The bromodomain and extraterminal domain (BET)-containing proteins are a class of epigenetic regulators known as “chromatin readers,” which bind to acetylated lysines on histones, recruit transcription factors and chromatin-modifying enzymes, and can function as co-activators or co-repressors of gene transcription depending on the context.1,2 BRD4 (bromodomain containing 4) is a conserved member of the BET family protein that includes other subfamily members such as BRD2, BRD3, and testis-specific BRDT. Studies with the small molecules, JQ1 and I-BET762, were the first to indicate the druggability of the BET bromodomain family.1,2 In addition to the NUT–BRD3/4 fusion in NUT midline carcinomas, MLL fusions in leukemias and MYC overexpression in variety of cancers have been suggested as targets of BET bromodomain inhibitors.1-3 Importantly, JQ1 has exhibited little or no toxicity in mice treated with high levels (50 mg/kg daily) over a month. While JQ1 itself is not a clinical lead compound, trials with BET inhibitors GSK525762 (GlaxoSmithKline), CPI-0610 (Constellation Pharmaceuticals), TEN-010 (Tensha Therapeutics), and OTX-015 (Oncoethix) have been initiated for a variety of malignancies, and several other BET inhibitors are in pre-clinical development.4 Due to their favorable side-effect profiles, BET inhibition has even been contemplated in non-disease indications such as male contraception.5
In the context of prostate cancer, we provided evidence that BET inhibitors may mediate their anti-neoplastic effects through ablation of AR signaling.3 Maintenance of AR signaling has been identified as the most common resistance mechanism that patients with advanced prostate cancer develop after conventional hormonal treatments.6 AR amplification, mutation, and alternative splicing have all been suggested as potential resistance mechanisms to anti-androgen treatments.6 In our study we showed that these mechanisms of AR signaling can be attenuated by BET inhibition as the 3 tested AR-positive cell lines sensitive to JQ1 each harbor unique mechanisms to maintain AR signaling as follows: (1) VCaP cells have amplification of AR; (2) LNCaP cells have the gain-of-function mutation in AR (T877A); and (3) 22RV1 cells have a constitutively active AR splice variant. Over half of CRPC patients have at least one of these aberrations in the AR pathway.6
While there has been much excitement around the development of second generation therapeutics targeting the androgen axis, such as abiraterone and enzalutamide, the responses to these agents are often not durable. Resistance mechanisms to these drugs include those that develop with conventional hormonal treatment (e.g., AR amplification or mutation) as well as novel mechanisms involving compensatory steroid hormone signaling such as glucocorticoid receptor (GR) activation.7 Although not formally addressed in our study, we predict that GR signaling, in the context of AR resistance, will also be susceptible to BET bromodomain inhibition, since GR likely engages a similar co-transcriptional machinery.
We provided evidence that JQ1 functions in prostate cancer to abrogate AR signaling downstream of the receptor itself. We show that BRD2/3/4 physically interacts with the N terminus of AR, which is required for its transcriptional activity.3 Most current AR-directed therapies (e.g., enzalutamide) are dependent on the presence of the C-terminal ligand-binding domain; however, novel approaches targeting the N-terminal domain have been considered without much success. We show that JQ1 can disrupt the AR–BRD4 interaction in cell-free systems and in the endogenous setting. By functioning downstream of AR, BET bromodomain inhibition is less likely to be affected by acquired resistance mechanisms associated with AR antagonists, including the recently identified F876L mutation of AR in acquired enzalutamide-resistant prostate cancer cells.7 While both enzalutamide and JQ1 block AR recruitment to target loci on a genome-wide scale (the “AR cistrome”), we found that JQ1 likely has an enhanced effect by fully abrogating co-recruitment of BRD4, which is required for mobilization of the transcriptional machinery including RNA Pol II. In vitro and in vivo models both demonstrated the enhanced effect of JQ1 compared with enzalutamide, and we further showed the effect of JQ1 in castrated mice growing hormone refractory VCaP xenografts.3
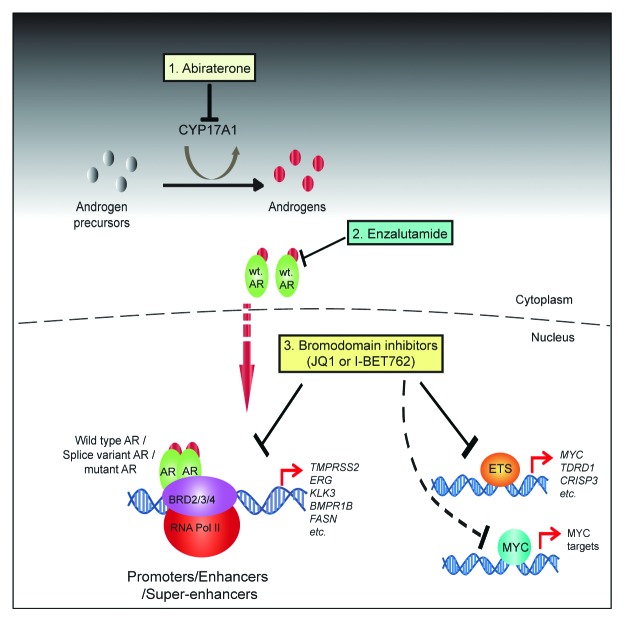
In addition to blocking AR signaling downstream of ligand and at the level of AR and BRD2/3/4 recruitment to chromatin, BET bromodomain inhibitors also negatively regulate the expression and oncogenic activity of TMPRSS2–ETS gene fusion products.3 This is very intriguing, as the oncogenic driver ETS transcription factors have been notoriously difficult to target therapeutically. Furthermore, it appears that in prostate cancer, ETS transcription factors bind to an enhancer region that regulates MYC expression, and when the ETS factors are inhibited, this leads to attenuation of oncogenic MYC expression. Taken together, this suggests a model in which BET bromodomain inhibitors may simultaneously block the functional activity and/or expression of multiple oncogenic transcriptional drivers in prostate cancer, including AR, ETS, and MYC (Fig. 1). Our data suggest that BET bromodomain inhibitors could be a promising therapeutic option for the treatment of castration-resistant prostate cancer. As clinical trials are being actively pursued to evaluate the toxicity and efficacy of novel BET inhibitors in the context of NUT mid-line carcinomas and leukemias, it will be interesting to see how castration-resistant prostate cancer, and potentially other common solid tumors will respond. There remains much to be learned in terms of the remarkable preferential mechanism of BET bromodomain inhibitors in cancer vs. normal tissues.
Figure 1. Schematic depicting varying mechanisms to block AR signaling in CRPC. (1) Abiraterone inhibits androgen biosynthesis by inhibiting CYP17A1. (2) Enzalutamide competitively antagonizes androgen binding to AR. (3) BET bromodomain inhibitors blocks AR (whether mutated/amplified) and BRD2/3/4 interaction, and co-recruitment to target gene loci as well as the activity and/or expression of the oncogenic transcription factors, including ETS fusions and MYC.
References
- 1.Dawson MA, et al. Nature. 2011;478:529–33. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filippakopoulos P, et al. Nature. 2010;468:1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asangani IA, et al. Nature. 2014 doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lou K-J. Science Business eXhange. 2014 [Google Scholar]
- 5.Matzuk MM, et al. Cell. 2012;150:673–84. doi: 10.1016/j.cell.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitsiades N. Cancer Res. 2013;73:4599–605. doi: 10.1158/0008-5472.CAN-12-4414. [DOI] [PubMed] [Google Scholar]
- 7.Arora VK, et al. Cell. 2013;155:1309–22. doi: 10.1016/j.cell.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]