Abstract
Background
Impairment in instrumental activities of daily living (IADL) heralds the transition from mild cognitive impairment (MCI) to dementia and is a major source of burden for both the patient and caregiver.
Objective
To investigate the relationship between IADL and regional cortical thinning and cerebrospinal fluid (CSF) Alzheimer disease (AD) biomarkers cross-sectionally and longitudinally in clinically normal (CN) elderly, MCI, and mild AD dementia subjects.
Methods
Two hundred and twenty nine CN, 395 MCI, and 188 AD dementia subjects participating in the Alzheimer's Disease Neuroimaging Initiative underwent baseline magnetic resonance imaging, baseline lumbar puncture, and clinical assessments, including the Functional Activities Questionnaire used to measure IADL, every 6 to 12 months up to 3 years. General linear regression and mixed effects models were employed.
Results
IADL impairment was associated with the interactions between lower inferior temporal cortical thickness and diagnosis (p<0.0001), greater lateral occipital cortical thickness and diagnosis (p<0.0001), and greater amyloid-beta 1-42 (Aβ1-42) and diagnosis (p=0.0002) at baseline (driven by AD dementia). Lower baseline supramarginal (p=0.02) and inferior temporal (p=0.05) cortical thickness, lower Aβ1-42 (p<0.0001), and greater total tau (t-tau) (p=0.02) were associated with greater rate of IADL impairment over time.
Conclusions
Temporal atrophy is associated with IADL impairment in mild AD dementia at baseline, while baseline parietal and temporal atrophy, lower CSF Aβ1-42, and greater t-tau predict worsening IADL impairment over time across the AD spectrum. These results emphasize the importance of assessing IADL at the stage of MCI and even at the transition from CN to MCI.
Keywords: Alzheimer's disease, cerebrospinal fluid, instrumental activities of daily living, magnetic resonance imaging, mild cognitive impairment
Introduction
Instrumental activities of daily living (IADL) consist of complex activities such as handling the finances, keeping track of appointments, driving or using public transportation, and performing household chores and hobbies. IADL worsen as mild cognitive impairment (MCI) progresses to Alzheimer disease (AD) dementia[1,2]. IADL impairment leads to loss of productivity by the affected individual, as well as increased caregiver mental, physical, and financial burden.
Impairment in basic ADL has been associated with increased global cerebral amyloid plaque and neurofibrillary tangle burden in post-mortem studies of patients with moderate to severe AD dementia[3,4]. Recent studies assessing less impaired individuals have shown that IADL impairment has been associated with greater in vivo global cortical amyloid burden in MCI visualized by Pittsburgh Compound B (PiB) positron emission tomography (PET)[5]. Additionally, lower cerebrospinal fluid (CSF) amyloid-beta 1-42 (Aβ1-42) and higher total tau (t-tau) and tau phosphorylated at the threonine 181 (p-tau181p) at baseline have been associated with greater IADL impairment over time in clinically normal elderly (CN) and MCI subjects[6].
Imaging studies have also shown an association between IADL impairment and typical AD neurodegenerative patterns: Global brain atrophy seen on magnetic resonance imaging (MRI) has been associated with greater IADL impairment over time in MCI[7], while an aggregate of temporal, lateral parietal, and posterior cingulate hypometabolism seen on 18F-fluorodeoxyglucose (FDG) PET has been associated with greater IADL impairment over time in MCI and mild AD dementia[8]. Other studies have attempted to more closely localize IADL impairment in the brain. Cross-sectional MRI studies showed an association between IADL impairment and temporoparietal and medial frontal atrophy in mild AD dementia[9,10], while cross-sectional functional imaging studies showed an association with inferior temporal, inferior parietal, and superior occipital hypometabolism in mild to moderate AD dementia[11], and medial frontal, dorsolateral prefrontal, lateral superior parietal, and occipital hypoperfusion in mild AD dementia[12].
The manifestation of disease progression that is usually most important to patients and caregivers is impairment in daily functioning, which can be captured by assessing IADL. There are multiple clinical features (cognition, behavior, demographics) and underlying pathophysiological changes (amyloid deposition and downstream neurodegeneration) that can contribute to future IADL decline. However, few studies have attempted to assess many of these features together. By evaluating these multiple complex features and their interactions, we will be able to determine which features significantly contribute to early functional impairment and to what extent, while adjusting for the other relevant features.
The objective of this study was to investigate the relationship between IADL and regional cortical thinning, CSF Aβ1-42, t-tau, and p-tau181p cross-sectionally and longitudinally in CN, MCI, and mild AD dementia subjects. We hypothesized that lower CSF Aβ1-42, higher t-tau, and higher p-tau181p, as well as temporal and parietal atrophy will all be associated independently with impairment in IADL at baseline and over time across the AD spectrum, while controlling for subject characteristics, including demographics, cognitive function, and behavior that are usually related to IADL impairment.
Materials and Methods
Participants
Data used in the analyses in this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI, PI Michael W. Weiner)[13] (see Supplementary Data).
Eight hundred and twelve subjects (229 CN, 395 MCI, 188 AD dementia) participating in ADNI underwent clinical assessments every 6 to 12 months up to 3 years. Of those subjects, 802 underwent MRI at baseline and 413 (114 CN, 198 MCI, 101 AD dementia) underwent lumbar puncture at baseline. Subject demographics and characteristics for those undergoing MRI and lumbar puncture resembled those of the entire ADNI population.
Subjects were assigned to diagnostic groups (CN, amnestic MCI, mild AD dementia) by site investigators at baseline as previously described[13,14] (see Supplementary Data for more detail). The Functional Activities Questionnaire (FAQ)[15], the dependent variable in our analyses used to assess IADL, was used in the determination of follow-up but not screening diagnoses.
The study was approved by the Institutional Review Board (IRB) of each participating site. Written informed consent was obtained from all subjects and study partners prior to initiation of any study procedures in accordance with local IRB guidelines.
Clinical Assessments
IADL were assessed with the FAQ[15] (higher scores indicate greater impairment; range 0-30). There is no established cut-off score for impairment on the FAQ. However, one study reported that a score of ≥ 6 is suggestive of functional impairment[16]. Moreover, studies have shown that FAQ can clearly distinguish between CN, MCI, and mild AD dementia subjects[14,17]. See Supplementary Data for other assessments used in this study.
Apolipoprotein E ε4 (APOE4) carrier status (homozygous carrier, heterozygous carrier, or non-carrier) was reported for subjects. Duration of AD dementia symptoms (in years) was reported as well. Duration was available only for subjects with a diagnosis of mild AD dementia at screening and was set to zero for CN and MCI subjects.
MRI Data
MRI scans were obtained at baseline according to a standardized protocol (http://www.loni.ucla.edu/ADNI/Research/Cores/index.shtml). In brief, the magnetization-prepared rapid gradient echo (MPRAGE) sagittal T1-weighted 3D sequence was used. Cortical reconstruction and automated thickness measures (in mm) were performed using Freesurfer (version 4.3.0, http://surfer.nmr.mgh.harvard.edu/).
Seven regions of interest (ROI) were chosen based on prior studies assessing the association with IADL[8-12]: bilateral inferior temporal, supramarginal (lateral parietal), precuneus (medial parietal), rostral anterior cingulate, medial orbitofrontal, rostral middle frontal (dorsolateral prefrontal), and lateral occipital cortices.
CSF Data
Subjects underwent lumbar puncture at baseline and CSF samples were obtained, processed, and stored according to a standardized protocol (http://www.adni-info.org)[18]. In brief, CSF assays of Aβ1-42,, t-tau, and p-tau181p were performed using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with Innogenetics (INNO-BIA AlzBio3; Ghent Belgium; for research use-only reagents) immunoassay kit-based reagents[18,19].
Statistical Analyses
All analyses in this study were performed with SAS Version 9.3. Associations between diagnostic groups and subject demographics and characteristics were evaluated using analysis of variance with Bonferroni correction for continuous variables and the chi-square test for categorical variables.
Cross-sectional analysis
A general linear regression model with backward elimination (p<0.01 retention requirement) was used to evaluate the cross-sectional association of the dependent variable, baseline FAQ, with the following baseline predictors: MRI regions (cortical thickness) and their interaction with diagnosis, CSF biomarkers and their interaction with diagnosis, sex, diagnosis, interaction of sex and diagnosis, age (linear and quadratic effects), APOE4 carrier status, duration of AD dementia symptoms, the American National Adult Reading Test intelligence quotient (AMNART IQ), the Rey Auditory Verbal Learning Test (RAVLT) total learning, Digit Symbol, and the Neuropsychiatric Inventory brief questionnaire form (NPI-Q) apathy and depression items. In addition to reporting partial regression coefficient estimates (β) with confidence intervals (CI), significance test results (p values) were complemented with effect size estimates such as covariate adjusted means and estimates of percent variance accounted for in the dependent variable uniquely by individual predictors, as well as by the model as a whole (R2). The inclusion of the interaction of MRI and CSF variables with diagnosis allowed us to test for any differential relation of any given MRI or CSF variable with FAQ across diagnostic groups. Model residuals were checked for conformance to assumptions and model fit.
Longitudinal analysis
A mixed random and fixed coefficient regression model was used with backward elimination (p<0.05 retention requirement) for the dependent variable, FAQ, on a large initial pool of fixed predictors and variances/covariances of random terms. The fixed predictors were diagnostic group and their interactions with time, the MRI regions and CSF biomarkers and their interactions with time, the CSF biomarkers and their interactions with diagnosis, and the baseline dependent variable (FAQ) and its interaction with time, which allowed for different trajectories depending on level of FAQ at entry as might be expected for example if there are floor or ceiling effects. The same additional covariates used in the cross-sectional analysis were also included. The random terms in all these models included correlated intercept and linear slopes of time. β values with CI were reported. The squared correlations of predicted values from fixed and random predictor sets vs. actual values were used to indicate the percent of variance of the dependent variable linearly accounted for by the predictors. Residuals from fixed and random components of models were checked for conformance to assumptions and model fit.
Results
Table 1 provides baseline demographic and clinical data for all subjects and for each of the three diagnostic groups (CN, MCI, mild AD dementia). There were significant differences between diagnostic groups for all variables in expected directions except for age, which was similar across all groups. Table 2 provides baseline regional cortical thickness and CSF biomarker data for all subjects and for each diagnostic group. There were significant differences between diagnostic groups for all variables in expected directions.
Table 1.
Baseline demographic and clinical data for subjects.
| Group | All subjects | CN | MCI | AD dementia |
|---|---|---|---|---|
| n | 812 | 229 | 395 | 188 |
| Age (years) | 75.3±6.9 | 76.0±5.0 | 74.8±7.5 | 75.3±7.5 |
| Sex (% male) | 57.9‡‡ | 52.0 | 64.3 | 51.6 |
| Education (years) | 15.5±3.1‡ | 16.0±2.9 | 15.7±3.1 | 14.7±3.1 |
| AMNART IQ | 117.2±11.6†† | 121.1±10.6 | 116.6±11.5 | 114.0±11.7 |
| Duration of AD dementia symptoms (years) | 3.5±2.5 | |||
| APOE4 (% non-carrier/heterozygous carrier/homozygous carrier) | 51.1/38.1/10.6† | 72.9/24.5/2.2 | 46.6/41.8/11.4 | 34.0/46.8/19.1 |
| MMSE | 26.8±2.7* | 29.1±1.0 | 27.04±1.8 | 23.3±2.0 |
| RAVLT Total Learning | 32.5±11.5* | 43.1±10.0 | 30.8±9.0 | 23.19±7.6 |
| Digit Symbol | 36.9±13.4* | 45.8±10.2 | 36.8±11.3 | 26.5±13.2 |
| NPI-Q Apathy (% present) | 0.2±0.6 (15.0)* | 0.01±0.1 (1.3) | 0.2±0.6 (13.9) | 0.5±0.7 (34.0) |
| NPI-Q Depression (% present) | 0.2±0.5 (18.8)* | 0.1±0.3 (5.7) | 0.2±0.5 (19.2) | 0.4±0.6 (34.0) |
| CDR-SB | 1.8±1.8* | 0.0±0.1 | 1.6±0.9 | 4.3±1.6 |
| FAQ | 4.9±6.6* | 0.1±0.6 | 3.8±4.4 | 13.1±6.9 |
AD (Alzheimer's disease), AMNART IQ (American National Adult Reading Test intelligence quotient), APOE4 (Apolipoprotein E ε4), CDR-SB (Clinical Dementia Rating sum of boxes), CN (clinically normal elderly), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NPI-Q (Neuropsychiatric Inventory brief questionnaire form), RAVLT (Rey Auditory Verbal Learning Test).
All values (except n, sex, APOE4) represent mean ± standard deviation.
p<0.0001 for CN vs. MCI, CN vs. AD and MCI vs. AD.
p<0.01 for CN vs. MCI, CN vs. AD and MCI vs. AD.
p<0.05 for CN vs. MCI, CN vs. AD and MCI vs. AD.
p<0.001 for CN vs. AD and MCI vs. AD.
p<0.01 for CN vs. MCI and MCI vs. AD.
Table 2.
Baseline regional cortical thickness and CSF biomarker concentrations.
| Group | All subjects | CN | MCI | AD dementia |
|---|---|---|---|---|
| MRI Variables (mm) | ||||
| n | 802 | 227 | 389 | 186 |
| Inferior temporal | 2.64±0.23* | 2.76±0.17 | 2.64±0.21 | 2.48±0.24 |
| Supramarginal (lateral parietal) | 2.22±0.19* | 2.31±0.17 | 2.22±0.18 | 2.13±0.19 |
| Precuneus (medial parietal) | 2.03±0.17* | 2.11±0.15 | 2.02±0.16 | 1.96±0.19 |
| Rostral anterior cingulate | 2.81±0.25†† | 2.86±0.22 | 2.79±0.24 | 2.78±0.30 |
| Medial orbitofrontal | 2.23±0.20* | 2.30±0.17 | 2.22±0.19 | 2.14±0.21 |
| Rostral middle frontal (dorsolateral prefrontal) | 2.12±0.17* | 2.20±0.15 | 2.11±0.16 | 2.04±0.18 |
| Lateral occipital | 1.96±0.15† | 2.01±0.14 | 1.96±0.15 | 1.91±0.17 |
| CSF Variables (pg/ml) | ||||
| n | 413 | 114 | 198 | 101 |
| Aβ1-42 | 170.3 ± 56.8* | 205.6 ± 55.1 | 163.9 ± 54.9 | 142.8 ± 40.9 |
| t-tau | 98.6 ± 56.7* | 69.7 ± 30.4 | 103.69 ± 61.0 | 121.8 ± 57.8 |
| p-tau181p | 34.1 ± 18.7* | 24.9 ± 14.6 | 35.6 ± 18.0 | 41.7 ± 19.9 |
Aβ1-42 (amyloid-beta 1-42 peptide), AD (Alzheimer's disease), CN (clinically normal elderly), CSF (cerebrospinal fluid), MCI (mild cognitive impairment), MRI (magnetic resonance imaging), p-tau181p (tau phosphorylated at threonine 181), t-tau (total tau). MRI values represent bilateral averaged cortical thickness by region.
All values (except n) represent mean ± standard deviation.
p<0.0001 for CN vs. MCI, CN vs. AD and MCI vs. AD.
p<0.005 for CN vs. MCI, CN vs. AD and MCI vs. AD.
p<0.001 for CN vs. MCI and CN vs. AD.
Cross-sectional analysis
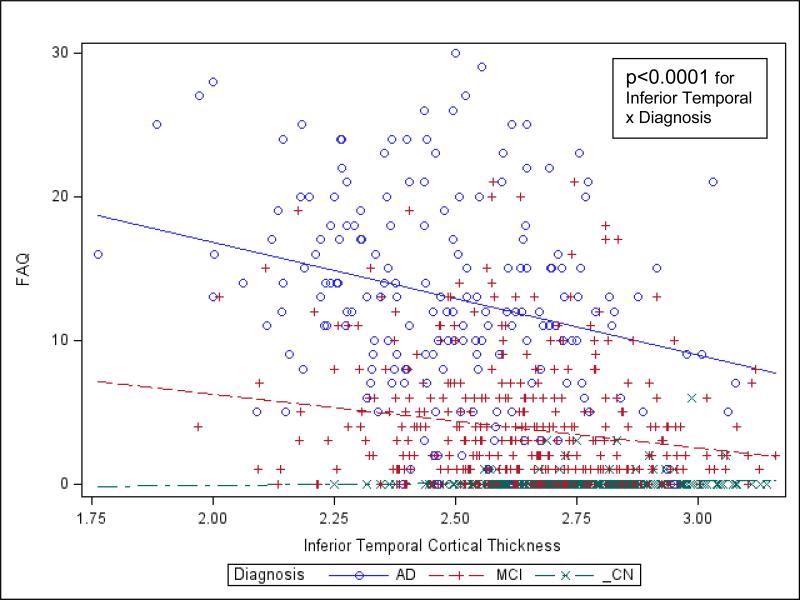
In the general linear regression model for all subjects, after backward elimination, there was a significant association between greater FAQ, representing greater IADL impairment, and the interaction between lower baseline inferior temporal cortical thickness and diagnosis (p<0.0001; driven by a relatively negative relation for the AD dementia group), the interaction between greater baseline lateral occipital cortical thickness and diagnosis (p<0.0001; driven by an unexpected positive relation for the AD dementia group), and the interaction between greater baseline Aβ1-42 and diagnosis (p=0.0002; driven by an unexpected positive relation for the AD dementia group), see Supplementary Table 1 and Figure 1.
Of note, lateral occipital and inferior temporal cortical thickness were moderately to strongly positively correlated (r=0.62, p<0.0001), and thus multi-collinearity may underlie the counterintuitive reversal of the sign of the coefficient for lateral occipital when inferior temporal cortical thickness was held constant (the unadjusted correlation of lateral occipital cortical thickness with FAQ was negative: r=−0.22, p<0.0001 like that of inferior temporal cortical thickness, which is consistent with an association between lower cortical thickness and greater IADL impairment). Similarly, the relation between Aβ1-42 and FAQ was reversed in the model with other predictors held constant (the unadjusted correlation of Aβ1-42 with FAQ was negative: r=−0.28, p<0.0001). However, in this case there was no suggestion of multi-collinearity of Aβ1-42 with other predictors. Therefore, the reason for the sign reversal for the association between Aβ1-42 and FAQ is unclear.
Other predictors significantly (p<0.01) associated with FAQ were duration of AD dementia symptoms, Digit Symbol, and NPI-Q apathy item (all in expected directions) (R2=0.64, p<0.0001 for overall model), see Supplementary Table 1. Residuals reasonably conformed to model assumptions.
Longitudinal analysis
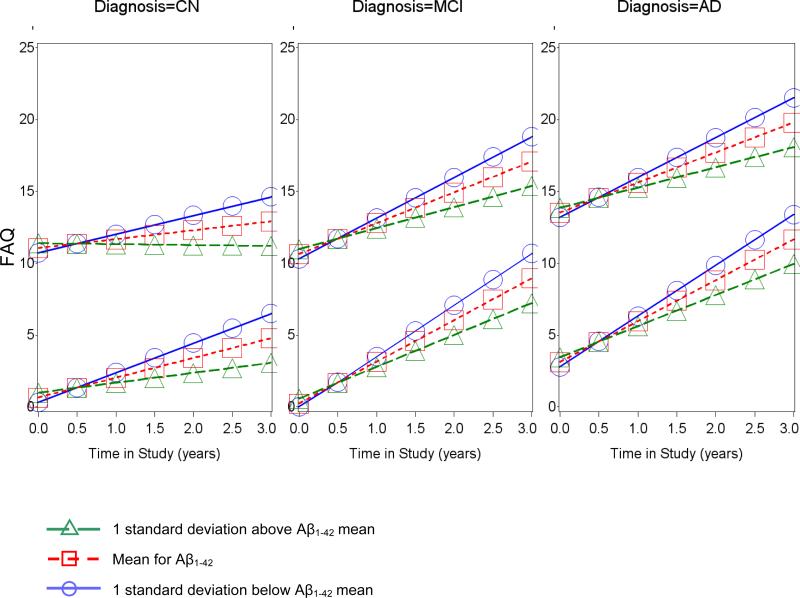
In the mixed random and fixed coefficient longitudinal regression model for all subjects, after backward elimination, lower baseline supramarginal cortical thickness, lower baseline inferior temporal cortical thickness, lower baseline Aβ1-42, and greater baseline t-tau were significantly associated with greater rate of increase in FAQ, representing greater IADL impairment, over time (for interactions with time for: Supramarginal: p=0.02; Inferior temporal: p=0.05; Aβ1-42: p<0.0001; t-tau: p=0.02), see Supplementary Table 2, Figures 2 and 3, and Supplementary Figure 1.
Figure 2.
Predicted values from fixed effects of best fitting longitudinal model of FAQ across time in the study vs. supramarginal cortical thickness for selected FAQ baselines by diagnostic groups. In each panel, one set of lines begins at a baseline FAQ one standard deviation below the mean (approximately zero) and the other starts at one standard deviation above the baseline FAQ mean (approximately 12). Other predictors were set to their mean and sex was set to female. AD (Alzheimer's disease), CN (clinically normal elderly), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment).
Figure 3.
Predicted values from fixed effects of best fitting longitudinal model of FAQ across time in the study vs. Aβ1-42 for selected FAQ baselines by diagnostic groups. In each panel, one set of lines begins at a baseline FAQ one standard deviation below the mean (approximately zero) and the other starts at one standard deviation above the baseline FAQ mean (approximately 12). Other predictors were set to their mean and sex was set to female. AD (Alzheimer's disease), CN (clinically normal elderly), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment).
Additional significant (p<0.05) fixed effect predictors were the interaction of diagnostic group with time and the interaction of the baseline dependent variable, FAQ, with time, and linear level effects for precuneus cortical thickness, sex (female > male), and RAVLT total learning (all in expected directions) (R2=0.75, p<0.0001 for overall model fixed effects; R2=0.95 including random terms, p<0.0001), see Supplementary Table 2. There was significant (p<0.0001) random variation in slope and intercepts of time trajectories across subjects and a significant (p<0.01) negative correlation between the two. Residuals reasonably conformed to model assumptions.
Discussion
These results suggest that lower inferior temporal cortical thickness suggestive of atrophy and possibly greater lateral occipital cortical thickness and greater CSF Aβ1-42 are associated with greater IADL impairment in mild AD dementia at baseline. Whereas, baseline lower supramarginal (lateral parietal) and inferior temporal cortical thickness, lower Aβ1-42, and greater t-tau are associated with worsening IADL impairment over time across the AD spectrum, including CN, MCI, and mild AD dementia subjects. Of note, the FAQ, which was used to assess IADL, was not used for the initial diagnoses of subjects, thus eliminating this potential confound. Moreover, these findings were independent of age, sex, cognitive reserve, duration of AD dementia symptoms, APOE4 status, apathy, depression, memory and processing speed performance, many of which were associated with IADL impairment. Furthermore, these results suggest that cross-sectionally an association between AD biomarkers and IADL impairment is seen primarily at the stage of mild dementia, while longitudinally it is possible to observe independent associations between markers of amyloid and neurodegeneration and IADL decline across the early AD spectrum, even in CN elderly at risk for AD.
Over the past decade multiple clinical trials of potentially disease-modifying drugs in mild-moderate AD dementia have failed to show a clinical benefit. Consequently, the field has been moving toward treatment earlier in the disease course, and the Food and Drug Administration recently issued guidelines for clinical trial design in early AD[20]. These guidelines noted that IADL tests are currently inadequately sensitive for capturing disease progression in early AD, especially in the transition from preclinical AD to MCI. Our results suggest that the FAQ is able to longitudinally capture IADL worsening in the early AD spectrum: even though about ½ of our sample consisted of late MCI and about ¼ of mild AD dementia subjects, about a ¼ of our sample consisted of CN elderly subjects, over a ⅓ of whom had significantly low Aβ1-42 (as previously defined[18]), which is suggestive of preclinical AD. Therefore, the current analyses suggest a potential utility for certain sensitive IADL measures in tracking disease progression even at the earliest stages of AD. Furthermore, we showed that amyloid pathology, regional neurodegeneration, and certain clinical features drive this IADL worsening.
Two previous studies used the ADNI database to investigate the relationship between IADL impairment over time and baseline global brain atrophy separately from baseline CSF AD biomarkers[6,7]. The study assessing global brain atrophy, used the ventricle-to-brain ratio, and found an association between greater baseline atrophy and IADL impairment over time in MCI, especially in APOE4 carriers[7]. The study assessing CSF AD biomarkers found an association between baseline lower CSF Aβ1-42 and greater baseline t-tau and p-tau181p and IADL impairment over time in CN and MCI, partially mediated by global cognitive performance[6]. Both of these studies used models adjusted for age and looked at MRI and CSF measures separately, while our study looked at MRI and CSF measures in the same model, targeted specific MRI regions rather than global atrophy, and adjusted for several other relevant covariates. We found associations between IADL impairment over time and baseline inferior temporal and lateral parietal (supramarginal) atrophy and baseline lower CSF Aβ1-42 and greater t-tau across all subject groups, including AD dementia. These results extend those of the previous studies referenced above and suggest that evidence of neurodegeneration, such as regional atrophy and greater CSF t-tau, as well as amyloid burden, independently and simultaneously contribute to longitudinal functional impairment across the early AD spectrum.
Another longitudinal study using FDG PET assessing IADL impairment, showed that a composite score of regional hypometabolism predicted longitudinal functional decline in MCI and mild AD dementia[8]. Two of the three regions in the composite score were temporal and lateral parietal, which are the regions of atrophy we found an association with; the third region was the posterior cingulate[8]. The FDG PET study used similar covariates to the ones we used in our analyses. Our results reinforce the cortical regional localization of progressive functional impairment in the AD spectrum, which matches the cortical neurodegeneration typically seen in AD.
Our cross-sectional results are partly in agreement, while our longitudinal results are more so in agreement with previous neuroimaging studies and post-mortem and in vivo studies of cortical amyloid deposition. Previous, smaller, cross-sectional, MRI studies showed an association between IADL impairment and temporal atrophy in mild AD dementia[9,10] similar to our results indicating an association with reduced inferior temporal cortical thickness signifying atrophy, driven by the mild AD dementia group; one of the other studies also showed an association with parietal and medial frontal atrophy[9]. Another cross-sectional study employing FDG PET showed an association between IADL impairment and inferior temporal hypometabolism in mild to moderate AD dementia similar to our regional results; it also showed an association with inferior parietal and superior occipital hypometabolism[11]. Yet another cross-sectional functional imaging study using single photon emission computed tomography showed an association between IADL impairment and lateral superior parietal, occipital, medial frontal, and dorsolateral prefrontal hypoperfusion in mild AD dementia[12]. In our study, the multivariate model indicated an independent association between greater rather than reduced lateral occipital cortical thickness and IADL impairment. However, the univariate unadjusted relationship was reversed, which could have been tied to significant multi-collinearity between the inferior temporal and lateral occipital regions, suggesting that in fact reduced rather than greater lateral occipital cortical thickness is more likely associated with IADL impairment.
Neither our cross-sectional nor longitudinal analyses showed an association with frontal atrophy. It is possible that in our early AD sample IADL associations with regional frontal changes would be seen with an earlier marker of neurodegeneration, such as FDG PET, rather than MRI cortical thickness, which was used in the current analyses.
Cross-sectional post-mortem studies have demonstrated that greater amyloid burden is associated with greater basic ADL impairment in moderate to severe AD dementia[3,4], and a cross-sectional in vivo study has shown that greater amyloid burden, as seen on PiB PET, is associated with greater IADL impairment in MCI[5]. Lower CSF Aβ1-42 has been associated with greater global cortical amyloid burden seen at post-mortem and in vivo[21,22]. Therefore, we anticipated that in our cross-sectional analysis lower CSF Aβ1-42 will be associated with greater IADL impairment, which was the result in the univariate unadjusted analysis. However, the relationship was reversed in the multivariate model—greater CSF Aβ1-42 was associated with greater IADL impairment (higher FAQ score), driven by the mild AD dementia group. Here there was no suggestion of multi-collinearity of Aβ1-42 with other predictors to possibly explain the reversed relationship. In the AD dementia group, there was a small cluster of high FAQ / high Aβ1-42 subjects, who were primarily responsible for the correlation/regression line becoming positive. It is possible that these subjects were less likely to harbor AD pathology as the cause of their dementia, leading to the unexpected results. Moreover, subjects with low Aβ1-42 values, low FAQ scores, and cognitive impairment in memory and another domain that could have potentially met criteria for MCI in the study, might have been classified as having mild AD dementia due to the overlapping cognitive impairment criteria between the two diagnostic groups. The addition of low FAQ / low Aβ1-42 subjects to the AD dementia group could have further contributed to this unexpected positive relation of FAQ to Aβ1-42.
The current study had several limitations. The ADNI population is not representative of the general population because the subjects are carefully selected to have limited general health issues, psychiatric conditions, and cerebrovascular disease; moreover, the subjects were highly intelligent premorbidly and had a high proportion of APOE4 carriers[13,14]. However, we adjusted for these elements in all analyses. Additionally, this population resembles that of most AD spectrum clinical trials, making it easier to compare our results to clinical trial outcomes. The IADL scale used in these analyses, the FAQ, has been shown to be a sensitive measure for differentiating between CN, MCI, and mild AD dementia, but nearly all CN subjects have a baseline score of 0, representing a major floor effect[14,17]. As such, the cross-sectional results were driven by the mild dementia group. However, the longitudinal results were significant across all diagnostic groups indicating that the FAQ is sensitive to the development of functional decline over time even in CN subjects. Although our intention was to adjust for cognitive impairment in terms of its known contribution to IADL impairment in the AD spectrum, the resulting association between biomarkers and IADL impairment independent of cognition could have been due to a non-neurodegenerative process. Significant cerebrovascular disease or traumatic brain injury were exclusionary in this study, but milder presentations, which could still contribute to IADL impairment, were not exclusionary and could have contributed to the associations we found. Finally, relatively few regions (seven) of cortical thickness were used in our regression models, and exploratory whole-brain voxel-based analyses were not performed. The latter would have had the potential to allow the data to drive the localization of IADL impairment. However, we picked the regions for these analyses based on prior imaging studies, and this approach allowed us to employ more sophisticated multivariate regression models.
In conclusion, baseline parietal and temporal atrophy and lower CSF Aβ1-42 and greater t-tau predict worsening IADL impairment over time across the early AD spectrum, while temporal atrophy is associated with IADL impairment in mild AD dementia cross-sectionally. These results, especially the longitudinal results, demonstrate the association between complex daily functioning decline in early AD and typical AD pathophysiological changes, including influence exerted by markers of amyloid and neurodegeneration independent of each other, as well as emphasize the importance of assessing for IADL impairment at the stage of MCI and even at the transition from CN to MCI.
Supplementary Material
Figure 1.
Values predicted from general linear model of baseline FAQ regressed on diagnostic group and inferior temporal cortical thickness. Lines indicate the predicted values for FAQ, and symbols denote corresponding actual values. For simplicity, the graph was not adjusted for the negligible effects of additional partialed significant predictors in the model. AD (Alzheimer's disease), CN (clinically normal elderly), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment).
Acknowledgements
This study was supported by R01 AG027435, K23 AG033634, K24 AG035007, the Rosalinde and Arthur Gilbert Foundation/AFAR New Investigator Awards in Alzheimer's Disease, the Massachusetts Alzheimer's Disease Research Center (P50 AG005134), the Harvard Aging Brain Study (P01 AGO36694), and the Alzheimer's disease Neuroimaging Initiative (ADNI) (NIH Grant U01 AG024904) (See Supplementary Data).
The authors have received research salary support from Janssen Alzheimer Immunotherapy (GAM, DMR), Wyeth/Pfizer Pharmaceuticals (GAM, DMR), and Bristol-Myers-Squibb (RAS).
References
- 1.Luck T, Luppa M, Angermeyer MC, Villringer A, Konig HH, Riedel-Heller SG. Impact of impairment in instrumental activities of daily living and mild cognitive impairment on time to incident dementia: results of the Leipzig Longitudinal Study of the Aged. Psychol Med. 2011;41(5):1087–1097. doi: 10.1017/S003329171000142X. [DOI] [PubMed] [Google Scholar]
- 2.Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, Stern Y, Devanand DP. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 3.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of activities of daily living in Alzheimer's disease. Alzheimer Dis Assoc Disord. 2006;20(1):56–59. doi: 10.1097/01.wad.0000201852.60330.16. [DOI] [PubMed] [Google Scholar]
- 4.Roth M, Tomlinson BE, Blessed G. Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature. 1966;209(18):109–110. doi: 10.1038/209109a0. [DOI] [PubMed] [Google Scholar]
- 5.Marshall GA, Olson LE, Frey MT, Maye J, Becker JA, Rentz DM, Sperling RA, Johnson KA, Initiative AsDN Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement Geriatr Cogn Disord. 2011;31(6):443–450. doi: 10.1159/000329543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okonkwo OC, Alosco ML, Griffith HR, Mielke MM, Shaw LM, Trojanowski JQ, Tremont G. Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol. 2010;67(6):688–696. doi: 10.1001/archneurol.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okonkwo OC, Alosco ML, Jerskey BA, Sweet LH, Ott BR, Tremont G. Cerebral atrophy, apolipoprotein E varepsilon4, and rate of decline in everyday function among patients with amnestic mild cognitive impairment. Alzheimers Dement. 2010;6(5):404–411. doi: 10.1016/j.jalz.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer's disease. J Alzheimers Dis. 2010;19(2):517–527. doi: 10.3233/JAD-2010-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Gois Vasconcelos L, Jackowski AP, Oliveira MO, Flor YM, Bueno OF, Brucki SM. Voxel-based morphometry findings in Alzheimer's disease: neuropsychiatric symptoms and disability correlations - preliminary results. Clinics (Sao Paulo) 2011;66(6):1045–1050. doi: 10.1590/S1807-59322011000600021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salmon E, Lespagnard S, Marique P, Peeters F, Herholz K, Perani D, Holthoff V, Kalbe E, Anchisi D, Adam S, Collette F, Garraux G. Cerebral metabolic correlates of four dementia scales in Alzheimer's disease. J Neurol. 2005;252(3):283–290. doi: 10.1007/s00415-005-0551-3. [DOI] [PubMed] [Google Scholar]
- 12.Nadkarni NK, Levy-Cooperman N, Black SE. Functional correlates of instrumental activities of daily living in mild Alzheimer's disease. Neurobiol Aging. 2012;33(1):53–60. doi: 10.1016/j.neurobiolaging.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8(1 Suppl):S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall GA, Rentz DM, Frey MT, Locascio JJ, Johnson KA, Sperling RA, Initiative AsDN Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2011;7(3):300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 16.Nitrini R, Caramelli P, Herrera E, Jr., Bahia VS, Caixeta LF, Radanovic M, Anghinah R, Charchat-Fichman H, Porto CS, Carthery MT, Hartmann AP, Huang N, Smid J, Lima EP, Takada LT, Takahashi DY. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2004;18(4):241–246. [PubMed] [Google Scholar]
- 17.Morris JC. Revised Criteria for Mild Cognitive Impairment May Compromise the Diagnosis of Alzheimer Disease Dementia. Arch Neurol. 2012 doi: 10.1001/archneurol.2011.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, Rosengren L, Vanmechelen E, Blennow K. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem. 2005;51(2):336–345. doi: 10.1373/clinchem.2004.039347. [DOI] [PubMed] [Google Scholar]
- 20.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer's disease. N Engl J Med. 2013;368(13):1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 21.Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, Pirttila T. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66(3):382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 22.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.