Abstract
Pharmacological inhibition of Drosophila Polo kinase with BI2536 has allowed us to re-examine the requirements for Polo during Drosophila male gametogenesis. BI2536-treated spermatocytes persisted in a pro-metaphase state without dividing and had condensed chromosomes that did not separate. Centrosomes failed to recruit γ-tubulin and centrosomin (Cnn) and were not associated with microtubule arrays that were abnormal and did not form proper bipolar spindles. Centrioles, which usually separate during the anaphase of the first meiosis, remained held together in a V-shaped configuration suggesting that Polo kinase regulates the proteolysis that breaks centriole linkage to ensure their disengagement. Despite these defects spermatid differentiation proceeds, leading to axoneme formation.
Keywords: BI2536, Polo kinase, centrosome maturation, spindle assembly, centriole disengagement, gametogenesis, Drosophila
Introduction
Proper chromosome segregation during cell division is crucial to ensure that each daughter receives the full chromosome complement, thus avoiding aneuploidy that represents a hallmark of cell transformation. The proper execution of this process relies on the correct assembly and functioning of the bipolar spindle, which is largely dependent upon the microtubule-nucleating activity of the duplicated centrosome. The Polo family of serine–threonine kinases plays crucial roles in regulating centrosome function and other aspects of mitotic progression. The founder member of the Polo family was originally discovered in Drosophila.1 Flies homozygous for the polo1 mutant exhibit broad and disorganized spindle poles in both syncytial embryos and larval neuroblasts.1,2 These phenotypes are associated with defects in centrosome organization and migration and a failure of several centrosomal proteins to be recruited to the spindle poles in polo mutant Drosophila embryos.3,4 polo1 mutant males exhibit chromosome non-disjunction and cytokinesis defects in male meiosis.1,5,6 There are also defects in the organization of the female meiotic spindle.7
Of the multiple Polo-like kinases (Plks) in vertebrates, Plk1 has multiple roles in mitosis that best match those of Polo in Drosophila.8-10 Interfering with enzyme activity has identified multiple roles for this kinase during cell cycle progression.11-17 Plk1 is highly expressed in proliferating cells, and its upregulation in human tumors can have prognostic value. This association of Plk1 with a broad range of human tumors has highlighted the kinase as an attractive target for cancer drug development.18 Depletion of Plk1 by antisense oligonucleotides and small interfering RNA inhibits cell proliferation and decreases viability in cancer cells both in vivo and in vitro.11,13,19 Among chemical inhibitors targeting Plk1, the dihydropteridinone derivative BI2536, an ATP-competitive kinase inhibitor, is the most extensively investigated. BI2536 treatment results in the mitotic phenotypes that characterize Polo inhibition.16,20 In cancer cell lines, inhibition of Plk1 activity by BI2536 perturbs spindle pole assembly, leading to mitotic arrest and apoptosis.21
Previous studies of the effects of polo mutants on male meiosis have been restricted to weak hypomorphic alleles, in which males survive long enough to attempt meiosis.1,5,6 Here we have overcome this limitation by studying the pharmacological inhibition of Polo kinase in these cells. This has also let us examine the consequences of BI2536 treatment upon both early and late events in meiosis, because Drosophila spermatocytes only become transiently delayed by the spindle assembly checkpoint.22 Because mature spermatocytes are about 25–30 times larger than somatic cells and have larger spindles and very long centrioles, they offer a particularly suitable system for the analysis of cytological consequences of factors affecting cell division. We find that in addition to the typical defects already described to result from reduction of polo function, such as failure of centrosome maturation, spindle assembly, and cytokinesis, BI2536-treated spermatocytes show defects in the separation of chromatids and centrioles.
Results
Centrioles of primary spermatocytes
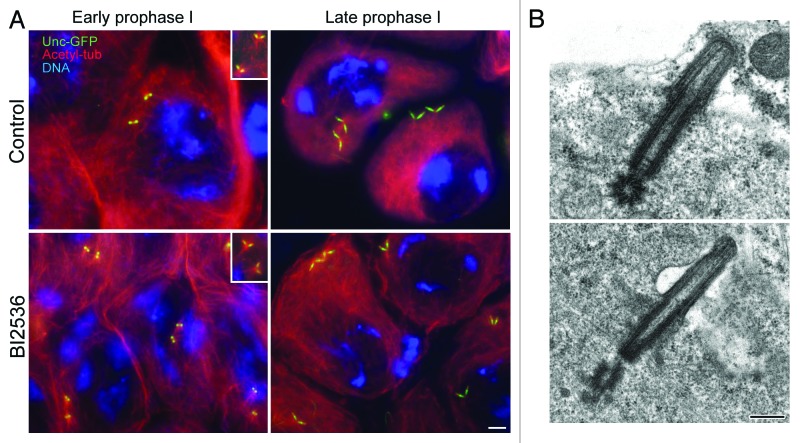
Our previous studies of the requirements for Polo in male meiosis utilized the hypomorphic mutant allele polo.1,5 We have been unable to study the effects of strong hypomorphic mutants upon meiosis, as these result in larval lethality.4 We first chose to examine the consequences of treating spermatocytes with BI2536 and following the effects upon centrioles monitored by expression of GFP-tagged Unc. The centrioles of primary Drosophila spermatocytes appear as small spots of Unc-GFP as they duplicate at the onset of the first meiotic prophase and gradually elongate during prophase (Fig. 1A). Thus, each primary spermatocyte has 2 pairs of connected centrioles that adopt a V-shaped configuration. At this stage, the distal ends of the centrioles push out the cell membrane to form short cilium-like projections (Fig. 1B). Unc-GFP is associated with this centriole–cilium complex from prophase I onwards. The labeling was localized in 3 distinct domains: the distal half region of the centriole, the whole cilium, and an intermediate dot-like zone (Fig. 1A). Centriole pairs were associated with small astral arrays of microtubules (Fig. 1A, inset). We could not find any notable consequences of treating primary spermatocytes with BI2536: they showed separated pairs of V-shaped centrioles (Fig. 1A) associated with small asters (Fig. 1A, inset); Unc-GFP showed a similar distribution on the centriole and cilium as seen in control spermatocytes; and the cilium-like projection had a similar organization (Fig. 1B).
Figure 1. Prophase of the first meiosis is unaffected by BI2536. (A) Primary spermatocytes expressing Unc-GFP (green) were stained for acetylated tubulin (red) and DNA (blue). Both control and BI2536-treated spermatocytes have 2 pairs of orthogonal centrioles that are associated to small asters of microtubules (insets). (B) Electron micrographs do not reveal significant differences between centriole/cilium complexes in control and treated mature primary spermatocytes. Scale bar = 2.5 μm in (A); 250 nm in (B).
Centriole separation
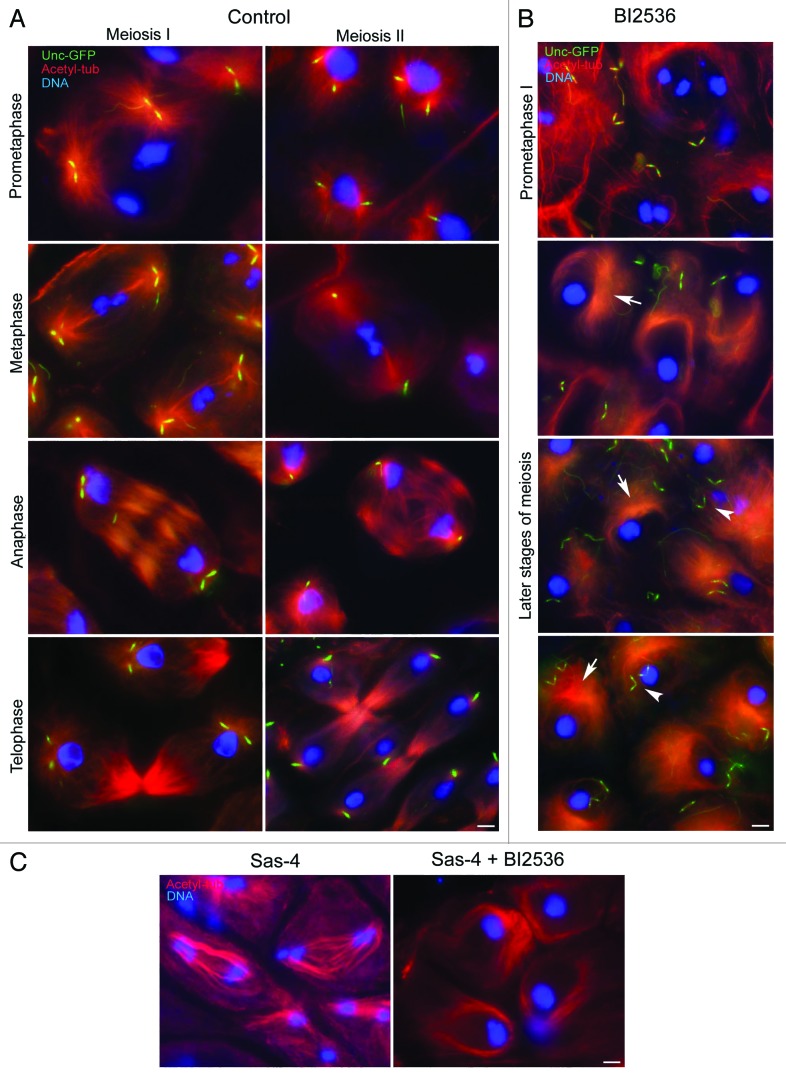
The centriole pairs moved apart during prometaphase in control primary spermatocytes, where they could be observed at the foci of 2 large asters (Fig. 2A) that organize the nascent metaphase spindle. During metaphase of the first meiosis, the centrioles within each pair maintain a V-shaped configuration with their proximal ends in close juxta-position (Fig. 2A). The centrioles disorient during the metaphase–anaphase transition of the first meiosis (Fig. 2A) and move apart. Thus, each daughter telophase nucleus in primary spermatocytes is associated with 2 widely separated centrioles (Fig. 2A). The centrosomal material splits into 2 aggregates and associates with each centriole to form new centrosomes, as it does during the embryonic divisions of the early embryo.23 Thus, 2 small asters can be observed at each opposite pole of the telophase spindles. Since centriole duplication does not occur during the second meiosis of Drosophila male germ cells, the spindle poles of the secondary spermatocytes contain only one centriole (Fig. 2A).
Figure 2. BI2536 affects centriole dynamics and spindle organization during meiosis. Post-prophase control (A) and BI2536-treated (B) spermatocytes expressing Unc-GFP (green) were stained for acetylated tubulin (red) and DNA (blue). The spindle poles of control primary spermatocytes (A) are organized by centrosomes containing 2 close centrioles that separate at anaphase and telophase; thus, the spindle poles contain only one centriole during the second meiosis. BI2536-treated spermatocytes (B) lack spindle poles both during prometaphase and later meiotic stages; the microtubules are disposed in asymmetric arrays (arrows) that resemble mono-polar spindles, but the centrioles do not associate with microtubules and are engaged in a V-shaped configuration (arrowheads). (C) Sas-4 spermatocytes assemble bipolar anastral spindles, but BI2536 induces the formation of monopolar-like structures. Scale bars = 2.5 μm.
Testes from mid-pupae mostly consist of dividing primary and secondary spermatocytes and post-meiotic spermatids. Thus, the incubation in BI2536 would give different phenotypes. Although we were unable to distinguish between the first and the second meiosis in the absence of distinct meiotic spindles, prophase, prometaphase, and post-prometaphase stages were inferred by the chromosome clustering that proceeds in the absence of functional spindles. The spermatid stage was identified by the formation of distinct onion nebenkerns and by the elongation of the axoneme.
BI2536-treated spermatocytes appeared normal during the first meiotic prophase, and chromatin condensed in 3 discrete masses as in control prometaphase cells (Fig. 2B). However, in contrast to untreated spermatocytes, asters of microtubules did not associate with centriole pairs in BI2536-treated spermatocytes (Fig. 2B). The chromatin compacted further to a single mass, as observed in metaphase or later stages of meiosis in control spermatocytes, but meiotic spindles did not form, and microtubules concentrated at one side of the cells in unfocused arrays that did not contain either γ-tubulin or Cnn. Closer examination of microtubules in 10-nm optical sections revealed that they appeared to concentrate and bend around one side of the spermatocyte nucleus. Two pairs of V-shaped centrioles were maintained in the post-prophase BI2536-treated spermatocytes randomly positioned with respect to the mass of microtubules.
To test if the centriole separation failure observed after treatment with BI2536 might be an indirect consequence of defects in cytokinesis we analyzed spermatocytes treated with latrunculin to inhibit cleavage furrow assembly. The resulting failure of cell division resulted in spermatids with supernumerary nuclei, but the process of centriole separation occurred as usual in control cells (Fig. S1). Therefore, the paired centrioles found in BI2536-treated spermatids are not simply the consequence of failed meiotic divisions.
To determine the extent to which microtubule arrays could form independently of centrioles, we examined spermatocytes of Sas-4 mutants that lack centrioles during gametogenesis.24 We found that these mutant spermatocytes assembled anastral bipolar spindles that partially supported chromosome movements (Fig. 2C). However, following BI2536 treatment of such mutant spermatocytes, microtubules never became organized into bipolar arrays but adopted an arrangement similar to that in BI-2536-treated wild-type cells (Fig. 2C). Thus Polo kinase is required to organize microtubules into bipolar arrays in the absence of functional centrosomes. This is likely to reflect the microtubule-organizing ability of chromosomes and kinetochores and is explored in detail elsewhere.25
Centrosome maturation
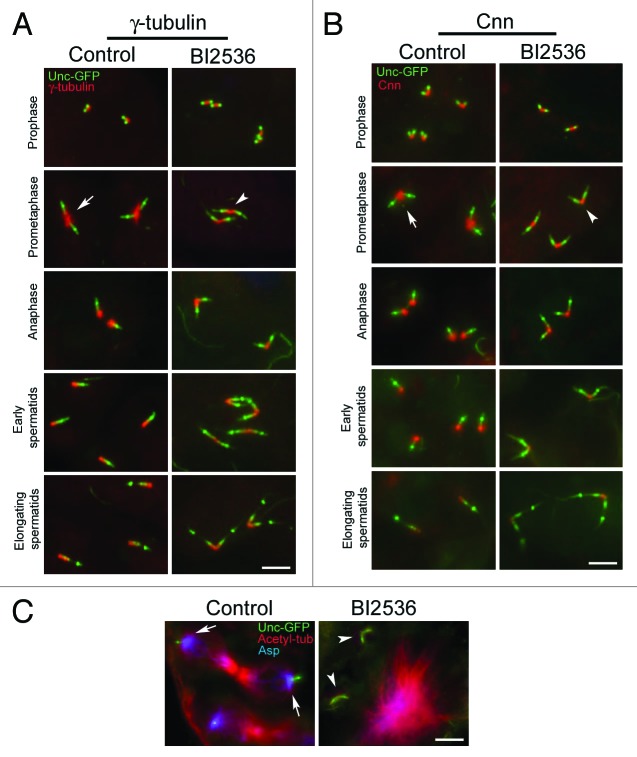
The failure of centrioles to associate with cytoplasmic microtubules in BI2536-treated spermatocytes suggested that inhibition of Polo kinase by BI2536 affects centrosome maturation as it does in mitotic cells. Since the nucleation of microtubules requires protein complexes containing γ-tubulin that are present at the centrosome in prophase and are enriched at the onset of cell division, we examined the distribution of this crucial protein in spermatocytes treated with BI2536. γ-tubulin was present in control cells at the proximal ends of the centrioles during prophase of the first meiosis (Fig. 3A), and then, as cells enter the first prometaphase, the amount of γ-tubulin between sister centrioles dramatically increased (Fig. 3A). γ-tubulin persisted around the proximal ends of newly separated centrioles after anaphase I and was diffusely present along the centriole wall during spermatid differentiation (Fig. 3A). BI2536 treatment did not affect the association of γ-tubulin with the centriole in primary spermatocytes (Fig. 3A), but it showed no significant increase upon completion of prophase (Fig. 3A). This strongly suggested a failure of the centrosomes to undergo maturation in the absence of Polo kinase function.
Figure 3. The recruitment of centrosomal proteins is impaired by BI2536. Control and BI2536 treated male germ cells expressing Unc-GFP (green) were counterstained for γ-tubulin (red; A) or Cnn (red; B). The accumulation of γ-tubulin and Cnn at the centrioles increases in control cells during the meiotic divisions (arrows), but does not augment after BI2536 incubation (arrowheads). (C) Localization of Asp protein (blue) in control and treated spermatocytes expressing Unc-GFP (green) and counterstained for acetylated tubulin (red). Asp is mainly found at the centrosomes in control cells (arrow) but does not associate with centrioles after drug treatment (arrowhead). Scale bars = 2.5 μm.
Since the recruitment of γ-tubulin and Centrosomin (Cnn) are mutually dependent in Drosophila26 and dependent upon Polo kianse in cultured cells27 we asked whether Cnn recruitment was also affected by BI2536-treatment of spermatocytes. We found that Cnn was present during the first prophase in both control and BI2536-treated testes (Fig. 3B). At the onset of meiosis in control cells, there was a significant enrichment of Cnn at the centrosome, and it was maintained at a high cencentration around the proximal ends of centrioles as they separated in anaphase I (Fig. 3B). Cnn was still present at the proximal ends of the centrioles of young control spermatids, but it gradually decreased as the sperm axoneme underwent elongation (Fig. 3B). By contrast, Cnn levels were reduced following BI2536 treatment (Fig. 3B) and were barely detectable during spermatid elongation (Fig. 3B).
Polo is also required for the activation of Abnormal spindle (Asp), a crucial factor for the centrosome nucleation of microtubules at their minus ends.28 We therefore asked whether BI2536 treatment would affect the distribution of the Asp protein. Whereas in control spermatocytes, Asp was localized in the peri-centrosomal region (Fig. 3C), it was not in BI2536-treated spermatocytes (Fig. 3C).
Otherwise, there appeared to be no effects of Polo inhibition upon the presence in centrioles of the Plk4-partner protein, Asterless, and Ana 1 (Fig. S2A and B), proteins required for centriole assembly.29,30 Accordingly, there was no effect of BI2536-= treatment upon centriole numbers, indicating that inhibition of Polo kinase did not affect centriole duplication.
Axoneme elongation
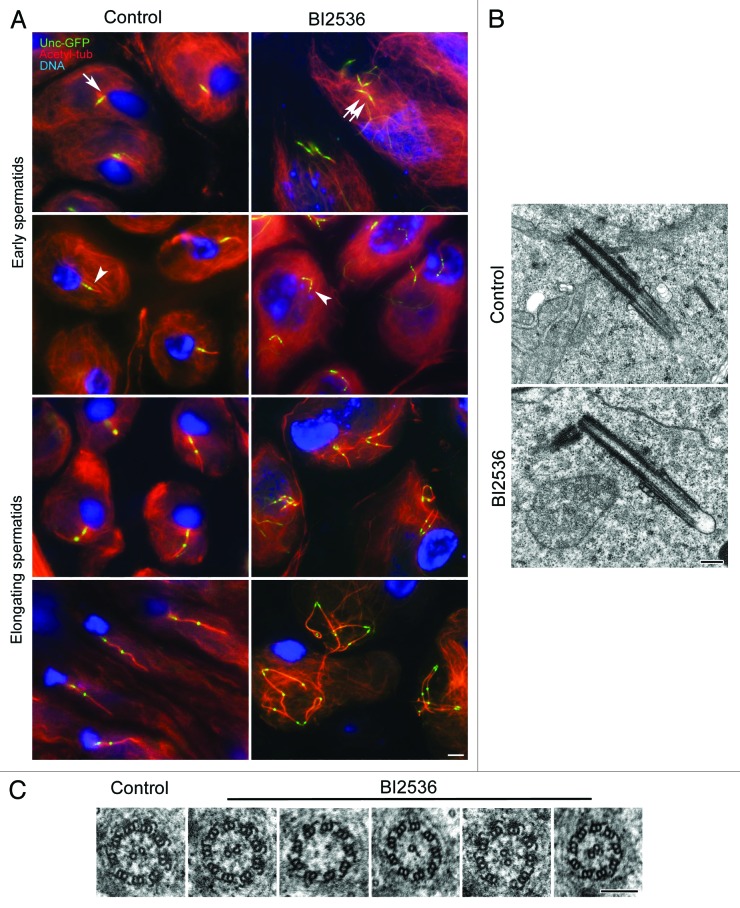
At the end of the second meiotic division in control testes, each spermatid inherits one centriole (Fig. 4A), from which extends a cilium-like projection (Fig. 4B). As spermatids differentiate and the axoneme begins to elongate, the distal dot of Unc-GFP appears to move progressively toward a more distal position (Fig. 4A).31 Although the spermatids in BI2536-treated testes had a single large nucleus, an unusual microtubule network, and 2 pairs of orthogonal centrioles, they still attempted to differentiate (Fig. 4A). EM analysis revealed that chromatin of BI2536-treated spermatids underwent further condensation, and mitochondria became organized in onion-like structures, and axonemes began to grow from each centriole. The centrioles in these cells had short cilium-like projections and were disposed in a V-shaped arrangement with their proximal ends close to each other (Fig. 4B). This suggests that centrioles remained engaged. Immunofluoresecnce microscopy indicated that the axonemes nucleated by each of the co-joined, paired centrioles elongated, and that the distal puncta of the Unc-GFP dot was displaced further distally as in control cells (Fig. 4A). These events could take place without disassembly of the arrays of cytoplamic microtubules in these cells. Moreover, spermatid differentiation and axoneme elongation also proceeded after latrunculin treatment as they did in control and BI2536-treated spermatids (Fig. S1).
Figure 4. Spermatid differentiation proceeds in the presence of BI2536. (A) Control and BI2536-treated spermatids expressing Unc-GFP (green) were counterstained for acetylated-tubulin (red) and DNA (blue). Control spermatids inherit only one centriole at the end of meiosis (arrow), whereas BI2536-treated spermatids inherit 2 pairs of orthogonal centrioles (double arrow). In both control and treated spermatocytes the beginning of axoneme formation is marked by the detachment of the distal Unc-GFP dots (arrowheads) that gradually move away from the centriole. (B) The centrioles have a distal cilium-like structure in both control and treated spermatids; treated spermatids display pairs of engaged centrioles. (C) Control spermatids have 9+2 axonemes, whereas treated spermatids display the conventional model together with abnormal axonemes. Scale bar = 2.5 μm in (A); 250 nm in (B); 100 nm in (C).
The axonemes of the elongating spermatids in BI2536-treated cells showed small differences with those of controls. Whereas axonemes of early control spermatids had the characteristic 9+2 microtubule architecture (Fig. 4C), BI2536-treated spermatids displayed axonemes with the usual 9+2 microtubule pattern and also axonemes showing structural defects: 9+0, 9+1, 9+3 patterns or with missing peripheral tubules (Fig. 4C). This suggests that Polo kinase participates in promoting the correct elongation of axonemal microtubules.
Discussion
Our findings support previous studies suggesting that Polo is required for the organization of the meiotic apparatus and for both chromosome segregation and cytokinesis in Drosophila males.1,5-7 The meiotic defects we find after treatment with BI2536 differ from those described for polo1 mutants in a manner that reflects the weak nature of this hypomorphic allele in contrast to the strong inhibition of Polo kinase activity by BI2536. The absence of a robust spindle assembly checkpoint arrest in spermatocytes enables the formation of late mitotic bipolar spindles that lack a distinct midzone and fail to properly execute cytokinesis in the polo1 mutant.5 Spindle organization defects are also seen in BI2536-treated spermatocytes, but only asymmetrically positioned monopolar-like spindles were found. The lack of typical pericentrosomal proteins, including γ-tubulin, Cnn, and Asp, in association with these cytoplasmic microtubules and the failure of these molecules to be recruited to centrioles, indicates that centrosome maturation is perturbed. This accords with previous findings following Polo depletion in P-element insertion mutants, RNAi, or treatment of cultured Drosophila cells with BI2536.4,27,32
We also find that BI2536 treatment resulted in a failure of microtubules to contact chromosomes. This is likely to reflect some deficiency in the acentrosomal spindle formation mechanism that requires microtubule nucleation at the chromosomes.33,34 This failure of microtubule–kinetochore interactions that we find after BI2536 treatment appears to reflect a requirement for Polo kinase to recruit Augmin to the kinetochores of primary spermatocytes (Savoian and Glover, unpublished data). It points to a key role for Polo kinase in acentrosomal spindle formation in spermatocytes.
A striking finding of our study is that Polo kinase is required for centriole disengagement. This process normally takes place during the metaphase/anaphase transition and in Drosophila spermatogenesis is essential to ensure that each spermatid has a single centriole to nucleate formation of the sperm axoneme. We observe that both spermatocytes and early spermatids have V-shaped configurations of paired centrioles following BI2536 treatment, indicating failure of centriole disengagement. This contrasts to the successful separation of centrioles in the first meiosis of polo1 mutants, in whom the defects in cytokinesis lead to elongating spermatids with 2 or 4 widely separated axonemes,5 again most likely reflecting the weak nature of the polo1 allele compared with the effective inhibition of Polo kinase by BI2536. The effect of the drug on centriole dynamics was dose-dependent. No effects were seen at 10 nM concentration, whereas at a concentration of 100 nM we find that approximately 20–30% of the centrioles were engaged in elongating spermatids. Increasing the concentration of BI2536 at 500 nM, we find about 50–60% of engaged spermatid centrioles. The most severe phenotype with 100% of engaged centrioles was found at a final concentration of 1 μM.
Centriole disengagement in vertebrate cells requires the proteolytic activity of separase,35 which releases engaged centrioles from a “glue” protein. Since separase cleaves cohesin components to allow sister chromatid separation at mitosis,36,37 cohesin itself may represent the ideal substrate for separase at the centrioles also. Cohesin was, indeed, found at the centrosome in cultured human cells,38-40 and depletion of cohesin causes premature separation of paired centrioles.41 Separase and meiosis-specific proteins have been implicated in centriole dynamics during C. elegans spermatogenesis.42 This suggests that cohesin may represent a part of the link that provides connection between the proximal ends of the centrioles.43 Moreover, the sSgo1 splice variant of Shugoshin 1 (Sgo1) that protects cohesin from separase cleavage at the chromosomes44,45 has been also found at the centrosomes,46 where it has been proposed to play a role in preventing cohesin degradation.47 Consistently, depletion of human sSgo1 through RNAi results in the premature separation of paired centrioles in cultured cells.47 Reduced Sgo1 amounts in murine embryonic fibroblasts and in adult bone marrow cells leads to multiple spindle poles.48,49
A short-lived pool of cohesin is transiently associated in Drosophila to centrosomes during metaphase and anaphase,60 and the product of MEI-S332, the Drosophila ortholog of the vertebrate cohesin protector Sgo1, seems to be localized at the spindle poles in male meiosis I cells.51 Polo localizes to centrosomes from metaphase I to metaphase II of Drosophila male meiosis,6 and the release of MEI-S332 in anaphase of the second meiosis and in anaphase of mitosis depends on the Polo kinase activity.52 Thus the finding of conserved proteins at the centrosome suggests that a mechanism of centriole disengagement like that used in vertebrate cells may also function in Drosophila. However, recent experiments in Drosophila have argued against a role for cohesin in maintaining centriole cohesion at least in syncytial embryos.53 This is consistent with the observation that, although sister chromatid separation is blocked in Drosophila separase mutant embryos, centrosomes duplicate correctly.54 Furthermore, the absence of the Drosophila counterpart of pericentrin, proposed as a separase substrate required for centriole cohesion in vertebrate cells, does not prevent the centrosome duplication cycle in flies.55-57 However, the role played by the separase substrate, cohesin, in centriole cohesion has been controversial. One report indicated that expression of non-cleavable cohesin in HeLa cells did not not prevent disengagement,58 whereas another using purified mammalian centrioles suggested centriole engagement did depend upon cohesin integrity.43 Recent observations in early C. elegans embryos point to multiple mechanisms contributing to centriole separation, with the removal of pericentriolar material by microtubule-based forces playing a central role.59 The separase activity becomes critical when the action of the cytoskeleton is reduced. Thus the conflicting roles of cohesin in centriole engagement may be in part explained by the different developmental stages of the systems examined.59 Plk1 promotes, indeed, a separase-independent removal of the centriolar “glue” at prophase,58 whereas in anaphase it stimulates a separase-dependent cleavage of centrosomal cohesin,43 likely by turning cohesin a better substrate for proteolysis after helping to remove sSgo1. This process is reminiscent of the roles of these proteins in removing cohesin from sister chromatids. Plk1 regulates the dissociation of the bulk of cohesin from the chromosome arms in early mitosis,60 whereas the remaining centromeric cohesin was removed at anaphase by the proteolytic activity of separase61 after phosphorylation of Sgo1 by Plk1.62
Although we cannot categorically rule out the involvement of multiple redundant mechanisms, one of which requires separase, the simplest explanation is that centriole disengagement is regulated through alternative proteins in Drosophila, although still under the control of Polo kinase. It has been demonstrated that centrioles can be connected in Drosophila spermatocytes through a link that has been postulated to depend upon the Sas-6–Ana2 complex and that may play a role in centriole engagement.63 Future work will be required to definitively determine whether this is the case.
Materials and Methods
Drosophila strains
The stocks containing the Unc-GFP transgene and the Ana1-GFP were described previously.29,64 Flies expressing Asl-Tomato (RFP) were kindly provided by Timothy Megraw. Flies were raised on standard Drosophila medium at 24 °C.
Antibodies and reagents
Rabbit anti-centrosomin (Cnn; 1:400) and anti-abnormal spindle (Asp; 1:50) have been previously described.65,66 Mouse anti-γ-tubulin-GTU88 (1:100) and mouse anti-acetylated tubulin (1:100) were from Sigma-Aldrich. Alexa Fluor 488-, 555-, and 647 secondary antibodies (1:800) were purchased from Invitrogen. BI2536 was purchased by Selleck. Dimethyl sulfoxide (DMSO), Latrunculin B, and Shields and Sang M3 Insect Medium were purchased from Sigma-Aldrich. BI2536 and Latruncilin B were dissolved in DMSO at stock concentration of 1000 μM and stored frozen at 20 °C. The stock solution was diluted to the desired concentration in culture medium prior to incubation with testes.
Culture and drug treatment experiments
Testes were dissected from pupae between 5–7 d in M3 medium. To inhibit Polo kinase, testes were incubated 24 h in M3 medium containing 10 nM, 100 nM, 500 nM, or 1 μM BI2536 for 24 h into a sterile 24-well plate at 24 °C.
To assess the effect of microfilament depolymerization on centriole dynamics the dissected testes were cultured at 24 °C for 24 h in M3 medium containing 0.5 μM Latrunculin B. Incubation of testes in M3 medium containing DMSO but lacking drugs had no effect on centrosome dynamics or on the organization of the cytoskeleton.
Indirect immunofluorescence staining
After incubation, the testes were washed in M3 medium for 10 min and then in phosphate-buffered saline (PBS) for 5 min. Then testes were placed in a small drop of 5% glycerol in PBS on a glass slide and squashed under a small cover glass and frozen in liquid nitrogen. After removal of the coverslip, the samples adhering to the slides were immersed in cold methanol for 10 min. For localization of centrosomal components, testes were washed for 15 min in PBS and incubated for 1 h in PBS containing 0.1% bovine serum albumin (PBS-BSA) to block non-specific staining. Testes were incubated overnight at 4 °C with the specific antisera against Cnn and Asp. For localization of γ-tubulin, testes were incubated in the GTU88 antibody. To visualize microtubules, testes were incubated with anti-acetylated tubulin antibody for 4–5 h at room temperature. After washing in PBS–BSA the samples were incubated for 1 h at room temperature with the appropriate secondary antibodies. In all cases, DNA was visualized with incubation of 3–4 min in Hoechst. Testes were mounted in small drops of 90% glycerol in PBS. Images were taken by using an Axio Imager Z1 (Carl Zeiss) microscope equipped with an AxioCam HR cooled charge-coupled camera (Carl Zeiss). Grayscale digital images were collected separately and then pseudocolored and merged using Adobe Photoshop 7.0 software (Adobe Systems).
Transmission electron microscopy
Testes incubated in M3 medium in the presence or absence of drugs were washed twice in the free-drug medium, then 5 min with PBS, and fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS) overnight at 4 °C. Samples were carefully rinsed in PBS and subsequently post-fixed in 1% osmium tetroxide in PBS for 1 h at 4 °C. After rinsing in PBS, the material was dehydrated through a graded series of ethanol, infiltrated with a mixture of Epon–Araldite resin and polymerized at 60 °C for 48 h. Serial ultrathin sections (65–75 nm) were cut with a Reichert ultramicrotome equipped with a diamond knife, collected with formvar-coated copper grids, and stained with uranyl acetate and lead citrate. TEM preparations were observed with a FEI Tecnai G2 Spirit transmission electron microscope operating at an accelerating voltage of 100 kV and equipped with a Morada CCD camera (Olympus).
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Eyal Schejter and Timothy Megraw for generously providing antibodies and stocks. This work was supported by a grant from PRIN2012 to G.C.
References
- 1.Sunkel CE, Glover DM. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci. 1988;89:25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Llamazares S, Moreira A, Tavares A, Girdham C, Spruce BA, Gonzalez C, Karess RE, Glover DM, Sunkel CE. polo encodes a protein kinase homolog required for mitosis in Drosophila. Genes Dev. 1991;5(12A):2153–65. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez C, Sunkel CE, Glover DM. Interactions between mgr, asp, and polo: asp function modulated by polo and needed to maintain the poles of monopolar and bipolar spindles. Chromosoma. 1998;107:452–60. doi: 10.1007/s004120050329. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson MM, Tavares AA, Ohkura H, Deak P, Glover DM. Metaphase arrest with centromere separation in polo mutants of Drosophila. J Cell Biol. 2001;153:663–76. doi: 10.1083/jcb.153.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmena M, Riparbelli MG, Minestrini G, Tavares AM, Adams R, Callaini G, Glover DM. Drosophila polo kinase is required for cytokinesis. J Cell Biol. 1998;143:659–71. doi: 10.1083/jcb.143.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrmann S, Amorim I, Sunkel CE. The POLO kinase is required at multiple stages during spermatogenesis in Drosophila melanogaster. Chromosoma. 1998;107:440–51. doi: 10.1007/pl00013778. [DOI] [PubMed] [Google Scholar]
- 7.Riparbelli MG, Callaini G, Glover DM. Failure of pronuclear migration and repeated divisions of polar body nuclei associated with MTOC defects in polo eggs of Drosophila. J Cell Sci. 2000;113:3341–50. doi: 10.1242/jcs.113.18.3341. [DOI] [PubMed] [Google Scholar]
- 8.Barr FA, Silljé HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–40. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 9.Petronczki M, Lénárt P, Peters JM. Polo on the rise-from mitotic entry to cytokinesis with Plk1. Dev Cell. 2008;14:646–59. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 10.van de Weerdt BC, Medema RH. Polo-like kinases: a team in control of the division. Cell Cycle. 2006;5:853–64. doi: 10.4161/cc.5.8.2692. [DOI] [PubMed] [Google Scholar]
- 11.Elez R, Piiper A, Kronenberger B, Kock M, Brendel M, Hermann E, Pliquett U, Neumann E, Zeuzem S. Tumor regression by combination antisense therapy against Plk1 and Bcl-2. Oncogene. 2003;22:69–80. doi: 10.1038/sj.onc.1206038. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Lei M, Erikson RL. Normal cells, but not cancer cells, survive severe Plk1 depletion. Mol Cell Biol. 2006;26:2093–108. doi: 10.1128/MCB.26.6.2093-2108.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spänkuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K. Effect of RNA silencing of Polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst. 2002;94:1863–77. doi: 10.1093/jnci/94.24.1863. [DOI] [PubMed] [Google Scholar]
- 14.Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human Polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–13. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian YW, Erikson E, Maller JL. Purification and cloning of a protein kinase that phosphorylates and activates the Polo-like kinase Plx1. Science. 1998;282:1701–4. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- 16.Lénárt P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of Polo-like kinase 1. Curr Biol. 2007;17:304–15. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 17.Santamaria A, Neef R, Eberspächer U, Eis K, Husemann M, Mumberg D, Prechtl S, Schulze V, Siemeister G, Wortmann L, et al. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and late stages of mitosis. Mol Biol Cell. 2007;18:4024–36. doi: 10.1091/mbc.E07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strebhardt K, Ullrich A. Targeting Polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–30. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 19.Giménez-Abián JF, Sumara I, Hirota T, Hauf S, Gerlich D, de la Torre C, Ellenberg J, Peters JM. Regulation of sister chromatid cohesion between chromosome arms. Curr Biol. 2004;14:1187–93. doi: 10.1016/j.cub.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 20.Lu B, Mahmud H, Maass AH, Yu B, van Gilst WH, de Boer RA, Silljé HH. The Plk1 inhibitor BI 2536 temporarily arrests primary cardiac fibroblasts in mitosis and generates aneuploidy in vitro. PLoS One. 2010;5:e12963. doi: 10.1371/journal.pone.0012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, Gürtler U, Garin-Chesa P, Lieb S, Quant J, et al. BI 2536, a potent and selective inhibitor of Polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316–22. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Rebollo E, González C. Visualizing the spindle checkpoint in Drosophila spermatocytes. EMBO Rep. 2000;1:65–70. doi: 10.1093/embo-reports/kvd011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callaini G, Riparbelli MG. Centriole and centrosome cycle in the early Drosophila embryo. J Cell Sci. 1990;97:539–43. doi: 10.1242/jcs.97.3.539. [DOI] [PubMed] [Google Scholar]
- 24.Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW. Flies without centrioles. Cell. 2006;125:1375–86. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Savoian MS, Glover DM. Drosophila Klp67A binds prophase kinetochores to subsequently regulate congression and spindle length. J Cell Sci. 2010;123:767–76. doi: 10.1242/jcs.055905. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Megraw TL. Proper recruitment of gamma-tubulin and D-TACC/Msps to embryonic Drosophila centrosomes requires Centrosomin Motif 1. Mol Biol Cell. 2007;18:4037–49. doi: 10.1091/mbc.E07-05-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu J, Glover DM. Structured illumination of the interface between centriole and peri-centriolar material. Open Biol. 2012;2:120104. doi: 10.1098/rsob.120104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.do Carmo Avides M, Tavares A, Glover DM. Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nat Cell Biol. 2001;3:421–4. doi: 10.1038/35070110. [DOI] [PubMed] [Google Scholar]
- 29.Blachon S, Cai X, Roberts KA, Yang K, Polyanovsky A, Church A, Avidor-Reiss T. A proximal centriole-like structure is present in Drosophila spermatids and can serve as a model to study centriole duplication. Genetics. 2009;182:133–44. doi: 10.1534/genetics.109.101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dzhindzhev NS, Yu QD, Weiskopf K, Tzolovsky G, Cunha-Ferreira I, Riparbelli M, Rodrigues-Martins A, Bettencourt-Dias M, Callaini G, Glover DM. Asterless is a scaffold for the onset of centriole assembly. Nature. 2010;467:714–8. doi: 10.1038/nature09445. [DOI] [PubMed] [Google Scholar]
- 31.Gottardo M, Callaini G, Riparbelli MG. The cilium-like region of the Drosophila spermatocyte: an emerging flagellum? J Cell Sci. 2013;126:5441–52. doi: 10.1242/jcs.136523. [DOI] [PubMed] [Google Scholar]
- 32.Bettencourt-Dias M, Giet R, Sinka R, Mazumdar A, Lock WG, Balloux F, Zafiropoulos PJ, Yamaguchi S, Winter S, Carthew RW, et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 2004;432:980–7. doi: 10.1038/nature03160. [DOI] [PubMed] [Google Scholar]
- 33.Maiato H, DeLuca J, Salmon ED, Earnshaw WC. The dynamic kinetochore-microtubule interface. J Cell Sci. 2004;117:5461–77. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- 34.Wadsworth P, Khodjakov A. E pluribus unum: towards a universal mechanism for spindle assembly. Trends Cell Biol. 2004;14:413–9. doi: 10.1016/j.tcb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–51. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- 36.Uhlmann F, Lottspeich F, Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- 37.Nasmyth K, Peters JM, Uhlmann F. Splitting the chromosome: cutting the ties that bind sister chromatids. Science. 2000;288:1379–85. doi: 10.1126/science.288.5470.1379. [DOI] [PubMed] [Google Scholar]
- 38.Guan J, Ekwurtzel E, Kvist U, Yuan L. Cohesin protein SMC1 is a centrosomal protein. Biochem Biophys Res Commun. 2008;372:761–4. doi: 10.1016/j.bbrc.2008.05.120. [DOI] [PubMed] [Google Scholar]
- 39.Wong RW, Blobel G. Cohesin subunit SMC1 associates with mitotic microtubules at the spindle pole. Proc Natl Acad Sci U S A. 2008;105:15441–5. doi: 10.1073/pnas.0807660105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong X, Ball AR, Jr., Sonoda E, Feng J, Takeda S, Fukagawa T, Yen TJ, Yokomori K. Cohesin associates with spindle poles in a mitosis-specific manner and functions in spindle assembly in vertebrate cells. Mol Biol Cell. 2009;20:1289–301. doi: 10.1091/mbc.E08-04-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura A, Arai H, Fujita N. Centrosomal Aki1 and cohesin function in separase-regulated centriole disengagement. J Cell Biol. 2009;187:607–14. doi: 10.1083/jcb.200906019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schvarzstein M, Pattabiraman D, Bembenek JN, Villeneuve AM. Meiotic HORMA domain proteins prevent untimely centriole disengagement during Caenorhabditis elegans spermatocyte meiosis. Proc Natl Acad Sci U S A. 2013;110:E898–907. doi: 10.1073/pnas.1213888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schöckel L, Möckel M, Mayer B, Boos D, Stemmann O. Cleavage of cohesin rings coordinates the separation of centrioles and chromatids. Nat Cell Biol. 2011;13:966–72. doi: 10.1038/ncb2280. [DOI] [PubMed] [Google Scholar]
- 44.McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 2005;3:e86. doi: 10.1371/journal.pbio.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salic A, Waters JC, Mitchison TJ. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell. 2004;118:567–78. doi: 10.1016/j.cell.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Gregson HC, Schmiesing JA, Kim JS, Kobayashi T, Zhou S, Yokomori K. A potential role for human cohesin in mitotic spindle aster assembly. J Biol Chem. 2001;276:47575–82. doi: 10.1074/jbc.M103364200. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Yang Y, Duan Q, Jiang N, Huang Y, Darzynkiewicz Z, Dai W. sSgo1, a major splice variant of Sgo1, functions in centriole cohesion where it is regulated by Plk1. Dev Cell. 2008;14:331–41. doi: 10.1016/j.devcel.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada HY, Yao Y, Wang X, Zhang Y, Huang Y, Dai W, Rao CV. Haploinsufficiency of SGO1 results in deregulated centrosome dynamics, enhanced chromosomal instability and colon tumorigenesis. Cell Cycle. 2012;11:479–88. doi: 10.4161/cc.11.3.18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricke RM, Jeganathan KB, Malureanu L, Harrison AM, van Deursen JM. Bub1 kinase activity drives error correction and mitotic checkpoint control but not tumor suppression. J Cell Biol. 2012;199:931–49. doi: 10.1083/jcb.201205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren WD, Steffensen S, Lin E, Coelho P, Loupart M, Cobbe N, Lee JY, McKay MJ, Orr-Weaver T, Heck MM, et al. The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Philos Trans R Soc Lond B Biol Sci. 2005;360:543–52. doi: 10.1016/s0960-9822(00)00806-x. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, Hayashi-Hagihara A, Orr-Weaver TL. Roles and regulation of the Drosophila centromere cohesion protein MEI-S332 family. J Cell Sci. •••;200:733–42. doi: 10.1098/rstb.2005.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke AS, Tang TT, Ooi DL, Orr-Weaver TL. POLO kinase regulates the Drosophila centromere cohesion protein MEI-S332. Dev Cell. 2005;8:53–64. doi: 10.1016/j.devcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Oliveira RA, Nasmyth K. Cohesin cleavage is insufficient for centriole disengagement in Drosophila. Curr Biol. 2013;23:R601–3. doi: 10.1016/j.cub.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Pandey R, Heidmann S, Lehner CF. Epithelial re-organization and dynamics of progression through mitosis in Drosophila separase complex mutants. J Cell Sci. 2005;118:733–42. doi: 10.1242/jcs.01663. [DOI] [PubMed] [Google Scholar]
- 55.Matsuo K, Ohsumi K, Iwabuchi M, Kawamata T, Ono Y, Takahashi M. Kendrin is a novel substrate for separase involved in the licensing of centriole duplication. Curr Biol. 2012;22:915–21. doi: 10.1016/j.cub.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 56.Lee K, Rhee K. Separase-dependent cleavage of pericentrin B is necessary and sufficient for centriole disengagement during mitosis. Cell Cycle. 2012;11:2476–85. doi: 10.4161/cc.20878. [DOI] [PubMed] [Google Scholar]
- 57.Martinez-Campos M, Basto R, Baker J, Kernan M, Raff JW. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J Cell Biol. 2004;165:673–83. doi: 10.1083/jcb.200402130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsou MF, Wang WJ, George KA, Uryu K, Stearns T, Jallepalli PV. Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev Cell. 2009;17:344–54. doi: 10.1016/j.devcel.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cabral G, Sans SS, Cowan CR, Dammermann A. Multiple mechanisms contribute to centriole separation in C. elegans. Curr Biol. 2013;23:1380–7. doi: 10.1016/j.cub.2013.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, Peters JM. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515–25. doi: 10.1016/S1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 61.Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM. Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 2005;3:e69. doi: 10.1371/journal.pbio.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakajima M, Kumada K, Hatakeyama K, Noda T, Peters JM, Hirota T. The complete removal of cohesin from chromosome arms depends on separase. J Cell Sci. 2007;120:4188–96. doi: 10.1242/jcs.011528. [DOI] [PubMed] [Google Scholar]
- 63.Stevens NR, Roque H, Raff JW. DSas-6 and Ana2 coassemble into tubules to promote centriole duplication and engagement. Dev Cell. 2010;19:913–9. doi: 10.1016/j.devcel.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baker JD, Adhikarakunnathu S, Kernan MJ. Mechanosensory-defective, male-sterile unc mutants identify a novel basal body protein required for ciliogenesis in Drosophila. Development. 2004;131:3411–22. doi: 10.1242/dev.01229. [DOI] [PubMed] [Google Scholar]
- 65.Vaizel-Ohayon D, Schejter ED. Mutations in centrosomin reveal requirements for centrosomal function during early Drosophila embryogenesis. Curr Biol. 1999;9:889–98. doi: 10.1016/S0960-9822(99)80393-5. [DOI] [PubMed] [Google Scholar]
- 66.Saunders RD, Avides MC, Howard T, Gonzalez C, Glover DM. The Drosophila gene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J Cell Biol. 1997;137:881–90. doi: 10.1083/jcb.137.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.