Abstract
Cdc55, a regulatory B subunit of the protein phosphatase 2A (PP2A) complex, plays various functions during mitosis. Sequestration of Cdc55 from the nucleus by Zds1 and Zds2 is important for robust activation of mitotic Cdk1 and mitotic progression in budding yeast. However, Zds1-family proteins are found only in fungi but not in higher eukaryotes. In animal cells, highly conserved ENSA/ARPP-19 family proteins bind and inhibit PP2A–B55 activity for mitotic entry.
In this study, we compared the relative contribution of Zds1/Zds2 and ENSA-family proteins Igo1/Igo2 on Cdc55 functions in budding yeast mitosis. We confirmed that Igo1/Igo2 can inhibit Cdc55 in early mitosis, but their contribution to Cdc55 regulation is relatively minor compared with the role of Zds1/Zds2. In contrast to Zds1, which primarily localized to the sites of cell polarity and in the cytoplasm, Igo1 is localized in the nucleus, suggesting that Igo1/Igo2 inhibit Cdc55 in a manner distinct from Zds1/Zds2.
Our analysis confirmed an evolutionarily conserved function of ENSA-family proteins in inhibiting PP2A-Cdc55, and we propose that Zds1-dependent sequestration of PP2A-Cdc55 from the nucleus is uniquely evolved to facilitate closed mitosis in fungal species.
Keywords: PP2A, Cdc55, Zds1, Igo1, mitosis
Introduction
In eukaryotic cells, activation of cyclin-dependent kinase 1 (Cdk1) complexed with mitotic cyclins promotes mitosis.1 Regulatory mechanisms of Cdk1 activation have been studied extensively;2 however, far less is understood about the control of the protein phosphatases that counteracts the mitotic Cdk1 activity. A key phosphatase that antagonizes Cdk1 activity and dephosphorylates Cdk1 substrates is the protein phosphatase 2A (PP2A) in complex with its regulatory subunit B55 (Cdc55 in budding yeast).3
The PP2A is a trimeric complex and consists of a scaffolding A subunit, a regulatory B subunit, and a catalytic C subunit.4 Among them, the regulatory B subunit is largely responsible for the subcellular localization and substrate specificity of PP2A.4
In budding yeast, cells lacking Cdc55 (a regulatory B subunit) exhibit multiple cell cycle defects, including mitotic entry,5-10 spindle assembly checkpoint (SAC),5,11-13 and precocious mitotic exit.12,14-18 We have proposed previously that PP2A-Cdc55 activity is largely controlled by its subcellular distribution.13,16 Cdc55 binding protein Zds1 (zillion different screens) and its homolog Zds2 are cytoplasmic proteins and sequester Cdc55 from the nucleus during mitosis.13,16 Sequestration of PP2A-Cdc55 in the cytoplasm by Zds1/Zds2 promotes mitotic entry and allows full activation of mitotic Cdk1 in the nucleus. Removal or inhibition of nuclear Cdc55 is also necessary for robust activation of APC-Cdc20 during metaphase–anaphase transition, as well as for the robust activation of Cdc14 phosphatase essential for mitotic exit.13,16
Because Zds1/Zds2 are important regulators of PP2A-Cdc55, zds1Δ zds2Δ cells exhibit pleiotropic cell cycle defects. First, zds1Δ zds2Δ cells are severely impaired in mitotic entry, and, as a consequence, they exhibit hyper-elongated cell morphology and cold temperature sensitivity.19-21 Second, zds1Δ zds2Δ cells are also impaired in mitotic exit, because nuclear PP2A-Cdc55 activity prevents activation of a Cdc14 phosphatase.14,15 These phenotypes of zds1Δ zds2Δ cells are due to nuclear accumulation of Cdc55, because either deletion or nuclear exclusion of Cdc55 rescues both phenotypes.8,14,15
On the other hand, overexpression of ZDS1 (or ZDS2) can inhibit nuclear functions of PP2A-Cdc55 by sequestering Cdc55 in the cytoplasm. For example, overexpression of ZDS2 promotes precocious mitotic entry.8 Overexpression of ZDS1 activates APC-Cdc20 and, as a consequence, causes SAC defects similar to cdc55Δ cells.13 Furthermore, overexpression of ZDS1 rescues the growth defect of late mitotic mutants such as cdc15–2.15 Although Zds1/Zds2 inhibit nuclear functions of PP2A-Cdc55, Zds1/Zds2 are not necessarily inhibitors of PP2A-Cdc55 catalytic activity; rather, it is formally possible that Zds1/Zds2 promote cytoplasmic functions of PP2A-Cdc55.
In animal cells, inhibition of PP2A–B55 activity is a prerequisite for mitotic entry.22 However, animal cells lack an apparent homolog of Zds1. Recent studies have identified α-endosulfine (ENSA) and Arpp19 as potent inhibitors of PP2A-B55.23,24 ENSA-family proteins bind directly to PP2A-B55 and inhibit its activity after phosphorylation by the Greatwall kinase.23,24
ENSA family proteins are widely conserved among eukaryotes. In budding yeast, Rim15 (a homolog of Greatwall kinase) phosphorylates ENSA family proteins Igo1 and Igo2 (initiation of G0) and promotes their binding to PP2A–Cdc55 complex. Rim15 and Igo1/Igo2 were originally identified as key regulators of entry into quiescence (G0) state.25 A recent study has shown that Rim15-Igo1/Igo2 can directly inhibit PP2A-Cdc55 activity in vitro,26,27 and that igo1Δ igo2Δ cells are defective in mitotic entry.27
However, relative contribution of ENSA-family proteins and Zds1-family proteins in regulation of PP2A-Cdc55-associated processes during mitosis and why Zds1-family proteins are uniquely evolved in fungal species remain interesting questions.
In this study, we performed a comparative genetic analysis of Zds1/Zds2 and Igo1/Igo2 to elucidate their relative contribution to mitotic progression via Cdc55 and to evaluate their differences. Our genetic analysis suggests that Igo1 and Igo2 serve as inhibitors of PP2A-Cdc55 in vivo, but their contribution is relatively minor compared with Zds1 and Zds2. We also found that Igo1 is enriched in the nucleus, in contrast to Zds1/Zds2, which are exclusively cytoplasmic. This suggests that, in agreement with the genetic data provided, Igo1/Igo2 are controlling PP2A-Cdc55 in a distinct mechanism from Zds1/Zds2.
Results
Zds1/Zds2 and Igo1/Igo2 prevent the toxic effects caused by CDC55 overexpression
It has been proposed that both Zds1/Zds2 and Igo1/Igo2 are inhibitors of PP2A–Cdc55 activity.13,16,27 To test this hypothesis in vivo, we examined the effects of CDC55 overexpression in the presence or absence of these proteins. We expected that overexpression of CDC55 is more toxic in the absence of its inhibitors.
Overexpression of CDC55 under strong GAL1 promoter was highly toxic to wild-type cells (Fig. 1C). This toxicity was most likely due to defects in mitotic entry, since cells overexpressing CDC55 exhibited elongated bud morphology (Fig. 1A). In budding yeast, Cdk1 activation upon mitotic entry is negatively regulated by Swe1 (wee1 homolog) and positively regulated by Mih1 (cdc25 homolog) (Fig. 1B). We found that deletion of SWE1 partially rescued GAL1–CDC55 toxicity, while deletion of MIH1 rendered the cells more sensitive to GAL1-CDC55 (Fig. 1C). These results suggest that GAL1-CDC55 delays mitotic entry in a manner dependent both on Swe1 and Mih1.
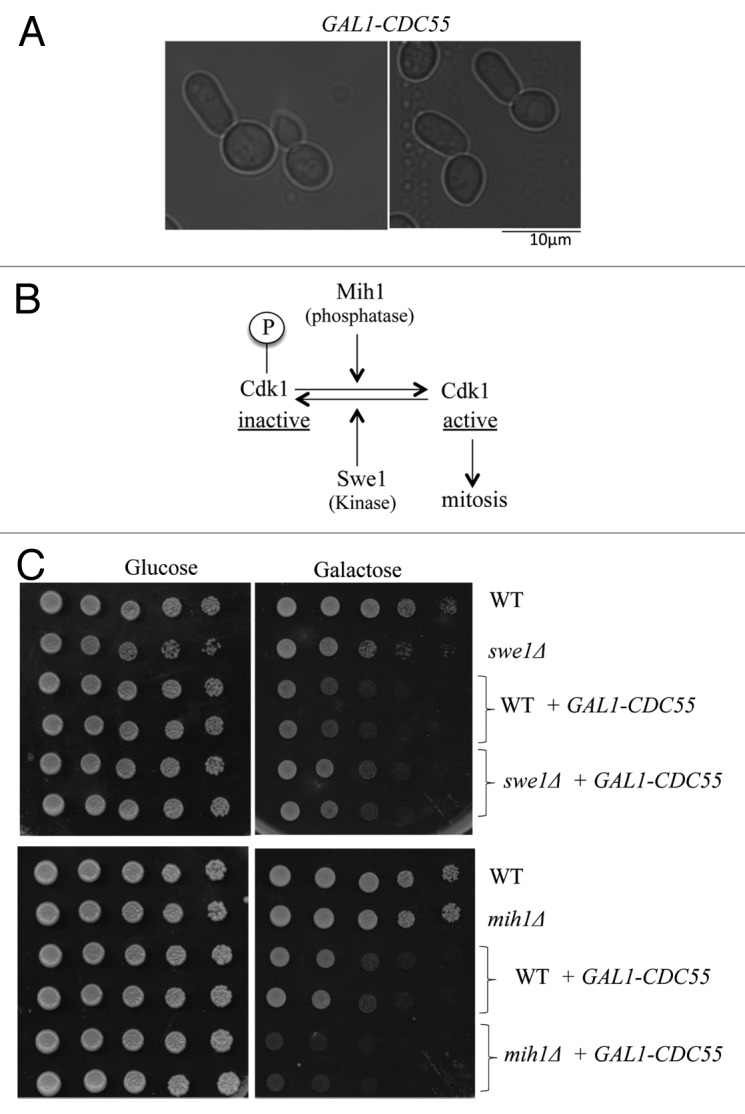
Figure 1. Overexpression of CDC55 delays mitotic entry (A) Representative images of cells overexpressing CDC55 under the strong GAL1 promoter. Cells were grown in YEP medium containing galactose (2%) for 3 h at 24 °C. (B) Schematic model of mitotic entry in budding yeast. (C) Wild-type (WT), swe1Δ or mih1Δ cells were transformed with either empty vector or GAL1-CDC55-containing plasmid. Saturated cultures were serially (10 x) diluted and spotted on SC (synthetic complete)-URA media containing either glucose (CDC55 off) or galactose (CDC55 on). Images were taken after 3 d at 24 °C.
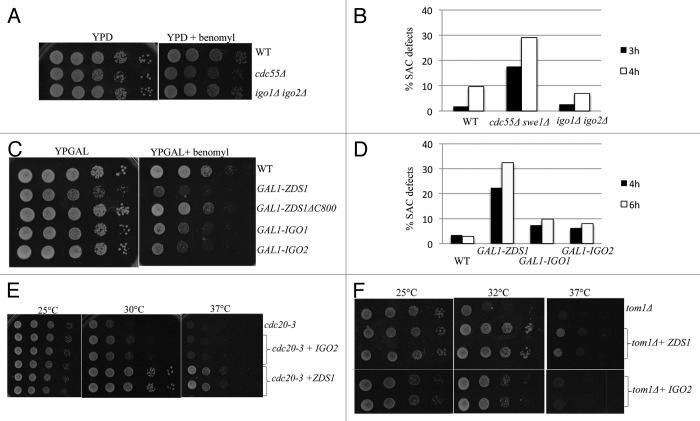
We found that the toxicity of GAL1-CDC55 is much stronger in zds1Δ zds2Δ cells compared with wild-type cells. This is consistent with the idea that Zds1/Zds2 can act as in vivo inhibitors of Cdc55 activity or function (Fig. 2A). We also found that overexpression of ZDS1 suppressed the toxicity of GAL1-CDC55 (Fig. 2B). Importantly, overexpression of ZDS1 lacking the Cdc55-binding domain (ZDS1ΔC800)16 failed to suppress the toxic effects of GAL1-CDC55 (Fig. 2B), confirming that Zds1 inhibits abnormal Cdc55 activity by direct interaction. These results suggest that Zds1/Zds2-dependent retention of Cdc55 in the cytoplasm is critical when Cdc55 is overexpressed.
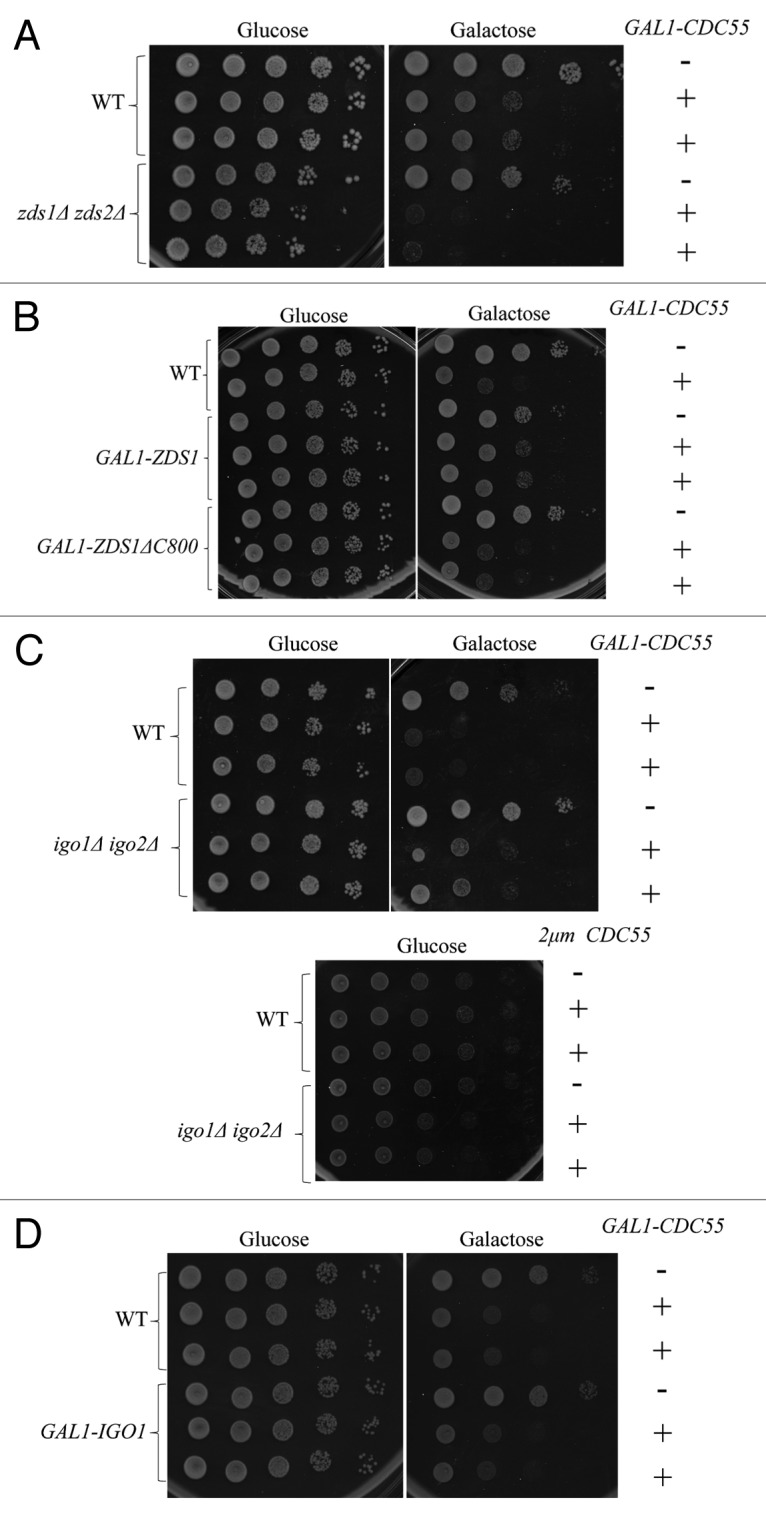
Figure 2.ZDS1/2 and IGO1/2 counteract with CDC55 overexpression Each saturated culture was serially (10x) diluted and spotted on glucose (CDC55 off) or galactose (CDC55 on). Images were taken after 3 d at room temperature. (A) GAL1-CDC55 is more toxic in zds1Δ zds2Δ cells. (B) Overexpression of ZDS1 rescued the toxicity caused by GAL1-CDC55. Note that overexpression of ZDS1ΔC800 lacking Cdc55-binding domain failed to rescue the toxicity of GAL1-CDC55. (C) Top: igo1Δ igo2Δ cells grew better than wild-type cells in galactose media. igo1Δ igo2Δ cells expressing GAL1-CDC55 grew better than wild-type cells with GAL1-CDC55. However, the better growth in the galactose media was associated with igo1Δ igo2Δ cells, not with GAL1-CDC55. Bottom: Overexpression of Cdc55 under its endogenous promoter with a multicopy plasmid is slightly more toxic to igo1Δ igo2Δ cells compared with the wild-type cells. (D) Overexpression of IGO1 did not rescue the toxicity caused by GAL1-CDC55.
In contrast to our prediction, GAL1-CDC55 was less toxic to igo1Δ igo2Δ cells compared with the wild-type cells in BY4741 strain background (Fig. 2C). Reduced toxicity of GAL1-CDC55 in igo1Δ, igo2Δ, and rim15Δ cells has also been previously reported in w303 background.27 In addition, overexpression of IGO1 failed to rescue the toxicity of GAL1-CDC55 (Fig. 2D). These results are inconsistent with our hypothesis that Igo1/Igo2 are inhibitors of PP2A-Cdc55.
Because we noticed that igo1Δ igo2Δ cells grow much better than wild-type cells in galactose media, we re-evaluated the effect of CDC55 overexpression in glucose media using a multicopy vector containing CDC55 under the control of its endogenous promoter. CDC55 expressed from a multicopy plasmid was slightly more toxic to igo1Δ igo2Δ cells compared with wild-type cells (Fig. 2C, bottom). We do not know why igo1Δ igo2Δ cells can grow better than wild-type cells in galactose, but it is interesting to note that cdc55Δ grows poorly in the galactose media (data not shown). The opposite phenotype of igo1Δ igo2Δ cells and cdc55Δ suggests that regulation of Cdc55 by Igo1/Igo2 is important for cell growth in different carbon sources. The reduced toxicity of GAL-CDC55 in igo1Δ igo2Δ cells was due to different carbon source in the media, thus we concluded that Igo1/Igo2 are serving as inhibitors of Cdc55 function in vivo.
IGO1/IGO2 have minor roles in mitotic entry and function in parallel with ZDS1/ZDS2
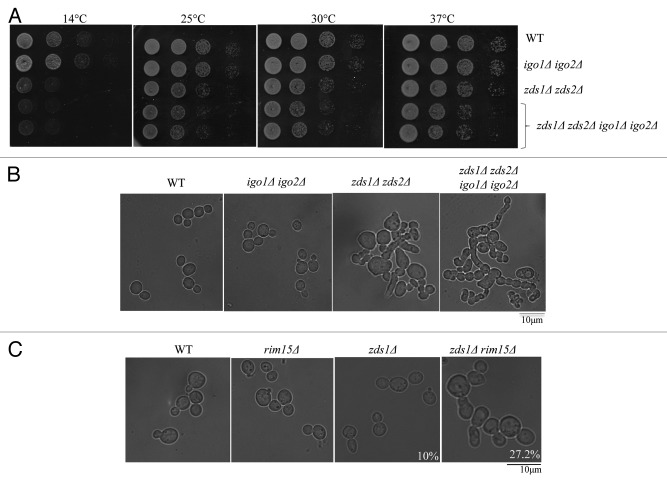
To gain insights into the role and relative contributions of Igo1/Igo2 in PP2A–Cdc55 regulation during the cell cycle, we re-evaluated the phenotype of igo1Δ igo2Δ cells in comparison to zds1Δ zds2Δ cells in our strain background (BY4741). zds1Δ zds2Δ cells are known to exhibit abnormally elongated cell morphology and show cold-sensitive growth defects.5,8,16,20 These phenotypes are associated with mitotic entry defects, because both abnormal morphology and cold sensitivity of zds1Δ zds2Δ cells are rescued either by deletion of SWE116 or by introduction of CDC28Y19F mutation.5 Moreover, we proposed that these phenotypes are due to an abnormal presence of Cdc55 in the nucleus. In our strain background (BY4741), igo1Δ igo2Δ cells did not exhibit any obvious morphological defects (Fig. 3B) or cold sensitivity (Fig. 3A) in contrast to zds1Δ zds2Δ cells.16 However, in agreement with the study using w303 background,27 igo1Δ igo2Δ cells caused synthetic morphological and growth defects when combined with the zds1Δ zds2Δ in BY4741 background (Fig. 3A and B).
Figure 3. Deletion of IGO1 and IGO2 has synthetic growth defects with deletion of ZDS1 and ZDS2. (A) Serial dilutions of the indicated strains were spotted on YPD at different temperatures (B) and (C) Morphology of the cells grown in YPD at 24 °C. The strains used are in BY4741 background (B) or in w303 (C). The percentage of elongated buds is 10% in zds1Δ cells (n = 100) and 27.2% in zds1Δ rim15Δ (n = 110).
Because Rim15 is known to phosphorylate Igo1/Igo2 to promote their binding to PP2A-Cdc55,26,27 we also tested the genetic interaction between RIM15 and ZDS1. Deletion of RIM15 did not give any obvious morphological defects in w303 background; however, rim15Δ zds1Δ cells exhibited a greater number of abnormally elongated buds (27%) compared with zds1Δ cells (10%) (Fig. 3C). These genetic interactions suggest that Rim15-Igo1/Igo2 function in a parallel pathway to Zds1/Zds2 for mitotic entry. To understand the relationship between Igo1/Igo2 and Zds1/Zds2, we further examined if Zds1/Zds2 can substitute for Igo1/Igo2 or Rim15. We used w303 background for this analysis, because both igo1Δ igo2Δ and rim15Δ cells exhibit stronger cold-sensitive growth phenotype than in our strain background (BY4741).27 We found that the cold-sensitive growth defects of igo1Δ, igo2Δ, and rim15Δ cells were efficiently suppressed by overexpression of ZDS1 (Fig. 4A). In contrast, overexpression of IGO2 failed to rescue the cold-sensitive growth defects of zds1Δ zds2Δ cells (Fig. 4B). These results suggest that although Igo1/Igo2 are potent inhibitors of PP2A-Cdc55 in vitro, IGO2 has limited or minor effect in vivo compared with ZDS1/ZDS2. We also found that cold-sensitive growth defects of rim15Δ cells were rescued by overexpression of IGO2, consistent with IGO2 being an important downstream target of the Rim15 kinase.26-28 The partial rescue of the cold sensitivity of rim15Δ cells by overexpression of IGO2 suggests that phosphorylation of Igo2 by Rim15 is essential for its full activation, or, alternatively, Rim15 may have other downstream targets than Igo1/Igo2.
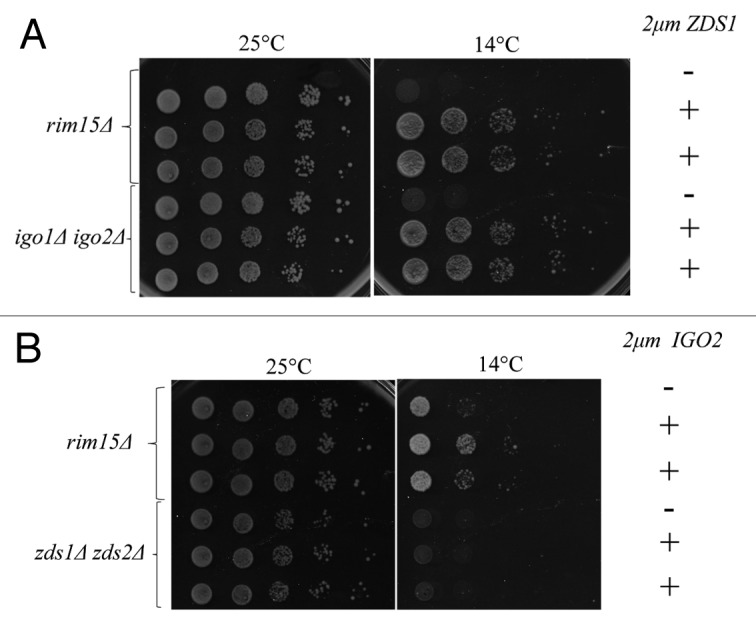
Figure 4. Genetic interactions between ZDS1/ZDS2 and RIM15-IGO1/IGO2 (A) Overexpression of ZDS1 rescued the cold-sensitive growth defects of rim15Δ and igo1Δ igo2Δ cells. Serial dilutions of the indicated strains were spotted on SC-URA medium at 14 °C and 24 °C. 2μm ZDS1 is a high copy number plasmid expressing ZDS1. (B) Overexpression of IGO2 rescued the cold-sensitive growth defect of rim15Δ but not zds1Δ zds2Δ cells. Serial dilutions of the indicated strains were spotted on SC-URA medium at 14 °C and 24 °C. 2μm IGO2 is a high copy number plasmid expressing IGO2.
Igo1/Igo2 can inhibit PP2A–Cdc55 activity in the SAC arrest
Because Cdc55 is essential for mitotic arrest by SAC activation through inhibition of APC-Cdc20,5,11,13 we tested whether Igo1/Igo2 are involved in the SAC and show genetic interaction with CDC20.
To test if Igo1/Igo2 have roles in the SAC, we first examined the effects of spindle damaging drugs. Unlike cdc55Δ, igo1Δ igo2Δ cells were not highly sensitive to microtubule-depolymerizing drug benomyl (Fig. 5A). SAC-defective mutants fail to maintain mitotic arrest in the presence of microtubule-depolymerizing drug nocodazole and proceed through the next round of the cell cycle, resulting in multi-budded phenotype.29,30 We confirmed that igo1Δ igo2Δ cells were tightly arrested in G2/M phase in the presence of nocodazole (Fig. 5B), using cdc55Δ swe1Δ as a SAC-defective positive control. Thus, igo1Δ igo2Δ cells are not defective in the SAC similar to zds1Δ zds2Δ cells.13 Because PP2A-Cdc55 is essential for the SAC, and because GAL1-ZDS1 causes SAC defects in a manner dependent on its Cdc55-binding domain (801–915 a.a.) (ref. 13; Fig. 5C), we tested whether overexpression of IGO1 or IGO2 can impair the SAC, and found that both GAL1-IGO1 and GAL1-IGO2 caused benomyl sensitivity (Fig. 5C). The rebudding assay confirmed that both GAL1-IGO1 and GAL1-IGO2 cells showed SAC defects (Fig. 5D), although the effect was milder compared with GAL1-ZDS1 cells.
Figure 5. Overexpression of IGO2 rescues the growth defects of cdc20–3, and tom1 cells and causes a mild SAC defects. (A) igo1Δ igo2Δ cells were not sensitive to benomyl (20 μg/ml). cdc55Δ cells were used as a control. (B) igo1Δ igo2Δ cells were not defective in the SAC. Each strain was grown in YPD medium containing 15 μg/ml of nocodazole. After 3 and 4 h, rebudded cells (indicator of the SAC defects) were counted. At least 150 cells for each strain were scored. (C) Overexpression of IGO1 or IGO2 caused mild benomyl sensitivity. Serial dilutions of the strains with the indicated genotypes were spotted on YP Galactose with or without benomyl (12.5 μg/ml) at 24 °C. (D) Overexpression of IGO1 or IGO2 caused mild SAC defects. Each strain was grown in YP Galactose medium containing 15 μg/ml nocodazole. After 4 and 6 h, rebudded cells were counted. At least 100 cells for each strain were scored. (E) Overexpression of IGO2 can weakly rescue the temperature-sensitive growth defects of cdc20–3 cells. Serial dilutions of the strains with the indicated genotypes were spotted on SC-URA at the indicated temperatures. (F) Overexpression of IGO2 can weakly rescue the temperature-sensitive growth defects of tom1Δ cells. Serial dilutions of the strains with the indicated genotypes were spotted on SC-URA at the indicated temperatures.
The target of PP2A-Cdc55 in the SAC is APC-Cdc20, whose activity is essential for anaphase onset. We have previously demonstrated that deletion of cdc55 or overexpression of ZDS1 can rescue the temperature-sensitive growth defects of cdc20–3.13 Although it is less efficient compared with that of ZDS1, overexpression of IGO2 partially rescued the growth defects of cdc20-3 cells (Fig. 5E), suggesting that Igo2 can inhibit PP2A–Cdc55 activity toward APC-Cdc20.
We also found that overexpression of IGO2 and ZDS1 partially rescues the temperature-sensitive growth defects of tom1Δ cells (Fig. 5F). TOM1 encodes an HECT type E3 ubiquitin ligase, and tom1Δ cells arrest in early mitosis at high temperature.13 Because removal of nuclear Cdc55 rescues the temperature-sensitive growth defect of tom1Δ,13 it is highly likely that overexpression of IGO2 and ZDS1 rescues tom1Δ by inhibition of nuclear PP2A-Cdc55.
These data suggest that, when overexpressed, Igo2 also can inhibit PP2A–Cdc55 function in early mitosis, and as a consequence, APC-Cdc20 is activated, leading to impairment in the SAC.
Igo1/Igo2 play no detectable role in mitotic exit
It is known that nuclear PP2A-Cdc55 prevents mitotic exit by inhibition of Cdc14.12,14,16,18 Thus, Cdc55 needs to be inactivated or removed from the nucleus for timely mitotic exit.14 Because Cdc55 accumulates in the nucleus throughout mitosis, zds1Δ zds2Δ cells are defective in Cdc14 activation and exhibit synthetic lethality with lte1Δ, which encodes an activator of mitotic exit.16 In contrast to zds1Δ zds2Δ, igo1Δ igo2Δ showed no synthetic growth defects with lte1Δ even at semi-permissive temperatures (Fig. 6A). This result suggests that Igo1/Igo2 are not required for timely mitotic exit and are not involved in inhibition of nuclear Cdc55 in anaphase.
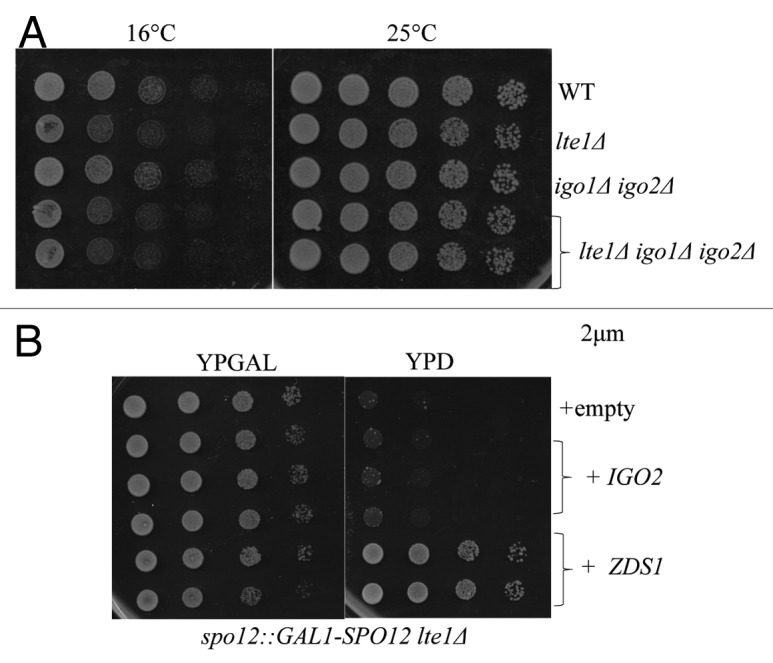
Figure 6. Igo1/Igo2 have no obvious defects in mitotic exit. (A) The deletions of IGO1 and IGO2 were not synthetic lethal with lte1Δ. Serial dilutions of the indicated strains were spotted on YPD medium at 14 °C and 24 °C. (B) Overexpression of ZDS1 but not IGO2 can rescue synthetic lethality of spo12Δ lte1Δ. Serial dilutions of the spo12 Δ::GAL1-SPO12 lte1 Δ strain whose viability is dependent on galactose. Indicated strains overexpressing either IGO2 or ZDS1 were spotted on SC-URA medium containing glucose or galactose medium at 24 °C.
Although lte1Δ is non-essential at the room temperature, it is partially defective in mitotic exit and shows synthetic lethality with spo12Δ, another mutant partially defective in mitotic exit.32 The synthetic lethality of spo12Δ lte1Δ is rescued by additional deletion of CDC55.12 We found that overexpression of ZDS1 efficiently rescued the lethality of spo12Δ::GAL1-SPO12 lte1Δ in glucose medium (Fig. 6B); however, overexpression of IGO2 failed to suppress the spo12Δ lte1Δ lethality. Thus, we conclude that IGO1/IGO2 do not play any detectable roles in mitotic exit.
Igo1-GFP is enriched in the nucleus
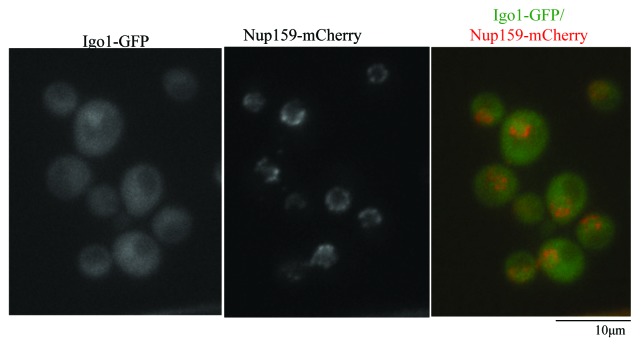
To better understand the function of Igo1, we examined the subcellular localization. In a clear contrast to Zds1-GFP, which localizes to the bud cortex, to the bud neck, and in the cytoplasm, Igo1 was enriched in the nucleus. (Fig. 7). This is consistent with the previous work28 that reported that Igo1/Igo2 proteins localize to both to the nucleus and cytoplasm. Nuclear accumulation of Igo1 is independent of Cdc55 binding, as nuclear localization of Igo1-GFP was unaffected in cdc55Δ cells (data not shown). This suggests that Igo1/Igo2 nuclear localization is independent of Cdc55.
Figure 7. Igo1-GFP is enriched in the nucleus and is not localized at the bud cortex or at the bud neck. (A) Igo1-GFP expressed at the endogenous locus was visualized together with a nuclear envelope marker Nup159-mCherry. Merged image is shown in the right panel.
Nuclear enrichment of Igo1 is consistent with the idea that Igo1 inhibits PP2A–Cdc55 activity in the nucleus. Little overlap of subcellular localization may explain the difference between Igo1/Igo2 and Zds1/Zds2.
Both Zds1/Zds2 and Igo1/Igo2 control nuclear accumulation of Cdc55, but only Zds1/Zds2 are essential for cortical localization of Cdc55
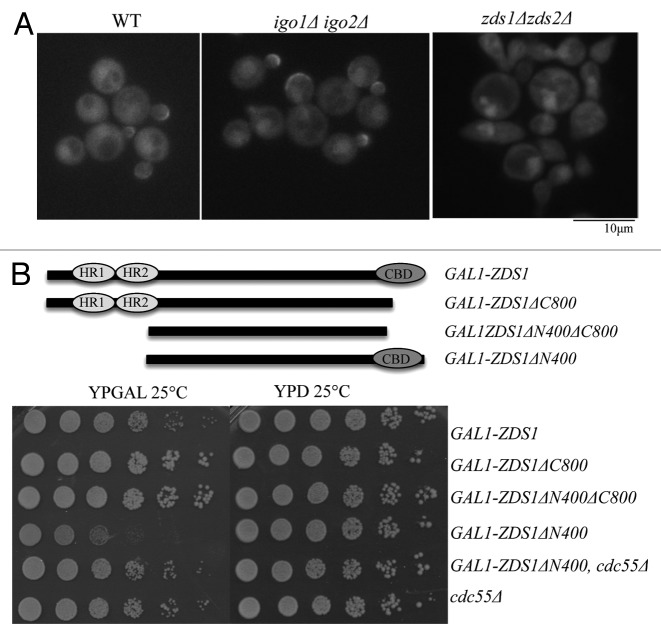
Lastly we examined if Igo1/Igo2 regulates subcellular localization of Cdc55. We used w303 background for this analysis, because igo1Δ igo2Δ cells exhibit a more severe phenotype than BY4741, as previously mentioned. In the wild-type cells, Cdc55-GFP is localized both in the nucleus and cytoplasm. In addition, Cdc55-GFP localized to the bud cortex in small to medium budded cells and at the bud neck in late mitotic cells16,33 as in BY4741. In zds1Δ zds2Δ cells, consistent with our previous report,16 both the cortical and bud neck localization of Cdc55-GFP were completely lost, and Cdc55-GFP accumulated in the nucleus (Fig. 8A). In igo1Δ igo2Δ cells, Cdc55 localization to the bud cortex and bud neck was unaffected; however, there was an increase in nuclear Cdc55–GFP signal (Fig. 8A). This observation is consistent with the previous report, which used the immunostaining of Cdc55 tagged with HA epitope.27 Thus, Igo1/Igo2 can partially affect nucleocytoplasmic distribution of Cdc55.
Figure 8.ZDS1 is essential for Cdc55 localization to the sites of cell polarity and can induce Cdc55-dependent toxicity. (A) Localization of Cdc55-GFP in igo1Δ igo2Δ and in zds1Δ zds2Δ. Note that cortical localization of Cdc55 was unaffected in igo1Δ igo2Δ but was completely lost in zds1Δ zds2Δ. (B) Top: Domain structure of the Zds1: HR, homology region; CBD, Cdc55-binding domain. Truncated constructs are as follows: full-length Zds1 (1–915 a.a.), Zds1ΔC800 (1–800 a.a.), Zds1ΔN400ΔC800 (401–800 a.a.), Zds1ΔN400 (401–915 a.a.). All the constructs were expressed under the control of the strong GAL1 promoter. Bottom: Each saturated culture were serially (5×) diluted and spotted on glucose (ZDS1 off) or galactose (ZDS1 on).
Nuclear accumulation of Cdc55-GFP in igo1Δ igo2Δ and in zds1Δ zds2Δ suggests that they may share similar functions in retaining or in exporting Cdc55 to the cytoplasm. We attempted to compare the nuclear localization of Cdc55-GFP in zds1Δ zds2Δ igo1Δ igo2Δ and in zds1Δ zds2Δ, but it was technically challenging because these cells suffered from highly abnormal morphology, and the nuclear signal of Cdc55-GFP is already very strong in zds1 zds2Δ cells.
Zds1/Zds2 are possible positive regulators of PP2A-Cdc55 in the cytoplasm
Essential requirement of Zds1/Zds2 in the cortical Cdc55 localization suggests that Zds1/Zds2 are not simply inhibiting nuclear Cdc55, but also do have distinct, positive roles in Cdc55 functions. Consistent with this idea, we found that overexpression of truncated Zds1 lacking the first 400 amino acids (GAL1-ZDS1ΔN400) caused toxic effects on cell growth (Fig. 8B).
The toxic effects of GAL1-ZDS1ΔN400 was due to abnormal PP2A–Cdc55 activation, because the toxicity was cancelled in cdc55Δ (Fig. 8B; note that cdc55Δ grew poorly in galactose media). Furthermore, the toxicity of GAL1-ZDS1ΔN400 was cancelled by the removal of its Cdc55-binding domain (GAL1-ZDS1ΔN400ΔC800; Fig. 8B). These results suggest that overexpression of active Zds1 cause hyperactivation of PP2A-Cdc55 that leads to growth defects. (Fig. 8B)
Discussion
Physiological function of ENSA-family proteins in budding yeast cell cycle
Our genetic data are in agreement with the idea that Igo1/Igo2 can function as inhibitors of PP2A-Cdc55. Although igo1Δ igo2Δ cells don’t have obvious defects in mitotic progression, they showed synthetic growth defects and prolonged G2 phase in combination with deletion of ZDS1 and ZDS2. Thus Igo1/Igo2 is regulating Cdc55-PP2A during mitotic entry in parallel with Zds1 and Zds2. A role of Igo1 and Igo2 in mitotic entry is consistent with the recent finding in a different strain background,27 although the phenotype of igo1Δ igo2Δ cells in our background was much weaker. The idea that Igo1/Igo2 and Zds1/Zds2 are sharing overlapping functions is also supported by our finding that the cold-sensitive growth defects of igo1Δ igo2Δ cells and rim15Δ (in w303) were rescued by overexpression of ZDS1. Other than in mitotic entry, we were not able to find detectable mitotic defects in igo1Δ igo2Δ. For example, igo1Δ igo2Δ cells are competent in the SAC arrest and in mitotic exit. These results suggest that Igo1/Igo2 are active toward Cdc55 only at the time of mitotic entry. Consistent with this idea, it has been reported that in vivo complex formation of Igo1 and Cdc55 is maximum at G2/M phase.27 Thus, compared with Zds1/Zds2, physiological contribution of Igo1/Igo2 in Cdc55 regulation during mitosis is either functionally or temporally limited.
Overexpressions of IGO1 and IGO2 also suggest that these proteins can inhibit nuclear functions of PP2A-Cdc55. We found that overexpression of IGO1 or IGO2 causes weak SAC defects and suppresses the temperature-sensitive growth defects of cdc20–3 and tom1Δ cells. These genetic interactions are similar to CDC55 deletion or overexpression of ZDS1. However, overexpression of IGO1 or IGO2 is not as effective as ZDS1 overexpression. Furthermore, overexpression of IGO1 or IGO2 failed to promote mitotic exit in GAL1-spo12 lte1Δ cells and failed to rescue the cold-sensitive growth defects of zds1Δ zds2Δ cells.
One possible explanation why overexpression of IGO1 and IGO2 has only a weak effect on Cdc55 function is because Igo1/Igo2 require Rim15-dependent phosphorylation for their full activity. It is of interest to test co-expression of Rim15 and Igo1/Igo2 or to overexpress a phosphomimetic mutant form of Igo1/Igo2.
Above all, our genetic data suggests that Igo1/Igo2 function as inhibitors of Cdc55 in vivo, but their effect is limited compared with that of Zds1 and Zds2.
How do Igo1/Igo2 inhibit Cdc55?
We confirmed that Igo1 is enriched in the nucleus but does not localize to the bud cortex or the bud neck. Given that Igo1/Igo2 can directly inhibit PP2A–Cdc55 phosphatase activity in vitro,26,27 it is highly likely that Igo1/Igo2 are mainly inhibiting nuclear PP2A-Cdc55 by direct binding. It is also possible that Igo1/Igo2 have additional regulatory function in PP2A-Cdc55. In igo1Δ igo2Δ cells, we found that cortical localization of Cdc55 was unaffected, but Cdc55 was accumulated in the nucleus. A similar observation was reported in the recent paper,26 suggesting that Igo1/Igo2 are not only inhibiting Cdc55 activity, but also regulating Cdc55 localization.
It is interesting that the localization pattern of Igo1/Igo2 is largely different from that of Zds1/Zds2, which localize to the bud cortex and the neck and are excluded from the nucleus.16,34 Distinct localization pattern predicts that the molecular mechanisms by which Igo1/Igo2 and Zds1/Zds2 regulate Cdc55 are different. If these proteins are regulating nucleocytoplasmic distribution of Cdc55, we speculate that the nuclear proteins Igo1/Igo2 can promote the export of Cdc55 from the nucleus, while cytoplasmic proteins Zds1/Zds2 retain Cdc55 in the cytoplasm by sequestration. Further analysis is required to fully understand the molecular mechanisms by which these Cdc55 interacting proteins regulate PP2A–Cdc55 functions, but we propose that although they share overlapping functions, Zds1/Zds2 and Igo1/Igo2 are controlling PP2A-Cdc55 in a distinct manner.
Zds1 is not necessarily an inhibitor of PP2A-Cdc55
We have previously proposed that Zds1/Zds2 inhibit nuclear Cdc55 functions by sequestrating Cdc55 in the cytoplasm.16 This proposal is based on the observation that Cdc55 accumulates in the nucleus in zds1Δ zds2Δ cells, and that overexpression of ZDS1 excludes Cdc55 from the nucleus. In our model, Zds1/Zds2 are not necessarily inhibitors of Cdc55; rather, Zds1/Zds2 should behave as activators of cytoplasmic Cdc55 functions.
Consistent with this model, we found that overexpression of GAL1-ZDS1ΔN400 causes toxicity to the cells in a manner dependent on Cdc55 interaction. Since PP2A-Cdc55 is localized to the bud cortex and to the bud neck in a manner exclusively dependent on Zds1/Zds2,16 cell polarity regulators are attractive candidate to be the targets of PP2A–Cdc55–Zds1/Zds2 complex in the cytoplasm. In agreement with this idea, Cdc55 and Zds1/Zds2 are known to regulate polarized growth and cell wall biogenesis.35,36 Identification of the molecular targets and functions of Cdc55 in the cytoplasm requires further investigation.
Why Zds1-family proteins are unique to fungal cells?
One interesting question is why yeasts, but not animal cells, require Zds1-family proteins in addition to ENSA-family proteins to control PP2A-Cdc55. Because Zds1 homologs are only found in fungal species, they are likely dedicated for fungal cell-specific regulations or functions of PP2A-Cdc55. One attractive idea is that Zds1-dependent exclusion of PP2A-Cdc55 during mitosis is critical only in fungal cells whose nuclear envelope does not break down. If exclusion of Cdc55 from the nucleus is the important function of Zds1-family proteins, they are not necessary in animal cells, because there is no nuclear envelope during mitosis.
Another, but not mutually exclusive, idea is that Zds1-family proteins are used for fungi-specific functions of PP2A. One attractive candidate of fungi-specific target of PP2A is cell wall biogenesis machinery because PP2A-Cdc55 and Zds1/Zds2 are known to promote cell wall assembly.36 Further investigation is required to fully understand the molecular mechanisms by which Zds1-family proteins and ENSA-family proteins regulate PP2A functions in vivo, but our analysis revealed that these PP2A regulators are functioning in a totally distinct fashion in the cell.
Materials and Methods
Yeast genetics
All yeast strains used in this study were isogenic or congenic to BY4741 (MATa leu2Δ0 his3Δ1 met15Δ0 ura3Δ0, obtained from Thermo Fisher Scientific) or to w303 (Mata, ade2–1, trp1–1, leu2–3,112, his3–11,15, ura3, ssd1). Standard yeast genetic was used to generate the strains. Yeast strains are listed in the Table 1. PY3295 and SY strains were gifts from D Pellman (Dana-Farber Cancer Institute). Gene deletions or modifications were performed with PCR-mediated one-step gene replacement using pFA6a vectors provided by J Pringle (Stanford University)38 and confirmed by PCR. The ZDS1 and IGO2 plasmids were obtained from the National Bio-Resource Project (NBRP). The GAL1-CDC55 plasmid was a gift from Y Kikuchi (Gakushuin University). Nocodazole was used at 15 µg/ml. Benomyl was used at 12.5 µg/ml or 20 µg/ml as indicated in the legends. For galactose induction, 2% of galactose was added to the medium.
Table 1.
| Strain | Mat | Genotype | Background | Source |
|---|---|---|---|---|
| PY 3295 | a | leu2Δ0 his3Δ1 met15Δ0 ura3Δ0 | BY4741 | Rossio and Yoshida 2011 |
| SY 1390 | a | swe1::LEU2 | BY4741 | Rossio and Yoshida 2011 |
| SY 069 | a | mih1::kanMX6 | BY4741 | This study |
| MH 35 | a | WT + [URA3-GAL1-CDC55, 2μm] | BY4741 | This study |
| MH 44 | a | SY1390+ [URA3, 2μm] | BY4741 | This study |
| MH 45 | a | SY1390 + [URA3-GAL1-CDC55, 2μm] | BY4741 | This study |
| MH 46 | a | SY069+ [URA3, 2μm] | BY4741 | This study |
| MH 47 | a | SY069 + [URA3-GAL1-CDC55, 2μm] | BY4741 | This study |
| VR 1291 | a | igo1::kanMX6, igo2::kanMX6 | BY4741 | Gift from N. Talarek |
| VR 1295 | a | VR 1291+ [URA3, 2μm] | BY4741 | This study |
| VR 1303 | a | VR 1291+ [URA3-GAL1-CDC55, 2μm] | BY4741 | This study |
| VR 36 | zds1Δ::kanMX6 zds2Δ::His3MX6 | BY4741 | Rossio and Yoshida 2011 | |
| VR 146 | a | zds1Δ::kanMX6-GAL1–3xHA-ZDS1-GFP-His3MX6 | BY4741 | Rossio et al., 2013 |
| VR148 | a | zds1Δ::His3MX6-GAL1–3xHA-zds1Δc800-GFP-kanMX6 | BY4741 | Rossio et al., 2013 |
| VR 200 | a | zds1Δ::His3MX6-GAL1–3xHA-ZDS1-GFP-kanMX6 + [URA3, 2μm] | BY4741 | This study |
| VR 196 | a | zds1Δ::His3MX6-GAL1–3xHA-ZDS1-GFP-kanMX6 + [URA3-GAL1-CDC55, 2μm] | BY4741 | This study |
| VR 202 | a | zds1Δ::His3MX6-GAL1–3xHA- zds1Δc800-GFP-kanMX6 + [URA3, 2μm] | BY4741 | This study |
| VR 198 | a | zds1Δ::HIS3-GAL1–3xHA-zds1Δc800-GFP-kanMX6 + [URA3-GAL1-CDC55, 2μm] | BY4741 | This study |
| VR 1541 | a | igo1Δ::His3MX6-GAL1-IGO1 | BY4741 | This study |
| VR 1542 | a | igo2Δ::His3MX6-GAL1-IGO2 | BY4741 | This study |
| VR 1980 | a | zds1Δ::kanMX6 zds2Δ::His3MX6 igo1Δ::natNT2 igo2Δ::hphR | BY4741 | This study |
| VR 1981 | α | zds1Δ::kanMX6 zds2Δ::His3MX igo1Δ::natNT2 igo2Δ::hphR | BY4741 | This study |
| VR 1293 | a | igo1Δ::natNT2 igo2Δ::hphR | BY4741 | Gift from N. Talarek |
| VR 1957 | a | rim15Δ::natNT2 | w303 | Juanes et al., 2013 |
| VR 1995 | a | zds1Δ::TRP1, CDC55-GFP::His3MX | w303 | This study |
| VR 1996 | α | zds1Δ::TRP1, rim15Δ::natNT2 CDC55-GFP::HIS3MX6 | w303 | This study |
| VR 1994 | α | rim15Δ::natNT2 CDC55-GFP::His3MX6 | w303 | This study |
| VR 1958 | a | igo1Δ::natNT2 igo2Δ::hphR | w303 | Juanes et al., 2013 |
| VR 1959 | a | zds1Δ::TRP1 zds2Δ::hphR | w303 | Juanes et al., 2013 |
| VR 81 | a | lte1Δ::kanMX6 | BY4741 | Rossio and Yoshida 2011 |
| VR 1334 | α | lte1Δ::kanMX6 igo1Δ::natNT2 igo2Δ::hphR | BY4741 | This study |
| VR 1335 | a | lte1Δ::kanMX6 igo1Δ::natNT2 igo2Δ::hphR | BY4741 | This study |
| VR 1474 | α | lte1Δ::kanMX6 spo12Δ::His3MX6-GAL1-SPO12 | BY4741 | This study |
| VR 31 | a | cdc55Δ::kanMX6 | BY4741 | Rossio and Yoshida 2011 |
| VR 40 | a | cdc55Δ::kanMX6 swe1Δ::kanMX6 | BY4741 | Rossio and Yoshida 2011 |
| K8029 | a | cdc20–3 | w303 | Gift from Kikuchi |
| VR 1556 | a | cdc20–3 + [URA3, 2μm] | w303 | Rossio et al. 2013 |
| VR 1557 | a | cdc20–3 + [ZDS1-URA3, 2μm] | w303 | Rossio et al. 2013 |
| VR 1761 | a | tom1–10::His3MX6 | w303 | Sasaki et al., 2000 |
| VR 1966 | a | IGO1-GFP::His3MX6 NUP159-mCherry-kanMX6 | BY4741 | This study |
| VR 1363 | a | CDC55-GFP::His3MX6 | w303 | This study |
| VR 1972 | a | CDC55-GFP::His3MX6 zds1Δ::TRP1 zds2Δ::hphR | w303 | This study |
| VR 1969 | a | igo1Δ::natR igo2Δ::kanMX6 CDC55-GFP::His3MX6 | w303 | This study |
| An70 | a | zds1Δ:: His3MX6-GAL1–3xHA-ZDS1-GFP-kanMX6 | BY4741 | This study |
| An16 | a | zds1Δ::kanMX6-GAL1–3xHA-ZDS1 ΔN400-GFP-His3MX6 | BY4741 | This study |
| An27 | a | zds1Δ:: kanMX6-GAL1–3xHA-ZDS1 ΔN400ΔC800-GFP-His3MX6 | BY4741 | This study |
| An56 | a | zds1Δ::kanMX6-GAL1–3xHA-ZDS1 ΔN400-GFP-His3MX6 cdc55:: kanMX6 | BY4741 | This study |
Spindle assembly checkpoint (SAC) assay
Yeast cells arrest as large budded cells in mitosis in response to SAC activation; however, the SAC mutants continue through the cell cycle and form a new bud (re-budding). Logarithmically growing cells were treated with 15 μg/ml nocodazole, and the cells were fixed in 70% ethanol at each pf the indicated time points at room temperature. The samples were then washed twice in phosphate-buffered saline (PBS), vortexed vigorously, and examined by bright-field microscopy.
Fluorescence microscopy
Fluorescence images were acquired with a fluorescence microscope (Eclipse E600; Nikon) equipped with a charge-coupled device camera (DC350F; Andor) and 100× (NA1.45) or 60× (NA1.4) oil objectives. The images were captured using appropriate filters and were analyzed with NIS-Elements software (Nikon).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs David Pellman, Jim Haber, Nicholas Talarek, John Pringle, Yoshiko Kikuchi, and Simonetta Piatti for the gift of the reagents. We also thank Simonetta Piatti for comments on the manuscript. This work was supported by a MLSC grant for S.Y. and by the American Italian Cancer fellowship for V.R.
Glossary
Abbreviations:
- SAC
spindle assembly checkpoint
References
- 1.Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–8. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 2.Lindqvist A, Rodríguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong AL, Yang Y. PP2A function toward mitotic kinases and substrates during the cell cycle. BMB Rep. 2013;46:289–94. doi: 10.5483/BMBRep.2013.46.6.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–84. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Burke DJ. Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:620–6. doi: 10.1128/mcb.17.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan JN, Sia RA, Bardes ES, Lew DJ. Phosphorylation-independent inhibition of Cdc28p by the tyrosine kinase Swe1p in the morphogenesis checkpoint. Mol Cell Biol. 1999;19:5981–90. doi: 10.1128/mcb.19.9.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Jiang W, Gentry M, Hallberg RL. Loss of a protein phosphatase 2A regulatory subunit (Cdc55p) elicits improper regulation of Swe1p degradation. Mol Cell Biol. 2000;20:8143–56. doi: 10.1128/MCB.20.21.8143-8156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasutis K, Vignali M, Ryder M, Tameire F, Dighe SA, Fields S, Kozminski KG. Zds2p Regulates Swe1p-Dependent Polarized Cell Growth in S. cerevisiae via a Novel Cdc55p Interaction Domain. Mol Biol Cell. 2010 doi: 10.1091/mbc.E10-04-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal G, Paraz MT, Kellogg DR. Regulation of Mih1/Cdc25 by protein phosphatase 2A and casein kinase 1. J Cell Biol. 2008;180:931–45. doi: 10.1083/jcb.200711014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wicky S, Tjandra H, Schieltz D, Yates J, 3rd, Kellogg DR. The Zds proteins control entry into mitosis and target protein phosphatase 2A to the Cdc25 phosphatase. Mol Biol Cell. 2011;22:20–32. doi: 10.1091/mbc.E10-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minshull J, Straight A, Rudner AD, Dernburg AF, Belmont A, Murray AW. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr Biol. 1996;6:1609–20. doi: 10.1016/S0960-9822(02)70784-7. [DOI] [PubMed] [Google Scholar]
- 12.Yellman CM, Burke DJ. The role of Cdc55 in the spindle checkpoint is through regulation of mitotic exit in Saccharomyces cerevisiae. Mol Biol Cell. 2006;17:658–66. doi: 10.1091/mbc.E05-04-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossio V, Michimoto T, Sasaki T, Ohbayashi I, Kikuchi Y, Yoshida S. Nuclear PP2A-Cdc55 prevents APC-Cdc20 activation during the spindle assembly checkpoint. J Cell Sci. 2013;126:4396–405. doi: 10.1242/jcs.127365. [DOI] [PubMed] [Google Scholar]
- 14.Queralt E, Lehane C, Novak B, Uhlmann F. Downregulation of PP2A(Cdc55) phosphatase by separase initiates mitotic exit in budding yeast. Cell. 2006;125:719–32. doi: 10.1016/j.cell.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Queralt E, Uhlmann F. Separase cooperates with Zds1 and Zds2 to activate Cdc14 phosphatase in early anaphase. J Cell Biol. 2008;182:873–83. doi: 10.1083/jcb.200801054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossio V, Yoshida S. Spatial regulation of Cdc55-PP2A by Zds1/Zds2 controls mitotic entry and mitotic exit in budding yeast. J Cell Biol. 2011;193:445–54. doi: 10.1083/jcb.201101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baro B, Rodriguez-Rodriguez JA, Calabria I, Hernáez ML, Gil C, Queralt E. Dual Regulation of the mitotic exit network (MEN) by PP2A-Cdc55 phosphatase. PLoS Genet. 2013;9:e1003966. doi: 10.1371/journal.pgen.1003966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Ng TY. Phosphatase 2A negatively regulates mitotic exit in Saccharomyces cerevisiae. Mol Biol Cell. 2006;17:80–9. doi: 10.1091/mbc.E04-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy AM, Zolnierowicz S, Stapleton AE, Goebl M, DePaoli-Roach AA, Pringle JR. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991;11:5767–80. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi E, Pringle JR. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5264–75. doi: 10.1128/mcb.16.10.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Jiang YW, Wellinger RJ, Carlson K, Roberts JM, Stillman DJ. Mutations in the homologous ZDS1 and ZDS2 genes affect cell cycle progression. Mol Cell Biol. 1996;16:5254–63. doi: 10.1128/mcb.16.10.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorca T, Castro A. The Greatwall kinase: a new pathway in the control of the cell cycle. Oncogene. 2013;32:537–43. doi: 10.1038/onc.2012.79. [DOI] [PubMed] [Google Scholar]
- 23.Gharbi-Ayachi A, Labbé JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–7. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 24.Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–3. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 25.Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–85. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bontron S, Jaquenoud M, Vaga S, Talarek N, Bodenmiller B, Aebersold R, De Virgilio C. Yeast endosulfines control entry into quiescence and chronological life span by inhibiting protein phosphatase 2A. Cell Rep. 2013;3:16–22. doi: 10.1016/j.celrep.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Juanes MA, Khoueiry R, Kupka T, Castro A, Mudrak I, Ogris E, Lorca T, Piatti S. Budding yeast greatwall and endosulfines control activity and spatial regulation of PP2A(Cdc55) for timely mitotic progression. PLoS Genet. 2013;9:e1003575. doi: 10.1371/journal.pgen.1003575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talarek N, Cameroni E, Jaquenoud M, Luo X, Bontron S, Lippman S, Devgan G, Snyder M, Broach JR, De Virgilio C. Initiation of the TORC1-regulated G0 program requires Igo1/2, which license specific mRNAs to evade degradation via the 5′-3′ mRNA decay pathway. Mol Cell. 2010;38:345–55. doi: 10.1016/j.molcel.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–31. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 30.Hoyt MA, Totis L, Roberts BTS. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–17. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 31.Utsugi T, Hirata A, Sekiguchi Y, Sasaki T, Toh-e A, Kikuchi Y. Yeast tom1 mutant exhibits pleiotropic defects in nuclear division, maintenance of nuclear structure and nucleocytoplasmic transport at high temperatures. Gene. 1999;234:285–95. doi: 10.1016/S0378-1119(99)00197-3. [DOI] [PubMed] [Google Scholar]
- 32.Stegmeier F, Visintin R, Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–20. doi: 10.1016/S0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 33.Gentry MS, Hallberg RL. Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol Biol Cell. 2002;13:3477–92. doi: 10.1091/mbc.02-05-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bi E, Pringle JR. ZDS1 and ZDS2, genes whose products may regulate Cdc42p in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5264–75. doi: 10.1128/mcb.16.10.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anastasia SD, Nguyen DL, Thai V, Meloy M, MacDonough T, Kellogg DR. A link between mitotic entry and membrane growth suggests a novel model for cell size control. J Cell Biol. 2012;197:89–104. doi: 10.1083/jcb.201108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekiya-Kawasaki M, Abe M, Saka A, Watanabe D, Kono K, Minemura-Asakawa M, Ishihara S, Watanabe T, Ohya Y. Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genetics. 2002;162:663–76. doi: 10.1093/genetics/162.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakura M, Ozoe F, Ishida H, Nakagawa T, Tanaka K, Matsuda H, Kawamukai M. zds1, a novel gene encoding an ortholog of Zds1 and Zds2, controls sexual differentiation, cell wall integrity and cell morphology in fission yeast. Genetics. 2006;172:811–25. doi: 10.1534/genetics.105.050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–61. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]