Abstract
The Hering–Breuer (HBR) reflex is considered a major regulatory feedback for the generation and patterning of respiratory activity. While HBR is important in neonates, its significance in adults is controversial. Previous experiments that investigated the plasticity of entrainment of the respiratory rhythm by vagal input demonstrated postnatal changes in HBR plasticity. Here we analyzed postnatal changes in the plasticity of HBR by mimicking the classic lung inflation tests with repetitive tonic vagal stimulation across different postnatal stages in an in situ perfused brainstem preparation of rat. The study shows that neonates stereotypically exhibit HBR stimulus-dependent prolongation of expiration while juvenile preparations (>postnatal day 16) showed significant habituation of HBR following repetitive stimulation. Subsequent experiments employing physiological lung inflation tests in situ confirmed HBR habituation in juveniles. We conclude that postnatal emergence of HBR habituation explains the weak contribution and high activation threshold of HBR in the regulation of eupnea.
Keywords: Postnatal development, Hebbian plasticity, Brainstem, Sensory processing
1. Introduction
The Hering–Breuer inflation reflex (HBR, Hering, 1868; Breuer, 1868) is a ‘classic’ reflex in respiratory control that is described in practically every physiology textbook. In brief, lung inflation activates slowly adapting pulmonary stretch receptors. The receptor input is relayed via the vagus nerve to ‘pump’ cells located in and around the ventro-lateral nucleus of the solitary tract (Clark and Euler, 1972; Berger and Dick, 1987; Bonham and McCrimmon, 1990; Bonham et al., 1993; Miyazaki et al., 1998, 1999). Pump cells project to and release inhibitory neurotransmitters on inspiratory neurons in the lateral respiratory column (Ezure and Tanaka, 2004; see Kubin et al., 2006); thus, contributing to the termination of inspiration (see Euler, 1981, 1983; Bianchi et al., 1995; Kubin et al., 2006).
The HBR is often considered as an inhibitory sensory feedback loop that shapes the respiratory motor pattern. Removing HBR feedback via cooling of the vagal nerve or vagotomy instantly transforms the respiratory pattern to a slower breathing rhythm with increased inspiratory amplitude and longer duration of expiration (Marckwald, 1887; Stella, 1938, Clark and Euler, 1972).
Although the HBR is an established mechanism, doubts about its physiological significance arose from findings that show little or no effect of the HBR in adult humans (summarized in Kubin et al., 2006). In particular, the HBR does not affect the respiratory pattern in humans until a high lung volume is reached. Thus, the threshold for HBR is well above end-inspiratory lung volume during resting breathing (Bechbache et al., 1979; Cunningham and Gardner, 1977; Duffin et al., 2000). In contrast, the HBR is important for the stabilization of the breathing pattern in neonatal rats (Fedorko et al., 1988). So, the HBR appears to becomes less significant with postnatal maturation of the cardio-respiratory control circuits as observed in humans (Gerhardt and Bancalari, 1981; Rabbette et al., 1991; Rabbette and Stocks, 1998; BuSha et al., 2002) and mammals in general (Trippenbach, 1994).
A plausible explanation for diminished role of the HBR in adults is provided by pioneering investigations of Chi-Sang Poon and co-workers demonstrating that the HBR habituates in rats (Siniaia et al., 2000; Poon, 2004; Song and Poon, 2004; MacDonald et al., 2009; Tadjalli et al., 2010). Thus, if postnatal maturation of brainstem circuitry is mandatory before these networks become permissive for synaptic plasticity (see Dutschmann et al., 2004, 2008, 2009; Dutschmann and Dick, 2012), then HBR habituation does not occur in the neonatal stages of brainstem development.
Therefore we investigated HBR habituation in juvenile rat by using repetitive brief electrical stimulation of the central vagus nerve across 3 different stages of postnatal brainstem development. Habituation is the decrease in strength of the response to a stimulus that is presented repeatedly. In the present study, the initial vagal stimuli suppressed inspiratory activity but as the stimulus was repeated, the phrenic bursting ‘broke-through’ during vagal stimulation. The phrenic bursting during the stimulus indicated habituation. Importantly, HBR habituation did not emerge until postnatal day 16. Subsequent experiments show an identical habituation of inspiratory suppression in response to repetitively applied sustained lung inflation in juvenile rat preparations. The experiments using physiological stimulus of sustained lung inflation are consistent with a postnatal emergence of synaptic plasticity linked to HBR habituation.
2. Materials and methods
Experiments were performed at the Georg August University Göttingen (Germany) and at the Florey Institute of Neuroscience and Mental Health (Australia). Approval for experiments was obtained from both Institutional Animal Care Ethics Committees. The experimental procedures were performed in accordance with international guidelines for the care and use of laboratory animals.
2.1. Perfused brainstem preparations
The arterially perfused brainstem preparation of rat at various postnatal stages was used, as previously described in detail (Dutschmann et al., 2009). Here phrenic nerve activity (PNA) was recorded as an index of the respiratory motor output. Respiratory motor output was optimized by adjusting the flow rate (5–22 ml min−1) and perfusion pressure (40–70 mmHg) depended on the age of the rat pup used for the preparation.
2.2. Electrical stimulation of the vagus and lung inflation tests
For vagus nerve stimulation, we used the stimulus parameters of our previous publication (Dutschmann et al., 2009). In brief, the effect of fictive feedback from pulmonary stretch receptors at different postnatal stages was simulated by electrical stimulation of central branch of the vagus nerve (Master 8, A.M.P.I., pulse duration: 50–100 μs; stimulus frequency: 20 Hz, stimulus duration: 10 s, stimulus intensity: 0.2–1 mA). The vagus nerve was stimulated 1.5× the stimulus threshold that terminated inspiration at end inspiration (Stanley et al., 1975). After threshold determination, we applied repetitive vagal stimulation: 15 consecutive trials separated by a 2 min interval.
We conducted these experiments in preparations of different postnatal ages which were divided into three age groups: (A) a neonatal group (postnatal day (P4–8), (B) an intermediate group (P9–15) and (C) a juvenile group (P16–21).
For sustained lung inflation tests in juvenile rat preparations, the right lung lobes were left intact during the initial surgery. The trachea was intubated and connected to a small rodent ventilator (SAR 830/P, CWE Inc., USA). Initially, we used rhythmic lung ventilation at a frequency of 30 breaths/min and increased ventilation pressure (mmH2O) until individual lung inflations terminated PNA bursts. After determining the threshold, the inflation pressure was increased by 50% (60–85 mmH2O) and 15 lung inflations of 10 s duration were delivered at 2-min intervals.
2.3. Data analysis
For both vagal stimulation and sustained lung inflation protocols, PNA was used to analyze the following respiratory variables: total respiratory cycle length (TTOT), durations of inspiration (TI) and expiration (TE). These variables were analyzed 1 min before, during and after the 10 s stimulations/inflations. Progressive changes in TE prolongation evoked by either vagal stimulation or lung inflation were tested for significance using regression analyses Analysis of Covariance (ANCOVA, Systat). ANCOVA was also used for the analysis of progressive changes in respiratory variables before and after vagal stimulation and lung inflations. The post-stimulus rebound (desensitization) was analyzed by calculating the time required until TTOT returned to baseline values determined from the pre-stimulus period. Changes in respiratory activity before (baseline) and after the vagal stimulation protocol were analyzed using a two-tailed, paired t-test.
3. Results
3.1. Developmental changes in inspiratory depression in response to Hering–Breuer inflation reflex (HBR)
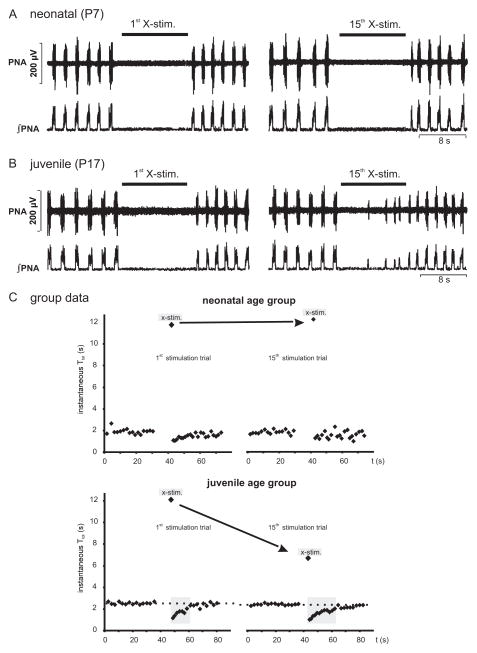
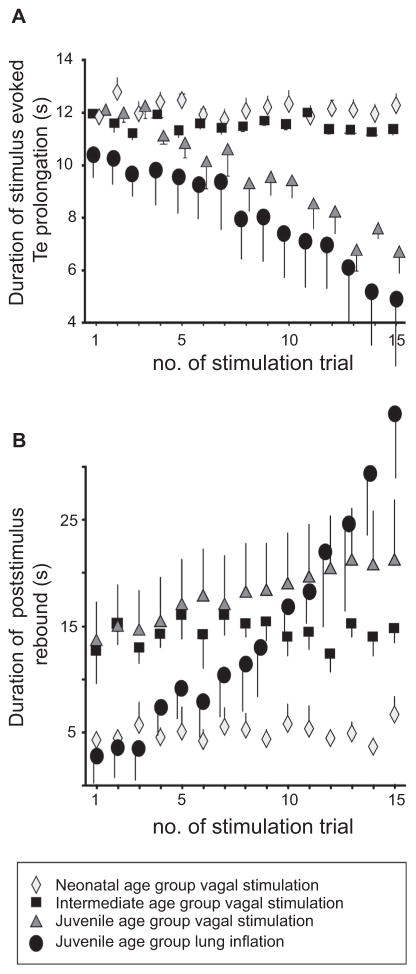
Postnatal maturation of HBR is illustrated by representative examples of the response to repetitive tonic vagal nerve simulation in a neonatal (Fig. 1A) and juvenile preparation (Fig. 1B). In each age group, the first stimulus consistently suppressed PNA for 10 s at least (see Fig. 1). In preparations of the neonatal age group (n = 5), analysis of vagally mediated TE prolongation was not significantly different between the 1st (11.82 ± 0.41 s) and 15th (12.26 ± 0.46 s) stimulus (see Fig. 2 ANCOVA, n.s.). A similar time course of vagally evoked TE prolongation was observed in the intermediate age group (1st stimulation: 11.96 ± 0.87 s vs. 15th stimulation: 11.37 ± 0.24 s; n = 5 preparations; Fig. 2 ANCOVA, n.s.). However, in the juvenile age group (n = 5), the repetitive vagal stimulation displayed habituation of the fictive HBR response because PNA bursts emerged during the acute vagal stimulation (see Fig. 1B). Consequently, vagally evoked TE prolongation became progressively shorter (1st stimulation: 12.1 ± 0.40 s; 15th stimulation: 6.69 ± 0.83 s, Fig. 2; ANCOVA, p < 0.001).
Fig. 1.
Developmental changes in response to train stimulation of the vagal nerve. (A) Examples of experiments that illustrate the effect of the first and last (15th) vagal stimulation (X-stim) on phrenic nerve activity (PNA) in a perfused brainstem preparation of neonatal rat at postnatal day 7 (P7). (B) Original traces illustrating the effect of first and last vagal stimulation in a juvenile rat (P17). Note that stimulus-evoked PNA suppression is disrupted by breakthrough of PNA during the 15th stimulation only in the juveniles but not in the neonates. (C) Mean instantaneous total respiratory cycle duration (TTOT ) 30 s before and after X-stim. Note that a post-stimulus rebound is characterized by at least 2 respiratory cycles after stimulus off switch with a TTOT shorter than baseline. The post-stimulus rebound in neonates was short and did not change with repetitive vagal stimulation. In contrast, the rebound in juveniles was more pronounced and increased in duration with repetitive vagal stimulations.
Fig. 2.
Group data illustrating developmental changes related to fictive (vagal stimulation) and physiological (lung inflation) HBR habituation and post-stimulus rebound. Diagrams illustrating group data across different postnatal age groups: (A) Progressive changes in TE prolongation evoked by electrical vagus stimulation or lung inflation. (B) Progressive changes in the duration of the post-stimulus rebound.
3.2. Post-stimulus rebound
In each experimental group, post-stimulus rebound activity was characterized by a transient increases in respiratory frequency (fR) compared to pre-stimulus fR after termination of vagal stimulation (indicated as decrease in TTOT illustrated in Fig. 1C). The time required for the fR returned to pre-stimulus values remained unchanged in the neonatal age group as shown in Fig. 2B (4.6 ± 0.7 s, 1st stimulation vs 6.7 ± 1.7 s, 15th stimulation). In the intermediate age group the rebound period was substantially longer compared to neonates (t-test, p < 0.05). However, repetitive vagal stimulus trials did not change the duration of the rebound significantly (12.6 ± 3 s, 1st trial vs. 14.8 ±1.4 s, 15th trail). Similar to habituation, only the juvenile age group showed plasticity of the post-stimulus rebound indicated by a significant prolongation of the rebound period (13.4 ± 3.9 s, 1st trial vs. 21 ± 5.8 s, 15th trail, Fig. 2B; ANCOVA p < 0.05).
3.3. Changes in baseline respiration
In neonates, the vagal stimulation protocol had no significant effect on timing of the respiratory phases after the trial; comparing the breathing pattern before (baseline) to that after the stimulation protocol (TTOT: 1.92 ± 0.04 s vs. 1.66 ± 0.26 s, TI: 0.36 ± 0.02 s vs. 0.32 ± 0.02 s, TE 1.56 ± 0.04 s vs. 1.34 ± 0.24 s). However, in the intermediate and juvenile groups, consecutive vagal stimulation periods caused a subtle but significant increase in respiratory drive. In the intermediate group, TTOT decreased from 1.92 ± 0.10 s to 1.61 ± 0.13 s, (p = 0.05) and TI, from 0.49 ± 0.05 s to 0.42 ± 0.04 s, p = 0.05); and similarly in the juvenile age group, TTOT decreased from 2.36 ± 0.26 s to 1.89 ± 0.16 s (p < 0.05) and TI, from 0.55 ± 0.04 s to 0.49 ± 0.05 s (p < 0.05).
3.4. Verification of HBR habituation with sustained lung inflation
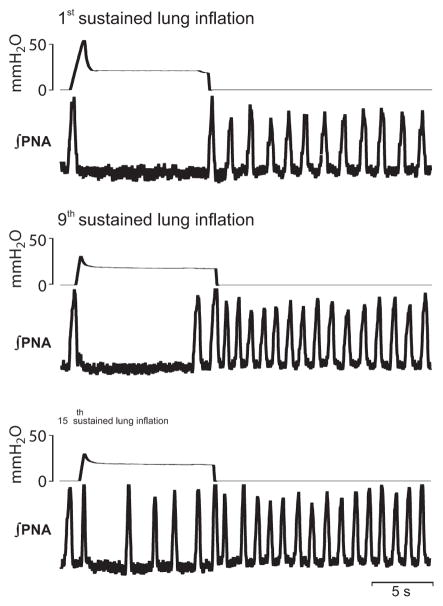
The habituation of HBR using vagal stimulation was verified with repetitive sustained lung inflation of 10 s duration in juvenile preparations (n = 5, Fig. 3). Using a comparable stimulation protocol (15 bouts of 10 s inflation at 2 min interval), we also observed a progressive shortening of lung inflation-evoked TE prolongation (1st inflation: 10.4 ± 0.67 s; 15th inflation: 4.9 ± 2.1 s, Fig. 2C; ANCOVA, p < 0.05). As with electrical stimulation of the vagal nerve, repetitive lung inflation also caused a progressive prolongation of the post-inflation rebound increase in respiratory frequency. The first inflation evoked only marginal rebound activity of 2.8 ± 1.8 s; whereas, repetitive lung inflation caused a robust rebound duration lasting 34.1 ± 5.6 s (after the 15th lung inflation Fig. 2; p < 0.05). The lung inflation protocol had no significant effect on baseline breathing parameters (TTOT: 2.82 ± 0.24 s vs. 2.2 ± 0.8 s, TI: 0.76 ± 0.12 s vs. 0.79 ± 0.22 s, all n.s.).
Fig. 3.
HBR habituation triggered by repetitive lung inflation. Example of an experiment that illustrates the effect of the first, the 9th and the last (15th) sustained lung inflation on PNA in a perfused brainstem preparation of neonatal rat (postnatal day 19). Note that inflation-mediated PNA suppression is disrupted by breakthrough of several PNA bursts during the 15th stimulation.
4. Discussion
4.1. Physiological significance of the Hering–Breuer reflex (HBR)
The results of the present study confirm previous observations that identified HBR habituation in the adult rat (Siniaia et al., 2000; Song, 2004; Song and Poon, 2004; MacDonald et al., 2007, 2009). HBR habituation was demonstrated using two different experimental approaches: repetitively applied short vagal stimulus trains and lung inflations. The novel finding in the present study is the postnatal emergence of HBR habituation and maturation as a mechanistic explanation for the observed developmental changes in strength and significance of the HBR in humans (Gerhardt and Bancalari, 1981; Rabbette et al., 1991; Rabbette and Stocks, 1998; BuSha et al., 2002). Confirmation of these results in other species is required due to species differences in the effect of HBR on the respiratory pattern (e.g. Phillipson et al., 1971; Bradley et al., 1975). Nevertheless, the present data highlights the need for future investigations to explore the physiological mechanisms that underlie HBR maturation during development e.g. developmental changes in NMDA receptor unit expression in nuclei that subserve the HBR (Liu and Wong-Riley, 2010). The importance of the HBR in neonatal humans (e.g. Rabbette and Stocks, 1998) and animals (e.g. rats, Fedorko et al., 1988) is the stabilization of a still vulnerable and slightly instable respiratory pattern generator by providing a breath-by-breath sensory feedback is undisputed (see Section 4.3). In contrast, significance of the HBR in respiratory control in adult life is controversial. The Hering–Breuer reflex is often argued as a significant mechanism that initiates and controls the inspiratory off-switch (IOS) because TI increases after bilateral vagotomy even in adult animals. Importantly however, the HBR habituates after brainstem maturation, as shown here. Ironically, a central pontine mechanism facilitating IOS was described shortly after the discovery of the HBR (see Marckwald, 1887). The central IOS mechanism that is mediated via the parabrachial and Kölliker-Fuse nuclei in the dorsolateral pons (see Dutschmann and Dick for recent review) has been considered as an alternative mechanism for the HBR in the absence of vagal input.
The growing evidence of HBR habituation and the struggle to assign a role for HBR at eupneic tidal volumes in both humans and cats (Clark and Euler, 1972) suggests that IOS at normal tidal volumes is controlled centrally by the dl pons and/or of neural influences (St John et al., 1972) rather than a sensory feedback. In other words, the physiological importance of the HBR in forming the respiratory pattern in juvenile and adult mammals may be secondary to central influences. Nevertheless, the HBR does have salient functions in juvenile/adult animals. Sensory feedback regarding the status of lung inflation (e.g. tidal volume) via this reflex appears to have an important role for the transformation of the breathing pattern in response to behavioral and emotional commands ranging from exercise to vocalization.
The role of HBR feedback in ‘learning’ respiratory patterns was investigated previously (Dutschmann et al., 2009). The key finding of the earlier study was that learning of sensory feedback patterns is indicated by anticipatory pontine-mediated IOS. In accordance with the present study, the learning of a sensory feedback pattern also emerged around postnatal day 16.
4.2. Plasticity of the post-stimulus rebound: implications for the postnatal maturation of the pontine respiratory group
In our study, a post-stimulus rebound of the PNA is evident as a transient increase in the respiratory frequency following vagal stimulation/lung inflation when compared to pre-stimulus values. An advantage of our study design is that the apnea caused by lung inflation or vagal stimulation did cause hypoxemia because oxygenation of the perfusate occurs externally. Further, the oxygenated perfusate is continually pumped through the animal so the brainstem remains well oxygenated regardless of the changes in central respiratory activity. Therefore, we can exclude hypoxia causing the post-stimulus tachypnea. Also, previous studies have rejected hypoxia as the cause for the post-stimulus rebound (Siniaia et al., 2000; MacDonald et al., 2007, 2009). These studies identified the post-stimulus rebound as independent form of plasticity and termed it HBR-reflex desensitization (Siniaia et al., 2000). The HBR desensitization is considered a form of vagal memory and its expression depends on NMDA-receptor dependent glutamatergic neurotransmission in the Kölliker-Fuse nucleus (Siniaia et al., 2000). Although we did not block neurotransmission in Kölliker-Fuse nucleus in the present study, the time course of the postnatal emergence of plasticity in post-stimulus rebound activity matches previous findings regarding the postnatal maturation the Kölliker-Fuse nucleus. Postnatal changes in neuron morphology, expression profile of NMDA-receptors and electrophysiology are strongly correlated temporally with the emergence of NMDA-receptor dependent memory around postnatal day 15 (Kron et al., 2008, see Dutschmann and Dick, 2012).
It is noteworthy that HBR-reflex desensitization is not only linked to the post-stimulus rebound activity but also apparently contributes to respiratory frequency changes during prolonged (1–2 min) vagal stimulation. However, post-stimulus rebound was identified in the intermediated age group, even though the acute reflex response remained unchanged. Thus, desensitization may not contribute to the plasticity that is evoked by HBR stimuli of short duration as used in the present study. On the other hand, the intermittent, repeated nature of the prolonged vagal stimulus/lung inflation trials used presently may itself contribute to the transient increase in respiratory frequency observed after our experimental protocol. A recent study in anaesthetized adult rat demonstrated the long term facilitation of respiratory motor output following repeated episodes of obstructive apneas is reliant on changes in vagal feedback (Tadjalli et al., 2010).
4.3. Developmental irregularities in Hering–Breuer reflex habituation (HBR) is linked to pathophysiology
The HBR feedback is considered to be important for the stabilization of the breathing pattern in neonatal rats (Fedorko et al., 1988). In this context, the absence of habituation to HBR-reflex may be protective in the neonate. An example of an ill-timed habituation is provided by studies of NMDA-R subunit knock-out mice (Poon et al., 2000). These mice display neonatal synaptic long-term depression (LTD) in the HBR relay circuits as a cellular equivalent of habituation. Such genetically caused neonatal LTD in the HBR relay pathways were associated with respiratory depression and premature neonatal death (Poon et al., 2000).
Another example of ill-timed HBR habituation is provided by studies of Mecp2 knock out mice as model for the human Rett syndrome. Both human patients and Mecp2 mice develop a severe respiratory disorder after they emerge from the neonatal period (see Katz et al., 2009). Investigators suggest that a lack of HBR habituation (Stettner et al., 2007; Song et al., 2011) may contribute to instability of the breathing pattern due to competing sensory and central mechanisms impinging on the IOS.
Acknowledgments
This study was supported by the Bernstein Center for Computational Neurosciences (BCCN, 01GQ0432, M. Dutschmann) and NHLBI (Cluster Grant R33 HL087377, M. Dutschmann, and T. E. Dick). M.D. is currently supported by an ARC Future fellowship and Florey Institute of Neuroscience and Mental Health.
References
- Bechbache RR, Chow HH, Duffin J, Orsini EC. The effects of hypercapnia, hypoxia, exercise and anxiety on the pattern of breathing in man. J Physiol. 1979;293:285–300. doi: 10.1113/jphysiol.1979.sp012889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ, Dick TE. Connectivity of slowly adapting pulmonary stretch receptors with dorsal medullary respiratory neurons. J Neurophysiol. 1987;58:1259–1274. doi: 10.1152/jn.1987.58.6.1259. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Coles SK, McCrimmon DR. Pulmonary stretch receptor afferents activate excitatory amino receptors in the nucleus tractus solitarii in rat. J Physiol. 1993;464:725–745. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer–Hering reflex in rat. J Physiol. 1990;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley GW, von Euler C, Marttila I, Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biol Cybern. 1975;19:105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- Breuer J. Die Selbststeurung der Athmung durch Nervus Vagus. Akad Wiss Wien (II) 1868;58:909–937. [Google Scholar]
- BuSha BF, Stella MH, Manning HL, Leiter JC. Termination of inspiration by phase-dependent respiratory vagal feedback in awake normal humans. J Appl Physiol. 2002;93:903–910. doi: 10.1152/japplphysiol.00153.2002. [DOI] [PubMed] [Google Scholar]
- Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol. 1972;222:267–295. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham DJC, Gardner WN. A quantitative description of the pattern of breathing during steady-state CO2 inhalation in man, with special emphasis on expiration. J Physiol. 1977;272:613–632. doi: 10.1113/jphysiol.1977.sp012063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J, Mohan RM, Vasiliou P, Stephenson R, Mahamed S. A model of the chemoreflex control of breathing in humans: model parameters measurement. Respir Physiol. 2000;120:13–26. doi: 10.1016/s0034-5687(00)00095-5. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol. 2012;2:2443–2469. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Morschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory–expiratory phase transition shifts from sensory-to central-dominated during postnatal development in rats. J Physiol. 2009;587:4931–4948. doi: 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Morschel M, Reuter J, Zhang W, Gestreau C, Stettner GM, Kron M. Postnatal emergence of synaptic plasticity associated with dynamic adaptation of the respiratory motor pattern. Respir Physiol Neurobiol. 2008;164:72–79. doi: 10.1016/j.resp.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Morschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kolliker-Fuse nucleus. Respir Physiol Neurobiol. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- von Euler C. The contribution of sensory inputs to the pattern generation of breathing. Can J Physiol Pharmacol. 1981;59:700–706. doi: 10.1139/y81-106. [DOI] [PubMed] [Google Scholar]
- von Euler C. On the central pattern generator for the basic breathing rhythmicity. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1647–1659. doi: 10.1152/jappl.1983.55.6.1647. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering–Breuer reflex pathway of the rat. Neuroscience. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Kelly EN, England SJ. Importance of vagal afferents in determining ventilation in newborn rats. J Appl Physiol. 1988;65:1033–1039. doi: 10.1152/jappl.1988.65.3.1033. [DOI] [PubMed] [Google Scholar]
- Gerhardt T, Bancalari E. Maturational changes of reflexes influencing inspiratory timing in newborns. J Appl Physiol. 1981;50:1282–1285. doi: 10.1152/jappl.1981.50.6.1282. [DOI] [PubMed] [Google Scholar]
- Hering E. Die Selbststeurung der Athmung durch den Nervus Vagus. Akad Wiss Wien (II) 1868;58:672–677. [Google Scholar]
- Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol. 2009;168:101–108. doi: 10.1016/j.resp.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Reuter J, Gerhardt E, Manzke T, Zhang W, Dutschmann M. Emergence of brain-derived neurotrophic factor-induced postsynaptic potentiation of NMDA currents during the postnatal maturation of the Kolliker-Fuse nucleus of rat. J Physiol. 2008;586:2331–2343. doi: 10.1113/jphysiol.2007.148916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal development of N-methyl-D-aspartate receptor subunits 2A, 2B, 2C, 2D and 3B immunoreactivity in brain stem respiratory nuclei of rat. Neuroscience. 2010;171:637–654. doi: 10.1016/j.neuroscience.2010.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SM, Tin C, Song G, Poon CS. Use-dependent learning and memory of the Hering–Breuer inflation reflex in rats. Exp Physiol. 2009;94:269–278. doi: 10.1113/expphysiol.2008.045344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SM, Song G, Poon CS. Nonassociative learning promotes respiratory entrainment to mechanical ventilation. PLoS ONE. 2007;2:e865. doi: 10.1371/journal.pone.0000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marckwald M. Die Athembewegungen und deren Innervation beim Kaninchen. Z Biol. 1887;23:149–283. [Google Scholar]
- Miyazaki M, Arata A, Tanaka I, Ezure K. Activity of rat pump neurons is modulated with central respiratory rhythm. Neurosci Lett. 1998;249:61–64. doi: 10.1016/s0304-3940(98)00402-9. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Tanaka I, Ezure K. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Exp Brain Res. 1999;129:191–200. doi: 10.1007/s002210050889. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Hickey RF, Graf PD, Nadel JA. Hering–Breuer inflation reflex and regulation of breathing in conscious dogs. J Appl Physiol. 1971;31:746–750. doi: 10.1152/jappl.1971.31.5.746. [DOI] [PubMed] [Google Scholar]
- Poon CS. Organization of central pathways mediating the Hering–Breuer reflex and carotid chemoreflex. Adv Exp Med Biol. 2004;551:95–100. doi: 10.1007/0-387-27023-x_15. [DOI] [PubMed] [Google Scholar]
- Poon CS, Zhou Z, Champagnat J. NMDA receptor activity in utero averts respiratory depression and anomalous long-term depression in newborn mice. J Neurosci. 2000;20:RC73. doi: 10.1523/JNEUROSCI.20-09-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbette PS, Costeloe KL, Stocks J. Persistence of the Hering–Breuer reflex beyond the neonatal period. J Appl Physiol. 1991;71:474–480. doi: 10.1152/jappl.1991.71.2.474. [DOI] [PubMed] [Google Scholar]
- Rabbette PS, Stocks J. Influence of volume dependency and timing of airway occlusions on the Hering–Breuer reflex in infants. J Appl Physiol. 1998;85:2033–2039. doi: 10.1152/jappl.1998.85.6.2033. [DOI] [PubMed] [Google Scholar]
- Siniaia MS, Young DL, Poon CS. Habituation and desensitization of the Hering–Breuer reflex in rat. J Physiol. 2000;523:479–491. doi: 10.1111/j.1469-7793.2000.t01-1-00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Poon CS. Functional and structural models of pontine modulation of mechanoreceptor and chemoreceptor reflexes. Respir Physiol Neurobiol. 2004;143:281–292. doi: 10.1016/j.resp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Song G, Tin C, Giacometti E, Poon CS. Habituation without NMDA receptor-dependent desensitization of Hering–Breuer apnea reflex in a Mecp2 mutant mouse model of Rett syndrome. Front Integr Neurosci. 2011;5:6. doi: 10.3389/fnint.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella G. On the mechanism of production and the physiological significance of apneusis. J Physiol. 1938;93:10–23. doi: 10.1113/jphysiol.1938.sp003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John WM, Glasser RL, King RA. Rhythmic respiration in awake vagotomized cats with chronic pneumotaxic area lesions. Respir Physiol. 1972;15:233–244. doi: 10.1016/0034-5687(72)90100-4. [DOI] [PubMed] [Google Scholar]
- Stanley NN, Altose MD, Cherniack NS, Fishman AP. Changes in strength of lung inflation reflex during prolonged inflation. J Appl Physiol. 1975;38:474–480. doi: 10.1152/jappl.1975.38.3.474. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2−/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjalli A, Duffin J, Peever J. Identification of a novel form of noradrenergic-dependent respiratory motor plasticity triggered by vagal feedback. J Neurosci. 2010;30:16886–16895. doi: 10.1523/JNEUROSCI.3394-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trippenbach T. Pulmonary reflexes and control of breathing during development. Biol Neonate. 1994;65:205–210. doi: 10.1159/000244054. [DOI] [PubMed] [Google Scholar]