Damage to normal tissue is a limiting factor for clinical radiation for cancer eradication. Ionizing radiation can induce a variety of cellular responses, including apoptosis, necrosis, necroptosis, autophagy, and accelerated senescence.1 The molecular mechanisms of radiation-induced cellular injury depend on a number of factors, including radiation dosage and dose rate, cell type, transformed status, and growth rate.1 Recent studies suggest that accelerated senescence is a primary response of normal, untransformed epithelial and endothelial cells to radiation exposure.2,3
Senescent cells display aberrant biological activities, and the induction of senescence may have important implications for the loss of normal tissue function following radiation damage.4,5 Research in aging cells posits that senescence develops in response to continuous mitogenic signaling in the presence of cell cycle blockade.6 Senescent cells have a variety of abnormal characteristics: genetic instability, reduced susceptibility to apoptosis, alterations in cellular morphology and polarity, changes in protein expression (especially increased expression of pro-inflammatory factors), and altered cell–cell contacts.5 Senescent cells do not proliferate or migrate, rendering them incapable of responding to tissue injury. Endothelial senescence contributes to monocyte adhesion, decreased nitric oxide production, and increased pro-inflammatory factor secretion associated with radiation-induced vascular dysfunction.4
The mammalian target of rapamycin (mTOR) is a central integration point for a number of cell signaling pathways, including proliferation and homeostasis. mTOR has been identified as a molecular target for the inhibition of aging-associated and stress-induced cellular senescence.6 Indeed, treatment with the mTOR inhibitor rapamycin prevents accelerated senescence in cells exposed to DNA-damaging agents. A recent study demonstrated mTOR inhibition prevented radiation-induced mucositis and inflammation in mice following head and neck irradiation.3 This study demonstrated that rapamycin blocked radiation-induced senescence, but not apoptosis, both in primary keratinocyte cultures and in the adult stem cell population mice in vivo following head and neck irradiation.
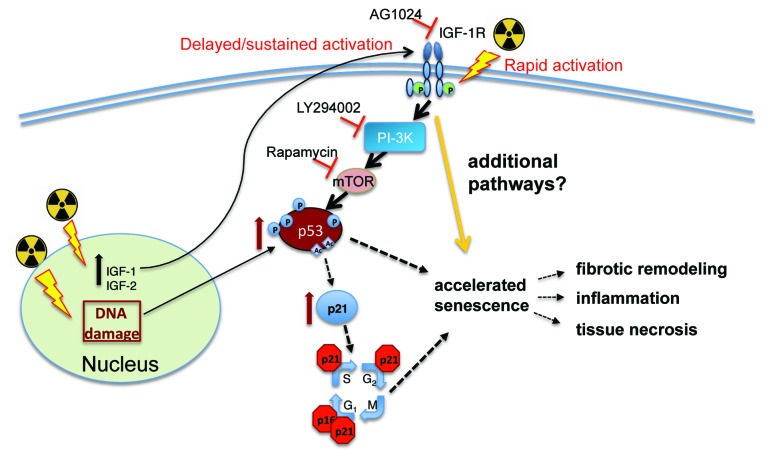
Because mTOR integrates myriad signals from multiple cellular stimuli, it is important to identify specific molecules associated with senescence induction, upstream of PI-3K and mTOR activation, within the context of radiation-induced injury. Studies of the receptor tyrosine kinase insulin-like growth factor receptor (IGF-1R) have revealed a conundrum in function following radiation exposure. Under normal conditions, IGF-1R induces cellular proliferation and survival through the activation of both the PI-3K and p42/p42 MAPK signaling pathways. In response to radiation, IGF-1R has been linked to both improved cell survival, most notably in tumor cell lines, as well as radiation-induced senescence in primary cells.7 In cancer cells, ligand-independent IGF-1R phosphorylation can be triggered by ATM activation in response to radiation-induced DNA damage, resulting in cytoprotective signaling. Conversely, IGF-1R activation in normal cells undergoing redox stress has been demonstrated to result in premature senescence through p53 activation and upregulation of the p21/waf1 cyclin-dependent kinase inhibitor. In studies of human pulmonary arterial endothelial cells (HPAEC) undergoing radiation-induced senescence, our laboratory detected IGF-1R phosphorylation within 24 h post-irradiation. We hypothesized that IGF-1R signaling contributed to accelerated senescence in HPAEC. To test this, we exposed primary HPAEC to X-rays (10 Gy) in the presence of AG1024 (a pharmacological IGF-1R inhibitor), and investigated whether inhibition of IGF-1R would delay the onset of and/or attenuate cellular senescence. AG1024 significantly reduced cellular alterations associated with radiation-induced senescence.7 Normal cellular morphology was maintained in a large percentage of irradiated cells treated with AG1024, and senescence-associated β-galactosidase activity was suppressed. IGF-1R inhibition also prevented radiation-induced activation of p21/waf1 and p53 (Fig. 1). These findings suggest that IGF-1R signaling is required for the induction of senescence in some primary cell types. This may further indicate that accelerated senescence is not an inevitable result of radiation-induced molecular damage, but that specific signaling cascades can be interrupted to rescue cells destined for senescence. The ultimate fate of these rescued cells remains to be determined.
Figure 1. IGF1R activation by ionizing radiation is necessary for radiation-induced accelerated senescence in some primary cell types. Ionizing radiation (indicated by radiation symbol) initiates a number of events in cells through the induction of molecular damage and the generation of ROS. DNA damage by radiation has been demonstrated to activate p53 and p21/waf (p21). The cell cycle is blocked by p21/waf activation, while p53 activation can have multiple effects on cellular activities, including the induction of repair mechanisms, cell death mechanisms, and senescence.1 Our laboratory also found that ionizing radiation can directly induce the activation of the IGF-1R receptor for early activation and induce the expression of IGF-1R ligands IGF-1 and IGF-1, which may mediate delayed and sustained activation of the receptor.7 Inhibition of IGF-1R, PI-3K, or mTOR blocks radiation-induced accelerated senescence in primary pulmonary endothelial cells.7
Although all forms of cell death impact tissue function, it is possible that accelerated cellular senescence in response to ionizing radiation might be more damaging to surrounding tissues.1,3 Heightened production of pro-inflammatory molecules and the loss of adult stem cells required for the execution of normal repair function are key pathological characteristics of delayed radiation tissue damage. In contrast to apoptotic cells that are readily cleared from the tissue by processes such as efferocytosis, senescent cells may persist in the tissue. Radiation-induced senescence may be a critical driver of the delayed, unpredictable cycles of inflammation observed following radiation exposure and may contribute to late-stage radiation tissue damage that extends the initial region of radiation tissue injury, both in surface area and tissue depth, resulting in cycles of failed repair and fibrotic remodeling. The development of therapeutic approaches for targeting IGF-1R and related signaling pathways for accelerated cellular senescence may reduce radiation-mediated tissue injury.
Panganiban RA, et al. PLoS One. 2013;8:e78589. doi: 10.1371/journal.pone.0078589.
References
- 1.Panganiban RA, et al. Int J Mol Sci. 2013;14:15931–58. doi: 10.3390/ijms140815931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panganiban RA, et al. Int J Radiat Biol. 2013;89:656–67. doi: 10.3109/09553002.2012.711502. [DOI] [PubMed] [Google Scholar]
- 3.Iglesias-Bartolome R, et al. Cell Stem Cell. 2012;11:401–14. doi: 10.1016/j.stem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller M. Antioxid Redox Signal. 2009;11:59–98. doi: 10.1089/ars.2008.2104. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, et al. Am J Physiol Heart Circ Physiol. 2006;290:H1729–39. doi: 10.1152/ajpheart.01103.2005. [DOI] [PubMed] [Google Scholar]
- 6.Demidenko ZN, et al. Cell Cycle. 2008;7:3355–61. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- 7.Panganiban RA, et al. PLoS One. 2013;8:e78589. doi: 10.1371/journal.pone.0078589. [DOI] [PMC free article] [PubMed] [Google Scholar]