Abstract
Formin proteins were recognized as effectors of Rho GTPases some 15 years ago. They contribute to different cellular actin cytoskeleton structures by their ability to polymerize straight actin filaments at the barbed end. While not all formins necessarily interact with Rho GTPases, a subgroup of mammalian formins, termed Diaphanous-related formins or DRFs, were shown to be activated by small GTPases of the Rho superfamily. DRFs are autoinhibited in the resting state by an N- to C-terminal interaction that renders the central actin polymerization domain inactive. Upon the interaction with a GTP-bound Rho, Rac, or Cdc42 GTPase, the C-terminal autoregulation domain is displaced from its N-terminal recognition site and the formin becomes active to polymerize actin filaments. In this review we discuss the current knowledge on the structure, activation, and function of formin-GTPase interactions for the mammalian formin families Dia, Daam, FMNL, and FHOD. We describe both direct and indirect interactions of formins with GTPases, which lead to formin activation and cytoskeletal rearrangements. The multifaceted function of formins as effector proteins of Rho GTPases thus reflects the diversity of the actin cytoskeleton in cells.
Keywords: formin, Cdc42, mDia1, mDia2, mDia3, Daam1, Daam2, FMNL1, FMNL2, FMNL3, FHOD1, FHOD3, Rac, Rho, ROCK, stress fiber, filopodia, lamellipodium
Introduction
Many cellular functions such as migration, adhesion, and changes in cell shape are regulated by remodeling of the actin cytoskeleton. The dynamic actin structures play key roles during tissue regeneration, immune responses, embryonic development, and wound healing in eukaryotic organisms. Among a wide array of cytoskeletal structures, three main categories of actin filament assemblies can be distinguished that play fundamental roles in cell migration of multicellular organisms. First, there is the lamellipodium as a veil-like membrane protrusion at the leading edge of a cell, which contains a meshwork of branched actin filaments. Secondly, filopodia and microvilli appear as finger-like outgrowths of the plasma membrane that are stabilized by an actin filament bundle of varying thickness. Lastly, actin stress fibers occur in the cytoplasm of the cell that can form at least three different assembly categories such as dorsal stress fibers, actin arcs, and ventral stress fibers. These actin structures are dynamically regulated by small GTPases of the Rho family, which has been phenotypically shown more than two decades ago.1
The assembly of actin monomers into filamentous structures does not occur spontaneously but requires factors which help to overcome the kinetic barrier of nucleation.2 These actin nucleation factors can be classified into three groups: the Arp2/3 complex and its nucleation promoting factors, WH2 domain-containing nucleators, and formin proteins.3-7 Members of these three groups employ different mechanisms to accomplish the nucleation and elongation of actin filaments. The Arp2/3 complex binds to the sides of pre-existing actin filaments and generates branched actin networks. Spir, as an example for WH2 domain-containing proteins, nucleates the assembly of straight actin filaments by its four WH2 domains.8 The WH2 elements are lined up at defined distances to accomplish binding to one G-actin molecule each and doubled through Spir dimerization. Formins finally nucleate actin molecules from the barbed end and remain associated with the barbed end during filament elongation. In a landmark study, the formin mDia as the mammalian homolog of Drosophila Diaphanous was found as a downstream effector of Rho that selectively interacts with the triphosphate bound form of RhoA GTPase.9 In this review we discuss the current knowledge on the structure, function, and activation mechanism of formins as downstream effectors of Rho GTPases.
Formin Effector Proteins of Rho-GTPases
In mammals, there are 15 formins that group into eight different sub-families based on their sequences and domain architectures.5,10 A part of these formins were found to be autoinhibited, which gave rise to the classification as Diaphanous-related formins, or DRFs,11,12 named after the product of the Drosophila gene diaphanous.13 The intramolecular interaction between the C-terminal Diaphanous autoregulatory domain (DAD) and its N-terminal recognition domain, termed FH3 or DID, leads to the autoinhibition of DRF proteins.11,12,14-16 For some of the DRFs it is now well established that the autoinhibition is relieved upon the interaction with an active Rho GTPase, such as Rho, Rac, or Cdc42.
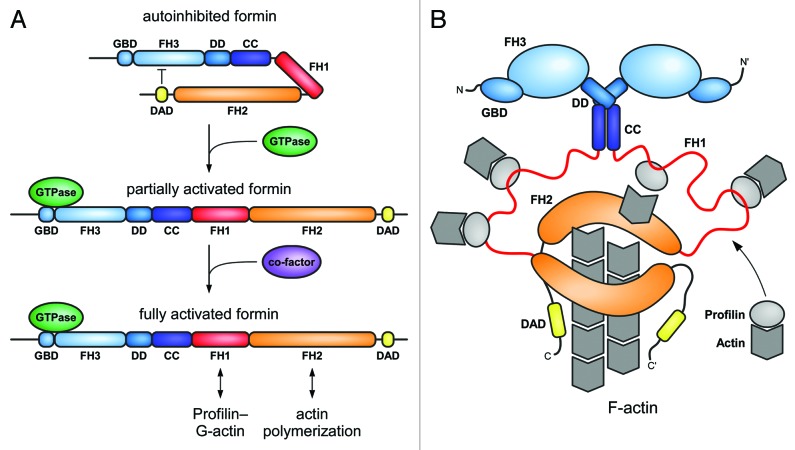
Formins are multi-domain proteins of typically more than 140 kDa in weight that are defined by the presence of a formin homology 2 (FH2) domain. The flanking regions of the FH2 domain vary considerably between individual formins, reflecting the different cellular functions and regulatory mechanisms of the actin polymerization factors. A molecular scheme of the domain architecture of human DRFs is shown in Figure 1. The FH2 domain binds directly to G- and F-actin and has been shown for many formins to nucleate actin molecules and elongate actin filaments.17 The approximately 400 amino acid long domain forms a doughnut-shaped head-to-tail dimer that remains associated with the fast-growing actin filament barbed end.18 The formin thereby prevents binding of capping proteins during the elongation procedure.19,20 In most formins a proline-rich FH1 domain that interacts with profilin for the recruitment of G-actin molecules precedes the FH2 domain, thus accelerating the actin polymerization rate of the formin.21,22 N-terminal to the FH1 domain is the FH3 domain, which is the least conserved module in the overall domain architecture and involved in the regulation of formin activity. In the resting state of the formin, the FH3 domain recognizes the C-terminal DAD generating an intramolecular, autoinhibited complex. In some formins, the FH3 domain is N-terminally merged with a GTPase-binding domain (GBD), whereas an additional dimerization element can be found C-terminal to the FH3 domain. As DAD and FH3 domains are on average about 800 residues apart, it is not clear if the DAD binds intramolecular or intermolecular in the dimer assembly. A cartoon displaying the conformational changes from the autoinhibited to the active state of the DRF is shown in Figure 2.
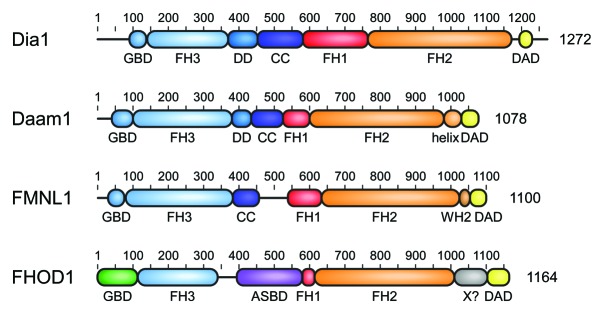
Figure 1. Domain architecture of mammalian Diaphanous-related formins. The multidomain proteins comprising more than 1000 amino acids contain a central proline rich FH1 domain followed by the actin polymerizing FH2 domain. DRFs contain in addition a C-terminal DAD autoregulation domain that interacts in the autoinhibited state with its N-terminal FH3 recognition domain. Additional dimerization elements DD (dimerization domain) and CC (coiled coil) contribute to the overall structure assembly of the formins. DRF activation occurs through interaction of the N-terminal GTPase-binding domain (GBD) with a Rho GTPase. Additional elements as the WH2 motif in FMNLs or the actin side-binding domain (ASBD) in FHOD1 contribute to the specificity of each DRF family.
Figure 2. Cartoon of the regulation of a Diaphanous related formin. (A) In the autoinhibited state, the C-terminal DAD interacts with the N-terminal FH3 domain. Binding to a GTP-bound Rho GTPase leads to relief of the autoinhibited state by a partial displacement of the DAD and formin activation. Possible co-factors as e.g., kinases for formin phosphorylation, additional interaction factors of the DAD (as described for Daam), or interactions with membrane compartments for proper orientation might be required for full activation of the formin. GBD, GTPase-binding domain, FH1/2/3, formin-homology domains, DD, dimerization domain, CC, coiled coil domain, DAD, Diaphanous-autoregulation domain. (B) Cartoon of the activated formin dimer. The proline-rich FH1 domain recruits profilin–actin complexes in close proximity to the FH2 domain. G-actin molecules are polymerized to F-actin by the dimeric FH2 domain.
The Diaphanous-related formins encompass the four mammalian families mDia, Daam, FMNL, and FHOD, that largely share a similar domain organization. Their interactions with Rho GTPases described today are listed in Table 1 and will be discussed in the following.
Table 1. Interactions between formins and Rho GTPases.
| Formin | binding domain | Rho GTPase | References |
|---|---|---|---|
| mammalia | |||
| mDia1, mDia2 | GBD-FH3 | RhoA | 9, 11, 131 |
| mDia1, mDia2 | GBD-FH3 | RhoB | 11, 70 |
| mDia1 | GBD-FH3 | RhoC | 11 |
| mDia2, mDia3 | GBD-FH3 | Cdc42 | 71, 131, 132 |
| mDia1, mDia2 | GBD-FH3 | Rac1,2 | 34, 69 |
| hDia2 | n.d. | RhoD | 133 |
| mDia2 | n.d. | Rif | 66 |
| Daam1 | N-terminus (aa 41–477) | RhoA, -B, -C | 33, 75 |
| Daam1 | N-terminus (aa 1–698) | Rac1 | 79 |
| Daam1 | n.d. | Cdc42 | 56 |
| FMNL1 | n.d. | Rac1 | 86, 88, 100 |
| FMNL1 | N-terminus (aa 1–450) | Cdc42 | 37 |
| FMNL2 | N-terminus (aa 27–276) | RhoC | 102 |
| FMNL2 | GBD-FH3 (aa 1–379) | Cdc42 | 95 |
| FMNL3 | n.d. | RhoC | 110 |
| FHOD1 | helical domain-FH1 (aa 422–717) | Rac1 | 113, 134 |
| INF2 | FH3 (aa 1–340) | Cdc42 | 135 |
| Drosophila melanogaster | |||
| Capu | N-terminus (aa 125–250) | Rho1 | 136, 137 |
| dmDia | N-terminus (aa 1–464) | Rho1 | 138 |
| dmDAAM | n.d. | RhoA | 76 |
| Dictyostelium discoideum | |||
| ForH (dDia2) | n.d. | Rac1A | 139 |
| Schizosaccharomyces pombe (fission yeast) | |||
| Cdc12p | N-terminus (aa 1–524) | Cdc15p | 140 |
| For3p | N-terminus (aa 149–488) | Rho3p | 141 |
| For3p | N-terminus (aa 149–488) | Cdc42p | 141, 142 |
| Saccharomyces cerevisiae (Baker’s yeast) | |||
| Bni1p | GBD-FH3 (aa 90–343) | Rho1p | 143, 144 |
| Bni1p | n.d. | Rho3p | 145, 146 |
| Bni1p | n.d. | Rho4p | 146, 147 |
| Bni1p | n.d. | Cdc42p | 148, 149 |
| Bnr1p | n.d. | Rho4p | 146, 147 |
n.d., not determined
Regulation of Diaphanous Related Formins
The resting, autoinhibited complex
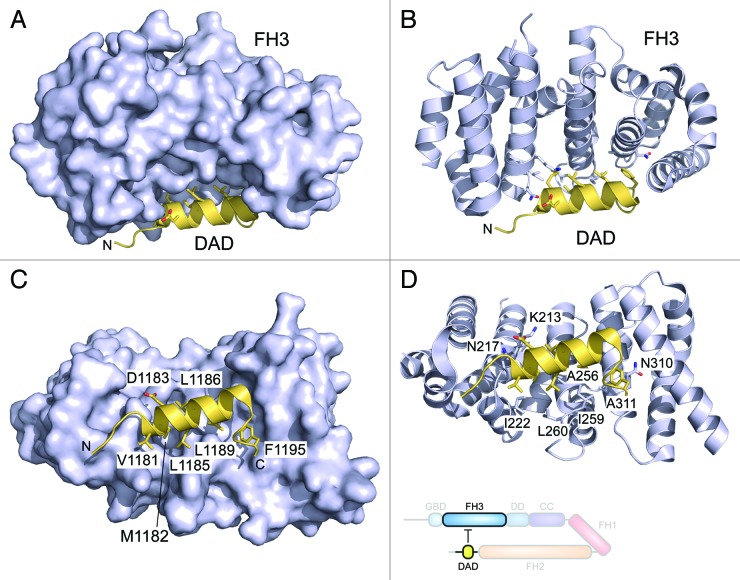
An autoinhibitory intramolecular interaction between the C- and N-terminal regions has been described for all mammalian DRFs,12,23-26 but only the autoregulatory interaction of mDia1 is known at structural detail to date.15,16 The C-terminal DAD of mDia1 is composed of an amphipathic helix with the central consensus motif MDxLLxxL followed by an unstructured, basic region of variable sequence and length (Fig. 3).12,27 While the DAD helix is essential for the binding to a hydrophobic surface patch at the concave side of the FH3 armadillo repeat structure,15,16 the basic region seems to be important for the affinity of the interaction.15,27 The interaction of the basic region with the FH3 domain has not been determined by structural means yet, but large, negatively charged patches were identified in mDia1 and FHOD1 adjacent to the MDxLLxxL recognition site.15,28 The basic region of the DAD likely interacts with an acidic groove located between the FH3 domain and the elongated α-helix at the C-terminus of the FH3 domain in mDia1, which connects the FH3 domain with the DD domain.29,30 In addition, the DAD has been shown to exhibit dual functions in autoinhibition and actin assembly as it enhances actin nucleation by recruiting actin monomers.31 This function is achieved without altering the filament elongation rate of the FH2 domain and independently of the FH1 domain.31
Figure 3. Structure of the autoinhibited FH3–DAD complex of mDia1. The helical DAD binds into the concave site of the FH3 domain armadillo repeat structure. (A) The DAD consensus motif MDxLL extends to VMDxLLxxLx5F in the binding interface to the FH3 domain. (B) All five armadillo repeats participate in the interaction of the N-terminal FH3 domain with the C-terminal autoregulatory domain. Mutation of the central A256 residue to aspartate on the last turn of the third armadillo repeats leads to relief of the autoinhibition and activation of the formin.15 Displayed is the structure 2F31 (ref. 16). A cartoon of the interaction scheme is shown below the atomistic model.
The activating complex
The interaction of Rho GTPases with formin effectors has been first described for mDia1 by the Narumiya laboratory.9,11 The specific binding of active RhoA•GTP to mDia1 was confirmed in the following years exhibiting dissociation constants in the nanomolar affinity range.32,33 The structural characterization of the activating complex of mDia1 with RhoC revealed that formin binding is mediated essentially through the switch regions of the GTPase, similar to that of other Rho effectors.32 While the switch I region (also named the “effector loop”) exclusively interacts with the GBD, the switch II region forms contacts with the GBD and the FH3 domain (Fig. 4). The GTPase interacts with the mDia1 GBD through a complementary hydrophobic surface, whereas mainly electrostatic driven interactions are formed with the first armadillo motif on the concave side of the FH3 domain.32,34 Since all formin-interacting residues in the switch regions of Rho GTPases are conserved, the specificity of the GTPase–formin interaction remains elusive. Two aromatic residues C-terminal to the α3 helix of the GTPase were shown to be involved in binding and contribute to the specificity of the interaction.34 Mutation of three interacting asparagine residues located in the first Armadillo repeat of mDia1 from 164NNN to the corresponding residues TSH found in mDia2 and mDia3 increased the binding affinity to Cdc42.34
Figure 4. Structure of the RhoC–mDia1 complex. (A) Assembly of the N-terminal dimer structure in mDia1. The GBD-FH3-DD subdomains are displayed as cartoon representation in blue shadings. The five armadillo repeats of the central FH3 domain are labeled. The last armadillo repeat leads into a bundle of four interweaved helices forming the dimerization domain. The second molecule of the dimer is shown as surface representation. (B) Complex structure between RhoC•GppNHp and mDia1. RhoC mostly interacts with hydrophobic residues in the GBD of the formin. The two switch regions of the GTPase whose conformation is changed upon the nucleotide change are highlighted. Displayed is the structure 1Z2C (ref. 32). A cartoon of the interaction schemes is shown below each atomistic model.
Activation through displacement
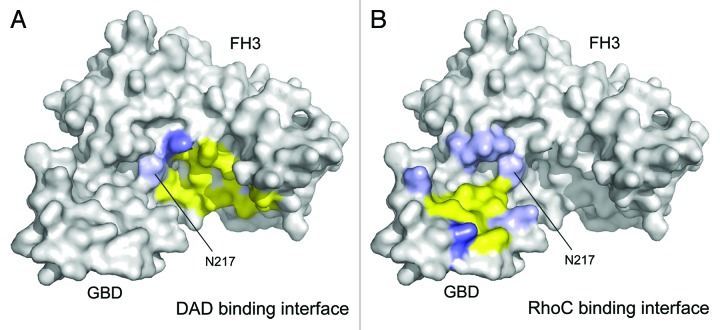
The mechanism how Rho GTPases displace the autoregulatory DAD domain from the FH3 domain is not fully understood today. Our molecular insights are currently based on the available complex structures of the N-terminus of mDia1 with either the DAD or active RhoC, respectively. Although the binding interfaces displayed on the surfaces of the FH3 domain for both, the DAD and the GTPase, only slightly overlap (Fig. 5), a simultaneous binding is excluded.15,16,32,35 Therefore, a two-step binding mechanism to abolish the autoinhibition has been suggested.15 The Rho GTPase might first bind in an initially weak complex to the GBD of the mDia1 formin, followed by the formation of a stronger interaction with the GBD-FH3 interface, which subsequently could result in the dislocation of the DAD from the FH3 domain.15
Figure 5. Display of the autoinhibitory and activating binding interfaces on mDia1 GBD-FH3 domains. (A) DAD binding interface on mDia1. Displayed are interacting residues derived from the mDia1 FH3–DAD complex structure 2F31 (ref. 16) and highlighted on the GBD-FH3 structure 1Z2C (ref. 32). Hydrogen bonds are formed between N217, N310, and Q352 (colored light blue) of the FH3 domain and the DAD. A salt bridge to D1183 of the DAD is mediated by K213 (colored blue) and hydrophobic interactions to the DAD motif are contributed by I222, K252, L253, A256, I259, L260, Q307, A311, T314, V351, and V355 (colored yellow) of the FH3 domain. (B) Display of the RhoC binding interface on mDia1 GBD-FH3 based on the evaluation provided in the 1Z2C structure.32 Polar interactions to the GTPase are formed by K100 and Q118 of the GBD and N164, N165, N166, and N217 of the FH3 domain. A salt bridge is mediated by K107 and hydrophobic interactions are formed by M90, M94, N95, L96, P103, L104, and M115. Only N217 on the second armadillo repeat of the FH3 domain is in the intersection of the binding interface between the inhibiting and activating complex.
However, there is increasing evidence that binding of a Rho GTPase is not sufficient for full activation of a DRF. Whereas constitutively active RhoA is able to completely displace a small DAD peptide from an N-terminal construct of mDia1,15,32 such active GTPase only partially relieved the autoinhibited complex between the dimeric N-terminus (GBD-FH3-DD or GBD-FH3-DD-CC) and the C-terminus (FH2-DAD).14,35 In vitro polymerization assays using near to full-length mDia1 protein exhibited only partial activation even in the presence of a three orders of magnitude higher excess of constitutively active RhoA.36 These observations led to the conclusion that additional formin family-specific regulation mechanisms might be required for full activation.36,37 For example, two studies demonstrated that phosphorylation events could interfere with the FH3-DAD interaction contributing to DRF regulation,38,39 and likewise association to membrane compartments is suggested to strengthen the active state. These additional activators are displayed as co-factors bridging the partially activated to the fully activated state of the DRFs shown in Figure 2A.
Formin inhibiting co-regulators often bind directly to the FH2 domain and thereby block the actin polymerization activity as it has been shown for the interaction of DIP-1 with mDia1.40 In contrast, activating co-regulators may prevent the autoinhibitory interaction between the C-terminal DAD and the N-terminal FH3 domain. For example, the competitive binding of Anillin to the N-terminus of mDia2 effects its DAD release resulting in formin activation.41 On the other hand, the activating Fli-1 protein has been reported to bind to the C-terminal DAD and to interfere with the FH3–DAD autoinhibition of mDia1.42 As another regulation mechanism, the autoinhibition of mDia2 is reversed by ROCK1-mediated posttranslational phosphorylation near the DAD domain, which leads to formin activation.39
Overall domain assembly
First studies addressed the assembly of full-length mDia1 in the autoinhibited state, yet the overall structure of the 140 kDa protein is not fully understood.29,30,36 Structural assemblies of the N- and C-terminal regions lacking the GBD and the FH1 domain reveal a tetrameric conformation29,30 that might result from crystal packing as full-length mDia1 is a dimer in solution.29,30,36 First insights into the structure of the dimeric, almost full-length mDia1 formin in the autoinhibited state were reported by Maiti et al. using electron microscopy.36 In this reconstruction the dimeric, fork-shaped N-terminus folds over the doughnut-shaped FH2 domain and inhibits F-actin elongation by steric hindrance of actin filament binding. Likewise, the mDia1 FH2 domain in the autoinhibited state of the crystal structure seems to be accessible for G-actin, but not F-actin due to steric hindrance.29 In the activated state, the elongated mDia1 molecule might not easily drop back into the autoinhibited conformation, due to large conformational transitions between the active and inactive conformations of the formin. It is indeed conceivable that the DAD interacts in the activated state with the F-actin filament preventing renewed autoinhibition, as interaction of the mDia1 DAD with actin has been described.31
Subcellular localization
Besides GTPase-mediated activation, the subcellular localization of the formin is also part of its regulation. This has been first described for the S. pombe formin Fus1,43 followed by mammalian formins FMNL1 and mDia1.37,44 As early as 2001, it has been assumed that the FH3 domain of mDia1 regulates its subcellular recruitment.45 Membrane recruitment of formins occurs either through the direct interaction with a prenylated, membrane-associated Rho GTPase or includes other, GTPase independent localization mechanisms. mDia1 and mDia2, but not mDia3, contain an additional membrane binding motif composed of polybasic clusters N-terminal to the GBD that is thought to sustain the interaction with phospholipids through electrostatic interactions.46,47 A Rho GTPase independent localization mechanism was confirmed for the N-terminus of Daam1 and the yeast formins Cdc12p and Bnr1p.48-50 Meanwhile, it has been shown that a region inside the FH3 domain of mDia1 mediates binding to the scaffolding protein IQGAP1.51 Furthermore, scaffolding proteins that contain membrane-anchored BAR domains represent crucial interfaces between signal transduction and actin cytoskeleton dynamics.52,53 Consequently, some formins were described to be recruited by FH1–SH3 interactions with BAR proteins to specific membranes, such as mDia by IRSp5354,55 or Daam by Cip4/Toca-1.56
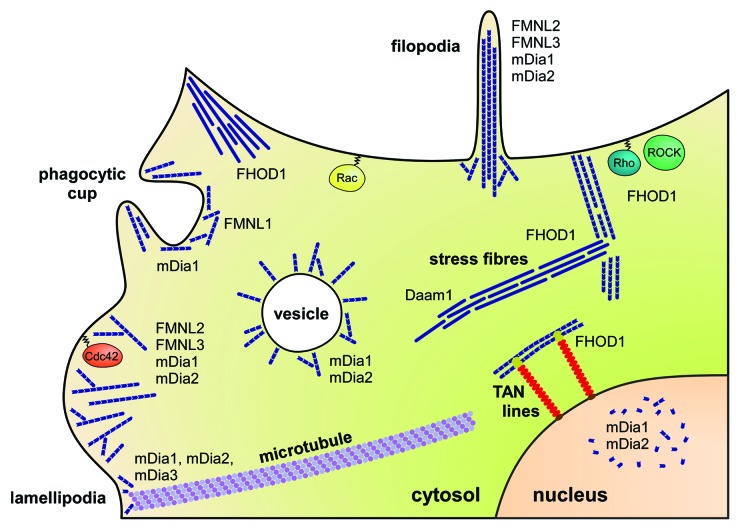
Overall, the combination of formin localization either by intrinsic targeting motifs or external recruitment factors and the interaction with activating factors of the Rho family GTPases determines the regulation of DRFs in cells. An overview about the expression profiles, specific function in actin remodeling, cellular functions, and binding interactions and localization of the DRFs is provided in Table 2. In the following we will describe the four mammalian DRF families with regard to their function and mechanism of activation by Rho GTPases as known to date. A model figure summarizing the function and localization of DRFs in cells is shown in Figure 6.
Table 2. Expression profiles, functions, and interactions of DRFs.
| mDia1/DRF1 | mDia2/DRF3 | mDia3/DRF2 | Daam1, Daam2 | |
|---|---|---|---|---|
| Expression | several cell types and tissues | several cell types and tissues | several cell types and tissues | Daam1: expressed in early developmental stages112 Daam2: high expression in neuronal cells,112 high expression in later development stages of central nervous system112 |
| Actin regulation | F-actin nucleation, elongation14,22 | F-actin nucleation, elongation17,22 | F-actin nucleation, elongation, bundling163,164 | F-actin polymerization33,167,168 |
| Actin structures | Stress fibers11,150 | Filopodia65-67,160,169 | Stress fibers71 | Filopodia79 |
| Function | Mechanotransduction59-61 Cell polarization62,151 Cell migration51,151 Phagocytosis51 Cell motility of T cells152 Axogenesis of neurons63 Endosome trafficking153 Exocrine vesicle secretion64 Microtubule stabilization154-156 Cell signaling, transcriptional regulation157 |
Cytokinesis41, 68,161 Nucleation of erythroblasts69 Cell movements during gastrulation162 Endosome trafficking70 |
Cell mitosis165 Mitotic chromosome alignment71,72 Endosome trafficking133 Apical-basal polarity of neuroepithelial cells166 |
Non-canonical Wnt/PCP pathway75 Cell development: Vertebrate gastrulation75,169 Tracheal development76 Axonal morphogenesis79 Asymmetric morphogenesis81 Neural-tube closure during embryogenesis82 Spinal cord development83 Heart morphogenesis80 |
|
Interactions, localization |
Rho GTPase RhoA9,158 Polybasic N-terminal clusters46 IQGAP151 IRSp5354,55 CLIP-170159 Nuclear localization172 |
Rho GTPase Rif47 Polybasic N-terminal clusters47 Abi167 Anillin41 Nuclear localization172 |
n.d. | Cip456 Toca-156 |
| FMNL1 | FMNL2, FMNL3 | FHOD1 | FHOD3 | |
| Expression | Macrophage-enriched86 Hematopoietic cells and tissues (thymus, spleen, peripheral blood leukocytes)98,99 Overexpressed in lymphoma cells98,99 |
Cells of nervous system, epithelium, lymphoid tissue94 Overexpressed in colorectal carcinoma101 |
high expression in several cell types112 mesenchymal cells119 |
low average expression levels, specific expression in skeletal and cardiac muscle112 highly expressed in heart123,170,171 |
| Actin regulation | F-actin polymerization, severing, bundling106 | F-actin polymerization, bundling95,109 | F-actin bundling, capping115 | F-actin acceleration123 |
| Actin structures | Lamellipodium, filopodia86 | Lamellipodium, filopodia94,95,107 | Stress fibers113 enriched in transversal actin arcs, mature stress fibers116 |
Stress fibers124,170 |
| Function | Cell proliferation100 Cell adhesion, growth, and migration86,100 Centrosome polarity88 Cytotoxic T cell activation88 Recognition of the antigen presenting cell88 Phagocytosis37,89 Regulation of podosomes90 Golgi complex stabilization91 Non-apoptotic membrane blebbing92 |
Cell motility and cell migration94,95,97,101-103,110 Cell proliferation101,105 Endothelial cell elongation during angiogenic morphogenesis96 |
Cell division118 Cell migration119 |
Regulation of sarcomere organization122 Heart developement125 Myofibril maintenance123 |
|
Interactions, localization |
Rho GTPase Cdc4237 srGAP2111 |
Rho GTPase Cdc4295 N-terminal myristoylation95 |
Recruitment by Rho GTPase Rac1113 Phosphorylation by ROCK38,126 Association with Nesprin-2-giant173 |
Phosphorylation by CK2123 |
n.d., not determined
Figure 6. Model of Diaphanous-related formin function and localization in cells. Shown are formins involved in filopodia and lamellipodia generation, the stabilization of actin stress fibers, interactions with microtubuli cytoskeletal structures or transmembrane actin-associated (TAN) lines, and vesicle formation and trafficking. The activation by Rho family GTPases as known today is indicated. Of note, FHOD3 is highly expressed in cardiac and skeletal muscle tissue and not displayed in this model scheme.
mDia
The mammalian Dia formin family with the three isoforms mDia1, mDia2, and mDia3 is a major effector of Rho GTPases.9,11 mDia proteins induce actin filaments upon activation and cooperatively work with ROCK (Rho-associated coiled-coil kinase) to regulate the formation of actin stress fibers in cultured cells. mDia1 is the mouse ortholog of human Dia1 or DRF1 that shares 90.3% sequence identity to its human counterpart. mDia1 binds to the barbed ends of actin filaments and promotes strong polymerization activity, as seen by the processive movement of mDia molecules at the filament barbed ends in living cells.57 In a recent study Breitsprecher and colleagues used single-molecule fluorescence microscopy techniques to image actin filament polymerization in vitro by differentially labeling the adenomatous polyposis coli (APC) and the FH1-FH2-DAD domain assembly of mDia1.58 Upon filament polymerization, the complexes separated as visualized in the fluorescence images, with mDia1 moving processively on growing barbed ends while APC remained at the site of nucleation.
The best studied isoform of the Dia family is mDia1, which is involved in a variety of cellular processes including mechanotransduction,59-61 cell polarization and migration of certain cell lines,51,62 axonal outgrowth in primary cell cultures of cerebellar granule neurons,63 and exocrine vesicle secretion in the apical membrane.64 mDia2 instead is involved in filopodia formation,65-67 and cytokinesis in cultured cells.68 mDia2 was also ascribed a function in the formation of the contractile ring during asymmetric cell division of erythroblasts and endosome trafficking in fibroblasts.69,70 mDia3 finally was shown to be required for chromosome alignment in HeLa cells,71 potentially by phosphorylation and regulation through the kinase Aurora B.72 A comprehensive overview of mDia function and the phenotypes resulting from mDia1 and mDia3 knockout in mice was recently provided by the Narumiya laboratory.73 Diseases associated with the roles of formins in cell division, migration, immunity, and microvesicle formation imply various types of cancer, deafness, and mental retardation.74 The misregulation of formins is suggested to loosen adhesion of cancer cells, migration and ultimately invasion.74
The majority of biochemical and structural data to this day results from mDia1-Rho GTPase interactions. The FH3 domain (also called DID for “Diaphanous inhibitory domain”), encompassing amino acids 133–377 in mDia1, is located in the N-terminal regulatory part of DRF proteins. Up to now, FH3 domain structures of mDia116,32,35 and FHOD128 have been determined. The FH3 domain is exclusively helical and composed of five armadillo (ARM) repeats (Fig. 4).28,35 ARM repeats consist of three α helices arranged in a rectangular triangle, with each repeat rotated against another by 15–20° forming an elongated, banana-shaped structure with a convex and concave site of the superhelical domain fold. High sequence variations make a prediction of the helical assembly of other DRFs based on the structures of mDia1 or FHOD1 difficult and hence the prediction of interaction sites speculative. In mDia1, the extended helix of the last ARM motif leads into the following dimerization domain (DD, aa 377–452) that consists of three α-helices with two helices of each dimer chain forming a four helix bundle (Fig. 4A).32,35 A helical region that displays sequence features of a coiled coil structure is located C-terminal to the dimerization domain (CC, aa 452–570). It has been demonstrated that the DD of mDia1 is sufficient for dimerization and that the N-terminus of mDia1 represents a constitutive dimer which might only dissociate through unfolding.35 Not all DRFs necessarily contain this second dimerization element in addition to the C-terminal head-to-tail arrangement of the FH2 domain, as the N-terminus of FHOD1 was shown to contain a flexible linker region instead (Fig. 1).28 N-terminal to the FH3 domain of mDia1 is the GTPase-binding domain (GBD, aa 73–131). This short segment is constructed of three triangularly arranged helices that are connected by a short linker to the FH3 domain (Fig. 4).32,34 In the absence of the activating Rho GTPase, the GBD is presumably loosely folded but moves freely in solution,35 representing therefore a subdomain rather than an independent structural unit.
Daam
The protein Dishevelled-associated activator of morphogenesis 1 (Daam1) was identified as interaction factor of Dishevelled (Dvl), which mediates the non-canonical Wnt/PCP (planar cell polarity) signaling pathway.75 Early functional studies of Daam1 in lower species suggested an essential role in Xenopus gastrulation and Drosophila trachea formation.75,76 Daam1 localizes to the plasma membrane and cytoplasmic vesicles, and this pattern is tightly regulated by Wnt and Dvl.75,77-79 Recent studies in mammalian systems underline the role of the two Daam proteins, Daam1 and Daam2, in cell development.
Daam1 is highly expressed in developing murine organs, including the heart. Consistent with this expression pattern, Daam1-deficient mice show cardiac defects, including ventricular noncompaction, double outlet right ventricles, and ventricular septal defects. These animals die during embryonic development or at early postnatal days.80 The role of Daam1 in the nervous system has been analyzed in zebrafish.81 Here, Daam1 is enriched in the dorsal part of the asymmetric habenular neuropil. Loss of Daam1 in zebrafish embryos resulted in disturbed asymmetry and reduced neuropil formation. This can be explained by the finding that Daam1 regulates outgrowth of neuronal axons and dendrites.81 Another Daam1-dependent process is the closure of the neural tube during embryogenesis. This process involves a regulating cadherin, Dvl, Daam1, and the PDZ-RhoGEF to upregulate Rho kinase.82 Cellular forces of the ROCK stimulated actomyosin-dependent contraction promote the polarized bending of the neural plate.
Like Daam1, Daam2 seems to be involved in developmental processes regulated by Wnt-signaling. Two studies describe that Daam2 is important for asymmetric cell behavior. At first, loss-of-function studies revealed that Daam2 is required for dorsal progenitor identities and canonical Wnt signaling by its interaction with Dvl3, which modulates Wnt signal transduction during spinal cord development.83 In addition, initiation of a leftward tilt in gut morphogenesis is likewise a critical aspect of asymmetric cell behavior that was found to be modulated by Daam2. Effectors of the transcription factor Pitx2 responsible for the transfer of left-right information from early gastrulation to morphogenesis were found to mediate Wnt signaling to activate Daam2.84
The activation mechanisms of the two Daam proteins remain not well understood on a molecular level. The Daam proteins are autoinhibited by a C-terminal DAD, similarly as found in Dia formins, but activation is achieved through the interaction of the DAD with the PDZ domain of Dishevelled, releasing the autoinhibited state.26 As active Daam1 was reported to lead to RhoA activation, a positive feedback loop that amplifies the levels of active GTPase has been proposed.26,75 For Daam1 it has been speculated that either a RhoGEF is recruited to active Daam1 to increase the pool of GTP-loaded RhoA or that a RhoGAP might be silenced such that less RhoA-GTP is hydrolyzed. Yet, another study described that Daam1 does neither regulate cytoskeletal organization through RhoA nor Rac1 or Cdc42.80 Active Daam1 however is found in nonadhesive regions of cells bridging fibronectin-coated adhesive strips where it associated with actin networks containing myosin II and the cross-linker filamin A.85
FMNL
The “Formin-like” (FMNL) protein family represents the third family of mammalian DRFs and includes the members FMNL1 (alternative name FRL1), FMNL2 (also named FRL3), and FMNL3 (also named FRL2) with a total of eight splicing isoforms. The multidomain FMNL formins have been initially described as formin-related genes in leukocytes (FRL)86 and share about 23% sequence identity with Dia1.5 The functional roles of FMNL formins seem to be diverse and are only partly defined to date. The different members of the FMNL family appear to regulate similar processes during development based on overlapping expression patterns, but also seem to have independent functions based on distinct tissue expression.87 The macrophage-enriched FMNL1 is involved in the regulation of cell adhesion, growth and migration through the reorganization of the lamellipodial and filopodial actin cytoskeleton.86 In T lymphocytes, this formin has been identified as essential regulator of centrosome polarity and exhibits crucial functions in the activation of cytotoxic T cells.88 FMNL1 proteins display unique patterns circular around centrosomes and localize at the tip of filopodial structures that have been developed during the recognition of the antigen presenting cell.88 It has been furthermore described that FMNL1 is recruited to the phagocytotic cup and involved in the Fcγ receptor-mediated phagocytosis.37 FMNL1 also accumulates at the pseudopods of macrophages and regulates macrophage coiling phagocytosis since its depletion reduces the uptake of invading borrelia.89 Recently published studies have shown that FMNL1 may be involved in the regulation of podosomes and the structural stabilization of the Golgi complex.90,91 Activated FMNL1 might cause polarized non-apoptotic membrane blebbing independent of ROCK or Src activity.92 Additionally, first hints from proteomic screens point to a potential involvement of FMNL1 in calcium-dependent membrane plasticity.93
The second member of the FMNL formin family, FMNL2, generates protruding actin structures at the leading edge of a migrating cell, the lamellipodium and filopodia.94,95 FMNL3 in contrast regulates endothelial cell elongation during angiogenic morphogenesis by microtubule alignment96 and seems to be involved in cell motility.97 Both, FMNL2 and FMNL3 are predominantly associated with the plasma membrane and this localization depends on their N-terminal myristoylation (Table 2).95
The misregulation of FMNL formins has been implicated with severe diseases. FMNL1 is enriched in hematopoietic cells and tissues such as thymus, spleen and peripheral blood leukocytes and is overexpressed in malignant lymphomas of patients with chronic lymphocytic leukemia as well as in T cells from patients with malignant non-Hodgkin lymphoma.98,99 The depletion of FMNL1 reduces cell proliferation as well as migration of leukemia cells and tumor growth.100 The fmnl2 gene is expressed in many tissues with the highest expression level found in cells of the nervous system, the gastrointestinal as well as the breast epithelium and the lymphoid tissue.94 Overexpression of FMNL2 in cells of colorectal carcinoma causes a more aggressive tumor behavior associated with increased proliferation, motility, invasion, and metastasis.101 In breast melanoma cells, FMNL2 is likewise involved in their invasive cell migration.102 Furthermore, FMNL2 promotes the epithelial mesenchymal transition, which is associated with the loss of cell adhesions and enhanced migration ability.103 Besides a link to cancer progression, FMNL2 could also be involved in diseases of the nervous system. A sporadic 3.9 Mb deletion in gene locus 2q23.3 of an infant caused severe mental retardation, early onset of puberty, reduced stature and hand anomalies.104 This locus encompasses five genes including fmnl2. As a possible reason for these symptoms a morphological change of the dendritic spines based on disturbances of the actin cytoskeleton has been proposed.104 Like the other two FMNL proteins, also FMNL3 seems to participate in the proliferation of malignant tumor cells.105
Actin filament polymerization and bundling activity of FMNL formins was reported by several groups, while FMNLs also might sever actin filaments.25,95,106-108 It has been shown for FMNL1 that the dimeric FH2 domain associates with the barbed end of actin filaments, processively elongates them in the presence of profilin, reduces the elongation rate in the absence of profilin, and prevents binding of capping proteins.106 An FH1-FH2 protein construct of FMNL3 induces filopodia formation and accumulates at their tips, while the corresponding construct of FMNL1 does not.107 The region C-terminal to the FH2 domain accelerates the actin assembly activity of FMNL3 and this activity is mediated by an actin monomer and F-actin barbed end binding WASP homology 2 (WH2)-like sequence.109 FMNL2 represents an actin filament elongation factor promoting cell migration rather than a nucleation factor.95
FMNL1, -2 and -3 are autoinhibited by interactions between the N- and C-termini25,37,95 and also hetero-dimeric complex formation between N- and C-terminal domains of FMNL2 and FMNL3 appeared to be possible.25 The specificity of Rho GTPases for individual FMNL formins is still under debate as contradictory studies were reported in the past (Table 1). A nucleotide-independent binding mode of the N-terminus of FMNL1 to Rac1 has been described,86 as well as the nucleotide-dependent interaction with active GTPases Cdc42,37,95 Rac1,100 and Rho.88,102,110 RhoC, but not RhoA, was shown to specifically interact with FMNL3, which promotes polarized migration through FMNL3 by restricting lamellipodial broadening.110 In addition, Cdc42-induced recruitment of FMNL1 and FMNL2 to the plasma membrane has been demonstrated.37,95 FMNL1 might interact with the iBAR domain-containing protein srGAP2 and co-localizes with it at the phagocytotic cup of macrophages.111 srGAP2 is a Rac1-specific RhoGAP and might represent an inhibition mechanisms of formin activity.
FHOD
The mammalian FHOD family comprises two proteins, FHOD1 and FHOD3 (the name FHOD2 has been misleadingly assigned to a protein of a different formin family and is thus discontinued). Both FHOD1 and FHOD3 show considerably different expression profiles in cells. In a recent study, 22 different human cell and tissue types were analyzed by quantitative real-time PCR, showing on average highest expression levels for FHOD1 among all 15 formins.112 In contrast, FHOD3 was lowest on average but with a very specific expression profile in cardiac and skeletal muscle, outbalancing here its sister homolog FHOD1.
Expression of active FHOD1 leads to a phenotype of F-actin stress fibers.113 The protein contains an N-terminal F-actin side binding element and localizes to cellular stress-fiber structures.114 Yet FHOD1 is thought to poorly elongate actin filaments but rather acts as an actin bundling factor with capping activity toward the filament barbed end.115 FHOD1 thus stabilizes actin filaments by protecting barbed ends from depolymerization with its dimeric FH2 domain, whereas the region N-terminal to the FH1 domain mediates F-actin bundling by binding to the sides of adjacent F-actin filaments. The protein moves with the actin retrograde flow and enriches in actin arcs and more mature stress fibers,115 rather than staying at the leading edge and expanding cell migration as Dia and FMNL. FHOD1 stimulates the spatio-temporal organization of transversal arcs that are formed by fusion of short antiparallel actin filaments, which is critical for stress fiber maturation.116 The GBD-FH3 domains of FHOD1 are responsible for stress fiber association and co-localization with Myosin.116
FHOD1 was recently described to be recruited to integrin clusters, which results in actin assembly.117 Integrin binding to matrix ligands provides critical signals for cell growth or differentiation. Targeting of FHOD1 to the integrin sites depends on the direct interaction with Src family kinases and is upstream of the activation by Rho kinase. Functional studies showed that retention of the mitotic kinase Aurora-B at the cortex depends on FHOD1, which becomes phosphorylated by the kinase.118 Modulation of FHOD1 activity by Aurora-B thereby contributes to daughter cell spreading after mitosis. FHOD1 also appeared to be markedly upregulated upon epithelial-to-mesenchymal transition in cancer cells contributing to cell migration and invasion.119 FHOD1 in conjunction with Rac1 was furthermore described as novel regulators of vaccinia actin tail formation.120 Vaccinia virus thus integrates the activity of the N-WASP-ARP2/3 and Rac1-FHOD1 pathways to display robust actin-based motility. FHOD1 and Arp2/3 were also shown to cooperate in Salmonella invasion where both factors occupy distinct microdomains at the invasion site and control distinct aspects of membrane protrusion formation.121
FHOD3 was first described to regulate sarcomere organization in cardiomyocytes where it localizes to thin actin filaments in a striated pattern.122 Its depletion by siRNA resulted in a marked reduction in filamentous actin and disruption of the sarcomeric structure. A splice variant of FHOD3 specific for striated muscles promotes the polymerization of actin filaments in cardiomyocytes and downregulation of this isoform severely affects myofibril integrity.123 This specific FHOD3 variant is phosphorylated by casein kinase 2 (CK2), which is required for proper targeting of muscle FHOD3 to the myofibrils in embryonic cardiomyocytes being in the mature state restricted to the Z-disc proper in the adult heart.124 Knockout of fhod3 in mice resulted in disturbed myofibril maturation and embryonic lethality due to problems in heart development.125 Together, these studies demonstrate the different functions of FHOD1 and FHOD3 in cells, which is reflected by their different expression profiles.
Although FHOD is considered a DRF according to its domain architecture (Fig. 1), its interaction with GTPases and mechanism of activation remains still elusive. FHOD1 is autoinhibited by a C-terminal DAD24 and truncation of the C-terminus leads to an active phenotype.24,113 Structural studies showed that FHOD1 contains an N-terminal GTPase-binding domain composed of an ubiquitin superfold, yet a direct interaction of the GBD or the GBD-FH3 unit to Rac1 could not be confirmed.28 Instead FHOD1 was shown to become phosphorylated at three specific sites within the C-terminal DAD by the Rho effector kinase ROCK.38,126 This interaction places FHOD1 as a downstream effector of Rho, which is in line with the phenotype attributed to this GTPase and the observed function of active FHOD1 in stress fiber formation (Fig. 6). The ubiquitin superfold found in FHOD1 is known as GTPase-binding domain from Ras family effectors such as Raf, PI3 kinases or RalGDS.28 A similar N-terminal domain structure is found as F0 domain in Kindlin and Talin,127,128 moving the FHOD domain assembly close to integrin co-factors. The Dictyostelium discoideum protein Formin C (ForC) as the closest homolog to mammalian FHOD1 contains a similar N-terminal ubiquitin domain structure, whose positively charged surface area mediates localization to specific membrane patches.129 Likewise, ForC binds to actin filaments and crosslinks them into loose bundles of mixed polarity.130 The association of FHOD1 with the growing actin filament as bundling and capping factor however makes a stable interaction with a GTPase unlikely but fits well to the activation mechanism via phosphorylation, e.g., by ROCK. The possible interaction partners for recruitment and activation of FHOD1 and FHOD3 are not yet clear.
Conclusions
The analysis of the interaction between Rho GTPases and formin effector proteins is only at the beginning, as the specificity (or promiscuity) of these GTPases for effectors of the actin cytoskeleton is not yet well understood. For some formin families such as Dia and FMNL, all three major Rho GTPase subfamilies Rho, Cdc42, and Rac have been reported to interact with these effectors (Table 1). However, as different biochemical techniques were employed for the analyses of these interactions, some of these results are difficult to compare. It is supposed that GTPase activation by guanine nucleotide exchange factors occurs at lipid membranes. The Rho, Cdc42, and Rac subfamilies all contain C-terminal prenylation motifs as either farnesylation or geranylgeranylation that target these signaling proteins to membrane compartments. The targeting of formins to specific cellular membranes is therefore a major determinant of function. This mechanistic condition correlates well with the observed phenotypes of some DRFs, as e.g., for the generation of filopodia and lamellipodia at the leading edge of a cell (Fig. 6). The spatial positioning of these cytoskeletal membrane protrusions appears secured by the association and activation of the complex at membranes. Other formins such as FHOD instead are activated through phosphorylation, which only indirectly requires Rho as the upstream factor of ROCK kinase. Likewise the combined interaction of a Rho GTPase and a DAD binding factor as in Daam might be required for full activation of the formin. This diversity requires the individual characterization of each formin and the consideration of multiple co-factors. Future functional and structural studies are therefore required to shed light on the versatile aspects of the modulation of the actin cytoskeleton by formins as downstream effectors of Rho GTPases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft to M.G. (GE-976/7–2). S.K. is a member of the International Max Planck Research School in Chemical Biology, Dortmund.
Glossary
Abbreviations:
- FH2
formin homology 2
- FH3
formin homology 3
- GAP
GTPase activating protein
- GBD
GTPase-binding domain
- GEF
guanine nucleotide-exchange factor
- Daam
Disheveled-associated activator of morphogenesis
- Dia
Diaphanous-related formin
- FHOD
FH1/FH2 domain-containing protein
- FMNL
Formin-like protein
References
- 1.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 2.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–77. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 3.Rottner K, Hänisch J, Campellone KG. WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 2010;20:650–61. doi: 10.1016/j.tcb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Renault L, Bugyi B, Carlier MF. Spire and Cordon-bleu: multifunctional regulators of actin dynamics. Trends Cell Biol. 2008;18:494–504. doi: 10.1016/j.tcb.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Schönichen A, Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim Biophys Acta. 2010;1803:152–63. doi: 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–51. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerkhoff E. Cellular functions of the Spir actin-nucleation factors. Trends Cell Biol. 2006;16:477–83. doi: 10.1016/j.tcb.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich S, Weiß S, Pleiser S, Kerkhoff E. Structural and functional insights into the Spir/formin actin nucleator complex. Biol Chem. 2013;394:1649–60. doi: 10.1515/hsz-2013-0176. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–56. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitsprecher D, Goode BL. Formins at a glance. J Cell Sci. 2013;126:1–7. doi: 10.1242/jcs.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe N, Kato T, Fujita A, Ishizaki T, Narumiya S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–43. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- 12.Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276:2824–30. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- 13.Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–77. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–40. doi: 10.1016/S0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- 15.Lammers M, Rose R, Scrima A, Wittinghofer A. The regulation of mDia1 by autoinhibition and its release by Rho*GTP. EMBO J. 2005;24:4176–87. doi: 10.1038/sj.emboj.7600879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nezami AG, Poy F, Eck MJ. Structure of the autoinhibitory switch in formin mDia1. Structure. 2006;14:257–63. doi: 10.1016/j.str.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Moseley JB, Sagot I, Poy F, Pellman D, Goode BL, Eck MJ. Crystal structures of a Formin Homology-2 domain reveal a tethered dimer architecture. Cell. 2004;116:711–23. doi: 10.1016/S0092-8674(04)00210-7. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond SH, Evangelista M, Boone C, Yang C, Dar AC, Sicheri F, Forkey J, Pring M. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr Biol. 2003;13:1820–3. doi: 10.1016/j.cub.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 20.Moseley JB, Sagot I, Manning AL, Xu Y, Eck MJ, Pellman D, Goode BL. A conserved mechanism for Bni1- and mDia1-induced actin assembly and dual regulation of Bni1 by Bud6 and profilin. Mol Biol Cell. 2004;15:896–907. doi: 10.1091/mbc.E03-08-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119:419–29. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 22.Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–35. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Higgs HN. Dissecting requirements for auto-inhibition of actin nucleation by the formin, mDia1. J Biol Chem. 2005;280:6986–92. doi: 10.1074/jbc.M411605200. [DOI] [PubMed] [Google Scholar]
- 24.Schönichen A, Alexander M, Gasteier JE, Cuesta FE, Fackler OT, Geyer M. Biochemical characterization of the diaphanous autoregulatory interaction in the formin homology protein FHOD1. J Biol Chem. 2006;281:5084–93. doi: 10.1074/jbc.M509226200. [DOI] [PubMed] [Google Scholar]
- 25.Vaillant DC, Copeland SJ, Davis C, Thurston SF, Abdennur N, Copeland JW. Interaction of the N- and C-terminal autoregulatory domains of FRL2 does not inhibit FRL2 activity. J Biol Chem. 2008;283:33750–62. doi: 10.1074/jbc.M803156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105:210–5. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallar BJ, Stropich BN, Schoenherr JA, Holman HA, Kitchen SM, Alberts AS. The basic region of the diaphanous-autoregulatory domain (DAD) is required for autoregulatory interactions with the diaphanous-related formin inhibitory domain. J Biol Chem. 2006;281:4300–7. doi: 10.1074/jbc.M510277200. [DOI] [PubMed] [Google Scholar]
- 28.Schulte A, Stolp B, Schönichen A, Pylypenko O, Rak A, Fackler OT, Geyer M. The human formin FHOD1 contains a bipartite structure of FH3 and GTPase-binding domains required for activation. Structure. 2008;16:1313–23. doi: 10.1016/j.str.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Otomo T, Tomchick DR, Otomo C, Machius M, Rosen MK. Crystal structure of the Formin mDia1 in autoinhibited conformation. PLoS One. 2010;5:e12896. doi: 10.1371/journal.pone.0012896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nezami A, Poy F, Toms A, Zheng W, Eck MJ. Crystal structure of a complex between amino and carboxy terminal fragments of mDia1: insights into autoinhibition of diaphanous-related formins. PLoS One. 2010;5:e12992. doi: 10.1371/journal.pone.0012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould CJ, Maiti S, Michelot A, Graziano BR, Blanchoin L, Goode BL. The formin DAD domain plays dual roles in autoinhibition and actin nucleation. Curr Biol. 2011;21:384–90. doi: 10.1016/j.cub.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose R, Weyand M, Lammers M, Ishizaki T, Ahmadian MR, Wittinghofer A. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature. 2005;435:513–8. doi: 10.1038/nature03604. [DOI] [PubMed] [Google Scholar]
- 33.Higashi T, Ikeda T, Shirakawa R, Kondo H, Kawato M, Horiguchi M, Okuda T, Okawa K, Fukai S, Nureki O, et al. Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets. J Biol Chem. 2008;283:8746–55. doi: 10.1074/jbc.M707839200. [DOI] [PubMed] [Google Scholar]
- 34.Lammers M, Meyer S, Kühlmann D, Wittinghofer A. Specificity of interactions between mDia isoforms and Rho proteins. J Biol Chem. 2008;283:35236–46. doi: 10.1074/jbc.M805634200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18:273–81. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Maiti S, Michelot A, Gould C, Blanchoin L, Sokolova O, Goode BL. Structure and activity of full-length formin mDia1. Cytoskeleton (Hoboken) 2012;69:393–405. doi: 10.1002/cm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seth A, Otomo C, Rosen MK. Autoinhibition regulates cellular localization and actin assembly activity of the diaphanous-related formins FRLalpha and mDia1. J Cell Biol. 2006;174:701–13. doi: 10.1083/jcb.200605006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeya R, Taniguchi K, Narumiya S, Sumimoto H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J. 2008;27:618–28. doi: 10.1038/emboj.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staus DP, Taylor JM, Mack CP. Enhancement of mDia2 activity by Rho-kinase-dependent phosphorylation of the diaphanous autoregulatory domain. Biochem J. 2011;439:57–65. doi: 10.1042/BJ20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenmann KM, Harris ES, Kitchen SM, Holman HA, Higgs HN, Alberts AS. Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Curr Biol. 2007;17:579–91. doi: 10.1016/j.cub.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe S, Okawa K, Miki T, Sakamoto S, Morinaga T, Segawa K, Arakawa T, Kinoshita M, Ishizaki T, Narumiya S. Rho and anillin-dependent control of mDia2 localization and function in cytokinesis. Mol Biol Cell. 2010;21:3193–204. doi: 10.1091/mbc.E10-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higashi T, Ikeda T, Murakami T, Shirakawa R, Kawato M, Okawa K, Furuse M, Kimura T, Kita T, Horiuchi H. Flightless-I (Fli-I) regulates the actin assembly activity of diaphanous-related formins (DRFs) Daam1 and mDia1 in cooperation with active Rho GTPase. J Biol Chem. 2010;285:16231–8. doi: 10.1074/jbc.M109.079236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen J, Nielsen O, Egel R, Hagan IM. FH3, a domain found in formins, targets the fission yeast formin Fus1 to the projection tip during conjugation. J Cell Biol. 1998;141:1217–28. doi: 10.1083/jcb.141.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Copeland SJ, Green BJ, Burchat S, Papalia GA, Banner D, Copeland JW. The diaphanous inhibitory domain/diaphanous autoregulatory domain interaction is able to mediate heterodimerization between mDia1 and mDia2. J Biol Chem. 2007;282:30120–30. doi: 10.1074/jbc.M703834200. [DOI] [PubMed] [Google Scholar]
- 45.Kato T, Watanabe N, Morishima Y, Fujita A, Ishizaki T, Narumiya S. Localization of a mammalian homolog of diaphanous, mDia1, to the mitotic spindle in HeLa cells. J Cell Sci. 2001;114:775–84. doi: 10.1242/jcs.114.4.775. [DOI] [PubMed] [Google Scholar]
- 46.Ramalingam N, Zhao H, Breitsprecher D, Lappalainen P, Faix J, Schleicher M. Phospholipids regulate localization and activity of mDia1 formin. Eur J Cell Biol. 2010;89:723–32. doi: 10.1016/j.ejcb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Gorelik R, Yang C, Kameswaran V, Dominguez R, Svitkina T. Mechanisms of plasma membrane targeting of formin mDia2 through its amino terminal domains. Mol Biol Cell. 2011;22:189–201. doi: 10.1091/mbc.E10-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ang SF, Zhao ZS, Lim L, Manser E. DAAM1 is a formin required for centrosome re-orientation during cell migration. PLoS One. 2010;5:e13064. doi: 10.1371/journal.pone.0013064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yonetani A, Lustig RJ, Moseley JB, Takeda T, Goode BL, Chang F. Regulation and targeting of the fission yeast formin cdc12p in cytokinesis. Mol Biol Cell. 2008;19:2208–19. doi: 10.1091/mbc.E07-07-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao L, Liu W, Bretscher A. The yeast formin Bnr1p has two localization regions that show spatially and temporally distinct association with septin structures. Mol Biol Cell. 2010;21:1253–62. doi: 10.1091/mbc.E09-10-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007;178:193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frost A, Unger VM, De Camilli P. The BAR domain superfamily: membrane-molding macromolecules. Cell. 2009;137:191–6. doi: 10.1016/j.cell.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scita G, Confalonieri S, Lappalainen P, Suetsugu S. IRSp53: crossing the road of membrane and actin dynamics in the formation of membrane protrusions. Trends Cell Biol. 2008;18:52–60. doi: 10.1016/j.tcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Fujiwara T, Mammoto A, Kim Y, Takai Y. Rho small G-protein-dependent binding of mDia to an Src homology 3 domain-containing IRSp53/BAIAP2. Biochem Biophys Res Commun. 2000;271:626–9. doi: 10.1006/bbrc.2000.2671. [DOI] [PubMed] [Google Scholar]
- 55.Goh WI, Lim KB, Sudhaharan T, Sem KP, Bu W, Chou AM, Ahmed S. mDia1 and WAVE2 proteins interact directly with IRSp53 in filopodia and are involved in filopodium formation. J Biol Chem. 2012;287:4702–14. doi: 10.1074/jbc.M111.305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aspenström P, Richnau N, Johansson AS. The diaphanous-related formin DAAM1 collaborates with the Rho GTPases RhoA and Cdc42, CIP4 and Src in regulating cell morphogenesis and actin dynamics. Exp Cell Res. 2006;312:2180–94. doi: 10.1016/j.yexcr.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 57.Higashida C, Miyoshi T, Fujita A, Oceguera-Yanez F, Monypenny J, Andou Y, Narumiya S, Watanabe N. Actin polymerization-driven molecular movement of mDia1 in living cells. Science. 2004;303:2007–10. doi: 10.1126/science.1093923. [DOI] [PubMed] [Google Scholar]
- 58.Breitsprecher D, Jaiswal R, Bombardier JP, Gould CJ, Gelles J, Goode BL. Rocket launcher mechanism of collaborative actin assembly defined by single-molecule imaging. Science. 2012;336:1164–8. doi: 10.1126/science.1218062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higashida C, Kiuchi T, Akiba Y, Mizuno H, Maruoka M, Narumiya S, Mizuno K, Watanabe N. F- and G-actin homeostasis regulates mechanosensitive actin nucleation by formins. Nat Cell Biol. 2013;15:395–405. doi: 10.1038/ncb2693. [DOI] [PubMed] [Google Scholar]
- 60.Jégou A, Carlier MF, Romet-Lemonne G. Formin mDia1 senses and generates mechanical forces on actin filaments. Nat Commun. 2013;4:1883. doi: 10.1038/ncomms2888. [DOI] [PubMed] [Google Scholar]
- 61.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–86. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamana N, Arakawa Y, Nishino T, Kurokawa K, Tanji M, Itoh RE, Monypenny J, Ishizaki T, Bito H, Nozaki K, et al. The Rho-mDia1 pathway regulates cell polarity and focal adhesion turnover in migrating cells through mobilizing Apc and c-Src. Mol Cell Biol. 2006;26:6844–58. doi: 10.1128/MCB.00283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol. 2003;161:381–91. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geron E, Schejter ED, Shilo BZ. Directing exocrine secretory vesicles to the apical membrane by actin cables generated by the formin mDia1. Proc Natl Acad Sci U S A. 2013;110:10652–7. doi: 10.1073/pnas.1303796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beli P, Mascheroni D, Xu D, Innocenti M. WAVE and Arp2/3 jointly inhibit filopodium formation by entering into a complex with mDia2. Nat Cell Biol. 2008;10:849–57. doi: 10.1038/ncb1745. [DOI] [PubMed] [Google Scholar]
- 66.Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15:129–33. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 67.Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5:e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe S, De Zan T, Ishizaki T, Yasuda S, Kamijo H, Yamada D, Aoki T, Kiyonari H, Kaneko H, Shimizu R, et al. Loss of a Rho-regulated actin nucleator, mDia2, impairs cytokinesis during mouse fetal erythropoiesis. Cell Rep. 2013;5:926–32. doi: 10.1016/j.celrep.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Ji P, Jayapal SR, Lodish HF. Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat Cell Biol. 2008;10:314–21. doi: 10.1038/ncb1693. [DOI] [PubMed] [Google Scholar]
- 70.Wallar BJ, Deward AD, Resau JH, Alberts AS. RhoB and the mammalian Diaphanous-related formin mDia2 in endosome trafficking. Exp Cell Res. 2007;313:560–71. doi: 10.1016/j.yexcr.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 71.Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, Ishizaki T, Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature. 2004;428:767–71. doi: 10.1038/nature02452. [DOI] [PubMed] [Google Scholar]
- 72.Cheng L, Zhang J, Ahmad S, Rozier L, Yu H, Deng H, Mao Y. Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev Cell. 2011;20:342–52. doi: 10.1016/j.devcel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92:303–15. doi: 10.1016/j.ejcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 74.DeWard AD, Eisenmann KM, Matheson SF, Alberts AS. The role of formins in human disease. Biochim Biophys Acta. 2010;1803:226–33. doi: 10.1016/j.bbamcr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/S0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 76.Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihály J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133:957–66. doi: 10.1242/dev.02266. [DOI] [PubMed] [Google Scholar]
- 77.Kida YS, Sato T, Miyasaka KY, Suto A, Ogura T. Daam1 regulates the endocytosis of EphB during the convergent extension of the zebrafish notochord. Proc Natl Acad Sci U S A. 2007;104:6708–13. doi: 10.1073/pnas.0608946104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim GH, Han JK. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 2007;26:2513–26. doi: 10.1038/sj.emboj.7601688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matusek T, Gombos R, Szécsényi A, Sánchez-Soriano N, Czibula A, Pataki C, Gedai A, Prokop A, Raskó I, Mihály J. Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008;28:13310–9. doi: 10.1523/JNEUROSCI.2727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li D, Hallett MA, Zhu W, Rubart M, Liu Y, Yang Z, Chen H, Haneline LS, Chan RJ, Schwartz RJ, et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development. 2011;138:303–15. doi: 10.1242/dev.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Colombo A, Palma K, Armijo L, Mione M, Signore IA, Morales C, Guerrero N, Meynard MM, Pérez R, Suazo J, et al. Daam1a mediates asymmetric habenular morphogenesis by regulating dendritic and axonal outgrowth. Development. 2013;140:3997–4007. doi: 10.1242/dev.091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–97. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 83.Lee HK, Deneen B. Daam2 is required for dorsal patterning via modulation of canonical Wnt signaling in the developing spinal cord. Dev Cell. 2012;22:183–96. doi: 10.1016/j.devcel.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Welsh IC, Thomsen M, Gludish DW, Alfonso-Parra C, Bai Y, Martin JF, Kurpios NA. Integration of left-right Pitx2 transcription and Wnt signaling drives asymmetric gut morphogenesis via Daam2. Dev Cell. 2013;26:629–44. doi: 10.1016/j.devcel.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo W, Yu CH, Lieu ZZ, Allard J, Mogilner A, Sheetz MP, Bershadsky AD. Analysis of the local organization and dynamics of cellular actin networks. J Cell Biol. 2013;202:1057–73. doi: 10.1083/jcb.201210123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yayoshi-Yamamoto S, Taniuchi I, Watanabe T. FRL, a novel formin-related protein, binds to Rac and regulates cell motility and survival of macrophages. Mol Cell Biol. 2000;20:6872–81. doi: 10.1128/MCB.20.18.6872-6881.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santos-Ledo A, Jenny A, Marlow FL. Comparative gene expression analysis of the fmnl family of formins during zebrafish development and implications for tissue specific functions. Gene Expr Patterns. 2013;13:30–7. doi: 10.1016/j.gep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–90. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naj X, Hoffmann AK, Himmel M, Linder S. The formins FMNL1 and mDia1 regulate coiling phagocytosis of Borrelia burgdorferi by primary human macrophages. Infect Immun. 2013;81:1683–95. doi: 10.1128/IAI.01411-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mersich AT, Miller MR, Chkourko H, Blystone SD. The formin FRL1 (FMNL1) is an essential component of macrophage podosomes. Cytoskeleton (Hoboken) 2010;67:573–85. doi: 10.1002/cm.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colón-Franco JM, Gomez TS, Billadeau DD. Dynamic remodeling of the actin cytoskeleton by FMNL1γ is required for structural maintenance of the Golgi complex. J Cell Sci. 2011;124:3118–26. doi: 10.1242/jcs.083725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han Y, Eppinger E, Schuster IG, Weigand LU, Liang X, Kremmer E, Peschel C, Krackhardt AM. Formin-like 1 (FMNL1) is regulated by N-terminal myristoylation and induces polarized membrane blebbing. J Biol Chem. 2009;284:33409–17. doi: 10.1074/jbc.M109.060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han Y, Yu G, Sarioglu H, Caballero-Martinez A, Schlott F, Ueffing M, Haase H, Peschel C, Krackhardt AM. Proteomic investigation of the interactome of FMNL1 in hematopoietic cells unveils a role in calcium-dependent membrane plasticity. J Proteomics. 2013;78:72–82. doi: 10.1016/j.jprot.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 94.Gardberg M, Talvinen K, Kaipio K, Iljin K, Kampf C, Uhlen M, Carpén O. Characterization of Diaphanous-related formin FMNL2 in human tissues. BMC Cell Biol. 2010;11:55. doi: 10.1186/1471-2121-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Block J, Breitsprecher D, Kühn S, Winterhoff M, Kage F, Geffers R, Duwe P, Rohn JL, Baum B, Brakebusch C, et al. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr Biol. 2012;22:1005–12. doi: 10.1016/j.cub.2012.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hetheridge C, Scott AN, Swain RK, Copeland JW, Higgs HN, Bicknell R, Mellor H. The formin FMNL3 is a cytoskeletal regulator of angiogenesis. J Cell Sci. 2012;125:1420–8. doi: 10.1242/jcs.091066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bai SW, Herrera-Abreu MT, Rohn JL, Racine V, Tajadura V, Suryavanshi N, Bechtel S, Wiemann S, Baum B, Ridley AJ. Identification and characterization of a set of conserved and new regulators of cytoskeletal organization, cell morphology and migration. BMC Biol. 2011;9:54. doi: 10.1186/1741-7007-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Favaro PM, de Souza Medina S, Traina F, Bassères DS, Costa FF, Saad ST. Human leukocyte formin: a novel protein expressed in lymphoid malignancies and associated with Akt. Biochem Biophys Res Commun. 2003;311:365–71. doi: 10.1016/j.bbrc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 99.Favaro PM, Traina F, Vassallo J, Brousset P, Delsol G, Costa FF, Saad ST. High expression of FMNL1 protein in T non-Hodgkin’s lymphomas. Leuk Res. 2006;30:735–8. doi: 10.1016/j.leukres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 100.Favaro P, Traina F, Machado-Neto JA, Lazarini M, Lopes MR, Pereira JK, Costa FF, Infante E, Ridley AJ, Saad ST. FMNL1 promotes proliferation and migration of leukemia cells. J Leukoc Biol. 2013;94:503–12. doi: 10.1189/jlb.0113057. [DOI] [PubMed] [Google Scholar]
- 101.Zhu XL, Zeng YF, Guan J, Li YF, Deng YJ, Bian XW, Ding YQ, Liang L. FMNL2 is a positive regulator of cell motility and metastasis in colorectal carcinoma. J Pathol. 2011;224:377–88. doi: 10.1002/path.2871. [DOI] [PubMed] [Google Scholar]
- 102.Kitzing TM, Wang Y, Pertz O, Copeland JW, Grosse R. Formin-like 2 drives amoeboid invasive cell motility downstream of RhoC. Oncogene. 2010;29:2441–8. doi: 10.1038/onc.2009.515. [DOI] [PubMed] [Google Scholar]
- 103.Li Y, Zhu X, Zeng Y, Wang J, Zhang X, Ding YQ, Liang L. FMNL2 enhances invasion of colorectal carcinoma by inducing epithelial-mesenchymal transition. Mol Cancer Res. 2010;8:1579–90. doi: 10.1158/1541-7786.MCR-10-0081. [DOI] [PubMed] [Google Scholar]
- 104.Lybaek H, Ørstavik KH, Prescott T, Hovland R, Breilid H, Stansberg C, Steen VM, Houge G. An 8.9 Mb 19p13 duplication associated with precocious puberty and a sporadic 3.9 Mb 2q23.3q24.1 deletion containing NR4A2 in mentally retarded members of a family with an intrachromosomal 19p-into-19q between-arm insertion. Eur J Hum Genet. 2009;17:904–10. doi: 10.1038/ejhg.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martín-Rufián M, Segura JA, Lobo C, Matés JM, Márquez J, Alonso FJ. Identification of genes downregulated in tumor cells expressing antisense glutaminase mRNA by differential display. Cancer Biol Ther. 2006;5:54–8. doi: 10.4161/cbt.5.1.2238. [DOI] [PubMed] [Google Scholar]
- 106.Harris ES, Li F, Higgs HN. The mouse formin, FRLalpha, slows actin filament barbed end elongation, competes with capping protein, accelerates polymerization from monomers, and severs filaments. J Biol Chem. 2004;279:20076–87. doi: 10.1074/jbc.M312718200. [DOI] [PubMed] [Google Scholar]
- 107.Harris ES, Gauvin TJ, Heimsath EG, Higgs HN. Assembly of filopodia by the formin FRL2 (FMNL3) Cytoskeleton (Hoboken) 2010;67:755–72. doi: 10.1002/cm.20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Esue O, Harris ES, Higgs HN, Wirtz D. The filamentous actin cross-linking/bundling activity of mammalian formins. J Mol Biol. 2008;384:324–34. doi: 10.1016/j.jmb.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 109.Heimsath EG, Jr., Higgs HN. The C terminus of formin FMNL3 accelerates actin polymerization and contains a WH2 domain-like sequence that binds both monomers and filament barbed ends. J Biol Chem. 2012;287:3087–98. doi: 10.1074/jbc.M111.312207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193:655–65. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mason FM, Heimsath EG, Higgs HN, Soderling SH. Bi-modal regulation of a formin by srGAP2. J Biol Chem. 2011;286:6577–86. doi: 10.1074/jbc.M110.190397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krainer EC, Ouderkirk JL, Miller EW, Miller MR, Mersich AT, Blystone SD. The multiplicity of human formins: Expression patterns in cells and tissues. Cytoskeleton (Hoboken) 2013;70:424–38. doi: 10.1002/cm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gasteier JE, Madrid R, Krautkrämer E, Schröder S, Muranyi W, Benichou S, Fackler OT. Activation of the Rac-binding partner FHOD1 induces actin stress fibers via a ROCK-dependent mechanism. J Biol Chem. 2003;278:38902–12. doi: 10.1074/jbc.M306229200. [DOI] [PubMed] [Google Scholar]
- 114.Takeya R, Sumimoto H. Fhos, a mammalian formin, directly binds to F-actin via a region N-terminal to the FH1 domain and forms a homotypic complex via the FH2 domain to promote actin fiber formation. J Cell Sci. 2003;116:4567–75. doi: 10.1242/jcs.00769. [DOI] [PubMed] [Google Scholar]
- 115.Schönichen A, Mannherz HG, Behrmann E, Mazur AJ, Kühn S, Silván U, Schoenenberger CA, Fackler OT, Raunser S, Dehmelt L, et al. FHOD1 is a combined actin filament capping and bundling factor that selectively associates with actin arcs and stress fibers. J Cell Sci. 2013;126:1891–901. doi: 10.1242/jcs.126706. [DOI] [PubMed] [Google Scholar]
- 116.Schulze N, Graessl M, Blancke Soares A, Geyer M, Dehmelt L, Nalbant P. FHOD1 regulates stress fiber organization by controlling the dynamics of transverse arcs and dorsal fibers. J Cell Sci. 2014;127:1379–93. doi: 10.1242/jcs.134627. [DOI] [PubMed] [Google Scholar]
- 117.Iskratsch T, Yu CH, Mathur A, Liu S, Stévenin V, Dwyer J, Hone J, Ehler E, Sheetz M. FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev Cell. 2013;27:545–59. doi: 10.1016/j.devcel.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Floyd S, Whiffin N, Gavilan MP, Kutscheidt S, De Luca M, Marcozzi C, Min M, Watkins J, Chung K, Fackler OT, et al. Spatiotemporal organization of Aurora-B by APC/CCdh1 after mitosis coordinates cell spreading through FHOD1. J Cell Sci. 2013;126:2845–56. doi: 10.1242/jcs.123232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gardberg M, Kaipio K, Lehtinen L, Mikkonen P, Heuser VD, Talvinen K, Iljin K, Kampf C, Uhlen M, Grénman R, et al. FHOD1, a formin upregulated in epithelial-mesenchymal transition, participates in cancer cell migration and invasion. PLoS One. 2013;8:e74923. doi: 10.1371/journal.pone.0074923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Alvarez DE, Agaisse H. The formin FHOD1 and the small GTPase Rac1 promote vaccinia virus actin-based motility. J Cell Biol. 2013;202:1075–90. doi: 10.1083/jcb.201303055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Truong D, Brabant D, Bashkurov M, Wan LC, Braun V, Heo WD, Meyer T, Pelletier L, Copeland J, Brumell JH. Formin-mediated actin polymerization promotes Salmonella invasion. Cell Microbiol. 2013;15:2051–63. doi: 10.1111/cmi.12173. [DOI] [PubMed] [Google Scholar]
- 122.Taniguchi K, Takeya R, Suetsugu S, Kan-O M, Narusawa M, Shiose A, Tominaga R, Sumimoto H. Mammalian formin fhod3 regulates actin assembly and sarcomere organization in striated muscles. J Biol Chem. 2009;284:29873–81. doi: 10.1074/jbc.M109.059303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iskratsch T, Lange S, Dwyer J, Kho AL, dos Remedios C, Ehler E. Formin follows function: a muscle-specific isoform of FHOD3 is regulated by CK2 phosphorylation and promotes myofibril maintenance. J Cell Biol. 2010;191:1159–72. doi: 10.1083/jcb.201005060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Iskratsch T, Reijntjes S, Dwyer J, Toselli P, Dégano IR, Dominguez I, Ehler E. Two distinct phosphorylation events govern the function of muscle FHOD3. Cell Mol Life Sci. 2013;70:893–908. doi: 10.1007/s00018-012-1154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kan-O M, Takeya R, Abe T, Kitajima N, Nishida M, Tominaga R, Kurose H, Sumimoto H. Mammalian formin Fhod3 plays an essential role in cardiogenesis by organizing myofibrillogenesis. Biol Open. 2012;1:889–96. doi: 10.1242/bio.20121370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hannemann S, Madrid R, Stastna J, Kitzing T, Gasteier J, Schönichen A, Bouchet J, Jimenez A, Geyer M, Grosse R, et al. The Diaphanous-related Formin FHOD1 associates with ROCK1 and promotes Src-dependent plasma membrane blebbing. J Biol Chem. 2008;283:27891–903. doi: 10.1074/jbc.M801800200. [DOI] [PubMed] [Google Scholar]
- 127.Goult BT, Bouaouina M, Harburger DS, Bate N, Patel B, Anthis NJ, Campbell ID, Calderwood DA, Barsukov IL, Roberts GC, et al. The structure of the N-terminus of kindlin-1: a domain important for alphaiibbeta3 integrin activation. J Mol Biol. 2009;394:944–56. doi: 10.1016/j.jmb.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goult BT, Bouaouina M, Elliott PR, Bate N, Patel B, Gingras AR, Grossmann JG, Roberts GC, Calderwood DA, Critchley DR, et al. Structure of a double ubiquitin-like domain in the talin head: a role in integrin activation. EMBO J. 2010;29:1069–80. doi: 10.1038/emboj.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dames SA, Junemann A, Sass HJ, Schönichen A, Stopschinski BE, Grzesiek S, Faix J, Geyer M. Structure, dynamics, lipid binding, and physiological relevance of the putative GTPase-binding domain of Dictyostelium formin C. J Biol Chem. 2011;286:36907–20. doi: 10.1074/jbc.M111.225052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Junemann A, Winterhoff M, Nordholz B, Rottner K, Eichinger L, Gräf R, Faix J. ForC lacks canonical formin activity but bundles actin filaments and is required for multicellular development of Dictyostelium cells. Eur J Cell Biol. 2013;92:201–12. doi: 10.1016/j.ejcb.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 131.Alberts AS, Bouquin N, Johnston LH, Treisman R. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J Biol Chem. 1998;273:8616–22. doi: 10.1074/jbc.273.15.8616. [DOI] [PubMed] [Google Scholar]
- 132.Peng J, Wallar BJ, Flanders A, Swiatek PJ, Alberts AS. Disruption of the Diaphanous-related formin Drf1 gene encoding mDia1 reveals a role for Drf3 as an effector for Cdc42. Curr Biol. 2003;13:534–45. doi: 10.1016/S0960-9822(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 133.Gasman S, Kalaidzidis Y, Zerial M. RhoD regulates endosome dynamics through Diaphanous-related Formin and Src tyrosine kinase. Nat Cell Biol. 2003;5:195–204. doi: 10.1038/ncb935. [DOI] [PubMed] [Google Scholar]
- 134.Westendorf JJ. The formin/diaphanous-related protein, FHOS, interacts with Rac1 and activates transcription from the serum response element. J Biol Chem. 2001;276:46453–9. doi: 10.1074/jbc.M105162200. [DOI] [PubMed] [Google Scholar]
- 135.Madrid R, Aranda JF, Rodríguez-Fraticelli AE, Ventimiglia L, Andrés-Delgado L, Shehata M, Fanayan S, Shahheydari H, Gómez S, Jiménez A, et al. The formin INF2 regulates basolateral-to-apical transcytosis and lumen formation in association with Cdc42 and MAL2. Dev Cell. 2010;18:814–27. doi: 10.1016/j.devcel.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 136.Magie CR, Meyer MR, Gorsuch MS, Parkhurst SM. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development. 1999;126:5353–64. doi: 10.1242/dev.126.23.5353. [DOI] [PubMed] [Google Scholar]
- 137.Rosales-Nieves AE, Johndrow JE, Keller LC, Magie CR, Pinto-Santini DM, Parkhurst SM. Coordination of microtubule and microfilament dynamics by Drosophila Rho1, Spire and Cappuccino. Nat Cell Biol. 2006;8:367–76. doi: 10.1038/ncb1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grosshans J, Wenzl C, Herz HM, Bartoszewski S, Schnorrer F, Vogt N, Schwarz H, Müller HA. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development. 2005;132:1009–20. doi: 10.1242/dev.01669. [DOI] [PubMed] [Google Scholar]
- 139.Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol. 2005;7:619–25. doi: 10.1038/ncb1266. [DOI] [PubMed] [Google Scholar]
- 140.Carnahan RH, Gould KL. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J Cell Biol. 2003;162:851–62. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakano K, Imai J, Arai R, Toh-E A, Matsui Y, Mabuchi I. The small GTPase Rho3 and the diaphanous/formin For3 function in polarized cell growth in fission yeast. J Cell Sci. 2002;115:4629–39. doi: 10.1242/jcs.00150. [DOI] [PubMed] [Google Scholar]
- 142.Martin SG, Rincón SA, Basu R, Pérez P, Chang F. Regulation of the formin for3p by cdc42p and bud6p. Mol Biol Cell. 2007;18:4155–67. doi: 10.1091/mbc.E07-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kohno H, Tanaka K, Mino A, Umikawa M, Imamura H, Fujiwara T, Fujita Y, Hotta K, Qadota H, Watanabe T, et al. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 1996;15:6060–8. [PMC free article] [PubMed] [Google Scholar]
- 144.Qi M, Elion EA. Formin-induced actin cables are required for polarized recruitment of the Ste5 scaffold and high level activation of MAPK Fus3. J Cell Sci. 2005;118:2837–48. doi: 10.1242/jcs.02418. [DOI] [PubMed] [Google Scholar]
- 145.Robinson NG, Guo L, Imai J, Toh-E A, Matsui Y, Tamanoi F. Rho3 of Saccharomyces cerevisiae, which regulates the actin cytoskeleton and exocytosis, is a GTPase which interacts with Myo2 and Exo70. Mol Cell Biol. 1999;19:3580–7. doi: 10.1128/mcb.19.5.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dong Y, Pruyne D, Bretscher A. Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J Cell Biol. 2003;161:1081–92. doi: 10.1083/jcb.200212040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Imamura H, Tanaka K, Hihara T, Umikawa M, Kamei T, Takahashi K, Sasaki T, Takai Y. Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 1997;16:2745–55. doi: 10.1093/emboj/16.10.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–22. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- 149.Ozaki-Kuroda K, Yamamoto Y, Nohara H, Kinoshita M, Fujiwara T, Irie K, Takai Y. Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:827–39. doi: 10.1128/MCB.21.3.827-839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173:383–94. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, Siminovitch KA. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol. 2009;182:3837–45. doi: 10.4049/jimmunol.0803838. [DOI] [PubMed] [Google Scholar]
- 152.Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem. 2007;282:25152–8. doi: 10.1074/jbc.M703243200. [DOI] [PubMed] [Google Scholar]
- 153.Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J Cell Sci. 2005;118:2661–70. doi: 10.1242/jcs.02384. [DOI] [PubMed] [Google Scholar]
- 154.Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol. 2001;3:723–9. doi: 10.1038/35087035. [DOI] [PubMed] [Google Scholar]
- 155.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–30. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 156.Eng CH, Huckaba TM, Gundersen GG. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol Biol Cell. 2006;17:5004–16. doi: 10.1091/mbc.E05-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tominaga T, Sahai E, Chardin P, McCormick F, Courtneidge SA, Alberts AS. Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol Cell. 2000;5:13–25. doi: 10.1016/S1097-2765(00)80399-8. [DOI] [PubMed] [Google Scholar]
- 158.Rousso T, Shewan AM, Mostov KE, Schejter ED, Shilo BZ. Apical targeting of the formin Diaphanous in Drosophila tubular epithelia. Elife. 2013;2:e00666. doi: 10.7554/eLife.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lewkowicz E, Herit F, Le Clainche C, Bourdoncle P, Perez F, Niedergang F. The microtubule-binding protein CLIP-170 coordinates mDia1 and actin reorganization during CR3-mediated phagocytosis. J Cell Biol. 2008;183:1287–98. doi: 10.1083/jcb.200807023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Block J, Stradal TE, Hänisch J, Geffers R, Köstler SA, Urban E, Small JV, Rottner K, Faix J. Filopodia formation induced by active mDia2/Drf3. J Microsc. 2008;231:506–17. doi: 10.1111/j.1365-2818.2008.02063.x. [DOI] [PubMed] [Google Scholar]
- 161.Watanabe S, Ando Y, Yasuda S, Hosoya H, Watanabe N, Ishizaki T, Narumiya S. mDia2 induces the actin scaffold for the contractile ring and stabilizes its position during cytokinesis in NIH 3T3 cells. Mol Biol Cell. 2008;19:2328–38. doi: 10.1091/mbc.E07-10-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lai SL, Chan TH, Lin MJ, Huang WP, Lou SW, Lee SJ. Diaphanous-related formin 2 and profilin I are required for gastrulation cell movements. PLoS One. 2008;3:e3439. doi: 10.1371/journal.pone.0003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Machaidze G, Sokoll A, Shimada A, Lustig A, Mazur A, Wittinghofer A, Aebi U, Mannherz HG. Actin filament bundling and different nucleating effects of mouse Diaphanous-related formin FH2 domains on actin/ADF and actin/cofilin complexes. J Mol Biol. 2010;403:529–45. doi: 10.1016/j.jmb.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 164.Koizumi K, Takano K, Kaneyasu A, Watanabe-Takano H, Tokuda E, Abe T, Watanabe N, Takenawa T, Endo T. RhoD activated by fibroblast growth factor induces cytoneme-like cellular protrusions through mDia3C. Mol Biol Cell. 2012;23:4647–61. doi: 10.1091/mbc.E12-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wiggan O, DeLuca JG. FORMIN stable kinetochore-microtubule attachments. Dev Cell. 2011;20:283–4. doi: 10.1016/j.devcel.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Thumkeo D, Shinohara R, Watanabe K, Takebayashi H, Toyoda Y, Tohyama K, Ishizaki T, Furuyashiki T, Narumiya S. Deficiency of mDia, an actin nucleator, disrupts integrity of neuroepithelium and causes periventricular dysplasia. PLoS One. 2011;6:e25465. doi: 10.1371/journal.pone.0025465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu J, Meng W, Poy F, Maiti S, Goode BL, Eck MJ. Structure of the FH2 domain of Daam1: implications for formin regulation of actin assembly. J Mol Biol. 2007;369:1258–69. doi: 10.1016/j.jmb.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barkó S, Bugyi B, Carlier MF, Gombos R, Matusek T, Mihály J, Nyitrai M. Characterization of the biochemical properties and biological function of the formin homology domains of Drosophila DAAM. J Biol Chem. 2010;285:13154–69. doi: 10.1074/jbc.M109.093914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, Habas R. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133:4219–31. doi: 10.1242/dev.02590. [DOI] [PubMed] [Google Scholar]
- 170.Kanaya H, Takeya R, Takeuchi K, Watanabe N, Jing N, Sumimoto H. Fhos2, a novel formin-related actin-organizing protein, probably associates with the nestin intermediate filament. Genes Cells. 2005;10:665–78. doi: 10.1111/j.1365-2443.2005.00867.x. [DOI] [PubMed] [Google Scholar]
- 171.Kan-o M, Takeya R, Taniguchi K, Tanoue Y, Tominaga R, Sumimoto H. Expression and subcellular localization of mammalian formin Fhod3 in the embryonic and adult heart. PLoS One. 2012;7:e34765. doi: 10.1371/journal.pone.0034765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science. 2013;340:864–7. doi: 10.1126/science.1235038. [DOI] [PubMed] [Google Scholar]
- 173.Kutscheidt S, Zhu R, Antoku S, Luxton GW, Stagljar I, Fackler OT, Gundersen GG. FHOD1 interaction with nesprin-2G mediates TAN line formation and nuclear movement. Nat Cell Biol. 2014;16:708–15. doi: 10.1038/ncb2981. [DOI] [PMC free article] [PubMed] [Google Scholar]