Many molecular events are terminated by rapid protein degradation via the ubiquitin–proteasome system. Most target proteins are recognized by E3 ubiquitin ligases, often after specific protein modifications, leading to their poly-ubiquitylation and subsequent degradation by the proteasome. One such ubiquitin ligase is Fbw7, a member of the F-box protein family that associates with Skp1, Cul1, and Rbx1 to form the SCFFbw7 ubiquitin ligase complex. SCFFbw7 controls the abundance of several important oncoproteins, including Myc, cyclin E, Notch, Jun, and others. Accordingly, Fbw7 is a tumor suppressor that is frequently mutated in many types of cancer. It is thus critical to understand how Fbw7 precisely functions in order to understand its mechanisms of tumor suppression and to develop targeted therapies.
All Fbw7 substrates share a short conserved amino acid motif, termed a CPD (Cdc4 phospho-degron),1 that mediates substrate degradation through interactions with Fbw7. However, the dogma of a single high-affinity degron driving substrate turnover appears to be an over-simplification of how Fbw7 interacts with its substrates. Although all identified degrons conform to the general CPD consensus, many degrons deviate from the ideal motif, leading to decreased affinity for Fbw7. While these types of CPDs may suffice for substrate turnover, their decreased affinity may impair substrate ubiquitylation when compared with optimal degrons.
Some CPDs, however, deviate so substantially from the optimal consensus that they cannot mediate substrate turnover. We addressed the function of degrons that are too weak to drive substrate degradation by using cyclin E as a model substrate. In addition to its high-affinity C-terminal degron, cyclin E also contains a weak N-terminal CPD that cannot support Fbw7 binding or cyclin E turnover by itself. Surprisingly, we found that this weak degron synergizes with the C-terminal optimal degron to generate even higher cyclin E binding affinity to Fbw7, and that both degrons cooperatively drive cyclin E turnover.
One scenario for cooperating CPDs has previously been suggested for the multiple low-affinity degrons found in the CDK inhibitor Sic1, which is degraded by Cdc4, the budding yeast Fbw7 ortholog.1-3 However, a key distinction of our work from that of Sic1 degradation is our finding that Fbw7 degron cooperativity also requires Fbw7 dimerization. Fbw7 naturally occurs as dimers, which was previously shown to aid the ubiquitylation process.4,5 We show a new function for dimerization in substrate interactions: to increase avidity through binding to 2 (suboptimal) degrons. We found that the 2 cyclin E degrons simultaneously engage 2 individual substrate-binding domains of an Fbw7 dimer, and suggest that this mode of substrate regulation is not only applicable to many other substrates, but also to other dimerizing ubiquitin ligases. Indeed, similar substrate interactions have been demonstrated for dimeric Cul3-based ubiquitin ligases.6,7
In contrast to turnover driven by a single degron, the simultaneous engagement of 2 CPDs with Fbw7 may alter the rate of substrate turnover, thus potentially making substrate degradation nonlinear and sensitive to multi-signal input. Thus, secondary degrons may have evolved in substrates that require either switch-like regulation or the integration of multiple signal transduction pathways.
Most Fbw7 substrates appear to contain only single identified suboptimal degrons, and we thus speculate they require secondary, cooperating degrons for their destruction. One possibility is that these putative secondary degrons are embedded within the substrate itself (as for cyclin E), thus adding complexity to the signals that govern their degradation (Fig. 1; left). However, an important implication of our model is that cooperating degrons can also be separated onto 2 interacting proteins, either as a homodimer (as we show for SREBP) or perhaps within a heterodimer composed of 2 different interacting proteins. The separation of weak degrons onto 2 proteins could, thus, prevent premature monomeric substrate destruction and restrict substrate turnover to specific protein complexes.
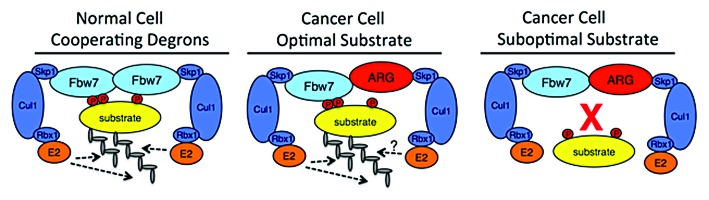
Figure 1. Models of Fbw7–substrate interactions in normal and cancer cells. In normal cells, an Fbw7 dimer binds to 2 separate cooperating degrons using the interaction domains on each protomer (middle). The high-affinity degron is depicted with 2 phosphorylations (red circles); the suboptimal degron is (for instance) mono-phosphorylated. Cancer-associated heterozygous Fbw7 missense mutations produce heterodimers composed of wt-Fbw7 and Fbw7ARG. While interactions of the wt-Fbw7 protomer with high-affinity degrons may suffice for substrate degradation (left), Fbw7 heterodimers are unable to bind substrates with suboptimal degrons (right).
We also found that Fbw7 dimerization adds remarkable robustness to substrate interactions, and that the increased affinity provided by cooperating degrons allows Fbw7 dimers to function with mutations in the Fbw7–substrate interface that disable Fbw7 monomers. Thus, besides regulating substrate affinity and specificity, Fbw7 dimerization may also have evolved to protect critical substrate degradation from potentially oncogenic mutations.
Our observations have important implications for understanding the selection and function of Fbw7 mutations in cancer. While many mutations in either substrates and Fbw7 can be buffered by Fbw7 dimerization (and therefore don’t occur in tumors), loss of the most critical contacts cannot be compensated, and these include Fbw7 arginine residues that form the central CPD phospho-binding pocket and are hotspot mutations in cancers (termed Fbw7ARG). Cancers with heterozygous Fbw7ARG mutations usually retain a wild-type Fbw7 allele, which allows the formation of crippled Fbw7:Fbw7ARG heterodimers. We suggest that substrates with high-affinity degrons can still be degraded via the binding affinity of the wild-type Fbw7 protomer of an Fbw7:Fbw7ARG dimer (Fig. 1; middle). In contrast, substrates with weak CPDs whose degradation relies on cooperating degrons and Fbw7 dimers cannot be targeted by Fbw7:Fbw7ARG heterodimers (Fig. 1; right). We thus speculate that substrate-specific dimer requirements may underlie the selective pressure for missense mutations in cancer, by retaining aspects of Fbw7 function advantageous to tumorigenesis while disabling critical tumor suppressor functions.
Welcker M, et al. Genes Dev. 2013;27:2531–6. doi: 10.1101/gad.229195.113.
References
- 1.Nash P, et al. Nature. 2001;414:514–21. doi: 10.1038/35107009. [DOI] [PubMed] [Google Scholar]
- 2.Feldman RM, et al. Cell. 1997;91:221–30. doi: 10.1016/S0092-8674(00)80404-3. [DOI] [PubMed] [Google Scholar]
- 3.Orlicky S, et al. Cell. 2003;112:243–56. doi: 10.1016/S0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 4.Tang X, et al. Cell. 2007;129:1165–76. doi: 10.1016/j.cell.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 5.Hao B, et al. Mol Cell. 2007;26:131–43. doi: 10.1016/j.molcel.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Tong KI, et al. Mol Cell Biol. 2006;26:2887–900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang M, et al. Mol Cell. 2009;36:39–50. doi: 10.1016/j.molcel.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


