Mean/median | SD | Range | ||
|---|---|---|---|---|
| ASD children (N = 355) | ||||
| Mean age (yr): | 9.06 | 6.38 | 2–47 | |
| Median VABS scores: (N = 140) | Communication | 77.0 | 23–221 | |
| Daily living skills | 72.0 | 19–180 | ||
| Socialization | 71.5 | 25–140 | ||
| Motor skills | 84.0 | 32–139 | ||
| Composite | 63.0 | 18–137 | ||
| N | Percent | |||
| Gender: | Male | 300 | 84.5% | |
| Female | 55 | 15.5% | ||
| M:F ratio | 5.5:1 | |||
| Family type: | Simplex | 324 | 94.7% | |
| Multiplex | 18 | 5.2% | ||
| DSM-IV diagnosis: | Autistic disorder | 274 | 77.2% | |
| Asperger’s disorder | 25 | 7.0% | ||
| PDD-NOS | 56 | 15.8% | ||
| I.Q. (N = 182): | >70 | 51 | 28.0% | |
| ≤70 | 131 | 72.3% | ||
| Unaffected siblings (N = 142) | ||||
| Mean age (yr): | 13.13 | 8.64 | 2-49 | |
| N | Percent | |||
| Gender: | Male | 68 | 47.9% | |
| Female | 74 | 50.7% | ||
| M/F ratio | 0.9:1 | |||
| Family type: | Simplex | 138 | 97.2% | |
| Multiplex | 4 | 2.8% | ||
| Mothers (N = 333) | ||||
| Mean age (yr): | 31.90 | 5.80 | 18-53 | |
| N | Percent | |||
| Family type: | Simplex | 318 | 95.5% | |
| Multiplex | 15 | 4.5% | ||
Finally, we investigated the association of antibrain antibodies with the ASD patient clusters identified by Sacco et al. (2012) using hierarchical clustering, namely ICS (“immune + circadian and sensory”), CS (“circadian and sensory”), S (“stereotypic behaviors”), and M (“mixed”).
2.2. Sample collection and processing
Blood samples collected in EDTA were centrifuged for 25 min at 4 °C and 140×g within 20 min of venipuncture; 1 ml of supernatant (i.e., platelet-rich plasma) was stored at −80 °C until use.
2.3. Protein preparation
Rhesus macaque brain tissue was placed in a 10-fold greater volume of 20 mM HEPES-OH, pH 7.5 buffer supplemented with (in mM) 1 EDTA, 5 DTT, 1 PMSF, 0.2 Na2VO3, 1 NaF and one tablet Complete (Roche, per 50 ml Buffer). The tissue was then mechanically homogenized using a Polytron 3000 homogenizer (Brinkman) and sonicated for 3 min. The sample was then centrifuged 10 min at 3000×g to remove insoluble material. The supernatant was concentrated 5-fold by ultrafiltration (Mr. cutoff 10,000) and diluted in 50 mM Tris–HCl pH 6.8 containing 25% glycerol and 1% LDS. The protein sample concentration was then adjusted to 3.5 mg/ml, as determined by BCA assay (Pierce). Human adult cerebellum protein extract was purchased from Abcam (Cambridge, MA, USA) and supplied as electrophoresis-ready purified protein sample. According to the manufacturer, the human adult proteins were prepared identically to the Rhesus samples described above.
2.4. Western blotting
Western blotting was performed as previously described (Cabanlit et al., 2007), with modifications detailed below. Briefly, SDS–PAGE was performed using pre-cast 4–12% Prep-well or multi-well mini-gels (Invitrogen). Gels were loaded with 300 lg protein (or 10 μg/lane for multi-well mini-gels) in electrophoresis buffer containing 1× NuPAGE LDS sample buffer (Invitrogen) and 100 mM DTT. The sample was heated to 95 °C for 10 min and centrifuged for 5 min at 10,000×g. 6 μl of MagicMark molecular weight standard (Invitrogen) was loaded into the marker well and protein sample(s) were loaded in the appropriate well(s). Electrophoresis was carried out at 150 V for 80 min. The gel was then transferred to 0.2 μm pore nitrocellulose membrane at 35 V for 16 h at 4 °C. Protein quality and loading was confirmed by staining the membrane with Ponceau S (Sigma). Membranes from preparative well gels were cut into strips and all blots were blocked with Blocker Casein in PBS (Thermo Fisher) for 10 min and probed with maternal or child plasma diluted 1:400 for 2 h at room temperature. After washing, strips were incubated with goat anti-human secondary antibody (Invitrogen), diluted 1:20,000 in PBS/0.05% Tween20 for 1 h. Blots were then washed and incubated with Super Signal West chemiluminescent substrate (Pierce) and imaged on a FluorChem 8900 imager (Alpha Innotech). Scoring for the presence and apparent molecular weight of a positive band was performed by individuals blinded to child diagnosis, using internal positive and negative controls on each blot. Examples of representative Western blots for both maternal and children are displayed in the Supplementary Figs. S1 and S2, respectively.
2.5. Statistical analysis
Data analysis was performed using SPSS 21.0 software. Categorical variables were analyzed by χ2 test, using Montecarlo permutations when more than 20% of cells had expected frequencies below N = 5. Fisher’s exact test was applied for pairwise post hoc analyses on language development. Continuous variables were tested using parametric Student t-tests or non-parametric Mann–Whitney U-tests depending on the normality of the distribution, as assessed using the Kolmogorov–Smirnoff statistics. The complete list of all variables is available in Supplementary Table S1. Nominal P-values < 0.05 were considered as significant and no correction for multiple testing was applied, in accordance with the exploratory nature of this study (Rothman, 1990; Perneger, 1998; Savitz and Olshan, 1998; Bender and Lange, 2001; Streiner and Norman, 2011). Data are reported as mean ± standard deviation (S.D.), unless otherwise specified.
3. Results
The prevalence of anti-adult cerebellum Abs in 355 ASD children and 142 unaffected siblings is shown in Fig. 1. No significant difference between the two groups was observed for presence of either the 45 kDa, or the 62 kDa Abs (P = 0.200 and 0.101, respectively). Only two autistic individuals and two unaffected siblings carried both Ab forms (Fig. 1). The clinical characteristics significantly associated with the presence of the 45 and 62 kDa Abs are listed in Tables 2 and 3, for categorical and continuous variables, respectively. We found a significant association of the 45 kDa Ab with cognitive impairment, and of the 65 kDa Ab with gross motor stereotypies observed by the physician during the first visit, at a time when the observer was obviously blind to the antibody status of the patients (P-values = 0.020 and 0.032, respectively) (Table 2). Statistical analyses performed on continuous variables confirmed the greater severity of autistic patients carrying anti-cerebellar autoantibodies, as evidenced by significantly lower adaptive scores at three of the four VABS subscales, i.e., Daily Living, Socialization, and Communication, as well as the Adaptive Behavior Composite score (P = 0.013–0.028). This result is primarily driven by the 45 kDa Ab, whereas the 62 kDa Ab is interestingly associated only with later birth order (Table 3). Significantly higher ADI-R Social Interaction and ADOS Play and Imagination subscores (Table 3), as well as greater percentages of autistic children not yet having achieved sphincter control (Table 2), also point toward anti-brain autoantibodies conferring greater clinical severity. The only negative associations pertain to self-injurious behavior, less likely to occur among Ab + autistics, and interestingly recurrent ear infections at autism onset as well as cumulative factor scores for principal component 2 “Immune Dysfunction” (Tables 2 and 3).
Fig. 1.
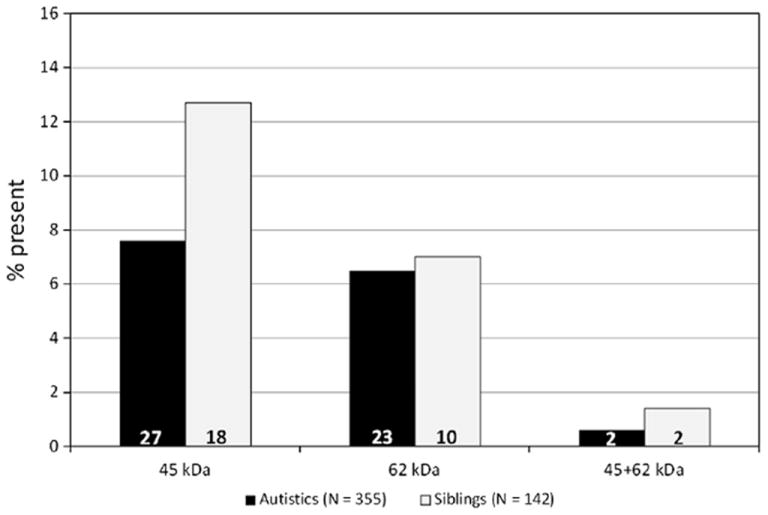
Prevalence of the 45 and 62 kDa antibrain autoantibodies in 355 autistic individuals and 142 unaffected siblings.
Table 2.
Clinical characterization of autistic individuals carrying either the 45 or the 62 kDa antibrain autoantibodies (Ab+). Only categorical variables reaching a nominal P < 0.05 are listed. Data are reported as N (%).
| Autoantibody | Categorical variables | Total N | Ab+ | Ab− | P | |
|---|---|---|---|---|---|---|
| 45 kDa | I.Q. | ≤70 | 182 | 13 (100.0) | 118 (69.8) | 0.020 |
| >70 | 0 (0.0) | 51 (30.2) | ||||
| History of recurrent otitis at autism onset | Present | 277 | 0 (0.0) | 52 (20.2) | 0.026 | |
| Absent | 20 (100.0) | 205 (79.8) | ||||
| 62 kDa | Motor stereotypies observed during the first visit | Present | 258 | 17 (94.4) | 170 (71.1) | 0.032 |
| Absent | 1 (5.6) | 69 (28.9) | ||||
| Either 45 or 62 kDa or both | I.Q. | ≤70 | 182 | 24 (92.3) | 107 (68.6) | 0.013 |
| >70 | 2 (7.7) | 49 (31.4) | ||||
| Motor stereotypies observed during the first visit | Present | 257 | 33 (86.8) | 154 (70.3) | 0.035 | |
| Absent | 5 (13.2) | 65 (29.7) | ||||
| Sphincter control | Typical development | 225 | 14 (43.8) | 122 (63.2) | 0.045 | |
| Delayed | 2 (6.3) | 17 (8.8) | ||||
| Never acquired | 16 (50.0) | 54 (28.0) | ||||
| Self-injurious behavior observed during the first visit | Present | 252 | 22 (64.7) | 175 (80.3) | 0.041 | |
| Absent | 12 (35.3) | 43 (19.7) |
Table 3.
Clinical characterization of autistic individuals carrying either the 45 or the 62 kDa antibrain autoantibodies (Ab+). Only continuous variables reaching a nominal P < 0.05 are listed.
| Autoantibody | Continuous variables | Ab+
|
Ab−
|
P | ||
|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | |||
| 45 kDa | Number of sisters | 23 | 1.04 ±1.02 | 268 | 0.53 ± 0.67 | 0.012 |
| VABS daily living | 14 | 57.9 ±31.7 | 128 | 79.0 ± 33.5 | 0.013 | |
| VABS socialization | 14 | 58.9 ±18.2 | 128 | 75.9 ± 24.0 | 0.014 | |
| VABS communication | 14 | 59.6 ±19.9 | 128 | 79.5 ± 32.0 | 0.016 | |
| VABS adaptive behavior composite | 11 | 52.0 ±16.0 | 109 | 70.3 ± 28.9 | 0.028 | |
| Principal component 2 “immune dysfunction” | 12 | −0.42 ± 0.980 | 126 | 0.11 ±1.06 | 0.044 | |
| 62 kDa | Order of birth | 19 | 2.3 ±1.4 | 271 | 1.6 ±0.7 | 0.040 |
| Either 45 or 62 kDa or both | ADI-R social interaction | 12 | 26.4 ± 5.8 | 82 | 21.0 ±7.1 | 0.023 |
| VABS communication | 24 | 65.0 ± 22.4 | 116 | 79.9 ± 32.6 | 0.025 | |
| Order of birth | 39 | 2.1 ±1.2 | 251 | 1.6 ±0.7 | 0.031 | |
| VABS socialization | 24 | 64.2 ± 22.3 | 116 | 75.9 ± 23.9 | 0.031 | |
| VABS daily living | 24 | 63.8 ± 30.4 | 117 | 79.4 ± 34.0 | 0.032 | |
| ADOS play and imagination | 13 | 3.3 ±1.0 | 100 | 2.4 ±1.5 | 0.042 | |
Maternal autoantibodies (mAbs) were screened in 333 mothers, who gave birth to 353 out of the 355 ASD children assessed here for antibrain autoantibodies. Prevalence of mAb 37, 39 and 73 kDa is shown in Fig. 2A, while the 37/73 and 39/73 combinations are shown in Fig. 2B. Overall, no difference in prevalence of maternal anti-fetal brain Abs was recorded between autistic individuals and unaffected siblings (for all single mAbs and combinations, P = 0.50–0.80), although a significant difference in birth order was detected for the presence of the 39 kDa mAb (see below). The clinical characteristics significantly associated with the presence of mAbs 37, 39 and 73 kDa are reported in Tables 4 and 5 for categorical and continuous variables, respectively. The autistic offspring of mothers carrying the 37 kDa antibrain antibody display an abnormal sleep/wake cycle more frequently (Table 4) and deficits both in non-verbal and in verbal language acquisition (Tables 4 and 5). In particular, the association with non-verbal language development (overall P = 0.018 in Table 4) is characterized by the absence of children exposed to maternal 37 kDa antibodies among those with “typical development” (post hoc Fisher’s exact test, P = 0.041), while most show a sizable “delay” (P = 0.063) or their non-verbal language was “never acquired” (P = 0.024). A negative effect on communication skills is not limited to non-verbal language, as also age at verbal language development is significantly higher among children exposed to the 37 kDa maternal antibody (Table 5). Meanwhile, also autistic children whose mothers carry the 73 kDa antibody, display verbal language deficits (overall P = 0.014 in Table 4), with significantly lower rates of typical language development (P = 0.046) paralleled by enhanced rates of language regression (P = 0.010) and lack of verbal language acquisition (P = 0.056). Verbal and non-verbal communication deficits may fall into a broader picture, since these children show significantly greater factor scores for principal component 3, “neurodevelopmental delay” of Sacco et al. (2010). Indeed these children also show other signs of abnormal and/or slow neurodevelopment, such as delayed onset of social smile and abnormal kissing (referring to perioral muscle weakness involved in oral dyspraxia, and not to kissing as a social behavior) (Table 4); furthermore, in the presence of both 39 and 73 kDa maternal antibodies, significantly lower VABS motor skills scores are recorded, likely reflecting broad motor coordination deficits (Table 6). The presence of both 39 and 73 kDa antibodies may also be associated with epilepsy, although small sample sizes here require caution (Table 6), while the 73 kDa was interestingly more frequent among patients belonging to the “immune-circadian-sensory” and “mixed” clusters described by Sacco et al. (2012) (Table 4). No variable was significantly associated with the co-presence of 37 and 73 kDa mAbs, likely due to the relatively few mothers in that category.
Fig. 2.
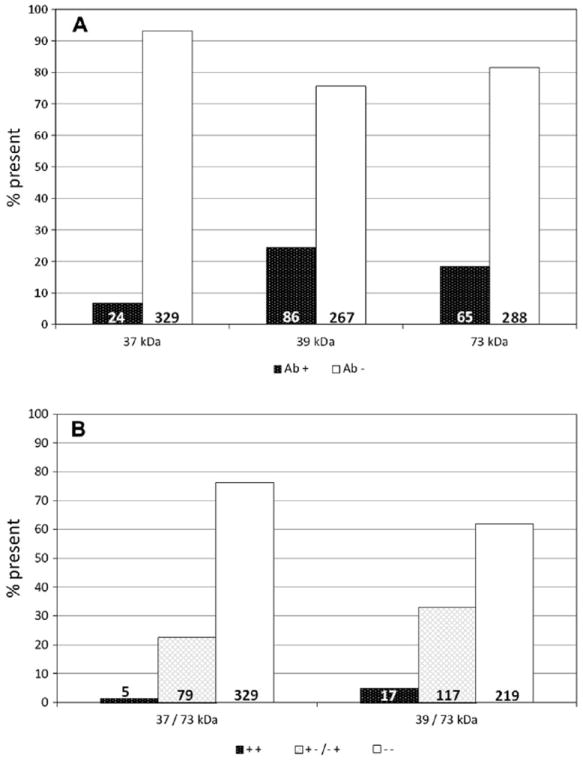
Percentage of autistic individuals born from 333 mothers positive for the 37, 39 and 73 kDa mAbs either (A) alone or (B) in combination.
Table 4.
Clinical characterization of autistic individuals, whose mothers carry either the 37, 39 or 73 kDa antibrain antibodies (mAb+). Only categorical variables reaching a nominal P < 0.05 are listed. Data are reported as N (%).
| Maternal antibody | Categorical variables | Total N | mAb+ | mAb− | P | |
|---|---|---|---|---|---|---|
| 37 kDa | Sleep/wake cycle | Normal | 232 | 4 (30.8) | 141 (64.4) | 0.015 |
| Abnormal | 9 (69.2) | 78 (35.6) | ||||
| Non-verbal language | Typical development | 149 | 0 (0.0) | 62 (43.7) | 0.018 | |
| Delayed | 5 (71.4) | 70 (49.3) | ||||
| Never acquired | 2 (28.6) | 10 (7.0) | ||||
| Autoimmune disorder in I degree relatives | Absent | 242 | 9 (60.0) | 188 (82.8) | 0.028 | |
| Present | 6 (40.0) | 39 (17.2) | ||||
| 39 kDa | I.Q. | ≤70 | 180 | 26 (56.5) | 99 (73.9) | 0.027 |
| >70 | 20 (43.5) | 35 (26.1) | ||||
| 73 kDa | Autoimmune disorder in I degree relatives | Absent | 242 | 24 (64.9) | 173 (84.4) | 0.005 |
| Present | 13 (35.1) | 32 (15.6) | ||||
| Verbal language | Typical development | 267 | 8 (18.2) | 74 (33.2) | 0.014 | |
| Delayed | 19 (43.2) | 99 (44.4) | ||||
| Regression | 4 (9.1) | 4 (1.8) | ||||
| Never Acquired | 13 (29.5) | 46 (20.6) | ||||
| Patient clusters | CS | 130 | 1 (3.6) | 19 (18.6) | 0.029 | |
| Mixed | 12 (42.9) | 26 (25.5) | ||||
| Stereo | 6 (21.4) | 38 (37.3) | ||||
| ICS | 9 (32.1) | 19 (18.6) | ||||
| Age at first smile | <5 months | 162 | 9 (32.1) | 72 (53.7) | 0.038 | |
| ≥5 months | 19 (67.9) | 62 (46.3) | ||||
| Kissing | Normal | 163 | 17 (58.6) | 103 (76.9) | 0.043 | |
| Abnormal | 12 (41.3) | 31 (23.1) |
Table 5.
Clinical characterization of autistic individuals, whose mothers carry either the 37, 39 or 73 kDa antibrain antibodies (mAb+). Only continuous variables reaching a nominal P < 0.05 are listed. Data are reported as N (%).
| Maternal antibody | Continuous variables | mAb+
|
mAb−
|
P | ||
|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | |||
| 37 kDa | VABS Adaptive behavior composite | 13 | 50.9 ± 15.8 | 106 | 71.8 ±24.4 | 0.008 |
| Numbers of brothers | 18 | 1.1 ±1.0 | 264 | 0.5 ± 0.6 | 0.012 | |
| Age at verbal language development (mo) | 19 | 29.6 ± 24.2 | 244 | 17.0 ±14.4 | 0.020 | |
| 39 kDa | ADOS language/communication | 31 | 5.3 ± 2.7 | 85 | 6.5 ± 3.0 | 0.020 |
| ADOS social interaction | 31 | 9.4 ± 3.0 | 85 | 10.6 ±3.1 | 0.041 | |
| 73 kDa | Principal component 3 “neurodevelopmental delay” | 28 | 0.37 ± 0.9 | 102 | −0.17 ±0.9 | 0.009 |
Table 6.
Clinical characterization of autistic individuals, whose mothers carry both the 39 and 73 kDa antibrain antibodies (mAb+/+), as compared to children whose mothers carry either mAb (+/−) or none (−/−). Only (A) categorical and (B) continuous variables reaching a nominal P < 0.05 are listed. Data are reported as N (%).
| N | Ab+/+ | Ab+/− | Ab−/− | P | ||
|---|---|---|---|---|---|---|
|
| ||||||
| (A) Categorical variables | ||||||
| Epilepsy | Absent | 284 | 12 (85.7) | 81 (95.3) | 178 (96.2) | 0.013 |
| Rare | 0 (0.0) | 4 (4.7) | 3 (1.6) | |||
| Frequent | 2 (14.3) | 0 (0.0) | 4 (2.2) | |||
| Verbal language | Typical development | 267 | 2 (16.7) | 22 (26.4) | 58 (33.9) | 0.015 |
| Delayed | 4 (33.3) | 39 (46.4) | 75 (43.9) | |||
| Regression | 2 (16.7) | 5 (6.0) | 1 (0.6) | |||
| Never Acquired | 4 (33.3) | 18 (21.4) | 37 (21.6) | |||
| Sleep/wake cycle | Normal | 232 | 3 (30.0) | 42 (57.5) | 100 (67.1) | 0.036 |
| Abnormal | 7 (70.0) | 31 (42.5) | 49 (32.9) | |||
| I.Q. | >70 | 180 | 2 (22.2) | 24 (43.6) | 29 (25.0) | 0.040 |
| ≤70 | 7 (77.8) | 31 (56.4) | 87 (75.0) | |||
| Ab+/+ | Ab+/+ | Ab−/− | P | ||||
|
|
|
|
|||||
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||
|
| |||||||
| (B) Continuous variables VABS (motor skills) | 6 | 69.3 ± 18.0 | 34 | 92.1 ± 24.5 | 72 | 84.5 ± 23.7 | 0.046 |
Autistic individuals carrying either the 45 or the 62 kDa antibrain autoantibodies have significantly larger head circumferences, whereas no effect on this parameter was detected for maternal antibodies (Table 7). The presence of anti-brain autoantibodies is associated with later birth order (62 kDa, Table 3), as well as greater numbers of sisters (45 kDa, Table 3). Overall, the distribution of mAbs, either alone or in combination, does not differ based on birth order within the ASD sample (Table S2). However the 37 kDa mAb is correlated with greater number of brothers (Table 5) and, perhaps more importantly, there is a significant difference between autistic and unaffected siblings for the 39 kDa mAb, with the latter significantly overrepresented among first-born and the former more frequently later-born (P < 0.001, Table S3). Interestingly, 45/242 (18.6%) ASD children have first-degree relatives diagnosed with an autoimmune disorder, but rates are significantly higher among children whose mothers carry either the 37 or the 73 kDa Abs (Table 4). Similarly, the presence of the 62 kDa autoantibody in an autistic individual predicts the presence of either the 39 or the 73 kDa antibodies in his/her mother (Table 8).
Table 7.
Head circumference and presence of antibrain antibodies in 222 autistic individuals. Data are presented as mean ± S.E.M. (N).
| Ab+ | Ab− | Statistics | P | |
|---|---|---|---|---|
|
| ||||
| Children | ||||
| 45 kDa | 81.2 ±5.8 (N = 17) | 69.8 ± 2.3 (N = 191) | t = 1.820 (206 df) | 0.082 |
| 62 kDa | 79.7 ± 6.7 (N = 9) | 70.3 ± 2.2 (N = 199) | t = 1.338 (206 df) | 0.214 |
| 45 and/or 62 kDa | 80.7 ± 4.4 (N = 26) | 69.3 ± 2.4 (N = 182) | t = 2.286 (206 df) | 0.027 |
| Mothers | ||||
| 37 kDa | 75.4 ± 9.1 (N = 14) | 71.0 ± 2.2 (N = 189) | t = 0.521 (201 df) | 0.603 |
| 39 kDa | 70.1 ± 4.5 (N = 48) | 71.7 ± 2.4 (N = 155) | t = −0.313 (201 df) | 0.775 |
| 73 kDa | 65.0 ± 5.0 (N = 39) | 72.8 ± 2.3 (N = 164) | t = −1.450 (201 df) | 0.149 |
| Ab +/+ | Ab +/− or Ab −/+ | Ab −/− | Statistics | P | |
|
| |||||
| 37 kDa and 73 kDa | 50.6 ± 21.5 (N = 4) | 69.7 ± 4.6 (N = 43) | 72.3 ± 2.4 (N = 156) | F = 1.078 (2,219 df) | 0.342 |
| 39 kDa and 73 kDa | 77.6 ± 8.2 (N = 12) | 64.6 ± 4.1 (N = 60) | 73.8 ± 2.6 (N = 131) | F = 2.172 (2,219 df) | 0.117 |
+ Present; − absent.
Table 8.
Familial clustering of antibrain antibodies in 328 ASD children (45 and 62 kDa) and their 312 mothers (37, 39, and 73 kDa).
| Maternal antibodies | Ab+/Ab− | ASD children antibodies
|
|||||
|---|---|---|---|---|---|---|---|
| 45 kDa+ | 45 kDa− | P | 62 kDa+ | 62 kDa− | P | ||
| 37/39/73 | + | 11 (47.8) | 125 (41.0) | 0.521 | 13 (61.9) | 123 (40.1) | 0.049 |
| − | 12 (52.2) | 180 (59.0) | 8 (38.1) | 184 (59.9) | |||
| 37 + 73 | + + | 0 (0.0) | 4 (1.3) | 0.415 | 3 (1.0) | 1 (4.8) | 0.180 |
| + −/− + | 7 (30.4) | 60 (19.7) | 61 (19.9) | 6 (28.6) | |||
| − − | 16 (69.6) | 241 (79.0) | 243 (79.2) | 14 (66.7) | |||
| 39 + 73 | + + | 3 (13.6) | 12 (3.9) | 0.126 | 4 (19.0) | 11 (3.6) | 0.001 |
| + −/− + | 6 (26.1) | 97 (31.8) | 9 (42.9) | 94 (30.6) | |||
| − − | 14 (60.9) | 196 (64.3) | 8 (38.1) | 202 (65.8) | |||
| 37 | + | 2 (8.7) | 20 (6.6) | 0.693 | 1 (4.8) | 21 (6.8) | 0.713 |
| − | 21 (91.3) | 285 (93.4) | 20 (95.2) | 286 (93.2) | |||
| 39 | + | 7 (30.4) | 73 (23.9) | 0.484 | 10 (47.6) | 70 (22.8) | 0.010 |
| − | 16 (69.6) | 232 (76.1) | 11 (52.4) | 237 (77.2) | |||
| 73 | + | 5 (21.7) | 50 (16.4) | 0.508 | 7 (33.3) | 48 (15.6) | 0.036 |
| − | 18 (78.3) | 255 (83.6) | 14 (66.7) | 259 (84.4) | |||
+ Present; − absent.
4. Discussion
In this study we investigated the clinical, cognitive, behavioral, morphological and familial correlates of anti-cerebellar and antifetal brain antibodies in a sample of Italian ASD children, unaffected siblings, and their mothers. Our results essentially show that, despite not increasing the probability of being affected over being unaffected within the same sibship, the 45 and 62 kDa antibrain antibodies present in many autistic individuals are indeed associated with significantly greater cognitive impairment (45 kDa), autism severity (45 kDa), and motor stereotypies (62 kDa) (Tables 2 and 3). Moreover, mAbs seemingly interfere with neurodevelopment, especially affecting verbal and non-verbal language, sleep/wake cycle and possibly social smile and motor development (Tables 4-6). Children’s autoantibodies, but not mAbs, are associated in our sample with macrocephaly, one of the best-characterized endophenotypes in autism research (Table 7) (Sacco et al., 2010). The anti-cerebellar autoantibodies and the maternal anti-fetal brain antibodies analyzed here do not seemingly confer a significantly greater risk of autism within the same sibship, since differences in Ab prevalence between patients and unaffected siblings are largely non-significant (Fig. 1). Goines et al. (2011) previously found an association of the 45 kDa Ab with strict autism, but not with the broader Autism Spectrum Disorder, which was instead assessed in our study. Wills et al. (2009) found a significantly increased prevalence of the 52 kDa band (further characterized as 45 kDa) in ASD children compared to unrelated controls. Several investigators also found mAbs significantly more often in mothers of affected children vs mothers of unaffected children (Braunschweig et al., 2008, 2012; Brimberg et al., 2013; Rossi et al., 2013). However, Rossi et al. (2013) found superimposable immunorectivity levels and Ab prevalence in age-matched typically developing and autistic children. Most importantly, here we compare autistic individuals with their unaffected siblings and not with population controls, as in other studies (Wills et al., 2009; Goines et al., 2011; Rossi et al., 2013). Hence, our results collectively do provide strong evidence of differential pathomorphic, albeit not pathogenic, roles in autism for anti-cerebellar antibodies produced either by the patient or fetal-brain specific autoantibodies produced by his/her mother.
The presence of the 45 kDa antibrain autoantibody is significantly associated with cognitive impairment and lower functioning, as shown by the VABS for daily living, socialization, communication, and composite scores. Similar results were reported by Goines et al. (2010), who also recorded in their sample significantly lower VABS composite scores. In contrast, the 62 kDa autoantibody is significantly associated with the presence of gross motor stereotypies observed by the child neuropsychiatrist during the first visit, a result again confirming previous findings by Goines et al. (2011) who reported aberrant behavior scores on the ABC Stereotypy subscale. In addition to these variables, the presence of either 45 or 62 kDa autoantibodies is also associated with higher ADI-R social interaction and ADOS play and imagination subscores, both pointing toward greater autism severity (Table 3). Also the acquisition of sphincter control is largely less successful among ASD children positive for either Ab (Table 2). Altogether, the consistency of present and previous results (Goines et al., 2011), obtained in independent and ethnically-distinct samples using partly different measures, strongly supports their reliability and underscores the biological influence exerted by the 45 and 62 kDa autoantibodies on autism severity.
Maternal antibodies (37, 39 and 73 kDa), either singly or in the 39 + 73 kDa combination, are most clearly associated in our sample with impaired verbal and non-verbal language acquisition and sleep/wake cycle disturbances (Tables 4-6). Carriers of the 37 kDa mAb display either delayed or absent non-verbal language development (Table 4), while parents of verbal children report mean onset of spoken language (i.e., initial five words in addition to “mama” and “papa”) at 29.6 vs 17.0 months in non-carriers (Table 5). Furthermore, autistic individuals carrying either the 73 kDa Ab or the 39 + 73 kDa combination show regression or lack of verbal language significantly more often than non-carriers (Tables 4 and 6). Several hints posing these language deficits as possibly the most prominent feature of a broader neurodevelopmental delay can be found in our data set. In fact, the 73 kDa antibody is associated also with (a) delayed appearance of social smile, as reported by parents, (b) oral dyspraxia as evidenced by abnormal kissing, and (c) significantly greater cumulative factor scores for principal component 3 “neurodevelopmental delay” (Tables 4 and 5). Variables filling this component include: level of verbal language development, age at verbal language development, age at non-verbal language development, age at walking, age at acquisition of bladder control at night (Sacco et al., 2010). In addition, the 39 + 73 kDa combination is associated with significantly lower VABS scores for Motor Skills (Table 6). These results largely replicate previous findings, whereby mAbs have been found associated with high rates of language regression and poor expressive language scores (Dalton et al., 2003; Braunschweig et al., 2012). Given the relatively small sample size of autistic individuals exposed to mAbs here and in previous published studies, influences on motor milestones will need to be specifically addressed in an independent and targeted replication study. It will be interesting to verify to what extent potential influences on motor development by mAbs may be autism-specific, or instead play a meaningful role in the broad spectrum of motor dyspraxias.
In addition, mAbs are significantly more prevalent in mothers of autistic children with either abnormal sleep/wake cycle or falling into the ICS “immune-circadian-sensory” cluster, encompassing autistic patients mainly characterized by immune, sleep/wake cycle, and sensory abnormalities (Sacco et al., 2012). Autoimmune mechanisms are indeed able to affect mechanisms underlying the sleep/wake cycle, as shown in narcolepsy (Mahlios et al., 2013; Cermakian et al., 2013). Interestingly, in the case of narcolepsy, as more generally in autoimmunity, disease risk increases with birth order (Watson et al., 2012), which is indeed associated with the presence of antibrain antibodies also in our autistic sample (Table 3). To the extent of our current knowledge, Abs and mAbs do not bind to antigens located in the suprachiasmatic nucleus. Hence the mechanism underlying this behavioral effect remains elusive.
Recently, Nordahl et al. (2013) analyzed the correlation between 37 + 73 kDa mAbs and total brain volume using structural magnetic resonance imaging (MRI), suggesting that these mAbs may impact brain development and be an important factor leading to abnormal brain enlargement in ASD. However, in our sample macrocephaly is associated with the presence of the 45 and/or 62 kDa autoantibodies and not with mAbs 37 and 73 kDa (Table 7). While it is plausible that autoantibodies may promote cell proliferation (see below), the reason behind this discrepancy requires further investigation. Age-dependent differences in head growth rates (Courchesne et al., 2007) may provide some contribution as the mean age of our patient sample is 9.06 years with a relatively broad range (Table 1), while the Nordahl et al. (2013) sample includes 131 ASD and 50 typically developing preschoolers.
The array of neuronal cells and antigens targeted by autoantibodies present in the blood of children, and mAbs is being actively investigated. The former bind several different cell types in the cerebral and cerebellar cortex, hippocampus, amygdala and striatum (Wills et al., 2011). Reactivity against a 52 kDa autoantibody, later more accurately identified as the 45 kDa Ab analysed here, was primarily detected in calbindin-positive Golgi interneurons located in the Purkinje cell layer of the cerebellar cortex (Wills et al., 2007, 2011). Golgi cells act as inhibitory interneurons upon Purkinje cells, modulating their outflow of neural activity from the cerebellar cortex to the deep cerebellar nuclei (De Schutter et al., 2000; Hirano et al., 2002). Interestingly, abnormal neurodevelopment or surgical lesions to the cerebellum have indeed been correlated with autistic behaviors, decreased cognitive function, language impairment and motor stereotypies (Fatemi et al., 2012; Riva and Giorgi, 2000). Molecular targets of these autoantibodies are still being sought, whereas the targets of maternal antibodies have been recently unveiled. Braunschweig et al. (2013) identified specific maternal IgG reactivity to six different proteins expressed in developing brain, namely LDH, YBH, cypin, STIP1, CRMP1 and CRMP2. In particular, LDH, YBX1 and STIP1 have been identified as targets of the mAbs analyzed in our study (37, 39 and 73 kDa, respectively), whereas cypin, CRMP1 and CRMP2 bind mAbs sized 44 and 70 kDa not assessed in our sample. LDH plays well-known roles in energy metabolism, although its relevance in the fetal brain has not been fully determined. STIP1 is a major ligand of the cellular prion protein: this interaction stimulates neurogenesis in cultured hippocampal neurons and its inhibition yields impaired memory formation in rodents (Coitinho et al., 2007). Anti-STIP1 autoantibodies have been detected in blood and cerebrospinal fluid (CSF) of patients with neuro-Behcet’s disease (Vural et al., 2011). Finally, YBX1 (also known as p50) is critically involved in the cell motility and migration required for successful neural tube formation; most importantly, YBX1 acts as a DNA- and RNA-binding protein with chaperone properties, interacting with MeCP2 and FMRP, notoriously involved in most forms of Rett and Fragile-X syndrome, respectively (Eliseeva et al., 2011). Though attempting to disentangle the contribution of each antigen to the clinical phenotype is at this stage too premature, the participation of YBX1 in protein complexes critical for synaptic physiology, as conclusively demonstrated in these neurodevelopment disorders, appears especially promising.
Curiously, autoantibody carriers appear protected against recurrent ear infections during early childhood and around the time of autism onset, an incident frequently reported by parents of autistic children (Konstantareas and Homatidis, 1987; Tajima-Pozo et al., 2010). Recurrent ear infections would be predicted to characterize a subset of ASD children unable to eradicate infectious agents, due to reduced immune reactivity and to a cytokine profile skewed toward anti-inflammation. On the contrary, the production of anti-brain autoantibodies would logically be associated with individuals excessively prone to produce an immune response and displaying a more pro-inflammatory cytokine profile. However, immune dysregulation with respect to immune homeostasis could affect both the response to bacteria agents as well as loss of immune regulation in the same individual. It would be interesting to verify this hypothesis exploring the cytokine profiles of these two subgroups of ASD children (i.e., autoantibody carriers vs positive for recurrent ear infections). Furthermore, cumulative factor scores for Principal Component 2, “Immune Dysfunction”, are significantly lower among 45 kDa Ab carriers (Table 3). Variables filling this component include: history of allergies in the patient, history of any allergic and/or autoimmune disease in first-degree relatives, history of any recurrent infectious disease at autism onset (ear, gastrointestinal, upper airways or other), obstetric complications or recurrent abortions in the mother, pregnancy duration (Table S1) (Sacco et al., 2010). Several immune anomalies have been described among individuals with ASD and their close relatives, involving altered cytokine profiles, immunoglobulin levels and neuroinflammation (Ashwood et al., 2006; Enstrom et al., 2009; Saresella et al., 2009; Goines et al., 2011). However, our results indicate that the mechanisms leading to the production of antibrain autoantibodies both in autistic individuals and in their mothers likely differ from those underlying the “immune dysfunction” defined by principal component 2 (Sacco et al., 2010).
This study presents at least two limitations, which must be duly acknowledged. First, despite assessing a large sample of autistic individuals, unaffected siblings and mothers, the relatively low prevalence of some Abs and incomplete clinical data sets result in relatively small numbers of carriers, especially for combinations of Abs. Secondly, our study does indeed possess an exploratory nature in reference not only to the ethnicity of the experimental sample, but also to many variables never assessed in prior studies. Hence the number of variables tested here, coupled with the relatively low prevalence of Abs, makes it impossible to reach P values < 0.05 when applying any strategy to correct for multiple comparisons. This would raise significant risk of a type II error, because two observations clearly speak in favor of the non-random nature of the vast majority of our nominally significant results: (a) they are consistent with previous observations obtained in other independent samples; (b) also within our data set, there is a clear convergence among different variables measuring the same or highly similar phenomena (as one example, see social communication-related variables in Tables 4 and 5). We have thus applied nominal significance at P < 0.05 with no correction for multiple testing. This strategy is appropriate for our study design according to many biostatisticians, who view correction for multiple testing as unnecessary in exploratory analyses (Bender and Lange, 2001; Savitz and Olshan, 1998; Streiner and Norman, 2011), while others even argue it should be avoided altogether, in order not to spuriously increase Type II error rates (Rothman, 1990; Perneger, 1998). On the other hand, the reliability of our results is most convincing when the same outcome is supported by two or more converging variables, while single positives (for example, epilepsy in Table 6) should be viewed with caution until independently replicated, as they may represent type I errors.
In summary, our results clearly indicate that, though not conferring autism risk within sibships, antibrain Abs do play an important pathoplastic role in autism. Prenatal and/or postnatal exposure to these Abs enhances autism severity by impairing cognitive processes and adaptive functioning, boosting motor stereotypies, altering the sleep/wake cycle, delaying or halting neurodevelopment especially in reference to verbal and non-verbal language. Our results, along with previous studies performed in independent samples, support the potential use of anti-brain antibodies as biomarkers predicting autism severity and clinical features of ASD, while possibly providing new avenues for preventive and therapeutic strategies (Careaga et al., 2010).
Supplementary Material
Acknowledgments
The Authors wish to thank all the patients and families who participated in this study. This work was supported by the Italian Ministry for University, Scientific Research and Technology (PRIN no. 2006058195 and no. 2008BACT54_002), the Italian Ministry of Health (RFPS-2007-5-640174, RF-2011-02350537 and CCM2012-Progetto NIDA), the Fondazione Gaetano e Mafalda Luce (Milan, Italy), Autism Aid ONLUS (Naples, Italy), Autism Speaks (Princeton, NJ), the Autism Research Institute (San Diego, CA), and the Innovative Medicines Initiative Joint Undertaking (EUAIMS, no. 115300), NIEHS 1 P01 ES11269-01, the U.S. Environmental Protection Agency (U.S.EPA) through the Science to Achieve Results (STAR) program (Grant R829388), NIEHS 1 R01-ES015359, the UC Davis M.I.N.D. Institute.
Footnotes
Conflict of interest
Drs. Van de Water is a member of the scientific advisory board for Pediatric Bioscience, a company that has licensed the maternal antibody technology from UC Davis. Pediatric Bioscience did not contribute in any way to the current studies. The remaining authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbi.2013.12.020.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth ed. American Psychiatric Publishing; Arlington, VA: 2013. pp. 5–25. [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Arch Neurol. 2012;69:693–699. doi: 10.1001/archneurol.2011.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J. Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord. 2012;42:1435–1445. doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, Hertz-Picciotto I, Pessah IN, Van de Water J. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry. 2013;9:e277. doi: 10.1038/tp.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry. 2013;18:1171–1177. doi: 10.1038/mp.2013.101. [DOI] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Ann N Y Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Careaga M, Van de Water J, Ashwood P. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermakian N, Lange T, Golombek D, Sarkar D, Nakao A, Shibata S, Mazzoccoli G. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int. 2013;30:870–888. doi: 10.3109/07420528.2013.782315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coitinho AS, Lopes MH, Hajjm GN, Rossato JI, Freitas AR, Castro CC, Cammarota M, Brentani RR, Izquierdo I, Martins VR. Short-term memory formation and long-term memory consolidation are enhanced by cellular prion association to stress-inducible protein 1. Neurobiol Dis. 2007;26:282–290. doi: 10.1016/j.nbd.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, Styles P, Vincent A. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- De Schutter E, Vos B, Maex R. The function of cerebellar Golgi cells revisited. Prog Brain Res. 2000;124:81–93. doi: 10.1016/s0079-6123(00)24009-0. [DOI] [PubMed] [Google Scholar]
- Eliseeva IA, Kim ER, Guryanov SG, Ovchinnov LP, Lyabin DN. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry (Mosc) 2011;76:1402–1433. doi: 10.1134/S0006297911130049. [DOI] [PubMed] [Google Scholar]
- Enstrom AM, Van de Water JA, Ashwood P. Autoimmunity in autism. Curr Opin Investig Drugs. 2009;10:463–473. [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Consensus paper: pathological role of the cerebellum in autism. Cerebellum. 2012;11:777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, Hansen R, Hertz-Picciotto I, Ashwood P, Van de Water J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Watanabe D, Kawaguchi SY, Pastan I, Nakanishi S. Roles of inhibitory interneurons in the cerebellar cortex. Ann N Y Acad Sci. 2002;978:405–412. doi: 10.1111/j.1749-6632.2002.tb07583.x. [DOI] [PubMed] [Google Scholar]
- Konstantareas MM, Homatidis S. Ear infections in autistic and normal children. J Autism Dev Disord. 1987;17:585–594. doi: 10.1007/BF01486973. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Ados, autism diagnostic observation schedule. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]; Tancredi R, Saccani M, Persico AM, Parrini B, Igliozzi R, Faggioli R, editors. Italian version. Florence: Organizzazioni Speciali; 2005. [Google Scholar]
- Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol. 2013;23:767–773. doi: 10.1016/j.conb.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Braunschweig D, Iosif AM, Lee A, Rogers S, Ashwood P, Amaral DG, Van de Water J. Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain Behav Immun. 2013;30:61–65. doi: 10.1016/j.bbi.2013.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123:1051–1061. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Rossi CC, Fuentes J, Van de Water J, Amaral DG. Brief Report: antibodies reacting to brain tissue in Basque Spanish children with Autism Spectrum Disorder and their mothers. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1859-y. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Rutter M, Le Couter A, Lord C. ADI-R, autism diagnostic interview—revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]; Faggioli R, Saccani M, Persico AM, Tancredi R, Parrini B, Igliozzi R, editors. Italian version. Florence: Organizzazioni Speciali; 2005. [Google Scholar]
- Sacco R, Curatolo P, Manzi B, Militerni R, Bravaccio C, Frolli A, Lenti C, Saccani M, Elia M, Reichelt KL, Pascucci T, Puglisi-Allegra S, Persico AM. Principal pathogenetic components and biological endophenotypes in autism spectrum disorders. Autism Res. 2010;3:237–252. doi: 10.1002/aur.151. [DOI] [PubMed] [Google Scholar]
- Sacco R, Lenti C, Saccani M, Curatolo P, Manzi B, Bravaccio C, Persico AM. Cluster analysis of autistic patients based on principal pathogenetic components. Autism Res. 2012;5:137–147. doi: 10.1002/aur.1226. [DOI] [PubMed] [Google Scholar]
- Saresella M, Marventano I, Guerini FR, Mancuso R, Ceresa L, Zanzottera M, Rusconi B, Maggioni E, Tinelli C, Clerici M. An autistic endophenotype results in complex immune dysfunction in healthy siblings of autistic children. Biol Psychiatry. 2009;66:978–984. doi: 10.1016/j.biopsych.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Olshan AF. Describing data requires no adjustment for multiple comparisons: a reply from Savitz and Olshan. Am J Epidemiol. 1998;147:813–814. doi: 10.1093/oxfordjournals.aje.a009532. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales-Survey form. American Guidance Service; Circle Pines, MN: 1984. [Google Scholar]
- Streiner DL, Norman GR. Correction for multiple testing: is there a resolution? Chest. 2011;40:16–18. doi: 10.1378/chest.11-0523. [DOI] [PubMed] [Google Scholar]
- Tajima-Pozo K, Zambrano-Enriquez D, De Anta L, Zelmanova J, De Dios Vega JL, Lopez-Ibor JJ. Otitis and autism spectrum disorders. BMJ Case Rep. 2010 doi: 10.1136/bcr.10.2009.2351. http://dx.doi.org/10.1136/bcr.10.2009.2351. [DOI] [PMC free article] [PubMed]
- Vural B, Uğurel E, Tüzün E, Kürtüncü M, Zuliani L, Cavuş F, Içöz S, Erdağ E, Gül A, Güre AO, Vincent A, Ozbek U, Eraksoy M, Akman-Demir G. Anti-neuronal and stress-induced-phosphoprotein 1 antibodies in neuro-Behçet’s disease. J Neuroimmunol. 2011;239:91–97. doi: 10.1016/j.jneuroim.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Watson NF, Ton TG, Koepsell TD, Longstreth WT., Jr Birth order and narcolepsy risk among genetically susceptible individuals: a population-based case-controlstudy. Sleep Med. 2012;13:310–313. doi: 10.1016/j.sleep.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Autoantibodies in autism spectrum disorders (ASD) Ann N Y Acad Sci. 2007;1107:79–91. doi: 10.1196/annals.1381.009. [DOI] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S, Rossi CC, Bennett J, Martinez Cerdeño V, Ashwood P, Amaral DG, Van de Water J. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Mol Autism. 2011;2:5. doi: 10.1186/2040-2392-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


