Abstract
Chromosome bipolar attachment is achieved when sister kinetochores are attached by microtubules emanating from opposite spindle poles, and this process is essential for faithful chromosome segregation during anaphase. A fundamental question in cell biology is how cells ensure that chromosome segregation only occurs after bipolar attachment. It is well documented that unattached kinetochores activate the spindle assembly checkpoint (SAC) to delay chromosome segregation. Therefore, the silencing of the SAC is thought to trigger anaphase onset, but how correct chromosome attachment is coupled with SAC silencing and the subsequent anaphase onset is poorly understood. The establishment of chromosome bipolar attachment not only results in the occupancy of kinetochores by microtubules but also applies tension on sister kinetochores. A long-standing debate is whether the kinetochore attachment (occupancy) or the tension silences the SAC. Recent work in budding yeast reveals the SAC silencing network SSN that prevents SAC silencing prior to tension generation at kinetochores. Therefore, this signaling pathway ensures that SAC silencing and the subsequent anaphase onset occur only after chromosome bipolar attachment applies tension on chromosomes. This review will summarize the recent advances in the understanding of the SAC silencing process.
Keywords: checkpoint control, chromosomes, mitosis, yeast biology
The Checkpoints that Monitor Kinetochore Attachments
The discovery of the spindle assembly checkpoint SAC
During mitosis, sister kinetochores are attached by microtubules emanating from opposite spindle poles, and the spindle elongation during anaphase pulls sister chromatids into daughter cells. Some microtubule poisons bind to tubulin subunits and cause microtubule depolymerization. Because treatment with these poisons disrupts the spindle structure in human cells and blocks mitosis, this group of compounds can inhibit cell growth, and they are widely used for cancer treatment. Yeast cells are also sensitive to these spindle poisons and arrest in metaphase after exposure. Two independent genetic screens were performed for the isolation of yeast mutants that fail to stop the cell cycle in response to the treatment with microtubule poisons, such as nocodazole and benomyl. One group of mutants mad1, mad2, and mad3 (mitotic arrest-deficient) was identified as they continue to grow on benomyl-containing plates and lose viability because of the failure to arrest in metaphase.1 Another group of mutants bub1, bub2, and bub3 (budding uninhibited by benzimidazole) was isolated as they recover poorly after exposure to a high concentration of benomyl (70 µg/ml) and rebud on benomyl plates.2 Another SAC component, Mps1 kinase, was subsequently identified as mps1 mutants fail to arrest in metaphase in response to dysfunctional spindle pole bodies or a disrupted spindle.3 Interestingly, overexpression of MPS1 causes Mad1 protein phosphorylation and arrests wild-type cells in metaphase without any noticeable spindle defect.4 Because these genes are essential for cell cycle arrest in response to spindle disruption, they were collectively named as the spindle assembly checkpoint (SAC).
In addition to the response to spindle disruption, these SAC proteins are also required for the cell cycle delay induced by dysfunctional kinetochores or mutated centromeric DNA, indicating that the SAC actually monitors kinetochore–microtubule interaction.5,6 A temperature-sensitive kinetochore mutant ctf13 arrests in metaphase with a short spindle structure when incubated at 37 °C, but the introduction of mad1, mad2, bub1, or bub3, but not bub2, allows the mutant cells to elongate the spindle for anaphase onset. Bub2 protein was later demonstrated to be a component of another checkpoint pathway that monitors the spindle position and delays mitotic exit.7-11 Therefore, the SAC includes Mad1, Mad2, Mad3, Bub1, Bub3, and Mps1, and these components are well-conserved in all eukaryotes.12
The activation of the spindle assembly checkpoint and its consequence
Studies from both yeast and mammalian cells indicate that some checkpoint proteins are recruited to unattached kinetochores to generate a signal for anaphase entry delay.13-15 The kinetochore protein Spc105/Knl1 was found to be the docking site for the SAC protein Bub1.16 The phosphorylation of multiple Met-Glu-Leu-Thr (MELT) motifs in Spc105/Knl1 by Mps1 kinase enables the recruitment of the Bub1–Bub3 complex.17-20 Recent work from the Biggins lab demonstrates that the phosphorylation of Bub1 by Mps1 leads to Bub1–Mad1 interaction in budding yeast. The interaction of Mad1 with Bub1 and kinetochores can be reconstituted in the presence of Mps1 and Mad2.21 These observations indicate that the kinetochore acts as a platform essential for SAC activation, and some protein phosphorylation events play a key role in SAC activation.
Once Mad1 binds to unattached kinetochores, it further recruits Mad2 and causes the conformational change of Mad2 from “open” to “closed” forms.22,23 The “closed” Mad2 proteins sequester Cdc20, the activator of APC/C (anaphase promoting complex or cyclosome), thereby preventing APCCdc20 activation.24 In addition to Mad2, other checkpoint proteins Mad3/BubR1 and Bub3 also bind to Cdc20 to form the mitotic checkpoint complex (MCC).25-27 Because APCCdc20 mediates the degradation of securin Pds1, the anaphase inhibitor active SAC delays anaphase onset by stabilizing Pds1.28 Compared with the understanding of SAC activation and its role in cell cycle control, the SAC silencing process is much less clear. Although a reasonable speculation is that chromosome bipolar attachment silences the SAC to allow anaphase onset, the link between chromosome attachment and SAC silencing is still missing at the molecular level.
Current SAC Silencing Models
The stripping of SAC proteins from kinetochores by the dynein module
It is well documented that SAC proteins localize at unattached kinetochores. Results from higher eukaryotic cells show that anaphase onset occurs several minutes after SAC protein Mad2 dissociates from the last kinetochore, indicating that SAC silencing occurs prior to anaphase onset.29 The minus-end-directed motor protein dynein also localizes to unattached kinetochores and dissociates upon microtubule attachment.30 The kinetochore localization of dynein depends on the Rod/Zw10/Zwilch (RZZ) complex, which directly binds to kinetochore protein Ndc80.31,32 Another protein, termed Spindly, mediates the interaction between dynein and the RZZ complex.33,34 Earlier data showed that the dynein module removes SAC proteins Mad1 and Mad2 from the kinetochore upon microtubule attachment, and depletion of dynein blocks cells in metaphase and leads to partial retention of Mad2 at bioriented kinetochores.29,35 Thus, the dynein module was proposed to silence the SAC by stripping the SAC proteins from kinetochores. In addition to its proposed role in SAC silencing, dynein also mediates the rapid poleward chromosome motion and stabilizes kinetochore microtubules. Cells with dysfunctional dynein show mis-orientated kinetochore pairs and destabilized kinetochore microtubule bundles.36,37 These observations indicate the function of the dynein motor in the stabilization of kinetochore–microtubule attachment.
Unlike direct dynein inhibition, depletion of Spindly in human cells does not block the removal of Mad2 from kinetochores, although this depletion abolishes dynein recruitment onto kinetochores.38 This observation raises doubts about the role of kinetochore dynein in SAC silencing. Further investigation suggests that kinetochore dynein is essential for SAC silencing in the presence of Spindly.39 More recent work demonstrates that the kinetochore dynein mediates the initial microtubule capture, which promotes the Ndc80-mediated end-on kinetochore attachment.31 Therefore, the metaphase block in dynein-depleted cells could be attributed to erroneous kinetochore attachment and/or the failure of SAC silencing. Nevertheless, the successful SAC silencing in Spindly-depleted cells suggests additional mechanisms for SAC silencing. Moreover, dynein-dependent removal of checkpoint proteins from kinetochores does not appear to be a conserved mechanism for SAC silencing, as no obvious Spindly/RZZ homologs are present in lower eukaryotes such as yeast.
The disassembly of the mitotic checkpoint complex MCC
The association of SAC proteins Mad2, Bub3, and Mad3 with the APC/C activator Cdc20 forms the MCC that prevents anaphase onset by inhibiting APC/CCdc20. Another SAC silencing mechanism involves MCC disassembly. p31comet protein was identified as a Mad2 interactor.40 This interaction stimulates MCC disassembly, and overexpression of p31comet protein results in less Mad2 bound to Cdc20.41,42 In addition, APC15/Mnd2 promotes Cdc20 auto-ubiquitination and the subsequent MCC disassembly in yeast and mammalian cells.43,44 A substrate of mitotic CDK, CEUDC2, also binds to Cdc20 once phosphorylated, and this binding promotes the release of Mad2 from Cdc20 and the subsequent activation of APC/CCdc20.45 However, no evidence indicates the direct link between these mechanisms and kinetochore attachment. It is likely that these mechanisms facilitate the robustness of SAC silencing once cells have initiated the SAC silencing process.
The role of protein phosphatase 1 (PP1) in SAC silencing
Among the SAC components, Mps1 is a protein kinase that phosphorylates the kinetochore protein Spc105/Knl1 to promote kinetochore recruitment of SAC proteins.17-20 If Mps1 kinase activates the SAC by phosphorylating some proteins at the kinetochore, the reversal of these phosphorylation events is likely required to silence/inactivate the checkpoint. Recent work shows that the kinetochore protein Spc105 also recruits protein phosphatase 1 (PP1) through a conserved RVSF motif, and this interaction is required to dephosphorylate the substrates of Ipl1/Aurora B at kinetochores to stabilize microtubule attachment.46 Results from budding yeast indicate that the binding of PP1 to Spc105 is essential for SAC silencing, but this binding plays a nonessential role for physical chromosome segregation.47 Consistently, high levels of PP1 promote SAC silencing in fission and budding yeast cells.48,49 Therefore, the balance of kinase/phosphatase activity at the kinetochore is likely the key to regulate SAC silencing. We speculate that either decreased kinase activity or increased PP1 activity at the kinetochore could trigger SAC silencing. One open question is how this balance is regulated during the cell cycle. Moreover, it is important to know which substrate of PP1 plays a key role in modulating SAC silencing.
Recent Evidence for Tension-Dependent SAC Silencing
A fundamental question in cell cycle control is which event triggers anaphase onset. It has been speculated that the silencing of the SAC allows anaphase onset, but a long-standing debate is whether kinetochore attachment (occupancy) or the tension on sister kinetochores silences the SAC to trigger anaphase entry.50 Recent evidence in budding yeast favors the tension model.
The checkpoint response to tension defects in higher eukaryotic cells
Bipolar attachment generates tension on chromosomes. The observation that applying tension on a mis-attached chromosome in grasshopper spermatocytes triggers anaphase onset suggests the role of tension in cell cycle progression.51 The 3F3/2 antibody detects phosphorylated kinetochore proteins. Interestingly, tension, whether from a micromanipulation needle or from normal mitotic forces, causes dephosphorylation of the kinetochore proteins recognized by 3F3, suggesting that tension controls the phosphorylation status at the kinetochore.52 The Salmon group further examined the 3F3/2 signal as well as the kinetochore localization of SAC protein Mad2 in PtK1 cells treated with taxol, which stabilizes microtubules and causes tension loss. Although the phosphoepitope 3F3/2 becomes phosphorylated in all the kinetochores after tension is reduced by taxol, very few kinetochores exhibit Mad2 localization, thereby arguing against the role of tension in checkpoint regulation.53 Further experiments show that tension promotes further kinetochore attachment by microtubules, which may cause the complete loss of Mad2 localization at the kinetochore.54 Therefore, the role of tension in SAC silencing remains controversial, as it may silence the SAC indirectly by strengthening kinetochore–microtubule interaction.
The checkpoint response to tension defects in budding yeast
The pulling force from the opposite spindle poles as well as cohesion between sister chromatids is necessary for tension generation. Cohesion loss or a complete block of DNA replication results in tensionless chromosomes. When incubated at 37 °C, temperature-sensitive yeast cohesin mutants (scc1/mcd1) show delayed anaphase onset as evidenced by the persistent protein levels of anaphase inhibitor Pds1, although the mutant cells can elongate spindles because of the lack of cohesion.55 Complete block of DNA replication in cdc6–1 temperature-sensitive mutants also delays anaphase onset due to the lack of sister chromatids.56 In addition to the SAC proteins, the kinetochore-associated Ipl1 kinase and a pericentromeric protein Sgo1 are required for the anaphase entry delay induced by these tension defects.55,57 In contrast, Ipl1 and Sgo1 are dispensable for the cell cycle arrest induced by unattached kinetochores, indicating their specific role in the response to tension defects.
In addition to the role in the response to tension defects, Ipl1 promotes the conversion of tensionless chromosomes to unattached ones.58,59 Thus, one explanation is that Ipl1-dependent destabilization of kinetochore attachment activates the SAC indirectly. However, this speculation is unable to explain the role of Sgo1 in the checkpoint response to tension defects, because Sgo1 does not promote the generation of unattached kinetochores.58 Therefore, additional mechanisms should also contribute to the anaphase entry delay induced by tension defects.
The checkpoint response to syntelic attachments in budding yeast
Computer-aided reconstruction from electron micrographs of mitotic yeast cells suggest that each kinetochore is attached by a single microtubule,60 which makes budding yeast an ideal organism to study the regulation of kinetochore–microtubule interaction. Syntelic attachment establishes when 2 sister kinetochores are attached by microtubules from the same spindle pole. Obviously, tension will be absent from chromosomes with syntelic attachment. Recently, our lab developed a genetic approach to induce syntelic attachment in budding yeast, which is a very useful tool to study the response to tension defects.
Cik1 and Kar3 form a motor complex that moves chromosomes along microtubules toward the minus end.61 In a genome-wide screen for yeast deletion mutants that are sensitive to stressful DNA replication, both cik1Δ and kar3Δ mutants were isolated, presumably due to the defect in chromosome bipolar attachment.62 Our further analysis indicates that the loss of function of Cik1/Kar3 increases the chance of syntelic attachment, although the mechanism for this incorrect attachment remains to be determined. Moreover, we found that overexpression of the coiled-coil domain of Cik1 (Cik1-CC) disrupts Cik1–Kar3 interaction, which allows us to conditionally inactivate the Cik1/Kar3 motor complex and induce syntelic attachments.63
While analyzing the checkpoint response to syntelic attachments, we found that dysfunctional SAC abolished the anaphase entry delay induced by CIK1-CC overexpression, resulting in sister chromatid co-segregation and viability loss. Similarly, ipl1 and sgo1Δ mutants also eliminate the CIK1-CC-induced anaphase entry delay, leading to chromosome missegregation.63 One important question is how a syntelic attachment delays anaphase onset. One possibility is that the sister kinetochores with syntelic attachment become unattached ones with the assistance of Ipl1 kinase, which subsequently activates the SAC. The second possibility is that the tension defect activates the SAC to delay anaphase onset through a specific signaling pathway (the tension checkpoint). Third, the absence of tension prevents SAC silencing, thereby maintaining the active status of the SAC prior to tension generation.
The absence of tension prevents SAC silencing
Sgo1 is essential for the anaphase entry delay induced by loss of cohesion or syntelic attachments, but it is not involved in generating unattached kinetochores when they are not under tension.58 To assess if Sgo1 activates the SAC or prevents SAC silencing in the absence of tension, we examined the SAC activation and silencing processes in sgo1Δ mutant cells in the absence of tension by analyzing Mad1 phosphorylation, as this modification indicates SAC activation.64,65 The absence of tension leads to sustained Mad1 phosphorylation, indicating SAC activation. Interestingly, sgo1Δ mutants show efficient Mad1 phosphorylation but fail to maintain this phosphorylation in the absence of tension. This result indicates that Sgo1 is dispensable for SAC activation but prevents SAC silencing in cells lacking tension. Therefore, Sgo1 is likely a component of the signaling pathway that prevents SAC silencing when tension is absent.
Ipl1 kinase is also required for the anaphase entry delay in response to tension defects,63 but the fact that Ipl1 destabilizes kinetochore attachment makes it difficult to define the role of Ipl1 in preventing SAC silencing.58,59 Before addressing this question, we first determined which Ipl1-dependent phosphorylation event is involved in the response to tension defects. One of the well-characterized Ipl1 substrates in budding yeast is the kinetochore protein Dam1, a subunit of the Dam1/DASH kinetochore complex.66 Substitution of 3 of the 4 Ipl1 consensus sites with alanine or aspartic acid generates viable nonphosphorylatable (dam1-3A) and phosphomimetic (dam1-3D) mutants.67 Strikingly, the dam1-3A mutant can also eliminate the anaphase entry delay induced by syntelic attachments. In addition, Mad1 is phosphorylated efficiently in dam1-3A cells lacking tension, but this phosphorylation disappears prematurely, a phenotype similar to sgo1Δ mutant.68 One possibility is that Dam1 phosphorylation by Ipl1 may also prevent premature SAC silencing. Alternatively, the stabilization of tensionless kinetochore attachment in dam1-3A cells may prevent SAC activation.
We further used the phosphomimetic dam1-3D mutant to distinguish these possibilities. Since dam1-3A mutant cells show premature SAC silencing in the absence of tension, we expect dam1-3D mutants to show difficulty in SAC silencing. Indeed, dam1-3D cells exhibit an obvious delay in anaphase entry. If the delay is due to destabilized kinetochore attachment, the combination with a SAC mutant will cause chromosome missegregation and viability loss. However, both dam1-3D mad1∆ and dam1-3D mad2∆ double mutants are viable, although the anaphase entry delay is abolished completely in these double mutants. Thus, the destabilized kinetochore attachment cannot fully explain the anaphase entry delay in dam1-3D mutants. To further test if a detachment-independent mechanism contributes to the anaphase entry delay in dam1-3D mutant cells, we used live-cell imaging to follow 2 successive cell cycles in dam1-3D and dam1-3D mad1∆ cells. Among the daughters of the 33 dam1-3D mad1∆ cells, 64 could finish the second round of cell division, as indicated by the successful chromosome segregation, suggesting that most of the mutant cells experienced faithful chromosome segregation in the first round of cell cycle.68 This result strongly supports a detachment-independent mechanism that prevents anaphase onset in dam1-3D mutant cells. Although Ipl1 may delay anaphase onset by generating unattached kinetochores that activate the SAC, our data indicate that the phosphorylation of Dam1 by Ipl1 also prevents SAC silencing in a manner independent of the destabilization of kinetochore attachment.
The SAC silencing network (SSN) coordinates anaphase onset with tension generation at kinetochores
Our data suggest that modulation of the phosphorylation of the kinetochore protein Dam1 plays a key role in the SAC silencing process. Previous work shows that Dam1 only becomes dephosphorylated when sister kinetochores are under tension.69 Therefore, Dam1 is an ideal candidate for the tension sensor. Prior to tension generation, its phosphorylation by Ipl1 kinase may prevent SAC silencing and anaphase onset. Indeed, the phosphomimetic dam1-3D mutant abolishes the premature anaphase onset in ipl1 mutants in response to tension defects, indicating that Ipl1 prevents anaphase onset through Dam1. Previous work also shows that high levels of PP1 induce SAC silencing, and PP1 dephosphorylates Dam1.70 We found that dam1-3D mutant blocks SAC silencing induced by PP1 overexpression, indicating that Dam1 also acts downstream of PP1. These results support the model that the tension at kinetochores can be converted into a biochemical signal through Dam1 phosphorylation, which further regulates SAC silencing. Therefore, the Ipl1 kinase, phosphatase PP1, and their substrate Dam1 constitute the SAC silencing network SSN that links tension generation to anaphase onset.
Then how to fit Sgo1 into this SSN? The comparison of Dam1 phosphorylation kinetics during the cell cycle in wild-type and sgo1 mutant cells did not reveal any difference. In contrast, deletion of SGO1 eliminates the cell cycle delay in dam1-3D phosphomimetic mutant cells, suggesting that Sgo1 functions downstream of Dam1 (Fig. 1A).68 Nevertheless, we cannot exclude the possibility that Sgo1 and Dam1 act in parallel to regulate SAC silencing. Taken together, recent advances in budding yeast support the tension model for SAC silencing. Prior to tension generation, the sustained phosphorylation of kinetochore protein Dam1 by Ipl1 prevents SAC silencing and anaphase onset. Once chromosome bipolar attachment applies tension on sister kinetochores, tension-induced Dam1 dephosphorylation by PP1 triggers SAC silencing and anaphase entry (Fig. 1B). Therefore, the SAC silencing network SSN couples SAC silencing with bipolar attachment-induced tension generation, ensuring that chromosome segregation only occurs after chromosome bipolar attachment.
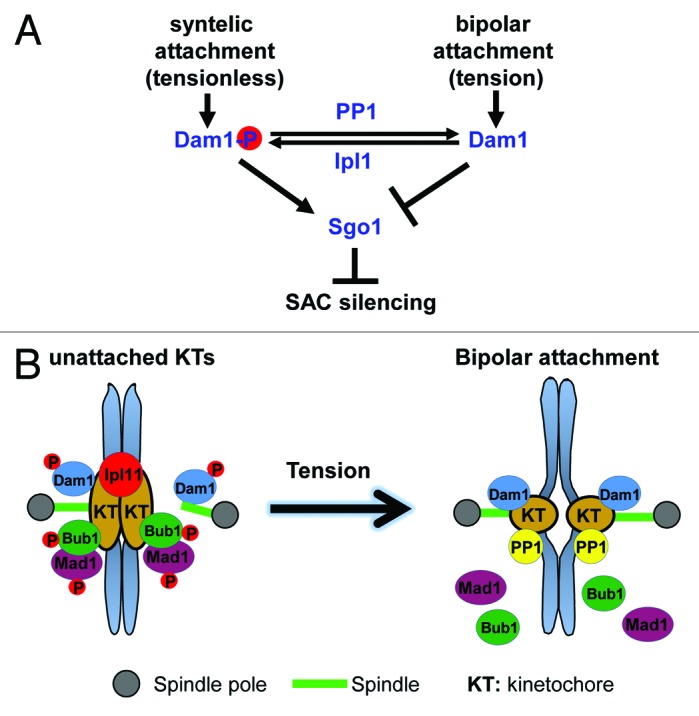
Figure 1. (A) The SAC silencing network SSN. Ipl1, PP1, Dam1, and Sgo1 constitute the SSN in budding yeast, which senses the tension at kinetochores and coordinates chromosome bipolar attachment and anaphase onset. (B) The working model for tension-induced SAC silencing. Chromosome bipolar attachment alters the balance of kinase/phosphatase at the kinetochore and triggers the dephosphorylation of Dam1, which induces the dephosphorylation of SAC proteins Mad1 and Bub1 to silence the SAC.
Further Directions
The occupancy vs. tension model?
An unresolved debate is whether the attachment of kinetochores (occupancy) or the tension silences the SAC. Recent work supports the tension model. If that is the case, does kinetochore occupancy play a role in SAC silencing? Although sgo1Δ and dam1-3A mutant cells can silence the SAC in the absence of tension, these mutant cells show proficient metaphase arrest in response to nocodazole treatment, which generates unattached kinetochores. Thus, we speculate that kinetochore occupancy is necessary but not sufficient for SAC silencing. SAC silencing could be a 2-step process. First, kinetochore attachment may decrease the capacity of SAC activation, for example, by compromising kinetochore binding of SAC protein Mad2.53 After chromosome bipolar attachment, the tension on sister kinetochores may silence the SAC by triggering the dephosphorylation of kinetochore protein Dam1 as well as some SAC proteins, such as Mad1 and Bub1. It will be informative to analyze the localization of the SAC components to unattached and attached but tensionless kinetochores. The results may reveal different SAC activation status in attached kinetochores with or without tension.
Does tension regulate the activity of kinases or phosphatases at the kinetochore?
The phosphorylation of some kinetochore or SAC proteins is essential for SAC activation. The reversal of these phosphorylation events is likely critical for SAC silencing. If tension at the kinetochore triggers SAC silencing, one important open question is how tension regulates the activity of kinases or phosphatases at the kinetochore. Using fluorescence biosensors to measure localized phosphorylation dynamics in living Hela cells, the Lens group found that phosphorylation of an Aurora B substrate at the kinetochore depends on its distance from the kinase at the inner kinetochore.71 Thus, one attractive model is that tension-induced kinetochore stretching separates Ipl1/Aurora B kinase from its substrates at the kinetochore, which may compromise phosphorylation and trigger SAC silencing. Recent work shows that deletion of the N terminus of Sli15, an Ipl1 interactor in yeast cells, abolished kinetochore binding of Ipl1, but the sli15-∆N cells grow normally.72 It will be interesting to examine if the elimination of Ipl1 kinase at kinetochores in sli15-∆N cells causes premature SAC silencing. Moreover, the checkpoint kinase Mps1 associates with the kinetochore through Ndc80,73 so tension on chromosomes may also regulate Mps1 kinase activity to control the timing of SAC silencing.
In addition to protein kinases, the kinetochore protein Spc105/Knl1 also recruits PP1 to the kinetochore, and the abolishment of PP1 recruitment in a spc105 yeast mutant blocks SAC silencing.46,47 It remains unclear if the Spc105–PP1 interaction is constitutive or regulated during the cell cycle. One possibility is that tension at kinetochores triggers recruitment of PP1 to the kinetochore to induce SAC silencing. Alternatively, tension enables PP1 to dephosphorylate its substrates through tension-induced kinetochore conformation change. Therefore, an important question regarding SAC silencing is how tension alters the kinase/phosphatase balance at the kinetochore.
Our results support the conclusion that the dephosphorylation of Dam1 is essential for SAC silencing. The key result supporting this conclusion is the observation that phosphomimetic dam1-3D mutant cells show compromised dephosphorylation of 2 SAC components, Mad1 and Bub1.68 However, it remains elusive how Dam1 phosphorylation by Ipl1 prevents the dephosphorylation of Mad1 and Bub1. Most of the dam1-3D cells are able to perform faithful mitosis, indicating the low frequency of unattached kinetochores.68 Nevertheless, results from in vitro assays suggest that Dam1 phosphorylation compromises the recruitment of the Ndc80 complex to microtubules.74,75 It is possible that Dam1 dephosphorylation induces a stronger Dam1–Ndc80 interaction, which may cause kinetochore conformation changes to trigger SAC silencing. This conformational change likely alters the distance between the substrates and its kinase or phosphatase. Alternatively, this change may promote the dissociation of kinases from the kinetochore or induce recruitment of PP1 to the kinetochore. In support of this speculation, mutation in the Ndc80 loop domain compromises Ndc80–Dam1 interaction and delays SAC silencing in yeast.76 This loop region in Ndc80 also mediates interaction with the Ska complex in mammalian cells.77 Moreover, results from the Salmon lab confirm the role of the loop domain in the conformational change of the Ndc80 complex.78 Further studies are needed to verify whether modulation of the phosphorylation of Dam1 or Ska proteins contributes to the conformation change of the Ndc80 complex and define the role of this change in SAC silencing.
Is the SSN a conserved mechanism for SAC silencing?
The Ipl1/Aurora B kinase destabilizes chromosome attachment in yeast and mammalian cells,58,59,79,80 but its role in checkpoint control remains controversial. Like yeast ipl1 mutant cells, mammalian cells treated with nocodazole as well as an Aurora B inhibitor arrest in mitosis, indicating proficient SAC function. Interestingly, Aurora B inhibition accelerates checkpoint exit after nocodazole washout.81,82 In addition, Aurora B inhibition overrides the checkpoint efficiently when cells are treated with taxol that stabilizes microtubules and compromises tension generation.82 A reasonable explanation is that Aurora B is also required to prevent premature SAC silencing in mammalian cells when tension is compromised by taxol treatment. Aurora B may also regulate SAC activation by promoting kinetochore detachment.
Our data suggest that Ipl1 prevents SAC silencing by phosphorylating Dam1, a subunit of the Dam1/DASH complex in budding yeast. Then what could the substrate of Aurora B kinase important for SAC silencing in mammalian cells be? Recent evidence indicates that the Ska complex in mammalian cells is likely the functional ortholog of the Dam1 complex.83,84 Unlike the 10-subunit Dam1 complex, only 3 components are present in the Ska complex. Both Dam1 and Ska complexes associate with the spindle microtubules prior to kinetochore–microtubule interaction.85 Moreover, both complexes contain some components that are phosphorylated by Ipl1/Aurora B, and this phosphorylation destabilizes kinetochore–microtubule interaction.74,75,86 Therefore, one untested possibility is that phosphorylation of the Ska complex by Aurora B also prevents SAC silencing in mammalian cells.
In summary, recent work reveals the SAC silencing network (SSN) that coordinates chromosome attachment, tension generation, and anaphase onset. In budding yeast, this network includes a kinetochore protein Dam1, its kinase Ipl1, phosphatase PP1, and a downstream component Sgo1, although more components remain to be identified. The SSN prevents SAC silencing prior to chromosome bipolar attachment that applies tension on sister kinetochores. Since premature SAC silencing leads to anaphase onset in the presence of incorrect chromosome attachments, deregulation of this pathway will increase the chance of chromosome missegregation and aneuploidy. Much more work is needed to elucidate the molecular details for this signaling pathway.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I apologize to those colleagues whose work was not cited in the review. This work was supported by R15GM097326 and RO1GM102115 from NIH/NIGMS to Y.W.
References
- 1.Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–31. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- 2.Hoyt MA, Totis L, Roberts BTS. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–17. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- 3.Weiss E, Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996;132:111–23. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–6. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Burke DJ. Checkpoint genes required to delay cell division in response to nocodazole respond to impaired kinetochore function in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:6838–44. doi: 10.1128/mcb.15.12.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer F, Hieter P. Centromere DNA mutations induce a mitotic delay in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992;89:8908–12. doi: 10.1073/pnas.89.19.8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Hu F, Elledge SJ. The Bfa1/Bub2 GAP complex comprises a universal checkpoint required to prevent mitotic exit. Curr Biol. 2000;10:1379–82. doi: 10.1016/S0960-9822(00)00779-X. [DOI] [PubMed] [Google Scholar]
- 8.Daum JR, Gomez-Ospina N, Winey M, Burke DJ. The spindle checkpoint of Saccharomyces cerevisiae responds to separable microtubule-dependent events. Curr Biol. 2000;10:1375–8. doi: 10.1016/S0960-9822(00)00780-6. [DOI] [PubMed] [Google Scholar]
- 9.Bardin AJ, Visintin R, Amon A. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 2000;102:21–31. doi: 10.1016/S0092-8674(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 10.Bloecher A, Venturi GM, Tatchell K. Anaphase spindle position is monitored by the BUB2 checkpoint. Nat Cell Biol. 2000;2:556–8. doi: 10.1038/35019601. [DOI] [PubMed] [Google Scholar]
- 11.Pereira G, Höfken T, Grindlay J, Manson C, Schiebel E. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol Cell. 2000;6:1–10. doi: 10.1016/S1097-2765(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 12.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–72. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 13.Gillett ES, Espelin CW, Sorger PK. Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J Cell Biol. 2004;164:535–46. doi: 10.1083/jcb.200308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen RH, Waters JC, Salmon ED, Murray AW. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science. 1996;274:242–6. doi: 10.1126/science.274.5285.242. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–8. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 16.Kiyomitsu T, Obuse C, Yanagida M. Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell. 2007;13:663–76. doi: 10.1016/j.devcel.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 17.London N, Ceto S, Ranish JA, Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol. 2012;22:900–6. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol. 2012;22:891–9. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Primorac I, Weir JR, Chiroli E, Gross F, Hoffmann I, van Gerwen S, Ciliberto A, Musacchio A. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. Elife. 2013;2:e01030. doi: 10.7554/eLife.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat Cell Biol. 2012;14:746–52. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- 21.London N, Biggins S. Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint. Genes Dev. 2014;28:140–52. doi: 10.1101/gad.233700.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo X, Fang G, Coldiron M, Lin Y, Yu H, Kirschner MW, Wagner G. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nat Struct Biol. 2000;7:224–9. doi: 10.1038/73338. [DOI] [PubMed] [Google Scholar]
- 23.Luo X, Tang Z, Rizo J, Yu H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol Cell. 2002;9:59–71. doi: 10.1016/S1097-2765(01)00435-X. [DOI] [PubMed] [Google Scholar]
- 24.Yu H. Structural activation of Mad2 in the mitotic spindle checkpoint: the two-state Mad2 model versus the Mad2 template model. J Cell Biol. 2006;173:153–7. doi: 10.1083/jcb.200601172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang G, Yu H, Kirschner MW. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 1998;12:1871–83. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardwick KG, Johnston RC, Smith DL, Murray AW. MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J Cell Biol. 2000;148:871–82. doi: 10.1083/jcb.148.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sczaniecka M, Feoktistova A, May KM, Chen JS, Blyth J, Gould KL, Hardwick KG. The spindle checkpoint functions of Mad3 and Mad2 depend on a Mad3 KEN box-mediated interaction with Cdc20-anaphase-promoting complex (APC/C) J Biol Chem. 2008;283:23039–47. doi: 10.1074/jbc.M803594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen-Fix O, Peters JM, Kirschner MW, Koshland D. Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev. 1996;10:3081–93. doi: 10.1101/gad.10.24.3081. [DOI] [PubMed] [Google Scholar]
- 29.Howell BJ, McEwen BF, Canman JC, Hoffman DB, Farrar EM, Rieder CL, Salmon ED. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J Cell Biol. 2001;155:1159–72. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King JM, Hays TS, Nicklas RB. Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J Cell Biol. 2000;151:739–48. doi: 10.1083/jcb.151.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheerambathur DK, Gassmann R, Cook B, Oegema K, Desai A. Crosstalk between microtubule attachment complexes ensures accurate chromosome segregation. Science. 2013;342:1239–42. doi: 10.1126/science.1246232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karess R. Rod-Zw10-Zwilch: a key player in the spindle checkpoint. Trends Cell Biol. 2005;15:386–92. doi: 10.1016/j.tcb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Griffis ER, Stuurman N, Vale RD. Spindly, a novel protein essential for silencing the spindle assembly checkpoint, recruits dynein to the kinetochore. J Cell Biol. 2007;177:1005–15. doi: 10.1083/jcb.200702062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gassmann R, Essex A, Hu JS, Maddox PS, Motegi F, Sugimoto A, O’Rourke SM, Bowerman B, McLeod I, Yates JR, 3rd, et al. A new mechanism controlling kinetochore-microtubule interactions revealed by comparison of two dynein-targeting components: SPDL-1 and the Rod/Zwilch/Zw10 complex. Genes Dev. 2008;22:2385–99. doi: 10.1101/gad.1687508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mische S, He Y, Ma L, Li M, Serr M, Hays TS. Dynein light intermediate chain: an essential subunit that contributes to spindle checkpoint inactivation. Mol Biol Cell. 2008;19:4918–29. doi: 10.1091/mbc.E08-05-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Z, Tulu US, Wadsworth P, Rieder CL. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr Biol. 2007;17:973–80. doi: 10.1016/j.cub.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varma D, Monzo P, Stehman SA, Vallee RB. Direct role of dynein motor in stable kinetochore-microtubule attachment, orientation, and alignment. J Cell Biol. 2008;182:1045–54. doi: 10.1083/jcb.200710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan YW, Fava LL, Uldschmid A, Schmitz MH, Gerlich DW, Nigg EA, Santamaria A. Mitotic control of kinetochore-associated dynein and spindle orientation by human Spindly. J Cell Biol. 2009;185:859–74. doi: 10.1083/jcb.200812167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gassmann R, Holland AJ, Varma D, Wan X, Civril F, Cleveland DW, Oegema K, Salmon ED, Desai A. Removal of Spindly from microtubule-attached kinetochores controls spindle checkpoint silencing in human cells. Genes Dev. 2010;24:957–71. doi: 10.1101/gad.1886810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habu T, Kim SH, Weinstein J, Matsumoto T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 2002;21:6419–28. doi: 10.1093/emboj/cdf659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westhorpe FG, Tighe A, Lara-Gonzalez P, Taylor SS. p31comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J Cell Sci. 2011;124:3905–16. doi: 10.1242/jcs.093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teichner A, Eytan E, Sitry-Shevah D, Miniowitz-Shemtov S, Dumin E, Gromis J, Hershko A. p31comet Promotes disassembly of the mitotic checkpoint complex in an ATP-dependent process. Proc Natl Acad Sci U S A. 2011;108:3187–92. doi: 10.1073/pnas.1100023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Foster SA, Morgan DO. The APC/C subunit Mnd2/Apc15 promotes Cdc20 autoubiquitination and spindle assembly checkpoint inactivation. Mol Cell. 2012;47:921–32. doi: 10.1016/j.molcel.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uzunova K, Dye BT, Schutz H, Ladurner R, Petzold G, Toyoda Y, Jarvis MA, Brown NG, Poser I, Novatchkova M, et al. APC15 mediates CDC20 autoubiquitylation by APC/C(MCC) and disassembly of the mitotic checkpoint complex. Nat Struct Mol Biol. 2012;19:1116–23. doi: 10.1038/nsmb.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao YF, Li T, Chang Y, Wang YB, Zhang WN, Li WH, He K, Mu R, Zhen C, Man JH, et al. Cdk1-phosphorylated CUEDC2 promotes spindle checkpoint inactivation and chromosomal instability. Nat Cell Biol. 2011;13:924–33. doi: 10.1038/ncb2287. [DOI] [PubMed] [Google Scholar]
- 46.Liu D, Vleugel M, Backer CB, Hori T, Fukagawa T, Cheeseman IM, Lampson MA. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol. 2010;188:809–20. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg JS, Cross FR, Funabiki H. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr Biol. 2011;21:942–7. doi: 10.1016/j.cub.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanoosthuyse V, Hardwick KG. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr Biol. 2009;19:1176–81. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinsky BA, Nelson CR, Biggins S. Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr Biol. 2009;19:1182–7. doi: 10.1016/j.cub.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maresca TJ, Salmon ED. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J Cell Sci. 2010;123:825–35. doi: 10.1242/jcs.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–2. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- 52.Nicklas RB, Ward SC, Gorbsky GJ. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J Cell Biol. 1995;130:929–39. doi: 10.1083/jcb.130.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waters JC, Chen RH, Murray AW, Salmon ED. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J Cell Biol. 1998;141:1181–91. doi: 10.1083/jcb.141.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicklas RB, Waters JC, Salmon ED, Ward SC. Checkpoint signals in grasshopper meiosis are sensitive to microtubule attachment, but tension is still essential. J Cell Sci. 2001;114:4173–83. doi: 10.1242/jcs.114.23.4173. [DOI] [PubMed] [Google Scholar]
- 55.Biggins S, Murray AW. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–29. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stern BM, Murray AW. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr Biol. 2001;11:1462–7. doi: 10.1016/S0960-9822(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 57.Indjeian VB, Stern BM, Murray AW. The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science. 2005;307:130–3. doi: 10.1126/science.1101366. [DOI] [PubMed] [Google Scholar]
- 58.Pinsky BA, Kung C, Shokat KM, Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat Cell Biol. 2006;8:78–83. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–29. doi: 10.1016/S0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 60.Winey M, Mamay CL, O’Toole ET, Mastronarde DN, Giddings TH, Jr., McDonald KL, McIntosh JR. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J Cell Biol. 1995;129:1601–15. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka K, Mukae N, Dewar H, van Breugel M, James EK, Prescott AR, Antony C, Tanaka TU. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–94. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 62.Liu H, Jin F, Liang F, Tian X, Wang Y. The Cik1/Kar3 motor complex is required for the proper kinetochore-microtubule interaction after stressful DNA replication. Genetics. 2011;187:397–407. doi: 10.1534/genetics.110.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin F, Liu H, Li P, Yu HG, Wang Y. Loss of function of the Cik1/Kar3 motor complex results in chromosomes with syntelic attachment that are sensed by the tension checkpoint. PLoS Genet. 2012;8:e1002492. doi: 10.1371/journal.pgen.1002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mirchenko L, Uhlmann F. Sli15(INCENP) dephosphorylation prevents mitotic checkpoint reengagement due to loss of tension at anaphase onset. Curr Biol. 2010;20:1396–401. doi: 10.1016/j.cub.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hardwick KG, Murray AW. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J Cell Biol. 1995;131:709–20. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y, Bachant J, Alcasabas AA, Wang Y, Qin J, Elledge SJ. The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev. 2002;16:183–97. doi: 10.1101/gad.959402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheeseman IM, Anderson S, Jwa M, Green EM, Kang Js, Yates JR, 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–72. doi: 10.1016/S0092-8674(02)00973-X. [DOI] [PubMed] [Google Scholar]
- 68.Jin F, Wang Y. The signaling network that silences the spindle assembly checkpoint upon the establishment of chromosome bipolar attachment. Proc Natl Acad Sci U S A. 2013;110:21036–41. doi: 10.1073/pnas.1307595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keating P, Rachidi N, Tanaka TU, Stark MJ. Ipl1-dependent phosphorylation of Dam1 is reduced by tension applied on kinetochores. J Cell Sci. 2009;122:4375–82. doi: 10.1242/jcs.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinsky BA, Kotwaliwale CV, Tatsutani SY, Breed CA, Biggins S. Glc7/protein phosphatase 1 regulatory subunits can oppose the Ipl1/aurora protein kinase by redistributing Glc7. Mol Cell Biol. 2006;26:2648–60. doi: 10.1128/MCB.26.7.2648-2660.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–3. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campbell CS, Desai A. Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature. 2013;497:118–21. doi: 10.1038/nature12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kemmler S, Stach M, Knapp M, Ortiz J, Pfannstiel J, Ruppert T, Lechner J. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 2009;28:1099–110. doi: 10.1038/emboj.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tien JF, Umbreit NT, Gestaut DR, Franck AD, Cooper J, Wordeman L, Gonen T, Asbury CL, Davis TN. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. J Cell Biol. 2010;189:713–23. doi: 10.1083/jcb.200910142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lampert F, Hornung P, Westermann S. The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J Cell Biol. 2010;189:641–9. doi: 10.1083/jcb.200912021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maure JF, Komoto S, Oku Y, Mino A, Pasqualato S, Natsume K, Clayton L, Musacchio A, Tanaka TU. The Ndc80 loop region facilitates formation of kinetochore attachment to the dynamic microtubule plus end. Curr Biol. 2011;21:207–13. doi: 10.1016/j.cub.2010.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang G, Kelstrup CD, Hu XW, Kaas Hansen MJ, Singleton MR, Olsen JV, Nilsson J. The Ndc80 internal loop is required for recruitment of the Ska complex to establish end-on microtubule attachment to kinetochores. J Cell Sci. 2012;125:3243–53. doi: 10.1242/jcs.104208. [DOI] [PubMed] [Google Scholar]
- 78.Varma D, Chandrasekaran S, Sundin LJ, Reidy KT, Wan X, Chasse DA, Nevis KR, DeLuca JG, Salmon ED, Cook JG. Recruitment of the human Cdt1 replication licensing protein by the loop domain of Hec1 is required for stable kinetochore-microtubule attachment. Nat Cell Biol. 2012;14:593–603. doi: 10.1038/ncb2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol. 2004;6:232–7. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 80.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–94. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kallio MJ, McCleland ML, Stukenberg PT, Gorbsky GJ. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr Biol. 2002;12:900–5. doi: 10.1016/S0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- 82.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–80. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hanisch A, Silljé HH, Nigg EA. Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J. 2006;25:5504–15. doi: 10.1038/sj.emboj.7601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaitanos TN, Santamaria A, Jeyaprakash AA, Wang B, Conti E, Nigg EA. Stable kinetochore-microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 2009;28:1442–52. doi: 10.1038/emboj.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR, 3rd, Cheeseman IM. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell. 2009;16:374–85. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan YW, Jeyaprakash AA, Nigg EA, Santamaria A. Aurora B controls kinetochore-microtubule attachments by inhibiting Ska complex-KMN network interaction. J Cell Biol. 2012;196:563–71. doi: 10.1083/jcb.201109001. [DOI] [PMC free article] [PubMed] [Google Scholar]


