Abstract
During cancer development, epithelial–mesenchymal transition (EMT) facilitates tumor dissemination and metastatic spread, which is characterized by morphologic changes from epithelial cells to fibroblast-like cells, disassembly of intercellular junction, and increased cell motility. Overexpression of astrocyte elevated gene-1(AEG-1) in various cancer cell lines and cancers has been found to be associated with aggressive tumor behavior. We found that AEG-1 expression was elevated in low differentiation cervical cancer specimens from patients. However, little is known about the AEG-1’s precise role in invasion and metastasis. Here we demonstrate that downregulation of AEG-1 by RNAi significantly decreased the invasion and migration of cervical cancer cells, suggesting that AEG-1 overexpression may enhance cancer cell motility by inducing EMT. Downregulation of AEG-1 also led to reduced expression of mesenchymal marker vimentin and the transcription factor Snail but upregulation of epithelial marker E-cadherin in HeLa cells. In addition, knockdown of AEG-1 decreased colony forming units and increased sensitivity to cancer drugs in vitro. Taken together, our results suggest that knockdown of AEG-1 could decrease EMT and chemoresistance in cervical cancer cells and attenuate their aggressive behavior.
Keywords: astrocyte elevated gene-1, AEG-1, chemoresistance, epithelial to mesenchymal transition, invasiveness, metastasis, cervical cancer
Introduction
Cervical cancer is the second most common cancer among women worldwide.1 Infection by human papilloma virus (HPV) is a necessary requirement for cervical cancer, but not all women infected by HPV develop cervical cancer, indicating that other factors contribute to the progression to cervical cancer.2 Poor prognosis is usually associated with pelvic lymph node involvement, indicating that the tumor cells have become metastatic.3 During invasive and metastatic progression, cancer cells acquire the ability to breach the basement membrane, and individual cancer cells or groups of cancer cells begin to invade the nearby stroma. In the initial stage of this process, morphogenetic changes due to conversion of polarized epithelial cells to motile mesenchymal cells, referred to as epithelial–mesenchymal transition (EMT), are associated with tumor dissemination and metastatic spread. How cervical cancer cells acquire the ability to invade surrounding tissues and metastasize, a property of cancer stem cells, is unclear. Little is known about the regulation of EMT in cervical cancer cells and tissues.
Astrocyte elevated gene-1 (AEG-1, also known as metadherin [MTDH] and lysine-rich CEACAM-1-associated protein [Lyric]), was originally identified as an oncogene whose expression can be induced in primary human fetal astrocytes by infection with human immunodeficiency virus type 1 (HIV-1) or treated with HIV envelope glycoprotein (gp120) or tumor necrosis factor-α (TNF-α).4-6 Recently, the AEG-1 expression levels have been found to be elevated in many types of human malignancies, such as prostate cancer, breast cancer, neuroblastoma, hepatocellular carcinoma, esophageal squamous cell carcinoma, gastric cancer, and non-small cell lung cancer7-13 and are associated with disease progression and poor clinical outcomes. Moreover, AEG-1 has been found to promote cancer chemoresistance, metastasis, invasion, and angiogenesis.5,11,12,14-17 Based on the previous studies, we hypothesize that AEG-1 might be associated with EMT in cervical cancer. In this study, we addressed this hypothesis by RNAi knockdown of AEG-1 in cervical cancer HeLa cell line and found that AEG-1 knockdown not only decreased EMT in cervical cancer cells, but also attenuated their aggressive behavior, as shown by reduced colony formation and increased sensitivity to cancer drugs.
Results
Elevated AEG-1 expression in poorly differentiated cervical cancer tissue samples
AEG-1 expression has been found to be elevated in various tumors,12,13 but its expression in cervical cancer has not been reported. To determine the level of expression of AEG-1 in cervical cancer, we performed immunohistochemistry analysis of 52 paraffin-embedded cervical cancer samples, including 27 high-differentiated samples and 25 low-differentiated samples, and 4 fresh surgical cervical cancer samples consisting of 2 low-differentiated and 2 high-differentiated samples. As shown in Figure 1A, low-differentiated samples (a, b) had stronger expression of AEG-1 than high-differentiated samples (c, d). To further confirm this, we performed western blot analysis and found that surgical samples from low-differentiated samples (p1 and p3) had higher expression of AEG-1 than those in high-differentiated samples (p2 and p4) (Fig. 1B).
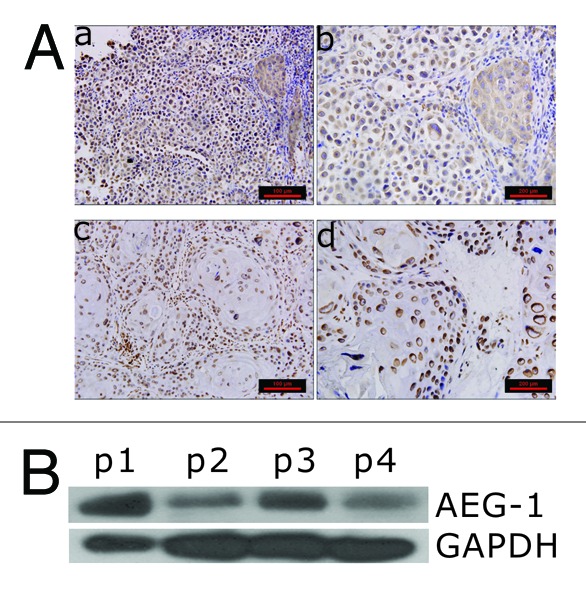
Figure 1. AEG-1 expression in different differentiated paraffin-embedded cervical cancer samples. (A) Immunohistochemistry staining with AEG-1 antibody of cervical cancer patient samples. (a and b) low-differentiated paraffin embedded samples; (c and d) high differentiated paraffin embedded samples. (B) Western blot analysis of AEG-1 expression in different differentiated surgical cervical cancer samples. p1 and p3 refer to low differentiated samples; p2 and p4 high differentiated samples.
Knockdown of AEG-1 led to reduction of mesenchymal markers
To determine whether AEG-1 plays any role in cervical cancer invasion by inducing EMT, we transfected the HeLa cells with AEG-1 shRNA and obtained stable cell lines. The expression of AEG-1 in HeLa stable cell lines and the markers of EMT, including E-cadherin, N-cadherin, vimentin, and snail, were examined by western blot. As shown in Figure 2A, cells transfected with AEG-1 shRNA1 had significantly decreased levels of AEG-1 compared with the control cells. Knockdown of AEG-1 downregulated the mesemchymal vimentin and EMT transcription factor Snail. N-cadherin was downregulated, while the level of E-cadherin was not significantly altered. However, western blot analysis further showed that the expression of E-cadherin was increased in those surgical specimens where the level of AEG-1 expression was lower (Fig. 2B). Since AEG-1 shRNA2 knockdown did not work as well as shRNA1 construct (Fig. 2), we only used AEG-1 shRNA1 construct as AEG-1 knockdown for subsequent assays.
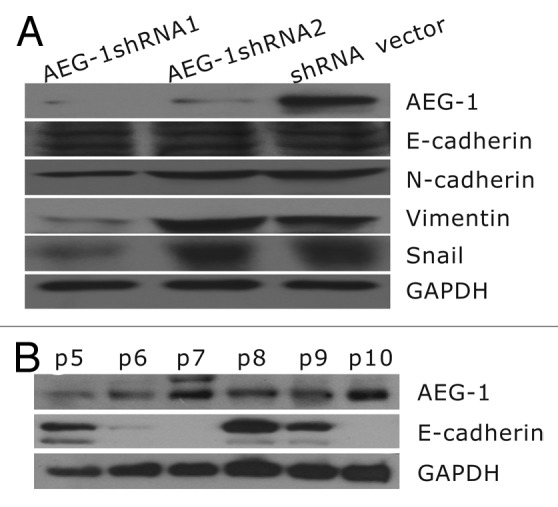
Figure 2. Expression of epithelial and mesenchymal markers in HeLa cells and patient samples. (A) Knockdown of AEG-1 led to reduction of mesenchymal markers in HeLa cells. (B) Expression of E-cadherin was increased in patient specimens where the level of AEG-1 was lower. In contrast, AEG-1 was increased in the surgical specimens (p5, p8, p9), where the level of E-cadherin was lower (p7, p10).
Knockdown of AEG-1 decreased migration and invasion of cervical cancer cells
Increased migration and invasion is a key feature of cells that undergo EMT.18 To assess whether stable AEG-1 shRNA affects migration and invasion of HeLa cells, we evaluated both cell migration by scratch assay and invasion by transwell assay. As shown in Figure 3, migration distance of AEG-1 shRNA cells was greatly reduced compared with the vector control cells by 24 h (P < 0.05). Moreover, AEG-1 shRNA cells (284.25 ± 39.9) showed significantly decreased invasion ability in the transwell assay in comparison with the shRNA vector cells (525.4 ± 83.7; P < 0.01) (Fig. 4).
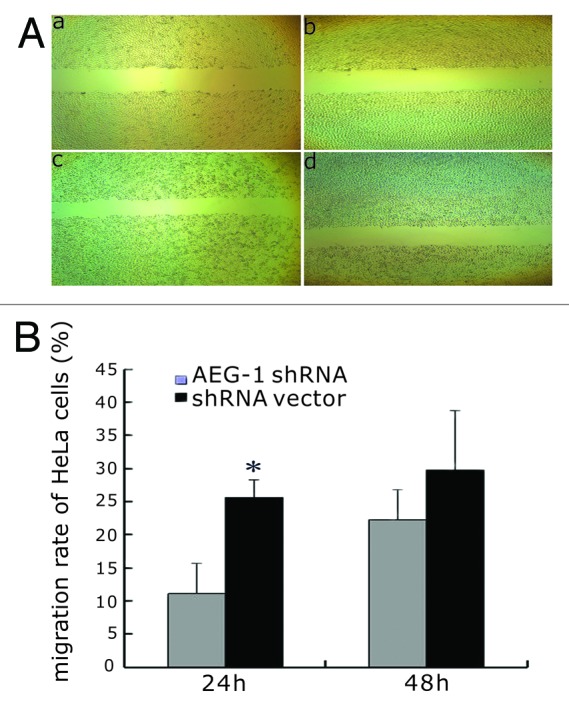
Figure 3. Effect of AEG-1 knockdown on cervical cancer cell migration. (A) The scratch assay showed that the migration distance of AEG-1 knockdown cells was greatly reduced compared with the vector control cells 24 h later; (a and c) shRNA vector 0 h, 24 h; (b and d) AEG-1 shRNA 0 h, 24 h). (B) The difference in migration distance of AEG-1shRNA knockdown cells compared with the vector control cells at 24 h and 48 h. The difference in migration between AEG-1 shRNA knockdown cells, and the vector control cells was statistically significant (*P < 0.05) at 24 h but not at 48 h.
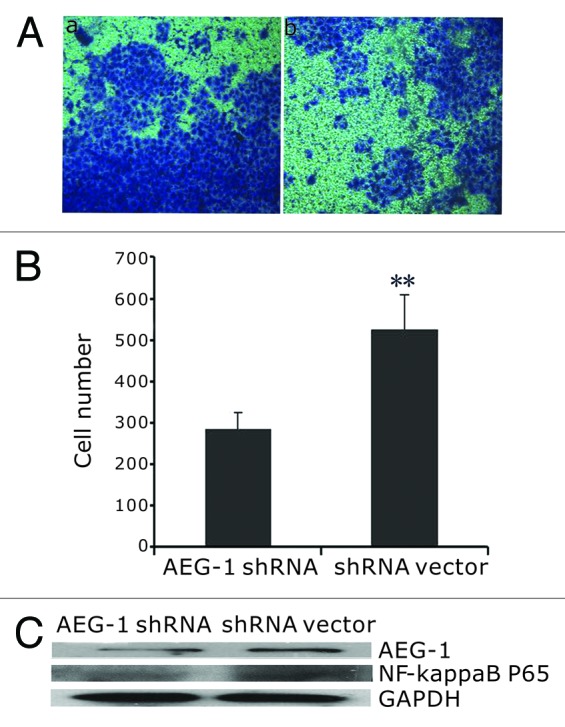
Figure 4. AEG-1 knockdown caused decreased invasive ability of HeLa cells. (A) Invasive cell numbers of shRNA vector (a) and AEG-1 shRNA (b); (B) AEG-1 shRNA (284.25 ± 39.9) cells showed significantly decreased invasive ability compared with the shRNA vector control cells (525.4 ± 83.7; **P < 0.01). (C) NF-κB p65 activity decreased in AEG-1 shRNA knockdown cells compared with shRNA vector control cells by western blot.
Since NF-κB is at the center of multiple pathways that promote an invasive phenotype,19 we wondered whether the EMT markers with significant changes promoted by AEG-1 in our study might be induced by activated NF-κB pathway. We then tested the NF-κB p65 activity by western blot in AEG-1 knockdown cells. We found that NF-κB p65 activity was decreased in AEG-1 shRNA cells compared with shRNA vector cells (Fig. 4C).
Knockdown of AEG-1 decreased colony formation and cell viability upon exposure of HeLa cells to chemotherapy drugs
Since colony formation is one of the characteristics of cancer stem cells, we also assessed the effect of AEG-1 knockdown on colony formation of HeLa cells. Knockdown of AEG-1 in HeLa cells decreased the colony-formation numbers in soft agar assay (Fig. 5A). AEG-1 shRNA knockdown cells formed 45 colonies/5000 cells, while shRNA vector control cells formed 102 colonies/5000 cells (P < 0.05) (Fig. 5A). We further evaluated the effects of 2 cancer drugs, paclitaxel and cisplatin, on AEG-1 knockdown cells and the vector control cells using colony-formation assay. Interestingly, AEG-1 knockdown cells were more sensitive to the 2 drugs than the vector control cells in colony-formation assay. As shown in Figure 5B, paclitaxel (2.5 nM) and cisplatin (5 μM) inhibited colony-formation ability of AEG-1 shRNA cells to about 3–8 colonies/5000 cells, but the same treatment produced about 30–170 colonies/5000 cells for the vector cells (P < 0.01).
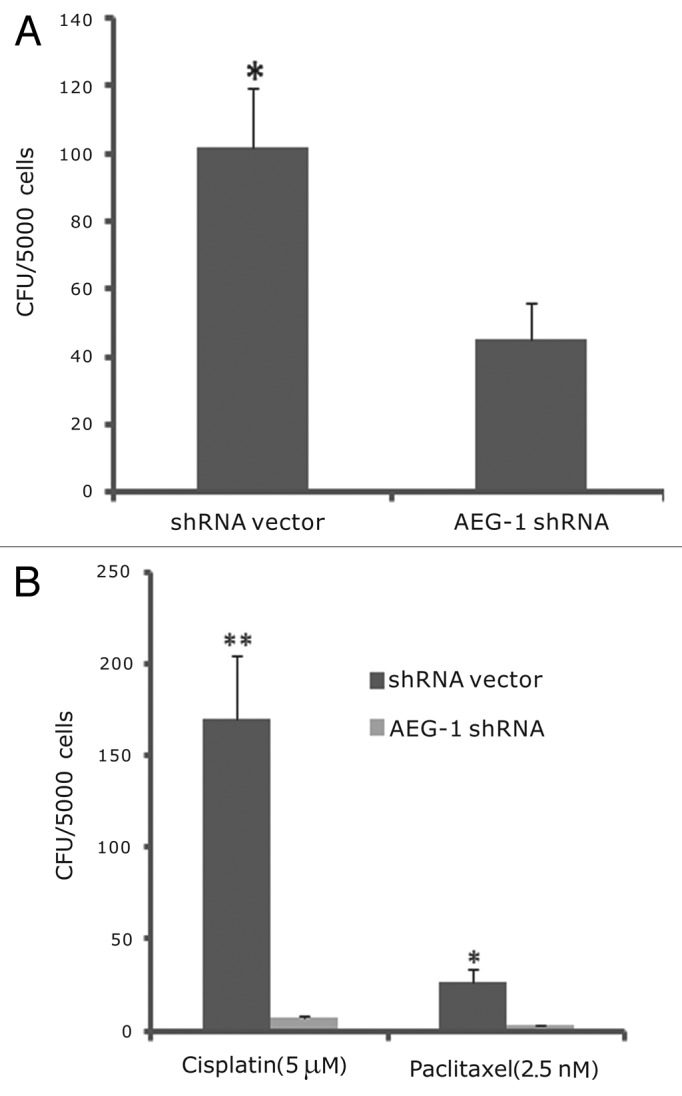
Figure 5. Knockdown of AEG-1 decreased colony formation in soft agar assay. (A) AEG-1 knockdown cells led to fewer colonies than vector control cells (*P < 0.05). (B) Paclitaxel and cisplatin caused more growth inhibition in AEG-1 shRNA cells than in shRNA vector control cells (*P < 0.05, ** P < 0.01, respectively).
Discussion
Understanding the molecular mechanisms leading to invasiveness and metastatic dissemination of carcinoma cells is important for development of new therapeutic strategies against cancer. Epithelial to mesenchymal transition (EMT) is believed to be one of the critical steps in progression to malignancy.18 EMT endows cells with migratory and invasive properties, but little is known about the regulation of EMT in cervical cancer cells. To date, only membrane KCl co-transporter-3, human papillomavirus, and transforming growth factor-β1 were reported as EMT inducers in cervical cancer cell lines.20-22 In the present study, we presented evidence showing that AEG-1 is involved in EMT in cervical cancer HeLa cells. We found that transfection of shRNA AEG-1 in HeLa cells could induce changes of EMT markers, as shown by upregulation of E-Cadherin and downregulation of vimentin and N-Cadherin in cervical cancer cells upon shRNA AEG-1 knockdown (Fig. 2). E-cadherin is a cell-adhesion molecule associated with epithelial cell-to-cell adhesion;23 its loss or reduced expression is a critical step in EMT process24 and has been used as a molecular biomarker to predict the clinical outcome for patients with esophageal carcinoma and other solid malignant tumors.25,26 Cells losing E-cadherin had increased expression of N-cadherin. In addition, Vimentin is one of the major mesenchymal intermediate filaments, and its expression has often been described as the end-stage progression in EMT, and it represents the completely dedifferentiated state in tumor cells that are highly proliferative and invasive. Consistent with this view, overexpression of vimentin in breast cancer cells led to enhanced migration of breast cancer cells.27 Conversely, knockdown of vimentin with antisense oligonucleotides reduced cell motility. Our finding that AEG-1 knockdown causing upregulation of E-cadherin and decreased expression of vimentin and N-cadherin suggests that AEG-1 is involved in EMT in HeLa cells.
We further showed that knockdown of AEG-1 could downregulate the transcription of Snail, which is considered to be a key regulator of EMT.28 Snail has been shown to correlate with E-cadherin downregulation29 and function to promote cell fate changes during development, leading to production of mesenchymal cells with greater migratory potential.30 Snail has been found to be expressed at the invasive front of carcinomas and linked to lymph node metastasis and tumor relapse, thus implying its important role in tumor progression.31 Our observation that knockdown of AEG-1 could significantly reduce the invasion and migration ability of cervical cancer cells in cell invasion and migration assays indicates that AEG-1 plays an important role in EMT and perhaps tumor progression.
AEG-1 is known to activate the NF-κB pathway and interacts with the p65 component of NF-κB in the nucleus of cells, which then induces invasiveness of tumor cells and increases expression of adhesion molecules. NF-κB has been shown to serve as a central mediator of EMT in a mouse model of breast cancer progression.32 Our observation that AEG-1 knockdown caused decreased NF-κB expression (Fig. 4C) is consistent with a role that AEG-1 plays in controlling the expression of NF-κB pathway.
We found that AEG-1 was overexpressed in cervical cancer patient specimens with low differentiation compared with those with high differentiation (Fig. 1). This is consistent with the observations that AEG-1 overexpression is associated with poor prognosis in several cancers, including gastric, breast, and prostate cancers and neuroblastoma.33 It has been shown that EMT-expressing cells have properties of stem cells,18 and that EMT is upregulated in cancer stem cells (CSC).34,35 We found that AEG-1 knockdown caused significant reduction in CFU-forming ability of HeLa cells. This finding is consistent with a previous study that showed knockdown of AEG-1 could decrease the colony formation in SiHa cells.36 Furthermore, that AEG-1 knockdown led to reduced NF-κB expression (Fig. 4C) is also consistent with our previous finding that NF-κB played important roles in more quiescent cancer stem-like cells than in the bulk cells.37 Cancer stem cells are known to be involved in tumorigenesis, chemoresistance, and invasion/metastasis.38,39 Our results that knockdown of AEG-1 caused decrease of cancer stem cell properties such as reduced CFU formation, decreased cell migration, and invasion as well as increased susceptibility to chemotherapy drugs suggest that AEG-1 is very likely a cancer stem cell marker.
In conclusion, we have shown that knockdown of AEG-1 could decrease EMT in cervical cancer cells. To the best of our knowledge, this is the first study to provide evidence that AEG-1 is involved in EMT in cervical cancer. In addition, we found that knockdown of AEG-1 weakened stem-like property of HeLa cells, as shown by reduced cell migration and invasion, decreased colony formation, and increased susceptibility to cancer drugs. Further studies are needed to investigate the mechanism by which AEG-1 controls EMT and confirm the reduced tumorigenicity in animal models. The findings that AEG-1 is involved in controlling EMT and diverse cancer stem cell-like properties suggest that AEG-1 may be a promising target for development of novel drugs for improved treatment of cervical cancer.
Materials and Methods
Tissue samples and immunohistochemistry
The streptavidin-peroxidase-biotin (SP) immunohistochemistry method was performed to study altered protein expression in 52 paraffin-embedded cervical cancer tissues, which were histopathologically diagnosed as high- or low-differentiated cervical cancer at the Department of Pathology, the First Hospital of Lanzhou University from 2009 to 2011. Paraffin-embedded specimens were cut into 5-μm sections and baked at 60 °C for 60 min. The sections were deparaffinized with xylene and rehydrated. Sections were submerged into antigenic retrieval buffer and microwaved for antigenic retrieval, and then cooled at room temperature for 20 min. The sections were treated with 3% hydrogen peroxide in methanol to quench the endogenous peroxidase activity, followed by incubation with normal serum to block nonspecific binding. Rabbit anti-AEG-1 (1:1000; Sigma-Aldrich) was incubated with the sections overnight at 4 °C. After washing, the tissue sections were treated with biotinylated anti-rabbit secondary antibody, followed by further incubation with streptavidin-horseradish peroxidase complexed with substrate diaminobenzidine (DAB). The sections were counterstained with hematoxylin. For negative controls, the rabbit anti-AEG-1 antibody was replaced with PBS buffer.
Cell cultures and surgical specimens
The human cervical carcinoma cell line HeLa (Chinese Academy of Sciences, Scsp-q07) was routinely maintained in Dulbecco modified Eagle medium (DMEM, Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 units/ml penicillin, and 100μg/ml streptomycin. In all experiments, cells were maintained at 37 °C, 5% CO2, and 95% air atmosphere. All of our experiments were performed on cultures that were 70% confluent. We selected 10 surgical specimens of cervical cancer patients from Department of Obstetrics and Gynecology, Gansu Provincial People’s Hospital, Lanzhou, China. Squamous cell carcinoma is the most common type of cervical cancer. The clinical staging was assessed based on the criteria of International Federation of Gynecology and Obstetrics classification. The experiment involving human participants was approved by the Human Research Ethics Committee of Lanzhou University and Gansu Provincial People’s Hospital. Each individual was informed about the nature of the research and given the study protocol, and they all signed the informed consents.
Construction of stably transfected cell lines
Endogenous AEG-1 was knocked down using the pLKO.1.puro vector containing short hairpin RNA (shRNA) against AEG-1. The cells were transfected with either shRNA against AEG-1 or a control vector using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions, and mixed resistant clones were collected and cultured in medium containing puromycin (Invitrogen). Western blot analysis was used to verify the downregulation of AEG-1expression in HeLa cells as described above.
Western blotting
The cells were washed in PBS and lysed in RIPA buffer (1% NP-40, 0.1% SDS, 5 mM EDTA, 0.5% sodium deoxycholate, and 1 mM sodium vanadate) containing protease inhibitors (1 mM PMSF, 1 mM sodium fluoride). The samples were incubated on ice for 30 min and centrifuged at 12 000 g for 15 min at 4 °C. The supernatants were collected, and the protein concentration was measured with the BCA Protein Assay Kit (Merck). An equal amount of total protein was separated by 10% sodium lauryl sulfate-PAGE (SDS-PAGE) and electroblotted onto a PVDF membrane (Millipore) using a semi-dry blotting apparatus (Bio-Rad Laboratories). Membranes were blocked in 5% non-fat milk in TBST at room temperature for 1 h, incubated overnight with primary antibodies at 4 °C, and then washed 3 times with TBST. The primary antibodies included AEG-1 (1:500, Sigma-Aldrich), E-cadherin (1:200, Santa Cruz Biotechnology), N-cadherin, vimentin, snail (1:200, Cell Signaling Technology, Inc), and GAPDH (1:1000) were used at appropriate dilutions for the western blot. This was followed by an additional 2 h of incubation with HRP-labeled secondary antibodies (Bioworld Technology CO, Ltd) in blocking buffer (1:5000). Finally, the membranes were washed 3 times with TBST, and the protein bands were detected using an ECL system (Merck) according to the manufacturer’s instructions.
Scratch assay
The scratch assay was performed as described.40 AEG-1shRNA cells or shRNA vector cells were cultured to 70–80% confluency in 6-well plates. Pipette tip was used to create a scratch of the cell monolayer. The plates were washed 3 times with Hanks Buffered Salt Solution and replenished with the DMEM (serum free). The plates were incubated at 37 °C in 5% CO2 atmosphere for 24 h and 48 h, respectively. Pictures were taken at 0 h, 24 h, and 48 h, respectively. Distance from scratch was measured from one side to the other side with Motic Images Advanced3.2.
Invasion assay
The invasion assay was performed in 24-well transwell chambers (Corning) containing polycarbonate filters with 8-μm pores coated with matrigel (BD Biosciences).41 First, the invasion chambers were rehydrated with DMEM (serum free) for 2 h at 37 °C in 5% CO2 atmosphere. Seven hundred µL of conditioned medium with 15% fetal bovine serum was added to the lower compartment as the chemotactic factor. Then, 1 × 105 shRNA-AEG-1 cells and shRNA-vector cells in serum-free DMEM were added to the upper compartment of the chamber. Each cell group was plated in 3 duplicate wells. After incubation for 48 h, the noninvasive cells were removed with a cotton swab. Cells that had migrated through the membrane and stuck to the lower surface of the membrane were fixed with methanol and stained with Crystal Violet. Finally, the cells in the lower compartment of the chamber were counted under a light microscope in at least 10 random visual fields.
Colony-formation assay
The colony-formation assay was performed in 35-mm dishes as described.42 Briefly, both AEG-1 shRNA and vector control cells were plated in 35-mm dishes at 5000 cells/well in 0.35% top agar in culture medium over a 0.5% agar layer. For drug sensitivity testing, drugs were added into the top agar at concentrations as indicated. Plates were incubated in cell culture incubator for 12 d, until colonies were large enough to be visualized. Colonies were stained with 200 μl MTT (3-[4.5-dimethylthiazol-2-yl]-2.5-diphenyl-tetrazoliumbromide) (5 mg/ml) for 30 min and counted. Experiments were done in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 for Windows. Student t test was used to analyze the statistical difference. Results were presented as mean ± standard deviation (SD). P < 0.05 was considered statistically significant and the experiments were repeated at least 3 times.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Yibin Kang for providing AEG-1 plasmid and Dr Yin Han for preparing AEG-1 shRNA constructs. This study was supported by Changjiang Scholar Program, Key Laboratory for Gastrointestinal Diseases of Gansu Province (gswcky-2012-002), and the National Natural Science Foundation of China (81171954 and 81160287).
Glossary
Abbreviations:
- AEG-1
astrocyte elevated gene-1
- CSC
cancer stem cells
- EMT
epithelial to mesenchymal transition
- NF-κB
nuclear factor kappaB
- RNAi
RNA interference
- shRNA
short hairpin RNA
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Castellsagué X, Bosch FX, Muñoz N. Environmental co-factors in HPV carcinogenesis. Virus Res. 2002;89:191–9. doi: 10.1016/S0168-1702(02)00188-0. [DOI] [PubMed] [Google Scholar]
- 3.Kim CJ, Jeong JK, Park M, Park TS, Park TC, Namkoong SE, Park JS. HPV oligonucleotide microarray-based detection of HPV genotypes in cervical neoplastic lesions. Gynecol Oncol. 2003;89:210–7. doi: 10.1016/S0090-8258(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 4.Britt DE, Yang DF, Yang DQ, Flanagan D, Callanan H, Lim YP, Lin SH, Hixson DC. Identification of a novel protein, LYRIC, localized to tight junctions of polarized epithelial cells. Exp Cell Res. 2004;300:134–48. doi: 10.1016/j.yexcr.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–74. doi: 10.1016/S1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- 6.Kang DC, Su ZZ, Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Zhang N, Song LB, Liao WT, Jiang LL, Gong LY, Wu J, Yuan J, Zhang HZ, Zeng MS, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res. 2008;14:3319–26. doi: 10.1158/1078-0432.CCR-07-4054. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Liu XX, He JR, Zhou CX, Guo M, He M, Li MF, Chen GQ, Zhao Q. Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis. 2011;32:2–9. doi: 10.1093/carcin/bgq209. [DOI] [PubMed] [Google Scholar]
- 9.Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, Place RF, Pookot D, Majid S, Igawa M, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene. 2007;26:7647–55. doi: 10.1038/sj.onc.1210572. [DOI] [PubMed] [Google Scholar]
- 10.Emdad L, Sarkar D, Su ZZ, Lee SG, Kang DC, Bruce JN, Volsky DJ, Fisher PB. Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration. Pharmacol Ther. 2007;114:155–70. doi: 10.1016/j.pharmthera.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo BK, Emdad L, Su ZZ, Villanueva A, Chiang DY, Mukhopadhyay ND, Mills AS, Waxman S, Fisher RA, Llovet JM, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest. 2009;119:465–77. doi: 10.1172/JCI36460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu C, Chen K, Zheng H, Guo X, Jia W, Li M, Zeng M, Li J, Song L. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis. 2009;30:894–901. doi: 10.1093/carcin/bgp064. [DOI] [PubMed] [Google Scholar]
- 13.Song L, Li W, Zhang H, Liao W, Dai T, Yu C, Ding X, Zhang L, Li J. Over-expression of AEG-1 significantly associates with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. J Pathol. 2009;219:317–26. doi: 10.1002/path.2595. [DOI] [PubMed] [Google Scholar]
- 14.Hu G, Chong RA, Yang Q, Wei Y, Blanco MA, Li F, Reiss M, Au JL, Haffty BG, Kang Y. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15:9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Song X, Liu C, Xie L, Wei L, Sun R. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. J Exp Clin Cancer Res. 2009;28:19. doi: 10.1186/1756-9966-28-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emdad L, Lee SG, Su ZZ, Jeon HY, Boukerche H, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci U S A. 2009;106:21300–5. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Wu Y, Peng D. Astrocyte elevated gene-1 regulates osteosarcoma cell invasion and chemoresistance via endothelin-1/endothelin A receptor signaling. Oncol Lett. 2013;5:505–10. doi: 10.3892/ol.2012.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–44. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 20.Hsu YM, Chen YF, Chou CY, Tang MJ, Chen JH, Wilkins RJ, Ellory JC, Shen MR. KCl cotransporter-3 down-regulates E-cadherin/beta-catenin complex to promote epithelial-mesenchymal transition. Cancer Res. 2007;67:11064–73. doi: 10.1158/0008-5472.CAN-07-2443. [DOI] [PubMed] [Google Scholar]
- 21.Yi JY, Hur KC, Lee E, Jin YJ, Arteaga CL, Son YS. TGFbeta1 -mediated epithelial to mesenchymal transition is accompanied by invasion in the SiHa cell line. Eur J Cell Biol. 2002;81:457–68. doi: 10.1078/0171-9335-00265. [DOI] [PubMed] [Google Scholar]
- 22.Veeraraghavalu K, Subbaiah VK, Srivastava S, Chakrabarti O, Syal R, Krishna S. Complementation of human papillomavirus type 16 E6 and E7 by Jagged1-specific Notch1-phosphatidylinositol 3-kinase signaling involves pleiotropic oncogenic functions independent of CBF1;Su(H);Lag-1 activation. J Virol. 2005;79:7889–98. doi: 10.1128/JVI.79.12.7889-7898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao L, Ha JR, Kuzel P, Garcia E, Persad S, Steiniche T. Cadherin switch from E- to N-cadherin in melanoma progression is regulated by the PI3K/PTEN pathway through Twist and Snail. Br J Dermatol. 2012;166:1184–97. doi: 10.1111/j.1365-2133.2012.10824.x. [DOI] [PubMed] [Google Scholar]
- 25.Berx G, Staes K, van Hengel J, Molemans F, Bussemakers MJ, van Bokhoven A, van Roy F. Cloning and characterization of the human invasion suppressor gene E-cadherin (CDH1) Genomics. 1995;26:281–9. doi: 10.1016/0888-7543(95)80212-5. [DOI] [PubMed] [Google Scholar]
- 26.Prognostic significance of CyclinD1 and E-Cadherin in patients with esophageal squamous cell carcinoma: multiinstitutional retrospective analysis. Research Committee on Malignancy of Esophageal Cancer, Japanese Society for Esophageal Diseases. J Am Coll Surg. 2001;192:708–18. doi: 10.1016/S1072-7515(01)00840-7. [DOI] [PubMed] [Google Scholar]
- 27.Hendrix MJ, Seftor EA, Chu YW, Trevor KT, Seftor RE. Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev. 1996;15:507–25. doi: 10.1007/BF00054016. [DOI] [PubMed] [Google Scholar]
- 28.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y, Zhou BP. New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin (Shanghai) 2008;40:643–50. doi: 10.1111/j.1745-7270.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–61. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 31.Guarino M. Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 2007;39:2153–60. doi: 10.1016/j.biocel.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Huber MA, Azoitei N, Baumann B, Grünert S, Sommer A, Pehamberger H, Kraut N, Beug H, Wirth T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–81. doi: 10.1172/JCI200421358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu HY, Liu CX, Han B, Zhang XY, Sun RP. AEG-1 is associated with clinical outcome in neuroblastoma patients. Cancer Biomark. 2012;11:115–21. doi: 10.3233/CBM-2012-0268. [DOI] [PubMed] [Google Scholar]
- 34.Kwon MJ. Emerging roles of claudins in human cancer. Int J Mol Sci. 2013;14:18148–80. doi: 10.3390/ijms140918148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng X, Brachova P, Yang S, Xiong Z, Zhang Y, Thiel KW, Leslie KK. Knockdown of MTDH sensitizes endometrial cancer cells to cell death induction by death receptor ligand TRAIL and HDAC inhibitor LBH589 co-treatment. PLoS One. 2011;6:e20920. doi: 10.1371/journal.pone.0020920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat. 2008;111:419–27. doi: 10.1007/s10549-007-9798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Zhang Y. Cancer stem cells: Models, mechanisms and implications for improved treatment. Cell Cycle. 2008;7:1360–70. doi: 10.4161/cc.7.10.5953. [DOI] [PubMed] [Google Scholar]
- 40.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 41.Li X, Kong X, Huo Q, Guo H, Yan S, Yuan C, Moran MS, Shao C, Yang Q. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci. 2011;102:1151–7. doi: 10.1111/j.1349-7006.2011.01919.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E, 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–63. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]


