Abstract
The aims of this study were to assess the effect of transarterial chemoembolization (TACE) on circulating tumor cells (CTCs) in the peripheral blood and right atrium of patients with HCC and to evaluate whether perioperative shedding of CTCs affects time to progression of HCC. Before and after TACE, peripheral and right atrial blood samples (7.5 mL) were collected from 42 patients with HCC. CTCs were enriched using EpCAM antibody-conjugated magnetic beads. The number of CTCs was 0–30 and 0–54 in peripheral blood before and after TACE, respectively (P=0.166), and 0–65 and 0–98 in the right atrium before and after TACE, respectively (P=0.102). The number of CTCs was significantly different between the two samples both before (P=0.007) and after (P=0.021) TACE. There was no difference in time to progression between patients with and without an increase in the number of CTCs after TACE in either sample (P>0.05 for both). There were more CTCs in right atrial blood than in peripheral blood. The numbers of CTCs in both samples remained unchanged after TACE. Shedding of tumor cells did not affect time to progression of disease in patients with HCC.
Keywords: hepatocellular carcinoma, transcatheter arterial chemoembolization, circulating tumor cells, metastasis, positive screening
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies, with increasing incidence and mortality rates in recent years.1 Because progression of HCC is relatively latent, it is not diagnosed until advanced stages in most patients, among whom only a small portion have the opportunity to undergo radical resection or liver transplantation for long-term survival. Nonsurgical palliative care, such as transcatheter arterial chemoembolization (TACE), is the most commonly used treatment for patients with HCC. TACE improves patient survival by inducing tumor necrosis and reducing lesion size.2 However, metastasis and recurrence severely impact patient survival and lead to high mortality in patients with HCC.3 This is mainly due to the fact that HCC is hematogenous metastasis to occur secondary malignant tumors and the tumor cells are capable of shedding into the blood circulatory system.4 It is known that shedding of tumor cells occurs during various treatments,5,6 but direct evidence for the dissemination of liver tumor cells during TACE is lacking.
The concept of circulating tumor cells (CTCs) was first introduced in 1869. CTCs play a major role in initiation of metastasis and tumor recurrence, and may act as a potential prognostic marker in patients with HCC. For examples, multicenter prospective studies have shown that the number of epithelial cell adhesion molecule (EpCAM)-positive CTCs in peripheral blood estimated by the CellSearch® system can be used to predict progression of disease and overall survival in metastatic breast cancer, metastatic colorectal cancer, and prostate cancer.7,8 Because quantitative cutoff values for the number of CTCs are used to distinguish between patients with a good and bad prognosis, a difference in the number of CTCs at different blood sampling sites might be clinically important. Further, a difference in the number of CTCs in different vascular compartments might offer an insight into the biology of these cells and the metastatic process. However, little is known about the kinetics of CTCs in the human bloodstream.
EpCAM is expressed in normal epithelial cells and overexpressed in malignant cells in a subset of human carcinomas.9 In this study, in order to evaluate whether TACE leads to shedding of tumor cells and if shedding of tumor cells affects disease progression in patients with HCC, we investigated and compared EpCAM-positive CTCs in peripheral blood and right atrial blood in patients with advanced HCC.
Materials and methods
Subjects
We prospectively recruited 42 patients with HCC and full clinical data from November 2012 to May 2013. Patients with HCC were included if they: met the diagnostic criteria outlined in “Diagnostic and Treatment Practices for Hepatocellular Carcinoma” (2011 edition, People’s Republic of China) and had an indication for TACE; had not been treated with radiochemotherapy or other antitumor therapies; had measurable lesions and no radiographic evidence of distant metastasis; and volunteered to participate and undergo regular follow-up. Exclusion criteria were: rupture of a liver tumor and significant shunt of the hepatic artery-portal vein or hepatic artery-hepatic vein; lack of blood supply to the tumor; widespread metastatic tumor with estimated survival of less than 3 months; cachexia or multiple organ failure; liver function of Child–Pugh level C; occlusion of the second hepatic hilum or inferior vena cava; severe anemia; aged older than 70 years. Ten patients with benign liver disease and ten healthy volunteers were also included as controls. The study was approved by the ethics committee of Zhongshan Hospital, Fudan University. Informed consents were obtained from the patients.
Reagents and equipment
Anticoagulant citrate dextrose blood collection tubes, fluorescein isothiocyanate-labeled mouse anti-human cytokeratin monoclonal antibody, and phycoerythrin-labeled mouse anti-human CD45 monoclonal antibody were purchased from Becton Dickinson (Franklin Lakes, NJ, USA). A magnetic bead-labeled anti-human EpCAM monoclonal antibody, a MACS® magnetic bead LS separation column, and a magnetic cell separator were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Lymphocyte separation medium was purchased from GE Healthcare (Waukesha, WI, USA). The nuclear fluorescent dye, DAPI (4, 6-diamidino-2-phenylindole) was obtained from Sigma (St Louis, MO, USA). Poly-lysine-coated slides were purchased from Thermo Electron (Pittsburgh, PA, USA). A fluorescent microscope (Olympus, Tokyo, Japan) was used for observation.
TACE and right atrial catheter placement
The right femoral artery was punctured after local anesthesia, and a 5 French RH catheter (or 3 French microcatheter if necessary) was inserted into the artery feeding the tumor to perform angiography. After the target vessel was identified, 30–50 mg of epirubicin (Pfizer, New York, NY, USA) and 100–150 mg of oxaliplatin (Jiangsu Hengrui Medicine Co, Ltd, Jiangsu, People’s Republic of China) were added to 5–20 mL of iodized oil for chemoembolization according to the tumor vasculature, preoperative liver function, and blood test results. If the remnants of the artery feeding the tumor were still abundant, embospheres with different diameters (Merit Medical Inc., South Jordan, UT, USA) were chosen for supplementary embolism. The right femoral vein was punctured to insert a 4F Ver or pigtail catheter, which was placed at the right atrium (Figure 1).
Figure 1.
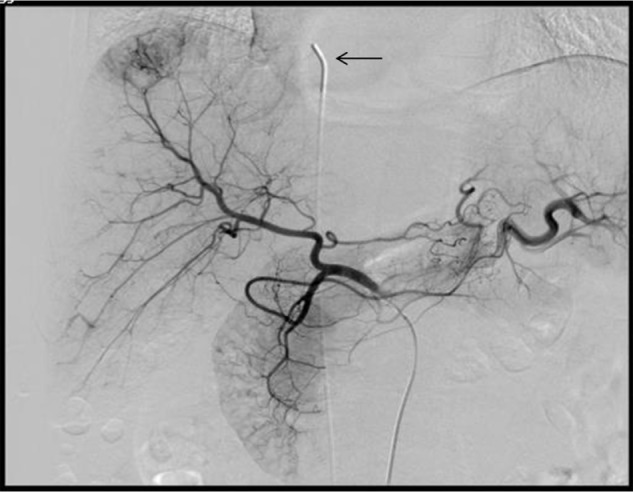
4F Ver catheter placed in the right atrium via a right femoral vein approach.
Notes: Blood samples (7.5 mL) were collected before and after transarterial chemoembolization. Arrow indicates 4F Ver catheter tip in the right atrium.
Blood sample collection and extraction of mononuclear cells
Three minutes before and after the TACE procedure, 7.5 mL blood samples were collected from the median vein at the right elbow and from the right atrium of the 42 patients with HCC. To avoid contamination by epithelial cells, each 7.5 mL blood sample was collected after the first 5 mL of the blood sample had been discarded. Lymphocyte separation medium was added to each sample. Mononuclear cells containing CTCs were isolated using density gradient centrifugation. Samples were processed within 4 hours of collection.
Enrichment, separation, and labeling of CTCs
First, 100 μL of the magnetic bead-labeled anti-human EpCAM monoclonal antibody was added to each 7.5 mL blood sample. Isolated mononuclear cells were then mixed with magnetic beads and incubated at 4°C for 30 minutes. The mixture was then washed twice by centrifugation using buffer. The cells were suspended in 1 mL of buffer. The magnetic separation column was placed into the magnetic field of the bead cell separation device and washed with 500 μL of buffer. The cell suspension was then added to the magnetic separation column and washed twice with buffer. The magnetic separation column was then removed from the magnetic field and placed the captured cells in a 1.5 mL Eppendorf tube. The cells captured by the column were eluted using buffer. The tumor cells were collected by centrifugation and fixed in 4% paraformaldehyde. After blocked with 5% bovine serum albumin, the cells were incubated with fluorescein isothiocyanate-conjugated anti-cytokeratin and phycoerythrin-conjugated anti-CD45 for one hour, stained in DAPI for 3 minutes, and then mounted for fluorescent microscopy.
Diagnostic criteria and counting of CTCs
The morphology of the enriched epithelial cells was investigated according to epithelium and leukocyte surface marker staining and nuclear staining under a fluorescent microscope. CTCs were stained with cytokeratin (pan-cytokeratin, CK8/18/19-fluorescein isothiocyanate) but not anti-leukocyte surface antigen (CD45), and had large nuclei (>8–10 μm in diameter). The nuclear staining (blue channel) and corresponding membrane or cytoplasmic antigen staining (green channel) must match in location for a diagnosis of CTC10 (Figure 2). The number of CTCs was determined by averaging the results obtained in a blinded manner by two experienced technicians.
Figure 2.
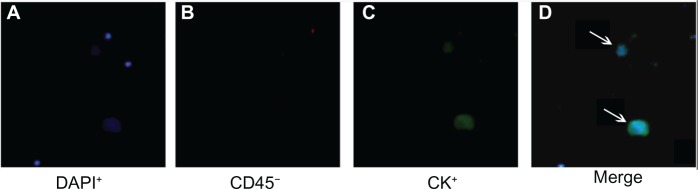
Circulating tumor cells from patients with hepatocellular carcinoma (DAPI+/CD45−/CK+).
Notes: (D) shows a merged image of (A–C). Arrows indicate circulating tumor cells.
Abbreviations: CK, cytokeratin; DAPI, 4, 6-diamidino-2-phenylindole dye.
Follow-up
Diagnosis of recurrence and metastasis after TACE during follow-up monitoring was based on the results of computed tomography, magnetic resonance imaging, digital subtraction angiography, and serum alpha-fetoprotein levels. Follow-up was completed on September 1, 2013. Time to progression was designated as the time from the start of the study to objective tumor progression, including progression of liver disease or extrahepatic metastasis. Evaluation of tumor status after TACE was in accordance with mRECIST (modified Response Evaluation Criteria In Solid Tumors). The study endpoint was set at progression of liver disease or extrahepatic metastasis. Radiographic evaluation of disease progression was performed by two experienced radiologists. Follow-up was complete for 42 cases (100%) with an average duration of 108.3 (40–186) days.
Statistical analyses
Data with a normal distribution are presented as the mean ± standard deviation, and data with a skewed distribution are presented as the median ± interquartile range. Disaggregated data were compared using the χ2 test or corrected χ2 test. Numbers of CTCs in peripheral and right atrial blood samples were compared before and after TACE using the Wilcoxon signed-rank test, and CTC-positive rates were compared using McNemar’s test. The probability of survival without disease progression in the two groups (group with increased CTCs and group with unchanged or decreased CTCs after TACE) was determined using the Kaplan–Meier technique, and the significance of the difference was analyzed using the log-rank test. A P-value <0.05 was considered to be statistically significant. Statistical analyses were performed using Statistical Package for the Social Sciences version 19.0 software (IBM Corporation, Armonk, NY, USA).
Results
Demographic and clinical characteristics of HCC patients
The 42 cases comprised 33 males and nine females, of mean age 53.6±7.4 (38–68) years. Imaging indicated 23 cases of liver cirrhosis, 21 cases of Child–Pugh level A HCC, and 21 cases of Child–Pugh level B HCC. Portal vein tumor thrombus was detected in 14 cases. The mean maximum tumor diameter was 6.7±2.2 (range 3–12) cm, and the mean number of tumor lesions was 3.4±1.7 (range 1–8). The ten cases of benign liver disease included two hepatic hemangiomas, three liver cysts, two hepatic adenomas, one case of focal liver nodular hyperplasia, and two cases of chronic hepatitis B.
Relationship between CTCs in peripheral and right atrial blood before TACE and clinical characteristics
The clinical characteristics investigated were age, gender, cirrhosis, serum alpha-fetoprotein level, Child–Pugh liver function, maximum tumor diameter, number of lesions, and portal vein invasion (Tables 1 and 2). The CTC-positive rate in peripheral blood was most closely related to the size of tumor lesions and serum alpha-fetoprotein level (P<0.05). For the right atrial blood samples, the CTC-positive rate was related not only to the size of tumor lesions but also to portal vein invasion (P<0.05).
Table 1.
Preoperative peripheral blood CTCs and clinical features of patients with HCC
| Total | CTC-positive | CTC-negative | P-value | |
|---|---|---|---|---|
| Number of patients | 42 | 22 | 20 | |
| Age, years | 0.899 | |||
| >50 | 15 | 14 | ||
| ≤50 | 7 | 6 | ||
| Sex | 1.000* | |||
| Male | 17 | 16 | ||
| Female | 5 | 4 | ||
| Cirrhosis | 0.067 | |||
| Positive | 15 | 8 | ||
| Negative | 7 | 12 | ||
| Serum AFP | 0.012 | |||
| >400 ng/mL | 14 | 5 | ||
| ≤400 ng/mL | 8 | 15 | ||
| Child–Pugh score | 0.537 | |||
| A | 12 | 9 | ||
| B | 10 | 11 | ||
| Tumor maximum diameter | 0.029 | |||
| >7 cm | 14 | 6 | ||
| ≤7 cm | 8 | 14 | ||
| Number of lesions | 0.204 | |||
| >3 | 12 | 7 | ||
| ≤3 | 10 | 13 | ||
| Portal vein tumor thrombus | 10/12 | 4/16 | 0.081 | |
| Positive | 10 | 4 | ||
| Negative | 12 | 16 |
Note:
Corrected χ2 test.
Abbreviations: AFP, alpha-fetoprotein; CTCs, circulating tumor cells; HCC, hepatocellular carcinoma.
Table 2.
Preoperative right atrial blood CTCs and clinical features of patients with HCC
| Total | CTC-positive | CTC-negative | P-value | |
|---|---|---|---|---|
| Number of patients | 42 | 31 | 11 | |
| Age, years | 0.148* | |||
| >50 | 19 | 10 | ||
| ≤50 | 12 | 1 | ||
| Sex | 0.463* | |||
| Male | 23 | 10 | ||
| Female | 8 | 1 | ||
| Cirrhosis | 0.712* | |||
| Positive | 18 | 5 | ||
| Negative | 13 | 6 | ||
| Serum AFP | 0.081* | |||
| >400 ng/mL | 17 | 2 | ||
| ≤400 ng/mL | 14 | 9 | ||
| Child–Pugh score | 0.726 | |||
| A | 16 | 5 | ||
| B | 15 | 6 | ||
| Tumor maximum diameter | 0.003 | |||
| >7 cm | 19 | 1 | ||
| ≤7 cm | 13 | 10 | ||
| Number of lesions | 0.298* | |||
| >3 | 16 | 3 | ||
| ≤3 | 15 | 8 | ||
| Portal vein tumor thrombus | 0.018* | |||
| Positive | 14 | 0 | ||
| Negative | 17 | 11 |
Note:
Corrected χ2 test.
Abbreviations: AFP, alpha-fetoprotein; CTCs, circulating tumor cells; HCC, hepatocellular carcinoma.
Immunomagnetic separation of CTCs from peripheral and right atrial blood before and after TACE
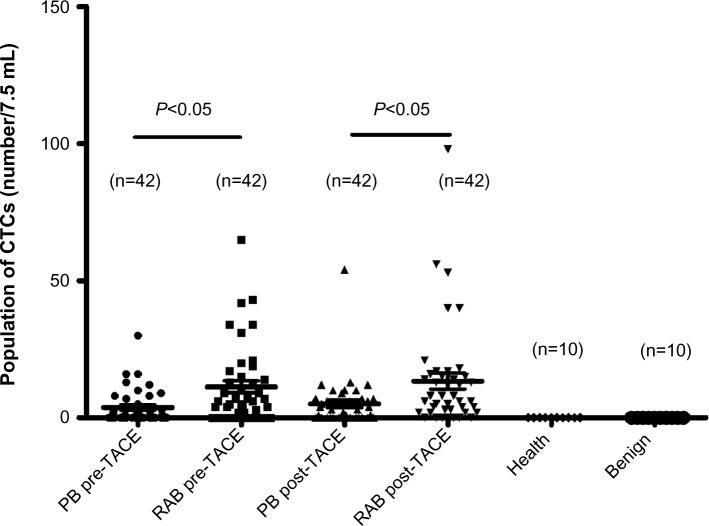
No CTCs were detected in the ten healthy volunteers or in the ten cases of benign liver disease using immunomagnetic separation. In patients with HCC, the number of CTCs ranged from 0 to 98. Therefore, CTCs ≥1 was defined as CTC-positive in this study. Twenty-two (52.4%) of the 42 peripheral blood samples were CTC-positive before TACE and 27 (64.3%) were CTC-positive after TACE, with no statistically significant difference between the two (P=0.267). Thirty-one (73.8%) of the 42 right atrial blood samples were CTC-positive before TACE and 35 (83.3%) were CTC-positive after TACE, with no statistically significant difference between the two (P=0.219). CTC-positive rates in atrial blood samples collected before (P=0.012) and after (P=0.039) TACE were higher than those in peripheral blood samples (Table 3). The number of CTCs in peripheral blood samples ranged from 0 to 30 before TACE and from 0 to 54 after TACE, with no statistically significant difference between the two (Z=−1.387, P=0.166). The number of CTCs in right atrial blood samples ranged from 0 to 65 before TACE and from 0 to 98 after TACE, with no statistically significant differences between the two (Z=−1.633, P=0.102). The difference in the number of CTCs between peripheral blood and right atrial blood before (Z=−2.714, P=0.007) and after (Z=−2.309, P=0.021) TACE treatment were both statistically significant (Table 4 and Figure 3).
Table 3.
CTC-positive rates in peripheral and right atrial blood and effect of TACE
| Peripheral blood | Right atrial blood | P-value | |
|---|---|---|---|
| Pre-TACE (+) | 22 | 31 | 0.012 |
| Post-TACE (+) | 27 | 35 | 0.039 |
| P-value | 0.267 | 0.219 |
Abbreviations: CTCs, circulating tumor cells; TACE, transarterial chemoembolization.
Table 4.
Number of CTCs in peripheral and right atrial blood and effect of TACE (median ± interquartile range)
| Peripheral blood | Right atrial blood | Z value | P-value | |
|---|---|---|---|---|
| Pre-TACE (+) | 1.00±5.50 | 6.00±15.50 | −2.714 | 0.007 |
| Post-TACE (+) | 3.50±7.00 | 7.00±13.25 | −2.309 | 0.021 |
| Z value | −1.387 | −1.633 | ||
| P-value | 0.166 | 0.102 |
Abbreviations: CTCs, circulating tumor cells; TACE, transarterial chemoembolization.
Figure 3.
CTCs in peripheral and right atrial blood before and after TACE. The difference between peripheral blood and right atrial blood in the number of CTCs both before (P=0.000) and after (P=0.000) TACE was statistically significant. No CTCs were detected in healthy volunteers or in patients with benign liver disease.
Abbreviations: CTCs, circulating tumor cells; PB, peripheral blood; RAB, right atrial blood; TACE, transarterial chemoembolization.
Impact of TACE-induced shedding of tumor cells on time to progression of HCC
During follow-up, six of the 42 cases had lung metastasis, three had bone metastasis, two had adrenal metastasis, and one had brain metastasis. To determine whether TACE-induced shedding of tumor cells affected time to progression in patients with HCC, we compared the time to progression of patients with increased CTCs (group 1) and those with unchanged or decreased CTCs (group 2). No difference in mean time to progression was observed between group 1 (106.9±8.6 days) and group 2 (125.6±8.9 days) when peripheral blood was considered (χ2=2.476, P=0.116, Figure 4). There was also no difference in mean time to progression between group 1 (109.2±10.0 days) and group 2 (123.9±8.0 days) when right atrial blood was considered (χ2=0.688, P=0.407, Figure 5).
Figure 4.
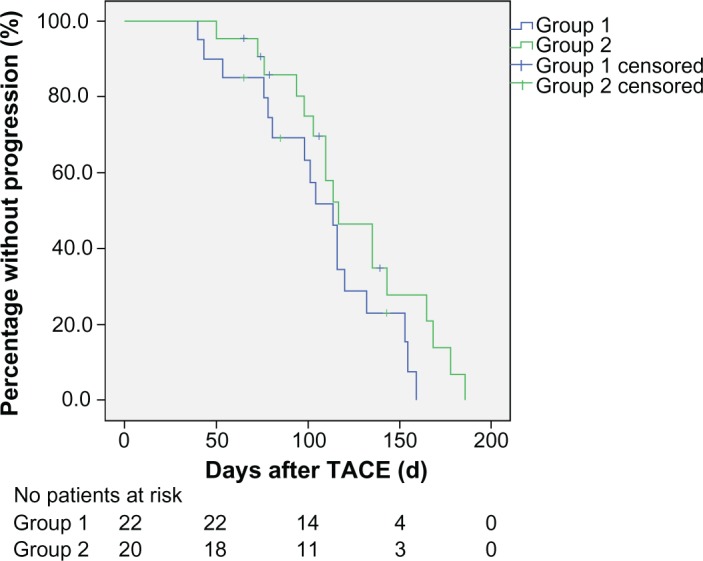
Kaplan–Meier curves for time to progression in groups of patients with increased and unchanged or decreased circulating tumor cells in peripheral blood after TACE (P>0.05).
Notes: Group 1, patients with increased circulating tumor cells; Group 2, patients with unchanged or decreased CTCs.
Abbreviation: TACE, transarterial chemoembolization.
Figure 5.
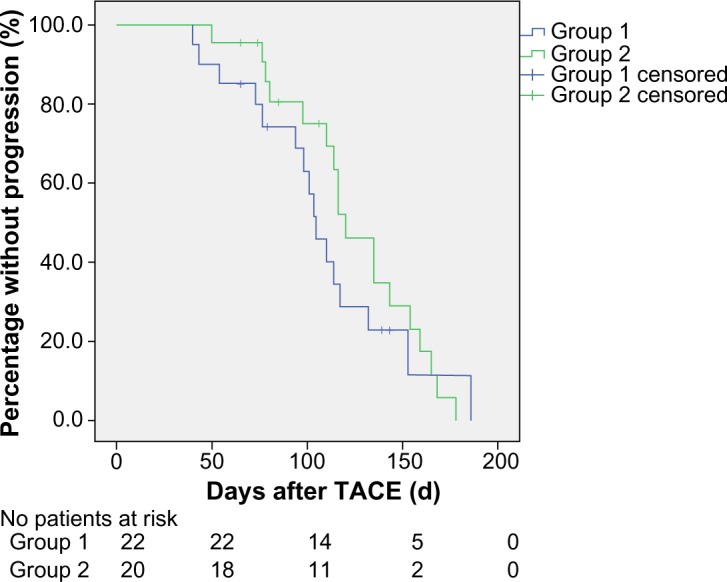
Kaplan–Meier curves for time to progression in patients with increased and unchanged or decreased circulating tumor cells in right atrial blood after TACE (P>0.05).
Notes: Group 1, patients with increased circulating tumor cells; Group 2, patients with unchanged or decreased circulating tumor cells.
Abbreviation: TACE, transarterial chemoembolization.
Discussion
With the advances in modern detection technology, CTCs can be measured using a variety of techniques. Currently, the two major types of CTC detection methods used are whole cell-based technology and nucleic acid-based technology.11,12 However, due to the complexity of the cellular components and the scarcity of CTCs in the peripheral circulation, there has been controversy about the reliability and reproducibility of CTC detection methods, and different conclusions can be reached according to the results obtained with different methods. In 2003, the CellSearch detection system was approved by the US Food and Drug Administration for the detection of CTCs in breast cancer, and has been widely used in a number of malignancies, including colon cancer, prostate cancer, and non-small cell lung cancer. This system is mainly based on semiautomatic isolation of epithelial tumor cells using immunomagnetic separation technology.13–15 In this study, to overcome the hurdle of the high cost of this system, we used techniques similar to the CellSearch system to isolate and detect EpCAM-positive CTCs.
Recurrence and metastasis of HCC result from hematogenous spread of tumor cells. In 1889, Paget et al published their “seed and soil” theory of tumor growth,16 according to which only a small number of CTCs (“seed”) with high metastatic potential and viability survive in the circulation and remain dormant in organs suitable for proliferation (“soil”). Upon activation by certain factors, these CTCs proliferate and form metastases. After radical resection or liver transplantation, distant metastases or recurrence still occur in some patients, indicating that these patients had CTCs before or during surgery.17 Therefore, screening for CTCs might provide important preoperative clinical data and compensate for the lack of clinical and pathological staging of HCC.
The number of CTCs is related to tumor burden, tumor blood supply, and the invasiveness of the tumor.17,18 In this study, we showed that tumor burden significantly affected CTC-positive rates in peripheral and right atrial blood. We also showed a positive correlation between the CTC-positive rate in the right atrium and portal vein tumor thrombus (vascular invasion), indicating that highly invasive tumors would generate more CTCs. There was no correlation between CTCs and age, gender, cirrhosis, Child-Pugh liver function level, or number of lesions (Tables 1 and 2).
The number of CTCs was greater in right atrial blood than in peripheral blood in this study, indicating that the CTCs shed from the primary liver lesion, entered the hepatic vein, and reached the right atrium. The pulmonary capillary bed acted as a filter to prevent the majority of CTCs from entering the peripheral circulation. Therefore, extrahepatic metastasis of HCC is most common in the lung3,19 but relatively rare in the limbs. For the other reason, CTCs are likely diluted because of the large volume of peripheral blood.20 Similarly, Wind et al detected and quantified CTCs in the peripheral and portal vein blood of patients with primary colon cancer during laparoscopy and open surgeries, which involved collection of peripheral and portal venous blood samples upon entering the abdominal cavity (T1), as well as stripping the tumor before (T2) and after (T3) tumor resection. The numbers of CTCs were significantly higher in portal venous blood samples than in peripheral blood samples at T1 (54% versus 7%), T2 (31% versus 4%), and T3 (26% versus 4%).10 Although the fate of these CTCs is unclear, the results of these studies have emphasized the critical role of CTCs in the spread of tumors.
It has been shown that a number of procedures, including surgery, radiofrequency ablation, and liver biopsy lead to an increase in CTCs in patients with HCC, likely because mechanical compression of the tumor during the procedure causes more CTCs to enter the blood circulation. Our study showed that the CTC numbers and positivity rates remained unchanged in peripheral and right atrial blood immediately after TACE. However, tumor necrosis and decomposition after TACE have been reported previously, and this facilitates the surviving tumor cells to shed and enter the blood circulation.21 A malignant phenotype such as epithelial-mesenchymal transition has also been reported due to hypoxia after TACE, and thus the number of CTCs in the blood circulation and tumor cell invasiveness would increase.22–24 These are also likely caused by activation of the coagulation system and surgical stress-induced immunosuppression after TACE.10 We were not sure whether shedding of CTCs during TACE could lead to metastasis or promoted local recurrence or disease progression previously. In this study, we found no significant difference in time to progression between patients with increased CTCs and those with unchanged or decreased CTCs in peripheral and right atrial blood samples. Because of the heterogeneity of tumor cells, especially CTCs, further investigations are required to determine whether CTCs shed during TACE have low invasiveness and fail to form metastases.
The method used in this study for identification of EpCAM-positive CTCs would likely miss cells that underwent epithelial-mesenchymal transition, but it has been reported that EpCAM-positive CTCs of HCC are tumor-initiating cells with stem cell features and are used to predict outcome.9,17
This was a non-randomized study, and there was a selection bias because of the small sample size and the different stages of the included patients with HCC. A larger sample size is required for further understanding of CTCs.
Conclusion
In summary, there were more CTCs in the right atrial blood than in the peripheral blood of patients with HCC. The positivity rates and numbers of CTCs in peripheral and right atrial blood did not change significantly after TACE, and TACE-induced shedding of tumor cells did not affect the time to progression of disease. Further understanding of the molecular biology of CTCs will increase our understanding of their role in tumor metastasis, and eliminating them may reduce rates of recurrence and metastasis.
Acknowledgments
This work was supported by two grants (81171432 and 81201170) from the National Natural Science Foundation of China.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 3.Lin SC, Shih SC, Kao CR, Chou SY. Transcatheter arterial embolization treatment in patients with hepatocellular carcinoma and risk of pulmonary metastasis. World J Gastroenterol. 2003;9(6):1208–1211. doi: 10.3748/wjg.v9.i6.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 5.Weitz J. Does intraoperative tumor-cell dissemination matter? J Am Coll Surg. 2007;205S(4):S31–S33. doi: 10.1016/j.jamcollsurg.2007.06.327. [DOI] [PubMed] [Google Scholar]
- 6.Park SY, Choi G, Park JS, Kim HJ, Ryuk JP, Choi WH. Influence of surgical manipulation and surgical modality on the molecular detection of circulating tumor cells from colorectal cancer. J Korean Surg Soc. 2012;82(6):356–364. doi: 10.4174/jkss.2012.82.6.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 8.Hauch S, Zimmermann S, Lankiewicz S, Zieglschmid V, Böcher O, Albert WH. The clinical significance of circulating tumour cells in breast cancer and colorectal cancer patients. Anticancer Res. 2007;27(3A):1337–1341. [PubMed] [Google Scholar]
- 9.Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69(14):5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 10.Wind J, Tuynman JB, Tibbe AG, et al. Circulating tumour cells during laparoscopic and open surgery for primary colonic cancer in portal and peripheral blood. Eur J Surg Oncol. 2009;35(9):942–950. doi: 10.1016/j.ejso.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Coumans FA, Doggen CJ, Attard G, de Bono JS, Terstappen LW. All circulating EpCAM+CK+CD45- objects predict overall survival in castration-resistant prostate cancer. Ann Oncol. 2010;21(9):1851–1857. doi: 10.1093/annonc/mdq030. [DOI] [PubMed] [Google Scholar]
- 12.Sergeant G, Roskams T, van Pelt J, Houtmeyers F, Aerts R, Topal B. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer. 2011;11(47) doi: 10.1186/1471-2407-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol. 2011;29(12):1556–1563. doi: 10.1200/JCO.2010.28.7045. [DOI] [PubMed] [Google Scholar]
- 14.Khan MS, Kirkwood A, Tsigani T, et al. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J Clin Oncol. 2013;31(3):365–372. doi: 10.1200/JCO.2012.44.2905. [DOI] [PubMed] [Google Scholar]
- 15.Liu MC, Shields PG, Warren RD, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27(31):5153–5159. doi: 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng R, Yao Y, Han M, et al. Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc Nephrol. 2008;19(2):380–387. doi: 10.1681/ASN.2006111194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun YF, Xu Y, Yang XR, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57(4):1458–1468. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- 18.Fan ZC, Yan J, Liu GD, et al. Real-time monitoring of rare circulating hepatocellular carcinoma cells in an orthotopic model by in vivo flow cytometry assesses resection on metastasis. Cancer Res. 2012;72(10):2683–2691. doi: 10.1158/0008-5472.CAN-11-3733. [DOI] [PubMed] [Google Scholar]
- 19.Liou TC, Shih SC, Kao CR, Chou SY, Lin SC, Wang HY. Pulmonary metastasis of hepatocellular-carcinoma associated with transarterial chemoembolization. J Hepatol. 1995;23(5):563–568. doi: 10.1016/0168-8278(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 20.Jiao LR, Apostolopoulos C, Jacob J, et al. Unique localization of circulating tumor cells in patients with hepatic metastases. J Clin Oncol. 2009;27(36):6160–6165. doi: 10.1200/JCO.2009.24.5837. [DOI] [PubMed] [Google Scholar]
- 21.Bonfil RD, Bustuoabad OD, Ruggiero RA, Meiss RP, Pasqualini CD. Tumor necrosis can facilitate the appearance of metastases. Clin Exp Metastastis. 1988;6(2):121–129. doi: 10.1007/BF01784843. [DOI] [PubMed] [Google Scholar]
- 22.Bonnomet A, Brysse A, Tachsidis A, et al. Epithelial-to-mesenchymal transitions and circulating tumor cells. J Mammary Gland Biol Neoplasia. 2010;15(2):261–273. doi: 10.1007/s10911-010-9174-0. [DOI] [PubMed] [Google Scholar]
- 23.Rofstad EK, Gaustad JV, Egeland TA, Mathiesen B, Galappathi K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int J Cancer. 2010;127(7):1535–1546. doi: 10.1002/ijc.25176. [DOI] [PubMed] [Google Scholar]
- 24.Azab AK, Hu JS, Quang P, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of endothelial to mesenchymal transition (EMT) features. Blood. 2011;118(21):218. doi: 10.1182/blood-2011-09-380410. [DOI] [PMC free article] [PubMed] [Google Scholar]