Abstract
Currently, maternal aging in women, based on mouse models, is thought to raise oocyte aneuploidy rates, because chromosome cohesion deteriorates during prophase arrest, and Sgo2, a protector of centromeric cohesion, is lost. Here we show that the most common mouse strain, C57Bl6/J, is resistant to maternal aging, showing little increase in aneuploidy or Sgo2 loss. Instead it demonstrates significant kinetochore-associated loss in the spindle assembly checkpoint protein Mad2 and phosphorylated Aurora C, which is involved in microtubule–kinetochore error correction. Their loss affects the fidelity of bivalent segregation but only when spindle organization is impaired during oocyte maturation. These findings have an impact clinically regarding the handling of human oocytes ex vivo during assisted reproductive techniques and suggest there is a genetic basis to aneuploidy susceptibility.
Keywords: spindle assembly checkpoint, oocyte, aging, cell cycle, aneuploidy, error correction, meiosis
Introduction
Aneuploidy is a major cause of infertility, early miscarriage, and birth defects.1-3 It is primarily brought about by the mis-segregation of chromosomes during the 2 meiotic divisions of the oocyte (meiosis I, MI; meiosis II, MII), and advanced maternal age is an important risk factor. The leading hypothesis to account for the maternal age effect, based on studies in mice that replicate this phenomenon, posits that aneuploidy is derived from chromosome cohesion loss during the long period of prophase I arrest, which is unique to mammalian oocytes and precedes the first of the 2 meiotic divisions.4,5 This is based on measurements of cohesion loss in oocytes from aged females and the inability of prophase I oocytes to replace cohesin components during the aging period.6-11
The spindle assembly checkpoint (SAC) is a near universal pathway that acts to prevent aneuploidy and involves members of the Mad and Bub families, Mps1 and Aurora B kinase.12,13 The SAC is active during prometaphase, and by assembly on unoccupied kinetochores, it generates a potent inhibitor of the anaphase-promoting complex.14 The SAC is satisfied and switched off when all kinetochores engage with spindle microtubules, and the kinetochore–microtubule (KT-MT) interaction causes chromosome alignment (biorientation) at the metaphase plate as a consequence of equal and opposite pulling forces from both poles. The SAC is certainly active in mouse oocytes, as its loss can raise aneuploidy rates considerably.1,15-18 However, the SAC is not as stringent in mouse oocytes as it is in somatic cells,19-23 and in human and mouse oocytes, aging is associated with a reduction in levels of SAC components such as Mad2.24-27 Although these data offer credence for a SAC basis to the maternal aging phenomenon, there are other studies demonstrating the SAC is active in aged oocytes.7,28 First, oocytes of aged and young mice seem to share the same ability to arrest in response to nocodazole-induced spindle damage. Second, the length of MI, which would be much shorter in the absence of the SAC, is observed to be no different in aged oocytes, and, further, there is no association between aneuploid eggs and the time taken to transit through MI, as there should be if the SAC were being bypassed, resulting in chromosome mis-segregation.28
Given recent work establishing the SAC is not so stringent in MI,19-23 and that the establishment of correct KT-MT for chromosomes (bivalents) is very poor,29 we decided to reexamine changes in the SAC with age. We used C57Bl6/J mice, because it is the standard laboratory mouse used in genome sequencing.
Results
C57Bl6/J oocytes have cohesion loss but no change in Sgo2 with maternal age
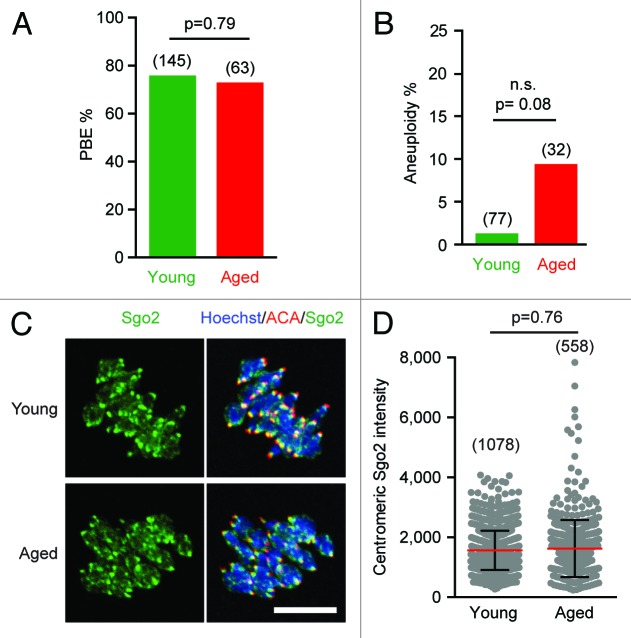
First, to examine if there is any age-related phenomenon in this mouse stain, chromosome spreads were performed on metaphase II (metII) oocytes from young and old mice following in vitro maturation. This was measured by rates of first polar body extrusion (PBE) and occurred at high rates independent of maternal age (Fig. 1A). Using this method, in a healthy normal metII oocyte, one would expect to observe 20 pairs of sister chromatids (dyads), which have been produced following the reductional division in MI, and this was the case in the vast majority of oocytes, with only a small number (n = 4/109) not containing 20 dyads (Fig. S1A). Unsurprisingly given its low incidence, there was no statistical significant rise in aneuploidy rate with maternal age, although it was modestly higher in aged mice (Fig. 1B). This suggests that this strain is in some way resistant to a maternal age effect when compared with other aged mice, which can have rates of between 20–60% at comparable or even younger ages.6,10,23,25,30
Figure 1. Maternal aging in C57Bl6/J oocytes does not significantly raise aneuploidy rates or lead to Sgo2 loss. (A) Polar body extrusion rates in oocytes from young vs. aged mice, showing no significant difference (Fisher test). (B) Percentage of aneuploid metII eggs following MI completion, showing no significant increase with age (Fisher test). (C) Sgo2 immunostaining in young and aged oocytes, 5 h after GVBD. Scale bars represent 10 µm. (D) Centromeric Sgo2 intensity is unchanged with age (mean ± s.d; Mann–Whitney U test; 27 young vs. 16 aged oocytes). (A and B) in parenthesis, number of oocytes examined; (D) in parenthesis, number of centromeres examined.
A loss in chromosome cohesion is thought to be a factor important in maternal age effect segregation errors in oocytes.6-8,10 Therefore, we examined if the lack of any pronounced maternal aging on aneuploidy was due to a resistance or tolerance to such cohesion deterioration. The kinetochore separation (interkinetochore or iKT separation) within dyads of metII oocytes were calculated; this distance tends to increase as the cohesion holding the dyad together diminishes. In agreement with others,6,7,10 we observed iKT increases in oocytes from aged mice (Fig. S1B). This suggests that in the C57Bl6/J strain, like other mice examined, and also in women,9 there is a general loss in chromosome cohesion, although in this particular strain it is not associated with an increase in aneuploidy to the same level observed elsewhere.
In addition to a loss in chromosome cohesion with maternal age, levels of centromeric Sgo2 have also been measured to decrease.7,31 So for example, we recorded a very significant drop in Sgo2 in Swiss mice at >12 mo as compared with 1 mo, which was associated with an increased aneuploidy rate with age.31 As suggested in the first study showing this association,7 such a decline is likely an important factor in the mis-segregation of bivalents during aging, because Sgo2 protects centromeric cohesin from separase-mediated degradation during MI. Its continued presence is therefore essential to maintain dyad integrity until MII.32,33 Any premature Sgo2 loss in MI would make centromeric cohesin vulnerable to separase-mediated cleavage, so generating single chromatids, which have been observed in other aging mouse strains.6,7,31 However, single chromatids were not found in the C57Bl6/J strain employed here, therefore we would predict no such Sgo2 drop if there was any causal relationship. Kinetochore-associated Sgo2 levels were measured by immunofluorescence from in situ chromosome of oocytes in MI (Fig. 1C), and in agreement with our prediction, no loss in Sgo2 staining occurred (Fig. 1D; Fig. S1C). However, when the same procedure was repeated on MI oocytes from a different mouse stain (Swiss CD-1), we found a significant reduction of centromere-associated Sgo2 with maternal age (Fig. S1D). These opposing observations on aneuploidy and centromeric Sgo2 localization between the 2 mouse stains suggest that the retention of this centromeric protector in aged C57Bl6/J mouse oocytes would account for the lack of observed single chromatids and supports a genetic basis to the age-related phenomenon.
Reduced Mad2, phospho-Aurora kinase, and ACA associated with aged bivalents
Although with maternal age there was no change in centromeric Sgo2, there was a rise in iKT separation, suggesting some relaxation in the cohesive forces holding chromosomes together. One possible interpretation for this is that aging results in a loss in the binding or retention of some chromosome-associated proteins but not others. To examine this idea further, we measured for changes, with respect to maternal age, in levels of 2 proteins: Mad2 and phosphorylated Aurora C (pAurora C). Aurora C is a substitute of Aurora B during meiosis,34-37 and its active form pAurora C is thought to be involved in destabilizing erroneous KT–MT attachment, as such contributing to the fidelity of bivalent division.34,38-40
One further reason for examining Mad2 is that previous studies have shown that levels of this transcript, like those of other SAC components, are lower in oocytes of both aged mice and humans.24,25 However, there appears to be no aging defect in SAC activity,7,28 as measured either by the length of MI, which would be predicted to be shorter in a situation of reduced SAC, or in the ability to arrest in response to a nocodazole challenge. It is therefore thought that the SAC does not reduce in functionality in aged oocytes.
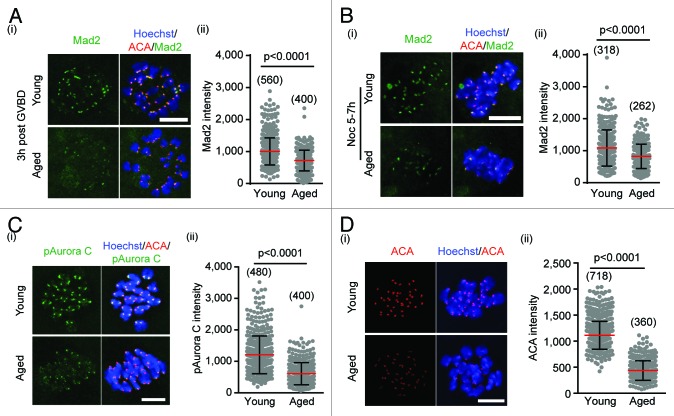
We assessed Mad2 on kinetochores at 3 h after resumption of MI, marked by germinal vesicle breakdown (GVBD), when the SAC is active,22 and found a significant ~30% reduction in mean levels in oocytes from aged mice (Fig. 2A). This reduction was apparent even when we attempted to maximally stimulate and re-engage the SAC activity by a 2-h, 10-µM nocodazole treatment at 5–7 h after GVBD (Fig. 2B), a later MI timepoint normally associated with low SAC activity. This procedure causes complete dissolution of all microtubule fibers and a re-engagement of Mad2 with all unoccupied kinetochores.22
Figure 2. Centromere-associated proteins are lost with age. (A) Kinetochore Mad2 immunostaining (i), and its quantification (ii), in oocytes at 3 h after GVBD (14 young vs. 10 aged oocytes). (B) As for (A) but in oocytes at 5–7 h and following nocodazole addition (13 young vs. 9 aged oocytes). Aged oocytes showed lower Mad2, independent of nocodazole addition. (C) pAurora C immunostaining (i), and its quantification (ii) at 5 h (12 young vs 10 aged oocytes). (D) ACA immunostaining (i) and its quantification (ii) at 5 h (18 young vs 9 aged oocytes). pAurora C (C) and ACA (D) were both significantly decreased in aged oocytes. (A–D; ii) mean ± s.d. (Mann–Whitney U test). Scale bars represent 10 µm. (A–D; ii) in parenthesis, number of centromeres examined.
When the same immunofluorescence analysis was performed for centromere-associated pAurora C, we similarly observed a significant fall in aged oocytes to a level about half that in young mice (Fig. 2C). This analysis was performed at 5 h after GVBD, a timepoint that would correspond to when error correction is active and needed.22,29
These data imply that with age, the centromere-kinetochore of a bivalent possesses reduced ability to recruit or retain both Mad2 and pAurora C. Indeed, this phenomenon of reduced levels may be more general, given that immunostaining with anti-centromeric antibody (ACA), which detects centromeric protein epitopes, was also found to be 61% lower in aged oocytes when compared with young (Fig. 2D). These data taken together suggest that many protein factors associated with the kinetochore and centromere may decline with age, although, as demonstrated with Sgo2, this decline is not universal.
Aged oocytes have a reduced ability to arrest with low-dose nocodazole
Reduced levels of key proteins in aged oocytes involved in the SAC at the kinetochore of the bivalent, such as Mad2 and pAurora C, may have an impact on their ability to undergo SAC activation and microtubule error correction during MI. This is notable given a recent study showing that the SAC response is likely to be graded rather than a binary on–off switch, and its strength depends on the amount of Mad2 recruited to kinetochores.41 In principle, a reduction in either Mad2 or pAurora C, could influence the fidelity of bivalent segregation at the end of MI. This is supported by the observations that when individual SAC proteins are reduced or knocked-out in oocytes, rates of aneuploidy in the resulting eggs are raised,1,15,17,42 and when young oocytes are cultured with the Aurora kinase inhibitor ZM447439, a significant reduction in kinetochore pAurora C (Fig. S2A) and a rise in aneuploidy at MI completion are both measured (Fig. S2B).40
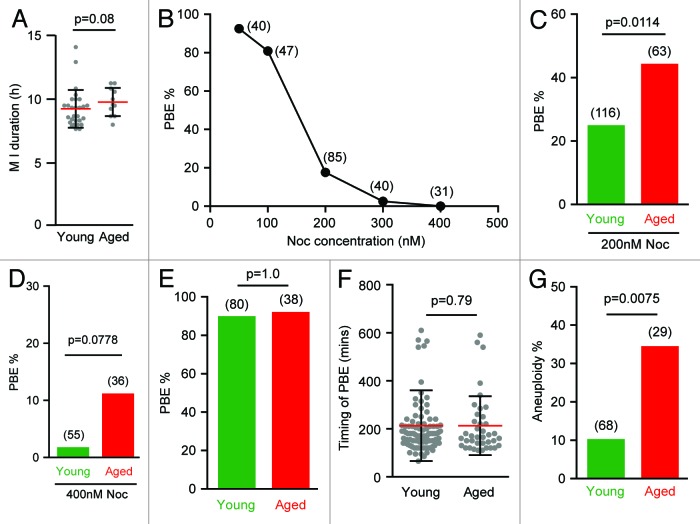
Despite the potential of reduced SAC activity being present in aged oocytes, it seemed unlikely that the SAC was actually non-functional, because there was no increase in aneuploidy with age (Fig. 1B) to a similar level achieved following loss of individual SAC proteins,15,17,42 and the duration of MI was not shortened (Fig. 3A). This lack of aging effect on duration is consistent with previous reports7,28,43 but would have occurred with an ineffectual SAC, because premature prometaphase APC activity shortens MI timing.17,44,45
Figure 3. Aged oocytes have reduced sensitivity to nocodazole and lowered microtubule error correction. (A) MI duration was similar between 2 age groups during in vitro maturation (mean ± s.d; Mann–Whitney U test). (B) Dose response of young oocytes to nocodazole, added at 5 h after GVBD. (C) Aged oocytes had a significantly higher PBE rate with 200 nM nocodazole treatment (Fisher test). (D) No significant difference was observed on PBE rate with age following 400 nM nocodazole treatment (Fisher test). (E and F) PBE rates (E; Fisher test) and timing (F; mean ± s.d; Mann–Whitney U test) were similar in young or aged oocytes after a brief, 400 nM nocodazole challenge at 5–7 h post GVBD. (G) Aneuploidy examined in resulting metII eggs showed a significantly higher increase in aged eggs after such challenge (Fisher test). (B–E and G) in parenthesis, number of oocytes examined.
One possibility not explored so far is that maternal aging may affect SAC activity, due to a lowering of Mad2 and pAurora C, but the reduction is not so dramatic as to compromise SAC function in the ways that have been measured previously. Oocytes proceed through MI with unattached and/or incorrectly attached bivalents19-23 that from equivalent mitotic chromosome studies are predicted to activate a checkpoint, and yet complete microtubule depolymerization leads to a meiotic arrest.17,45 These seemingly conflicting observations are resolved by the proposition that a small number of attachment errors are tolerated by the SAC, because it may not generate a sufficiently strong “wait-anaphase” signal, whereas when many bivalents are affected, the SAC is no longer weak and can act as a checkpoint. On this basis, we thought differences between young and aged oocytes may be uncovered if a weaker stimulus for the SAC was applied; one which is below the level induced by complete microtubule depolymerization. Under such circumstances it may be that a 25–50% fall in Mad2 and pAurora C on kinetochores becomes functionally significant.
To identify appropriate doses of nocodazole, oocytes from young mice were first exposed to a concentration series, and their ability to undergo PBE was assessed (Fig. 3B). We observed a very narrow dose range, over which the effects of nocodazole transitioned from being negligible to causing a near complete block to PBE. At a dose of 100 nM or less, oocytes underwent PBE at rates of >80%, similar to no additions, while at doses of 300 nM, oocytes remained arrested in MI (Fig. 3B). It was only at 200 nM that a partial effect could be observed, with 18% of oocytes undergoing PBE (Fig. 3B). Intriguingly, when oocytes from old mice were challenged to this dose, a significantly greater percentage—nearly double—failed to arrest, and went on to extrude their polar body (Fig. 3C). At the higher dose of 400 nM nocodazole, meiotic arrest was observed at ~90% in both young and aged mouse oocytes (Fig. 3D). Therefore, these observations show that with advancing maternal age the SAC responds less efficiently to low-level spindle disruption.
Aged oocytes have increased rates of aneuploidy following spindle disruption
Mad2 is recruited to unoccupied kinetochores to activate the SAC,15 and initial KT–MT attachments in mouse oocytes are predominantly incorrect.29 Therefore, 400 nM nocodazole treatment was used to examine for reduced SAC function in aged mouse oocytes as well as to examine its ability to repair erroneous KT–MT attachments, a process dependent on Aurora kinase.38,46,47 Our approach was to dissolve completely existing KT–MT attachments mid-way through MI, and then measure the extent to which the oocyte can establish new correct attachments by assessing for rates of aneuploidy in the resulting metII egg. If erroneous KT–MT attachments persist at anaphase onset, then this would result in a higher aneuploidy rate. Adding 400 nM nocodazole for a 2-h window, between 5–7 h after GVBD, is a dose sufficient to completely interrupt all existing microtubule fibers, but following its washout leads to new meiotic spindle formation. This procedure did not affect either the rate or the timing of polar body extrusion between young and aged oocytes (Fig. 3E and F), because >90% of oocytes underwent PBE at ~200 min after nocodazole washout. The similarities here in timing are consistent with our hypothesis that complete disruption of microtubules generates a sufficiently strong signal to fully activate the SAC, independent of maternal age, and so produce an effective “wait-anaphase” signal. Interestingly, however, when we analyzed the chromosome content of the resulting metII eggs, we observed a significantly higher, 3.5-fold, increase in the rate of aneuploidy with age (Fig. 3G). The majority of these aneuploidies (82%, n = 17) were in the form of one extra or missing dyad, which may have been due to a non-disjunction event in the bivalent at MI. Therefore, consistent with a recent report showing an association between KT–MT attachment and chromosome mis-segregation in aged oocytes,43 our data suggest that the processes associated with the establishment of correct KT–MT attachment following spindle disruption are in some way impaired with age.
Prevalence of non-aligned chromosomes accounting for the increased rate of aneuploidy
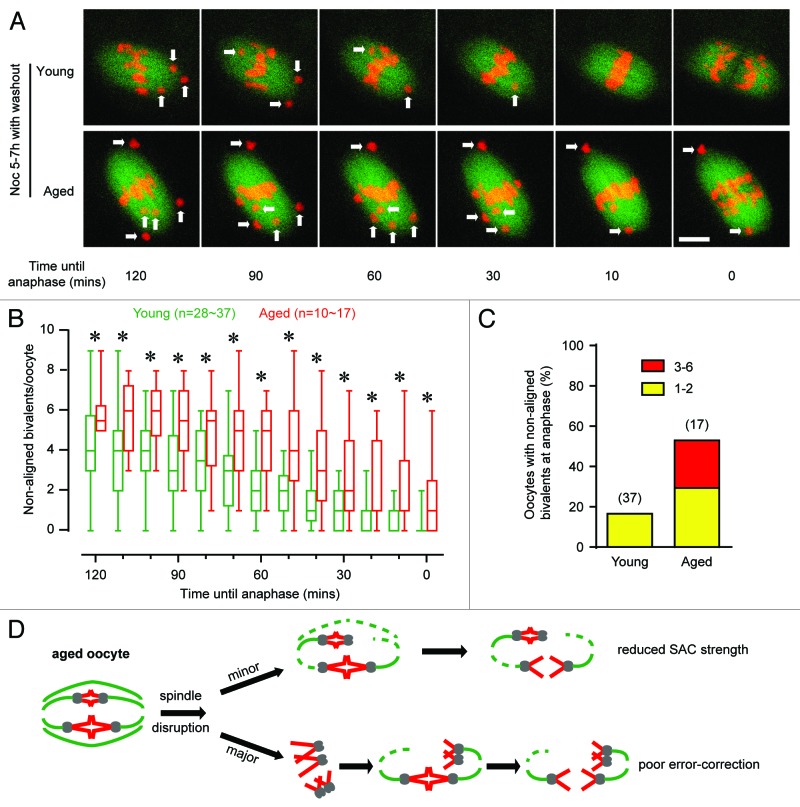
The increased aneuploidy observed following nocodazole treatment may have been caused by one of a number of factors, including an inability to repair incorrect KT–MT attachments that form following drug washout. To explore the possibilities more fully, it would be informative to visualize bivalents in real time on the meiotic spindle. Therefore, oocytes expressing EGFP-Map4 and H2B-mCherry, to label microtubules and chromosomes respectively, were imaged by 4D confocal microscopy following nocodazole washout until anaphase onset (Fig. 4A; Videos S1 and 2). Two hours before anaphase, many oocytes, independent of maternal age, possessed non-aligned bivalents—up to 9 per oocyte (Fig. 4B). However, even at this timepoint, the extent of bivalent non-alignment was significantly greater in the aged oocytes. Tracking chromosomes in young oocyte in the 2 h leading up to anaphase showed that the majority of non-aligned bivalents eventually achieved biorientation at anaphase onset (Fig. 4B and C; Video S1). In contrast, in aged oocytes bivalent non-alignment often persisted, such that they had significantly greater numbers at all timepoints measured (Fig. 4B; Video S2). At anaphase onset, very few young oocytes had non-aligned bivalents, whereas aged oocytes on average had 1–2, with a maximum of 6 (Fig. 4B and C).
Figure 4. Non-aligned bivalents fail to biorientate and mis-segregate at anaphase after spindle disruption. (A) Following a 2 h nocodazole incubation and washout in MI, non-aligned bivalents at different time points were examined from young and aged oocytes, marked with arrows. Spindle is labeled in green and chromosomes in red. Movies for both are available (Videos S1 and 2). Scale bar represents 10 µm. (B) Mean number of non-aligned bivalents per oocyte in the period leading up to anaphase (whiskers are min and max). Asterisks indicate significant differences (Sidak ANOVA test). (C) Percentage of oocytes with either 1 or 2, or 3 or more, non-aligned bivalents at anaphase onset. (D) Schematic showing reduced SAC strength and poor error correction in oocytes from aged mice. (B and C) in parenthesis, number of oocytes examined.
Discussion
The incidence of aneuploidy in both mouse and human oocytes increases with maternal age.1,2,4,5,48,49 However, here we observed that C57Bl6/J mice, at 17–19 months old, are largely resistant to this maternal-age related aneuploidy when compared with other strains. Sgo2 protects centromeric cohesion from degradation in MI,32,33 and there is evidence that loss of Sgo2 during the uniquely long meiotic arrest of mammalian oocytes may account for aneuploidy by promoting cohesion loss.7,31 In keeping with this hypothesis, and so explaining the resistance of C57Bl6/J mice to maternal-aging related aneuploidy, we observed little Sgo2 loss in oocytes in this strain. We did, however, observe maternal age-related losses in the SAC proteins Mad2 and pAurora C,35 as well as a drop in ACA staining that may reflect a more widespread loss in kinetochore–centromere proteins. In an unperturbed MI division such reductions in SAC proteins appeared to have little impact, but they did correlate with a lowered ability of aged oocytes to maintain arrest with nocodazole. Furthermore, aged oocytes were far less able to establish bivalent biorientation following spindle disruption. We hypothesize that these effects are due to reduced Mad2 and pAurora C, because they are known to influence these processes of SAC arrest and error correction.
Loss of kinetochore-associated proteins with maternal age
It is intriguing that oocytes from the mouse strain C57Bl6/J exhibited resistance to maternal age-related aneuploidy, since we and others have found a much higher incidence in other strains.6,7,10,23,25,31 In some strains, rates of aneuploidy up to 60% have been reported, which is equivalent to that seen in women.10,50,51 Such differences suggest there may well be a genetic susceptibility to age-related aneuploidy that initially could be investigated by interrogating gene expression profiles. However, the differential loss of kinetochore proteins Sgo2, Mad2, pAurora C, and ACA observed in our study provides an important starting point in understanding this phenomenon. It would be interesting to examine why Sgo2 protein is much lower in some aged strains than others. Furthermore, it needs to be determined why maternal aging in the strain used here can affect levels of ACA, Mad2, and pAurora C but leave Sgo2 unperturbed.
Shugoshins (Sgo1 and Sgo2) are a hub for regulating chromosome segregation and appear to have multiple functions.52,53 Recently in mouse oocytes Sgo2 was shown to be necessary for holding sister chromatids together during the division at MI, facilitating bivalent bi-orientation and alignment, and regulating the SAC through its recruitment or interactions with PP2A, MCAK or Mad2 on kinetochores.54 However, it seems that only heterochromatin protein 1 (HP1) and the SAC protein Bub1 affect Shugoshin recruitment to kinetochores.55-58 Therefore, one possibility may be that Sgo2 association with bivalents is not so readily impaired by maternal aging in C57Bl6/J as other chromosome-associated proteins. In contrast, the requirements for pAurora C and Mad2 to attach to kinetochores may be reliant on multiple proteins, whose assembly may be more easily perturbed in the process of aging. Mad2 recruitment appears to be dependent on kinetochore integrity,59 other SAC proteins, such as Mad1, Bub1, and Mps1,60,61 as well as the chromosomal passenger complex (CPC).62 Similarly, Aurora C, if like Aurora B, is dependent on the assembly of the CPC63 and haspin kinase activity.64,65 Or an alternative would be that Aurora C itself is normally recruited to kinetochores, but its activation mechanism may get perturbed in the process of aging, leading to the diminution of pAurora C. In support of a hypothesis where multiple proteins may determine Mad2 and pAurora C recruitment, it has been previously reported that the transcript of INCENP, a component of the CPC, is reduced in oocytes of aged mice.25 It remains to be determined whether other proteins are altered with maternal aging and how these changes affect the fidelity of bivalent segregation in oocytes.
Reduced SAC with maternal age
A functional SAC is essential for faithful chromosome segregation in both mitosis and meiosis,12,13 and interruption of SAC components in mouse oocytes, such as Mad2 or Aurora C, accelerates meiotic progression and increases aneuploidy rates.1,15 In fact we have recently observed that SAC proteins have functions that last the entire period of MI, not just the canonical period of prometaphase, and are essential in slowing down the time taken to complete MI—a phenomenon that promotes bivalent biorientation and reduces mis-segregation.66 However, whether a defective SAC is involved in aged mouse oocytes remains elusive. We have observed that with increased maternal age, the bivalent has a reduced ability to recruit or retain Mad2 and pAurora C proteins. This may reflect a general decline in whole-oocyte levels of these proteins, something that, with respect to Mad2, is supported by lowered transcript levels and is observed in both mice and humans.24,25,27 Considering levels of staining for ACA were also lower, we suggest their loss may mark a general decline in proteins associated with the centromere, kinetochore, and possibly all heterochromatin—proteins that likely include cohesins, a suggestion supported by the measured increases in iKT distances with age. It is interesting to note there might be some association between reduced SAC proteins and cohesion loss with maternal age, which is supported by a recent report showing that cohesin deterioration may compromise SAC function by impairing sister kinetochore biorientation and its SAC signal production in mouse oocytes.67
Despite a 25–50% fall in Mad2 and pAurora C on kinetochores that might indicate a reduced SAC activity in aged oocytes, it is highly unlikely that the SAC is completely non-functional. This is because MI duration was not shortened with age, a finding consistent with previous studies.7,28,43 An alternative conclusion is that aged oocytes possess reduced SAC functionality, but the extent of the reduction is not so large as to compromise SAC function in ways that have been measured so far. This appeared to be the case, because using a weaker stimulus for the SAC, a low dose of nocodazole, it was observed that the ability of the checkpoint to provide a brake on anaphase was compromised and, consequently, more oocytes completed MI and extruded a polar body (Fig. 4D, top pathway).
A failure of chromosomes to faithfully achieve biorientation is recognized as a contributing factor in their resulting mis-segregation both in somatic cells68 and aged oocytes.43 Consistent with this, we observed a much lower rate of bivalent biorientation in aged oocytes following brief spindle disruption, which consequently led to mis-segregation at completion of MI (Fig. 4D, lower pathway). It is interesting to note that in another strain of mouse this lower rate of biorientation was seen,43 but occurred normally in MI without any drug additions. So the same phenomenon appears to be observed in 2 different mouse stains, but in the present study it is less pronounced—requiring drug intervention for it to be uncovered. We hypothesize that the present findings are due to our observed 50% reduction in pAurora C, because this kinase has been reported to be the predominant isoform regulating meiotic progression and is known to be responsible for repairing erroneous KT–MT attachments in mouse oocytes.34,35,38 Recently it has been demonstrated that Sgo2 inhibits Aurora B/C activity at kinetochores, and that this inhibition may be required for chromosome bi-orientation in MI oocytes.54 However, we have observed that following spindle disruption at 5–7 h, young oocytes go on to complete MI with only ~10% aneuploidy rates, suggesting an active error correction is functioning during MI. Furthermore, our finding that Sgo2 levels do not decrease with maternal age suggests that this protein is not the major determinant for the observed changes in pAurora C kinase activity.
Mouse strain choice is a highly relevant factor in studying aneuploidy
One important conclusion from this study is that it supports a genetic basis to maternal age-related aneuploidy, because some mouse strains share the human phenomenon and others do not.6,7,10,31 Inroads to this genetic susceptibility are tractable and can be achieved within just a few years, since mRNA sequencing techniques on different strains are now feasible at the level of the single oocyte.69
We conclude it is important to appreciate that there appears differences between strains of mice that may make universal conclusions about the way oocytes behave with respect to maternal aging difficult to reach. So, for example, some strains show aging differences in bivalent biorientation,43 while others do not;31 some show loss in Sgo2,7,31 while others do not (present study). These appear to reflect true strain differences rather than discrepant results between laboratories.
Materials and Methods
Oocyte collection and RNA microinjection
Mice were used in accordance with ethics approved by the University of Newcastle Animal Care and Ethics Committee. For the aging study, either 1-mo- and >12-mo-old Swiss CD-1 outbred females or 1 mo and 17–19 mo C57Bl6/J ex-breeders were used. GV stage oocytes were collected from young or aged mice without hormone injection. A range of 20–30 oocytes for young, and 0–5 oocytes for old, were collected per mouse. Oocytes were handled in M2 media containing 2.5 µM milrinone (Sigma-Aldrich) under mineral oil at 37 °C, and maturation was stimulated by milrinone washout, timed relative to extrusion of the first polar body.70 Microinjections were performed on the heated stage of a Nikon TE300 inverted microscope as previously described.71 H2B-mCherry and EGFP-MAP4 (EUROSCARF, p30518) cRNAs were injected at a pipette concentration of 200 and 500 ng/μl respectively, with a measured 0.1–0.3% of oocyte volume.
Immunofluorescence
Oocytes were fixed and permeabilized in 2% formaldehyde in PHEM buffer with 0.5% TritonX-100 as described previously.31 For metII eggs, monastrol was used to spread chromosomes for aneuploidy analysis before fixation.40 Blocking was performed in 7% normal goat serum in PBS with 0.1% Tween-20. Primary antibodies were diluted in PBS with 3% BSA / 0.1% Tween-20 with overnight incubation at 4 °C: Sgo2, Mad2, phospho-Aurora C, or ACA. Secondary antibodies were Alexa-633 or Alexa-555 conjugated (Invitrogen). Oocytes were counterstained with Hoechst (20 µg/ml) prior to mounting in Citifluor (Citifluor Ltd). For intensity comparison experiments, young and aged mouse oocytes were collected and processed with the identical procedure, and then images were captured on the same day with the same confocal settings.
Confocal imaging and image analysis
An Olympus FV1000 confocal microscope was used on both fixed and live cells.22,31 For quantitative analysis of kinetochore Sgo2, Mad2, and pAurora C, oocytes were double stained with ACA, performed on the same day, and z-stacks of 1 μm z-resolution were acquired using identical settings. All images were analyzed using ImageJ (NIH, Bethesda), and fluorescence calculation was performed as previously described with modifications31,72 (Fig. S1C). Specifically, the single z-plane with greatest ACA was set to 0, then a z-projection with 3 successive planes (−1; 0; +1 μm) was acquired. All ACA and centromere-associated proteins fluorescence was captured by this z-sectioning. Kinetochore fluorescence intensity, a 2 μm diameter circle (red line), centered on the ACA (red), was background subtracted. The background was taken as the lowest value measured from 8 surrounding circles of the same size (B1-B8, dotted white line). For time-lapse imaging, oocytes were housed in a 37 °C temperature controlled environment, and ImageJ macros were used to track and maintain chromosomes within the center of field of view.31 Images were captured every 5 min, with z-resolution of 3.0 μm. In the EGFP-Map4- and H2B-mCherry-expressing oocytes, metaphase plate was defined as a drawn line, where >10 bivalents congressed, and non-aligned chromosomes were those clearly away from this line in all 3 dimensions.
Statistical analysis
Dichotomous data were analyzed using Fisher test. For multiple comparisons we employed Sidak ANOVA test. All of other means analysis was performed using Mann–Whitney U test. Data were processed using GraphPad Prism 6, with P < 0.05 set for significance. For most of the comparisons, more than 10 aged mice were tested from at least 2 independent experiments. All data are pooled.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge E Boon for his technical assistance; Y Watanabe for the Sgo2 antibody, RH Chen for the Mad2 antibody, and TK Tang for the phospho-Aurora C antibody. Y.Y. is the recipient of a China Scholarship Council and University of Newcastle joint scholarship award and J.E.H. is the recipient of an Australian Research Council Discovery Early Career Researcher Award. The work was supported by an Australian Research Council grant awarded to K.T.J. (DP120100946).
Author Contributions
All authors helped devise the study. The experiments were performed and figures prepared by Y.Y., supervised by K.T.J. and J.E.H. S.I.R.L. wrote the software for live cell imaging and kinetochore analysis. S.I.R.L., J.A.M., and E.A.M. provided critical assistance and advice during performing the whole project. The manuscript was written by Y.Y. and K.T.J., with input from all authors.
Glossary
Abbreviations:
- GVBD
germinal vesicle breakdown
- KT-MT
kinetochore-microtubule
- MetII
metaphase II
- MI
meiosis I
- MII
meiosis II
- PBE
first polar body extrusion
- SAC
spindle assembly checkpoint
References
- 1.Jones KT, Lane SI. Molecular causes of aneuploidy in mammalian eggs. Development. 2013;140:3719–30. doi: 10.1242/dev.090589. [DOI] [PubMed] [Google Scholar]
- 2.Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet. 2012;13:493–504. doi: 10.1038/nrg3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handyside AH. Molecular origin of female meiotic aneuploidies. Biochim Biophys Acta. 2012;1822:1913–20. doi: 10.1016/j.bbadis.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Jessberger R. Age-related aneuploidy through cohesion exhaustion. EMBO Rep. 2012;13:539–46. doi: 10.1038/embor.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang T, Schultz RM, Lampson MA. Meiotic origins of maternal age-related aneuploidy. Biol Reprod. 2012;86:1–7. doi: 10.1095/biolreprod.111.094367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–8. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–21. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505–16. doi: 10.1101/gad.605910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan FE, Hornick JE, Lampson MA, Schultz RM, Shea LD, Woodruff TK. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11:1121–4. doi: 10.1111/j.1474-9726.2012.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merriman JA, Jennings PC, McLaughlin EA, Jones KT. Effect of aging on superovulation efficiency, aneuploidy rates, and sister chromatid cohesion in mice aged up to 15 months. Biol Reprod. 2012;86:49. doi: 10.1095/biolreprod.111.095711. [DOI] [PubMed] [Google Scholar]
- 11.Revenkova E, Herrmann K, Adelfalk C, Jessberger R. Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol. 2010;20:1529–33. doi: 10.1016/j.cub.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–80. doi: 10.1016/j.cub.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Primorac I, Musacchio A. Panta rhei: the APC/C at steady state. J Cell Biol. 2013;201:177–89. doi: 10.1083/jcb.201301130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun SC, Kim NH. Spindle assembly checkpoint and its regulators in meiosis. Hum Reprod Update. 2012;18:60–72. doi: 10.1093/humupd/dmr044. [DOI] [PubMed] [Google Scholar]
- 16.Wei L, Liang XW, Zhang QH, Li M, Yuan J, Li S, Sun SC, Ouyang YC, Schatten H, Sun QY. BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte. Cell Cycle. 2010;9:1112–21. doi: 10.4161/cc.9.6.10957. [DOI] [PubMed] [Google Scholar]
- 17.Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 2005;19:202–7. doi: 10.1101/gad.328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuinness BE, Anger M, Kouznetsova A, Gil-Bernabé AM, Helmhart W, Kudo NR, Wuensche A, Taylor S, Hoog C, Novak B, et al. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr Biol. 2009;19:369–80. doi: 10.1016/j.cub.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 19.Nagaoka SI, Hodges CA, Albertini DF, Hunt PA. Oocyte-specific differences in cell-cycle control create an innate susceptibility to meiotic errors. Curr Biol. 2011;21:651–7. doi: 10.1016/j.cub.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gui L, Homer H. Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes. Development. 2012;139:1941–6. doi: 10.1242/dev.078352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolano A, Brunet S, Silk AD, Cleveland DW, Verlhac MH. Error-prone mammalian female meiosis from silencing the spindle assembly checkpoint without normal interkinetochore tension. Proc Natl Acad Sci U S A. 2012;109:E1858–67. doi: 10.1073/pnas.1204686109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane SI, Yun Y, Jones KT. Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension. Development. 2012;139:1947–55. doi: 10.1242/dev.077040. [DOI] [PubMed] [Google Scholar]
- 23.Sebestova J, Danylevska A, Novakova L, Kubelka M, Anger M. Lack of response to unaligned chromosomes in mammalian female gametes. Cell Cycle. 2012;11:3011–8. doi: 10.4161/cc.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steuerwald N, Cohen J, Herrera RJ, Sandalinas M, Brenner CA. Association between spindle assembly checkpoint expression and maternal age in human oocytes. Mol Hum Reprod. 2001;7:49–55. doi: 10.1093/molehr/7.1.49. [DOI] [PubMed] [Google Scholar]
- 25.Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–9. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 27.Steuerwald NM, Bermúdez MG, Wells D, Munné S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700–8. doi: 10.1016/S1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- 28.Duncan FE, Chiang T, Schultz RM, Lampson MA. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod. 2009;81:768–76. doi: 10.1095/biolreprod.109.077909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitajima TS, Ohsugi M, Ellenberg J. Complete kinetochore tracking reveals error-prone homologous chromosome biorientation in mammalian oocytes. Cell. 2011;146:568–81. doi: 10.1016/j.cell.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci U S A. 2011;108:12319–24. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yun Y, Lane SI, Jones KT. Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Development. 2014;141:199–208. doi: 10.1242/dev.100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol. 2008;10:42–52. doi: 10.1038/ncb1667. [DOI] [PubMed] [Google Scholar]
- 33.Llano E, Gómez R, Gutiérrez-Caballero C, Herrán Y, Sánchez-Martín M, Vázquez-Quiñones L, Hernández T, de Alava E, Cuadrado A, Barbero JL, et al. Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008;22:2400–13. doi: 10.1101/gad.475308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang KT, Li SK, Chang CC, Tang CJ, Lin YN, Lee SC, Tang TK. Aurora-C kinase deficiency causes cytokinesis failure in meiosis I and production of large polyploid oocytes in mice. Mol Biol Cell. 2010;21:2371–83. doi: 10.1091/mbc.E10-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindler K, Davydenko O, Fram B, Lampson MA, Schultz RM. Maternally recruited Aurora C kinase is more stable than Aurora B to support mouse oocyte maturation and early development. Proc Natl Acad Sci U S A. 2012;109:E2215–22. doi: 10.1073/pnas.1120517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernández-Miranda G, Trakala M, Martín J, Escobar B, González A, Ghyselinck NB, Ortega S, Cañamero M, Pérez de Castro I, Malumbres M. Genetic disruption of aurora B uncovers an essential role for aurora C during early mammalian development. Development. 2011;138:2661–72. doi: 10.1242/dev.066381. [DOI] [PubMed] [Google Scholar]
- 37.Slattery SD, Mancini MA, Brinkley BR, Hall RM. Aurora-C kinase supports mitotic progression in the absence of Aurora-B. Cell Cycle. 2009;8:2984–94. doi: 10.4161/cc.8.18.9591. [DOI] [PubMed] [Google Scholar]
- 38.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–3. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sessa F, Mapelli M, Ciferri C, Tarricone C, Areces LB, Schneider TR, Stukenberg PT, Musacchio A. Mechanism of Aurora B activation by INCENP and inhibition by hesperadin. Mol Cell. 2005;18:379–91. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Lane SI, Chang HY, Jennings PC, Jones KT. The Aurora kinase inhibitor ZM447439 accelerates first meiosis in mouse oocytes by overriding the spindle assembly checkpoint. Reproduction. 2010;140:521–30. doi: 10.1530/REP-10-0223. [DOI] [PubMed] [Google Scholar]
- 41.Collin P, Nashchekina O, Walker R, Pines J. The spindle assembly checkpoint works like a rheostat rather than a toggle switch. Nat Cell Biol. 2013;15:1378–85. doi: 10.1038/ncb2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Li S, Yuan J, Wang ZB, Sun SC, Schatten H, Sun QY. Bub3 is a spindle assembly checkpoint protein regulating chromosome segregation during mouse oocyte meiosis. PLoS One. 2009;4:e7701. doi: 10.1371/journal.pone.0007701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shomper M, Lappa C, Fitzharris G. Kinetochore microtubule establishment is defective in oocytes from aged mice. Cell Cycle. 2014;13:1171–9. doi: 10.4161/cc.28046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hached K, Xie SZ, Buffin E, Cladière D, Rachez C, Sacras M, Sorger PK, Wassmann K. Mps1 at kinetochores is essential for female mouse meiosis I. Development. 2011;138:2261–71. doi: 10.1242/dev.061317. [DOI] [PubMed] [Google Scholar]
- 45.Wassmann K, Niault T, Maro B. Metaphase I arrest upon activation of the Mad2-dependent spindle checkpoint in mouse oocytes. Curr Biol. 2003;13:1596–608. doi: 10.1016/j.cub.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 46.Lampson MA, Cheeseman IM. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–40. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maresca TJ, Salmon ED. Welcome to a new kind of tension: translating kinetochore mechanics into a wait-anaphase signal. J Cell Sci. 2010;123:825–35. doi: 10.1242/jcs.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang ZB, Schatten H, Sun QY. Why is chromosome segregation error in oocytes increased with maternal aging? Physiology (Bethesda) 2011;26:314–25. doi: 10.1152/physiol.00020.2011. [DOI] [PubMed] [Google Scholar]
- 49.Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, Wei YC, Yan J, Wang WH, Shen W, et al. The root of reduced fertility in aged women and possible therapentic options: Current status and future perspects. Mol Aspects Med. 2013 doi: 10.1016/j.mam.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Fragouli E, Alfarawati S, Goodall NN, Sánchez-García JF, Colls P, Wells D. The cytogenetics of polar bodies: insights into female meiosis and the diagnosis of aneuploidy. Mol Hum Reprod. 2011;17:286–95. doi: 10.1093/molehr/gar024. [DOI] [PubMed] [Google Scholar]
- 51.Kuliev A, Zlatopolsky Z, Kirillova I, Spivakova J, Cieslak Janzen J. Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod Biomed Online. 2011;22:2–8. doi: 10.1016/j.rbmo.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Gutiérrez-Caballero C, Cebollero LR, Pendás AM. Shugoshins: from protectors of cohesion to versatile adaptors at the centromere. Trends Genet. 2012;28:351–60. doi: 10.1016/j.tig.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Clift D, Marston AL. The role of shugoshin in meiotic chromosome segregation. Cytogenet Genome Res. 2011;133:234–42. doi: 10.1159/000323793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rattani A, Wolna M, Ploquin M, Helmhart W, Morrone S, Mayer B, Godwin J, Xu W, Stemmann O, Pendas A, et al. Sgol2 provides a regulatory platform that coordinates essential cell cycle processes during meiosis I in oocytes. Elife. 2013;2:e01133. doi: 10.7554/eLife.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427:510–7. doi: 10.1038/nature02312. [DOI] [PubMed] [Google Scholar]
- 56.Kitajima TS, Hauf S, Ohsugi M, Yamamoto T, Watanabe Y. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr Biol. 2005;15:353–9. doi: 10.1016/j.cub.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 57.Tang Z, Sun Y, Harley SE, Zou H, Yu H. Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc Natl Acad Sci U S A. 2004;101:18012–7. doi: 10.1073/pnas.0408600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamagishi Y, Sakuno T, Shimura M, Watanabe Y. Heterochromatin links to centromeric protection by recruiting shugoshin. Nature. 2008;455:251–5. doi: 10.1038/nature07217. [DOI] [PubMed] [Google Scholar]
- 59.Kline-Smith SL, Sandall S, Desai A. Kinetochore-spindle microtubule interactions during mitosis. Curr Opin Cell Biol. 2005;17:35–46. doi: 10.1016/j.ceb.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat Cell Biol. 2012;14:746–52. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- 61.Kim S, Sun H, Tomchick DR, Yu H, Luo X. Structure of human Mad1 C-terminal domain reveals its involvement in kinetochore targeting. Proc Natl Acad Sci U S A. 2012;109:6549–54. doi: 10.1073/pnas.1118210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–80. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol. 2012;13:789–803. doi: 10.1038/nrm3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang F, Ulyanova NP, Daum JR, Patnaik D, Kateneva AV, Gorbsky GJ, Higgins JM. Haspin inhibitors reveal centromeric functions of Aurora B in chromosome segregation. J Cell Biol. 2012;199:251–68. doi: 10.1083/jcb.201205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Antoni A, Maffini S, Knapp S, Musacchio A, Santaguida S. A small-molecule inhibitor of Haspin alters the kinetochore functions of Aurora B. J Cell Biol. 2012;199:269–84. doi: 10.1083/jcb.201205119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lane SI, Jones KT. Non-canonical function of spindle assembly checkpoint proteins after APC activation reduces aneuploidy in mouse oocytes. Nat Commun. 2014;5:3444. doi: 10.1038/ncomms4444. [DOI] [PubMed] [Google Scholar]
- 67.Tachibana-Konwalski K, Godwin J, Borsos M, Rattani A, Adams DJ, Nasmyth K. Spindle assembly checkpoint of oocytes depends on a kinetochore structure determined by cohesin in meiosis I. Curr Biol. 2013;23:2534–9. doi: 10.1016/j.cub.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson SL, Compton DA. Chromosome missegregation in human cells arises through specific types of kinetochore-microtubule attachment errors. Proc Natl Acad Sci U S A. 2011;108:17974–8. doi: 10.1073/pnas.1109720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xue Z, Huang K, Cai C, Cai L, Jiang CY, Feng Y, Liu Z, Zeng Q, Cheng L, Sun YE, et al. Genetic programs in human and mouse early embryos revealed by single-cell RNA sequencing. Nature. 2013;500:593–7. doi: 10.1038/nature12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holt JE, Tran SM, Stewart JL, Minahan K, García-Higuera I, Moreno S, Jones KT. The APC/C activator FZR1 coordinates the timing of meiotic resumption during prophase I arrest in mammalian oocytes. Development. 2011;138:905–13. doi: 10.1242/dev.059022. [DOI] [PubMed] [Google Scholar]
- 71.Madgwick S, Nixon VL, Chang HY, Herbert M, Levasseur M, Jones KT. Maintenance of sister chromatid attachment in mouse eggs through maturation-promoting factor activity. Dev Biol. 2004;275:68–81. doi: 10.1016/j.ydbio.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 72.Holt JE, Lane SI, Jennings P, García-Higuera I, Moreno S, Jones KT. APC(FZR1) prevents nondisjunction in mouse oocytes by controlling meiotic spindle assembly timing. Mol Biol Cell. 2012;23:3970–81. doi: 10.1091/mbc.E12-05-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.