Abstract
This study examined the extent to which rumination and depression share genetic and environmental influences in a community sample of adult twins (N = 663). Twins completed multiple rumination questionnaires, a depressive symptoms questionnaire, and a diagnostic interview. Rumination was moderately heritable (h2=.37–.41 for the latent variable) and substantially influenced by nonshared environmental factors, and these results were consistent across different measures. Nonshared environmental influences on rumination were larger for women than men. Depressive symptoms and diagnosis were influenced by genetic and nonshared environmental factors (h2=.30–.45). The genetic correlations between rumination and depression were moderate to large (rA=.40–.82), suggesting that a substantial proportion of the genetic influences on rumination overlap with those on depression. Results were similar when examining self-reported depressive symptoms and interview-based diagnosis of major depressive disorder. These results highlight the importance of rumination in the integration of cognitive and genetic models of depression risk.
Keywords: rumination, brooding, reflection, depression, heritability
Rumination is a pattern of repetitive, self-directed thought, focused on symptoms of distress and potential causes and implications of those symptoms. Existing research suggests that rumination increases distress, perpetuates symptoms, intensifies functional impairment, and increases risk for depression relapse; it also increases risk for multiple forms of psychopathology, interacts with other vulnerability factors in predicting depression, and is a primary treatment target in psychotherapy for depression (for a review, see Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Much less is known, however, about the heritability of rumination, and the extent to which its genetic and environmental influences overlap with those for depression. Using multivariate twin modeling, we examined these issues in adults in an effort to integrate genetic and cognitive theories of depression vulnerability. Specifically, we addressed three novel empirical questions: Is rumination heritable in adults? If so, to what extent is heritability measure-dependent? And how much do the genetic and environmental influences on rumination overlap with those for depression in adults?
Research on the etiology (defined here as the extent to which genetic and environmental influences contribute to individual differences) of rumination is limited. Existing research has largely focused on environmental risk factors for rumination, and suggests that perception of adverse experiences in childhood, including emotional maltreatment (Spasojević & Alloy, 2002) and overcontrolling parenting (Hilt, Armstrong, & Essex, 2012; Spasojević & Alloy, 2002), increase risk for rumination later in life. These results suggest that stressful environmental contexts in childhood may serve as distal risk factors for depression that “set the stage” for the development of a ruminative coping style (a proximal risk factor), and subsequently increase risk for depression (Nolen-Hoeksema & Watkins, 2011).
Very few studies have examined genetic influences on rumination, though there is considerable interest in this topic. Recent theoretical models have emphasized the interplay of genetic influences, cognitive vulnerabilities (e.g., rumination), and environmental contexts in risk for psychopathology (Gibb, Beevers, & McGeary, 2013). The twin design provides a robust framework to examine of the etiology of rumination and its relation to depression. Using data from monozygotic (MZ: identical) and dizygotic (DZ: fraternal) twins, twin modeling can estimate the magnitude of genetic and environmental contributions to individual differences in rumination and the extent to which they overlap with influences on depression.
The twin design is based on the fact that MZ twins share 100% of their genes whereas DZ twins share 50% of their genes on average, and both types of twins are reared together (i.e., have shared environmental influences). Larger correlations within MZ twin pairs versus DZ pairs (rMZ>rDZ) suggest genetic influences. An rMZ approximately twice the rDZ suggests additive genetic effects (A) whereas an rMZ greater than twice the rDZ indicates dominant genetic effects (D, which can also include other non-additive effects such as gene-gene interaction). Shared or common environmental influences (C) contribute to the similarity of twins (e.g., family socioeconomic status); they are indicated when rMZ is less than twice rDZ (DZ twins are more similar than genes can explain alone). In contrast, nonshared environmental influences (E; those that contribute to twins’ behavior being uncorrelated such as individual-specific life events) are indicated when rMZ<1.0 (differences between MZ twins must be due to the environment). The E estimate also includes measurement error, because it contributes to twins’ behavior being uncorrelated.
A limitation of the traditional twin design is that D and C cannot be estimated in the same model. Therefore, the pattern of twin correlations described above can be used to decide whether an ADE or ACE model (Neale & Cardon, 1992) is most appropriate. This general model can be extended to include two or more variables; here we use a Cholesky decomposition that partitions the covariance matrix into ACE (or ADE) components shared by rumination and depression and those unique to depression. This decomposition allows for the calculation of genetic and environmental correlations between phenotypes (i.e., the extent to which the phenotypes share genetic and environmental influences).
Only two twin studies, both focusing on adolescents, have examined rumination and depression. Moore et al. (2013) examined 12–14-year-old twins and found that rumination and depressive symptoms were both heritable (h2=.17 and .54, respectively), and that their association was largely genetic (genetic correlation[rA]=.83). These findings were supported by a study of Chinese twins ages 11–17 (Chen & Li, 2013), which found a low heritability for rumination (h2=.24) and substantial genetic overlap between rumination and depressive symptoms (rA=.99).
These studies provide preliminary evidence of etiological overlap between rumination and depression, but are limited in several ways. First, they only examined depressive symptoms, which may not generalize to clinical depression. Research has shown that individuals who have experienced a major depressive episode ruminate more than individuals who have never been depressed, even after remission (e.g., Moulds et al., 2008). Additionally, a meta-analysis of emotion regulation strategies found a stronger association between rumination and depression in individuals diagnosed with major depressive disorder (Aldao & Nolen-Hoeksema, 2011). Thus, examining etiological overlap between rumination and clinical depression is an important next step in this line of research.
Second, these two previous studies used single measures of rumination, which were unlikely to have fully captured the construct of rumination (Moore et al., 2013; p. 5). Researchers have suggested using multiple measures of rumination to overcome the methodological limitations that affect individual measures (Siegle, Moore, & Thase, 2004).
Third, although there are well established gender differences in rumination (Johnson & Whisman, 2013) and depression (Kessler, 2006) in adults, Moore et al. (2013) did not examine gender differences, and Chen and Li (2013) found no evidence of gender differences. This null finding is not surprising given that gender differences in depression and rumination are thought to emerge in late adolescence (Hankin & Abramson, 2001) and thus, may not have been present in their sample. However, examining gender differences is of primary importance in understanding the differential etiological pathways to depression in women and men.
Fourth, studies have shown that the heritability of vulnerabilities for depression can change over developmental periods, often with increased genetic influences in late adolescence (e.g., Johnson, Rhee, Whisman, Corley, & Hewitt, 2013). Thus, it cannot be assumed that results found with adolescent samples will generalize to adults.
The current study addresses these limitations by examining the heritability of rumination in adulthood, whether the heritability is measure and/or gender dependent, and the extent to which rumination and depression (measured with both a symptom questionnaire and a diagnostic interview) share etiological influences. Consistent with research on adolescents, we hypothesized that rumination would be significantly influenced by genetic and nonshared environmental factors, (although heritability may be higher in adults). Based on prior research, we hypothesized there would be substantial overlap in the genetic and environmental influences for rumination and depression. Finally, we examined gender differences in the magnitude of the genetic or environmental influences on rumination in this adult sample, as gender differences in rumination emerge in late adolescence.
Method
Participants
Participants were 663 twins from 347 families in the Colorado Longitudinal Twin Study (LTS). The LTS is an ongoing study of same-sex twins born between 1986 and 1990, recruited through the Colorado Department of Health. Of the parents initially contacted, more than 50% of the families who lived within a 3-hour drive of Boulder, Colorado enrolled in the study. The sample was 86.6% Caucasian, 8.5% Hispanic, 0.7% African-American, 1.2% Asian, and 2.9% other, which corresponds well to the ethnic and racial composition of Boulder County, Colorado in the 1990 United States Census (Rhea, Gross, Haberstick, & Corley, 2013).
These twins also participated in a multi-wave study conducted by the Center for Antisocial Drug Dependence (CADD) at the University of Colorado (CU). The CADD sample includes all LTS twins as well as twins and family members from other CU registries. Contemporaneously, participants completed measures of rumination for the LTS study, and diagnostic interviews and a measure of depressive symptoms for the CADD study. This analysis includes data collected during the third wave of the CADD study, when twins were 21–26 years old (mean=22.7; SD=1.12). There were 71 MZ and 68 DZ male pairs; 95 MZ and 82 DZ female pairs; and 31 singletons.
Zygosity was determined using ratings based on the similarity of 10 physical characteristics at several ages. These ratings were later confirmed using 11 polymorphic microsatellite markers. All research protocols were reviewed and approved by CU’s Institutional Review Board.
Measures
Rumination
Two measures of rumination were collected. The 10-item version of the 22-item Ruminative Response Scale (RRS; Nolen-Hoeksema & Morrow, 1991) was developed by Treynor, Gonzalez, and Nolen-Hoeksema (2003). Treynor et al. (2003) eliminated RRS items overlapping substantially with items on depression inventories and factor analyzed the remaining 10 items to obtain two factors: brooding (RRS-B) and reflection (RRS-R). Brooding represents passive, perseverative, maladaptive self-focused thought, whereas reflection represents less maladaptive self-reflective strategies. Both factors are associated positively with each other and with concurrent depression; however, brooding is a stronger predictor of depression and other negative psychosocial outcomes (Nolen-Hoeksema et al., 2008; Treynor et al., 2003). Thus, these two subscales represent variations of the same construct, rather than orthogonal forms of self-focused thought (e.g., Siegle et al., 2004). Scores for RRS-B and RRS-R reflect the average of the five items for each subscale. Both scales have been shown to have adequate psychometric properties (RRS-B, α=.74–.77; RRS-R, α=.66–.72; Siegle et al., 2004; Treynor et al., 2003).
The 24-item Rumination-Reflection Questionnaire (RRQ; Trapnell & Campbell, 1999) measures two types of self-focused thought: rumination (RRQ-Ru) and reflection (RRQ-Re). The RRQ-Ru measures “self-attentiveness motivated by perceived threat, losses or injustices to the self” (Trapnell & Campbell, 1999; p. 297) in a reliable fashion (α>.90) and is strongly associated with neuroticism (Trapnell & Campbell, 1999), and with depressive symptoms and the RRS subscales (Siegle et al., 2004). The RRQ-Re measures a construct distinct from rumination (e.g., Siegle et al., 2004), so it was not included in this analysis. Scores for the RRQ-Ru reflect the average of the 12 items in the subscale.
Depressive Symptoms
The Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977) is a widely used 20-item scale for measuring depressive symptoms, with strong psychometric properties (α=.85–.90; Radloff, 1977). Scores reflect the sum of the items.
MDD
The Diagnostic Interview Schedule – IV (DIS-IV; Robins et al., 2000) is a fully structured interview designed to diagnose in a reliable and valid fashion the major psychiatric disorders according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2000). The psychometric properties of the DIS have been studied extensively and diagnosis of MDD using the DIS has yielded good inter-rater reliability ratings in other samples (K=.67; for a review, see Compton & Cottler, 2004). Assessment of MDD was based on criteria endorsement in the past year and lifetime.
Statistical Analyses
Model Estimation
Mplus 6.1 (Muthén & Muthén, 1998–2010) was used to estimate phenotypic correlations, twin correlations, and twin models using maximum likelihood estimation. In phenotypic analyses, we corrected for the non-independence of the twin pairs using Mplus’ TYPE = COMPLEX option to obtain a scaled χ2 and standard errors robust to non-independence. The statistical significance of the parameters was determined by χ2difference tests (scaled for non-independence when appropriate; Satorra & Bentler, 2001). Given that the χ2 is sensitive to sample size, additional fit indices, including the Tucker-Lewis index (TLI; Bentler, 1990), and the root mean square error of approximation (RMSEA; Browne & Cudeck, 1989), were evaluated. A TLI>.95 and RMSEA<.06 indicate good model fit (Hu & Bentler, 1999).
Results
Descriptive Statistics
Means, standard deviations, and reliability coefficients for continuous measures are presented in Table 1. Rumination measures were normally distributed, with acceptable skewness and kurtosis values (between 1.00 and −1.00). The CES-D showed a skewed distribution, so scores were log transformed to achieve a normal distribution for subsequent analyses (transformed skewness=0.67, kurtosis=–0.05). As the LTS is a community sample, rates of past year MDD were low (6.6%), and only lifetime MDD (13%; n=77) was analyzed. The rate of lifetime MDD in our sample is consistent with the rates reported in large, population-based young adult samples (e.g., 15.4% by Kessler et al., 2005; 14.1% by Reichborn-Kjennerud et al., 2010).
Table 1.
Phenotypic Correlations (Off Diagonal) and Reliability (On Diagonal) for Men/Women, and Means (Standard Deviations)
| Measure | RRS-B | RRS-R | RRQ-RU | CES-D | MDD | Male Mean | Female Mean |
|---|---|---|---|---|---|---|---|
| RRS-Ba | .78/.83 | 1.90 (.57) | 2.03 (.64) | ||||
| RRS-Ra | .60/.55 | .81/.81 | 1.96 (.69) | 2.13 (.71) | |||
| RRQ-RUa | .70/.70 | .50/.43 | .88/.92 | 2.70 (.74) | 2.91 (.75) | ||
| CES-D | .47/.50 | .40/.31 | .49/.50 | .89/.90 | 10.37 (8.62) | 11.11 (8.91) | |
| MDDb | .31/.40 | .37/.42 | .52/.42 | .35/.42 | -- | N = 25 | N = 52 |
Note. All correlations account for missing data and nonindependence using Mplus (all p<.05). Internal reliability assessed with Chronbach’s alpha.
Mean for women significantly greater than for men (p<.05).
Biserial correlations. RRS-B=Ruminative Responses Scale-Brooding; RRS-R=Ruminative Responses Scale-Reflection; RRQ-Ru=Rumination-Reflection Questionnaire-Rumination; CES-D=Center for Epidemiological Studies-Depression; MDD=Major Depressive Disorder diagnosis (lifetime). CES-D means are for the untransformed total score; CES-D was log transformed for subsequent analyses.
Men scored lower in rumination (see Table 1) and had lower rates of MDD (odds ratio=0.55, 95% CI=0.33–0.91, p=.02). Regression analyses indicated no significant interactions between gender and rumination in predicting depressive symptoms or MDD. None of the measures correlated significantly with age (r=.00–.06).
Phenotypic Associations between Rumination and Depression
Phenotypic correlations are presented separately by gender in Table 1, although gender differences in these correlations were not significant. Four items on the CES-D that measure cognitive symptoms (e.g., “I had trouble keeping my mind on what I was doing”) may tap rumination and could inflate the correlations, so we examined whether removing these four items affected the correlations. Because the correlations hardly changed (Δr=.007–.011), we use the full scale in all analyses. The three rumination measures were moderately to highly correlated, consistent with the literature suggesting they measure a common construct.
Given the considerable overlap between the three measures of rumination, we used these measures as indicators of a rumination latent variable (RLV). This model was just identified (zero degrees of freedom), so there was no test of overall model fit. Each indicator loaded significantly on the latent factor and a χ2 difference test indicated significant gender differences (χ2diff(3)=9.61, p=.02) between the factor loadings for men and women. However, these gender differences (shown later for the genetic analyses) were small and the pattern of factor loadings was similar across genders, suggesting that, phenotypically, the RLV was qualitatively similar for men and women. Subsequent analyses include models using this RLV as well as models using the individual measures.
Genetic and Environmental Influences on Rumination and Depression
To evaluate our hypothesis that rumination is heritable in adulthood, we examined twin correlations and fit univariate twin models. MZ and DZ twin correlations are presented separately by gender in Table 2 (full twin correlation matrix in Appendix A1). Results suggested genetic influences on all constructs, with MZ correlations greater than DZ correlations. Moreover, the pattern of twin correlations suggested that ADE models would be more appropriate than ACE models.
Table 2.
Univariate Twin Results and Twin Correlations
| Measure | χ2 | df | TLI | RMSEA | A | E | rMZ | rDZ | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | W | M | W | M | W | M | W | |||||
| RRS-Ba | 16.70 | 14 | .96 | .047 | .63(.07) | .47(.10) | .78(.05) | .88(.05)a | .46(.08)* | .25(.08)* | .16(.13) | .04(.13) |
| RRS-Ra | 11.98 | 14 | 1.00 | .000 | .70(.06) | .47(.10) | .71(.05) | .89(.05)a | .53(.07)* | .21(.10)* | .20(.10)* | .09(.10) |
| RRQ-RU | 17.74 | 14 | .96 | .056 | .59(.08) | .58(.07) | .81(.06) | .82(.05) | .45(.08)* | .31(.08)* | −.07(.10) | .22(.11)* |
| RLVa | 98.26 | 84 | .99 | .044 | .64(.07) | .61(.08) | .77(.06) | .79(.06) | -- | -- | -- | -- |
| CES-D | 20.30 | 14 | .93 | .073 | .63(.07) | .55(.08) | .78(.06) | .83(.05) | .48(.07)* | .31(.09)* | −.04(.15) | .19(.10)* |
| MDDb | 9.97 | 7 | .82 | .051 | .67 | .72 | .46(.15)* | .27(.23) | ||||
Note. A and E values are standardized path coefficients (all p<.05; standard errors in parentheses). M=Men; W=Women; RRS-B=Ruminative Responses Scale-Brooding; RRS-R=Ruminative Responses Scale-Reflection; RRQ-Ru=Rumination-Reflection Questionnaire-Rumination; CES-D=Center for Epidemiological Studies-Depression; MDD=Major Depressive Disorder diagnosis (lifetime); rMZ/rDZ=MZ/DZ twin correlation.
p<.05.
Statistically significant gender difference (p<.05); for the RLV, gender differences were found only in the measure specific loadings (see Figure 1).
Analyses include gender as a covariate. When using the WLSMV estimator, Mplus does not provide standard errors for standardized parameters in models with covariates.
We allowed unstandardized parameter estimates to differ for men and women given theoretical interest in gender differences in rumination and depression, mean gender differences (Table 1), and gender differences in the RLV factor loadings. In models including MDD, the number of depressed individuals in each group was too small (e.g., n=3 for DZ men) to allow for grouping by gender, so gender was entered as a covariate.
Univariate ADE models were compared to more parsimonious univariate AE models that constrained D to zero. The AE model did not fit significantly worse than the ADE model for any variable, all χ2diff(2)<1.99, p>.36. Therefore, we focus on the AE models (univariate ADE results in Appendix A2).1 Most of the AE models fit the data well, and the model for MDD provided an acceptable fit to the data (see Table 2).
Rumination was moderately heritable in men (RLV h2=.41) and women (RLV h2=.37). Estimates of nonshared environmental influences on rumination were substantial and consistent across measures (e2=.50–.79). Univariate results for depressive symptoms and MDD were consistent with the literature on these constructs, indicating significant additive genetic and nonshared environmental effects (for a review and meta-analysis, see Sullivan, Neale, & Kendler, 2000).
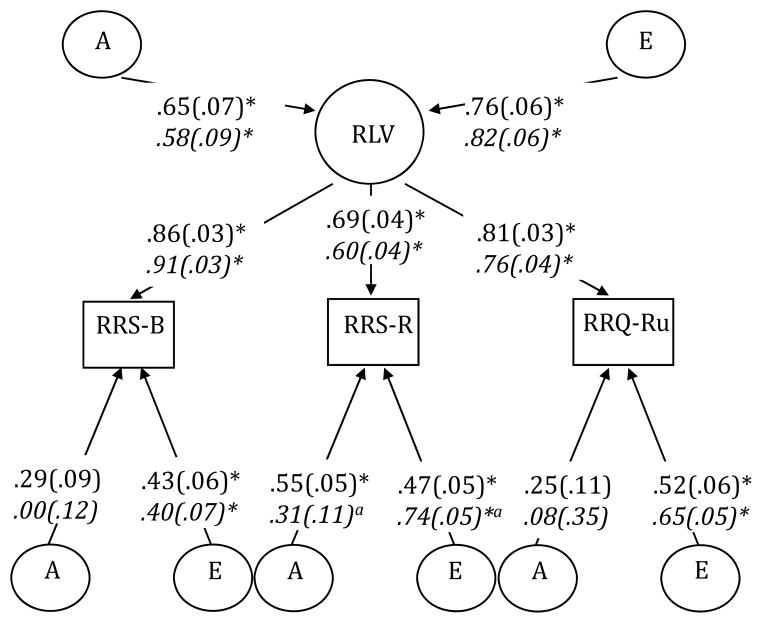
Significant gender differences were found for RRS-B, χ2diff(2)=7.35, p=.03; RRS-R, χ2diff(2)=6.54, p=.04; and RLV, χ2diff(8)=24.54, p<.01. For RRS-B and RRS-R, nonshared environmental influences were significantly greater in women than in men. For the RLV model (Figure 1), gender differences manifested in the measure-specific genetic and environmental effects for the RRS-R scale; there were no gender differences for the AE influences on the latent variable itself. The magnitude of genetic and environmental influences were nearly identical in a model where the RLV factor loadings were constrained to be equal across gender, suggesting that results were not affected by gender-specific factor loadings. These results suggest that, in addition to mean differences (i.e., women ruminate more), nonshared environmental influences on rumination may be larger for women than for men. Because reliability of the measures was not higher in men than in women, it is unlikely that these additional nonshared environmental influences in women reflect differences in measurement error.
Figure 1.
AE model for the rumination latent variable (RLV) with standardized parameter estimates (standard errors in parentheses). Parameters for women in italics. RRS-B=Ruminative Responses Scale-Brooding; RRS-R=Ruminative Responses Scale-Reflection; RRQ-Ru=Rumination-Reflection Questionnaire-Rumination; A=genetic influences; E=nonshared environmental influences. * p<.05. aStatistically significant gender difference (p<.05) in the unstandardized parameter estimates.
Genetic and Environmental Influences on the Association Between Rumination and Depression
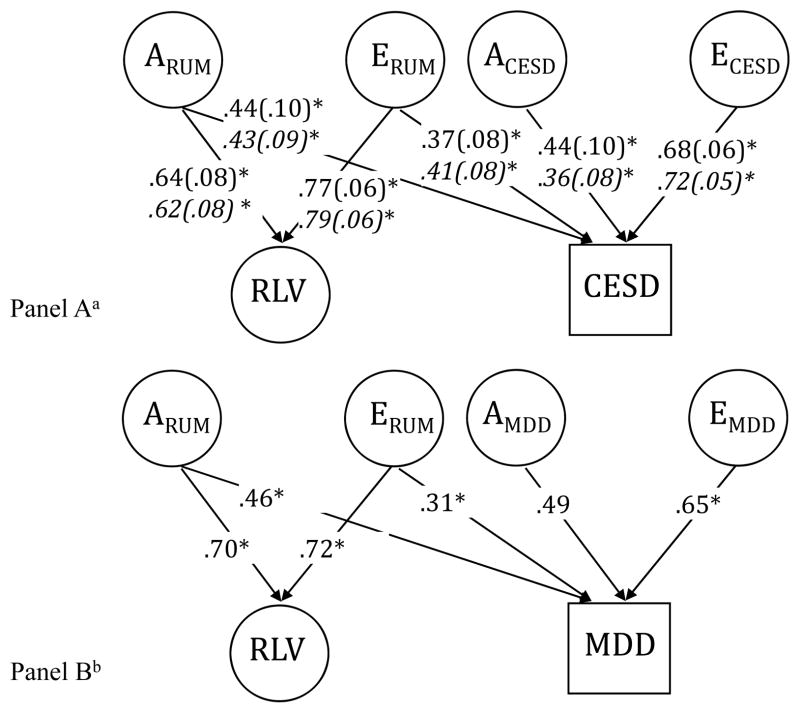
The univariate analyses establish that both rumination and depression have significant genetic and nonshared environmental influences, and the phenotypic correlations reported in Table 1 suggest that rumination and depression are moderately associated. To what extent is the phenotypic overlap between rumination and depression due to genetic and environmental influences? We estimated a bivariate AE Cholesky decomposition model to test our hypothesis that there would be substantial genetic and environmental overlap between rumination and depression.
Figure 2 presents bivariate AE Cholesky models of RLV and depression measures and Table 3 presents additional bivariate results for each rumination measure separately. Models showed good fit (all TLI>.95, RMSEA<.07, χ2(142)<179.33, p>.02). There was significant overlap in the genetic influences for rumination and depression, and this pattern of results did not depend on the measurement used or gender. There were also unique genetic influences on depressive symptoms and MDD above those shared with rumination, suggesting distinct etiologies for these constructs. However, this parameter did not reach statistical significance in all models, possibly due to low power. Nonshared environmental influences on rumination also significantly influenced depression, and those unique to depression were substantial in magnitude and significant in all models. The genetic and environmental influences unique to depression indicate separability of the rumination and depression constructs. Furthermore, approximately 25% of the genetic variance in depression was unshared with rumination, so measurement error (captured in E) cannot account for their separability.
Figure 2.
Standardized estimates for the AE Cholesky model of RLV and CESD (A) and MDD (B) (standard errors in parentheses). Parameters for women in italics. For simplicity, the indicators for the RLV are not shown. RLV=Rumination Latent Variable; CES-D=Center for Epidemiological Studies-Depression; MDD=Major Depressive Disorder diagnosis (lifetime); ARUM=additive genetic influences predicting rumination and depression; ACESD/MDD=additive genetic influences unique to depression; ERUM=nonshared environmental predicting rumination and depression; ECESD/MDD=nonshared environmental influences unique to depression. *p<.05. aStatistically significant gender difference (p<.05) in the unstandardized parameter estimates; for the RLV, gender differences were found only in the measure specific loadings. bGender included as a covariate. Using the WLSMV estimator, Mplus does not provide standard errors when a covariate is included.
Table 3.
Bivariate Twin Results
| Measures Predicting |
ARUM | ADEP | ERUM | EDEP | ||
|---|---|---|---|---|---|---|
| Rum | Dep | Dep | Rum | Dep | Dep | |
| RRS-B & CES-Da | .63(.07)* | .40(.10)* | .48(.09)* | .78(.05)* | .29(.08)* | .73(.06)* |
| .48(.09)* | .45(.11)* | .34(.07)* | .88(.05)*a | .34(.11)* | .76(.05)* | |
| RRS-R & CES-Da | .70(.06)* | .31(.10)* | .54(.08)* | .71(.05)* | .24(.08)* | .75(.06)* |
| .47(.10)* | .16(.14) | .52(.07)* | .89(.05)*a | .27(.07)* | .79(.05)* | |
| RRQ-Ru & CES-D | .59(.08)* | .37(.11)* | .52(.08)* | .81(.06)* | .34(.08)* | .69(.06)* |
| .59(.07)* | .46(.09)* | .32(.11) | .81(.05)* | .28(.07)* | .77(.05)* | |
| RRS-B & MDDb | .56* | .42* | .53 | .83* | .16 | .71* |
| RRS-R & MDDb | .58* | .27* | .61* | .81* | .32* | .65* |
| RRQ-Ru & MDDb | .59* | .51* | .44 | .81* | .21* | .69* |
Note. Parameters for women presented in italics for CES-D analyses (standard errors in parentheses). RRS-B=Ruminative Responses Scale-Brooding; RRS-R=Ruminative Responses Scale-Reflection; RRQ-Ru=Rumination-Reflection Questionnaire-Rumination; CES-D=Center for Epidemiological Studies-Depression; MDD=Major Depressive Disorder diagnosis (lifetime); Rum=rumination; Dep=depression. ARUM=additive genetic influences predicting rumination and depression; ADEP=additive genetic influences unique to depression; ERUM=nonshared environmental predicting rumination and depression; EDEP=nonshared environmental influences unique to depression;
p<.05.
Statistically significant gender difference (p<.05).
Analyses include gender as a covariate. When using the WLSMV estimator, Mplus does not provide standard errors for standardized parameters in models with covariates.
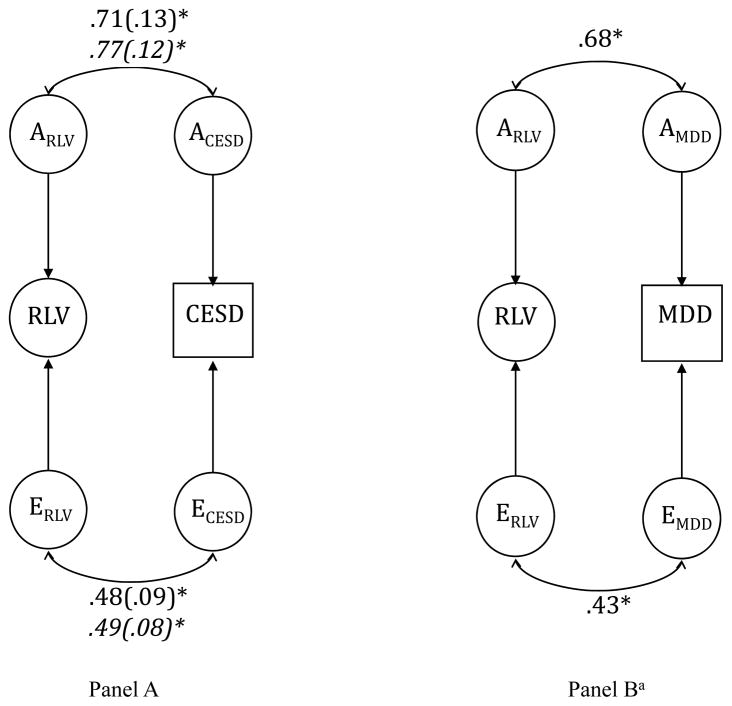
Another way to represent these results is through genetic (rA) and environmental (rE) correlations, which can be derived from the Cholesky model. Figure 3 presents results for RLV and depression measures, and additional results for the individual measures are presented in Appendix A3: rA ranged from .40–.82, and rE ranged from .22–.49. Genetic and environmental correlations can be used to compute the phenotypic correlations predicted by the model, and to calculate the percentages of those phenotypic correlations that are due to overlapping genetic and environmental influences. Results of these calculations (Appendix A3) suggest that genetic and nonshared environmental influences account for approximately equal proportions (~50%) of the phenotypic correlation between rumination and depression.
Figure 3.
AE correlational model for RLV and CESD (A) and MDD (B) (standard errors in parentheses). Parameters for women in italics. For simplicity, the indicators for the RLV are not shown. RLV=Rumination Latent Variable; CESD=Center for Epidemiological Studies-Depression; MDD=Major Depressive Disorder diagnosis (lifetime). *p<.05. aGender included as a covariate. Using the WLSMV estimator, Mplus does not provide standard errors when a covariate is included.
Discussion
We examined genetic and environmental influences on rumination, depression, and their association in a community sample of adult twins. Three novel findings have important implications for our understanding of the etiology of rumination. First, we found that rumination is moderately heritable, with the remaining variance due to nonshared environmental influences. Furthermore, results were similar across multiple rumination measures, suggesting that the genetic structure of rumination is not measure-dependent. Second, nonshared environmental influences on rumination (as measured by the RRS) were significantly larger for women. Third, there was a large overlap in the genetic influences on rumination and depression, suggesting a shared genetic etiology. Importantly, this finding was consistent across gender and across measures of depressive symptoms and diagnosis of MDD, suggesting this etiological overlap is not dependent on a continuous or categorical definition of depression.
Etiology of Rumination
Our results that rumination is moderately heritable in adulthood extend those from prior research (Chen & Li, 2013; Moore et al., 2013) in two ways. First, we examined multiple measures of rumination, including a latent variable, so we are confident that our results extend beyond a specific measure of rumination. Second, results suggest that the heritability of rumination may be higher in adulthood than early adolescence. Our heritability estimate for the RLV in men (h2=.41) was outside the confidence intervals reported for the two prior studies of rumination (h2=.21%, CI=.07–34 for Moore et al., and h2=.24, CI=.07–39 for Chen & Li). Our RLV estimate for women (h2=.37) was also outside the CI obtained by Moore et al., but was in the upper range of Chen and Li’s CI. This finding was evident only for measures of rumination, and was not found when examining measures of reflection strategies (our RLV h2 estimates were within the CI obtained by Moore et al. for the RRS-R: h2=.37, CI=.24–.49).
Our finding that rumination is heritable suggests it may be worthwhile to search for genes that contribute to variance in rumination. However, based on evidence from many large genome wide association studies of psychiatric disorders and phenotypes (for a review, see Sullivan, Daly, & O’Donovan, 2012), we should expect there to be many genetic variants, each with a very small effect on individual differences in rumination, rather than variants with large effects.
The remaining variability in rumination appears to be due to nonshared environmental influences. These results are consistent with prior research that has found that rumination is associated with retrospective reports of perceptions of adverse experiences in childhood, including emotional maltreatment (Spasojević & Alloy, 2002) and overcontrolling parenting (Hilt et al., 2012; Spasojević & Alloy, 2002), the effects of which likely vary between twins. The finding that variation in rumination was substantially due to nonshared environmental influences supports continued investigation into these and other experiences that may contribute to the development of rumination.
Etiology of the Association Between Rumination and Depression
Measures of depressive symptoms and MDD diagnosis were moderately heritable and influenced by nonshared environmental factors, consistent with existing literature (Sullivan et al., 2000). Our findings suggesting a common genetic etiology between rumination and depression (rA=.68–.77 for RLV) are consistent with results from two studies of adolescent twins that indicated substantial genetic overlap between rumination and depressive symptoms (Chen & Li, 2013; Moore et al., 2013). Importantly, our study builds on prior research to suggest that this etiological overlap is consistent across multiple measures of rumination and extends to adults with MDD. In particular, we found similar levels of etiological overlap with a dimensional versus a categorical (diagnostic) measure of depression, suggesting that the nature of this overlap is not dependent on a specific conceptualization of depression.
Although some genetic overlap between related constructs is to be expected (Plomin & Kovacs, 2005), the large genetic correlations between rumination and depression in our sample (rA up to .82) suggest that rumination may serve as a cognitive mediator between genetic risk for depression and the onset and course of depression. This interpretation is consistent with recent theoretical models of risk for psychopathology, in which individuals’ genetic predispositions for depression interact with cognitive risk factors (e.g. rumination) and environmental exposure to trigger the onset of depression (Gibb et al., 2013; Nolen-Hoeksema & Watkins, 2011). Our results support these models, suggesting that certain individuals are genetically predisposed to depression and also at risk for rumination, thus further increasing their risk for depression.
There are plausible mechanisms linking genetic predispositions to the development of rumination and depression. First, high stress reactivity (e.g., hypothalamic-pituitary-adrenal axis dysfunction) has been linked to depression and low resilience to stress in adults, and depressive behaviors in mice and rats (for a review, see Southwick, Vythilingam, & Charney, 2005). Individuals with a genetic propensity for high stress reactivity who are exposed to adverse events may be more likely to engage in rumination in an attempt cope with this intense reactivity, increasing their risk for depression (Disner, Beevers, Haigh, & Beck, 2011). Second, deficits in executive function (EF) may also increase risk for rumination and depression. Highly heritable (Friedman et al., 2008) EF deficits have been linked to rumination, in that ruminators have more difficulty disengaging from stimuli that is no longer relevant or rewarding (for a review, see Whitmer & Gotlib, 2013). Deficits in EF have also been linked to depression (Snyder, 2013), and recent theoretical models (Whitmer & Gotlib, 2013) provide a strong framework to examine EF deficits in the context of shared etiology between rumination and depression. These genetic mechanisms represent some of the exciting areas for future research efforts.
In comparison to genetic effects, environmental influences seem to be more construct-specific, as indicated by smaller environmental correlations between depression and rumination (rE=.22–.49). This result is consistent with findings from prior twin studies (Chen & Li, 2013; Moore et al., 2013) and with several studies suggesting some overlap in the environmental risk factors for cognitive vulnerabilities and depression, but also independent effects of the environment on these constructs (for a review, see Alloy, Abramson, Smith, Gibb, & Neeren, 2006). Additionally, the inclusion of measurement error in the estimate of nonshared environmental influences may deflate environmental correlations. It should be noted that genes and the environment can exert their influences on behavior in both an interdependent (e.g. gene-environment interaction, epigenetics; Kofink Boks, Timmers & Kas, 2013) and independent fashion. Our results should be considered in light of the dynamic nature of these influences.
Gender Differences
Finally, we found evidence for gender differences in the environmental influences on rumination, namely that nonshared environmental influences were greater in magnitude for women than for men for the two RRS measures.2 Because gender differences have not been found in previous studies of rumination in adolescents (e.g., Chen & Li, 2013) these results are particularly noteworthy. Gender differences in environmental influences are also important in light of the fact that the response styles theory was developed, in part, to account for gender differences in depression (Nolen-Hoeksema et al., 2008). Insofar as many stressful events linked to rumination are more commonly encountered by women (e.g., Harkness et al., 2010), gender differences in exposure or reaction to environmental adversities could contribute to gender differences in nonshared environmental influences on rumination.
Limitations
The results of our study should be considered with some limitations in mind. First, our sample was relatively small for twin analyses and replication in a larger sample would be useful in terms of generalizability. Although we did find significant gender differences in mean levels and the magnitude of nonshared environmental influences on rumination, our sample size may also have led to relatively low power for examination of gender differences (particularly with respect to MDD). Thus, further examination of gender differences is an important priority for future research.
Second, though our results were largely consistent across two measures of depression, the majority of our community sample was not clinically depressed at the time of assessment, reflecting the low base rates of current MDD in young adults (e.g., Kessler et al., 2005). The relatively small number of MDD cases may have limited our statistical power to detect significant, independent genetic influences on MDD in some bivariate models. Thus, the finding that MDD (but not CES-D) did not have significant genetic influences over and above those shared with rumination should be interpreted with caution.
Third, data were cross-sectional, so we cannot make inferences about the temporal association between rumination and depression in our sample. Additionally, our finding that the heritability of rumination may be larger in adulthood should be replicated in a longitudinal design. Rather than comparing estimates across studies, a longitudinal design would allow for consistency in study design, the sample, the measures of rumination, and the examination of gender differences, variation in which could affect heritability estimates.
Finally, although the twin design provides a powerful method to assess the etiology of depression and rumination, there are limitations to this method (for a review, see Tenesa & Haley, 2013). The twin design relies on several assumptions, which, if not met, may lead to biased heritability estimates. The most serious of these is the equal environments assumption, which states that the environments of MZ twins are no more similar than those of DZ twins. However, studies of this assumption have generally found little evidence for its violation influencing twin similarities (e.g., Kendler, Kessler, Neale, Heath, & Eaves, 1993). Moreover, our results are largely consistent with prior studies examining MDD (for a meta-analysis, see Sullivan et al., 2000). There is also speculation that the unique prenatal (and possibly postnatal) environments experienced by twins limit the generalizability of results to the general population of nontwins. However, studies examining twins and their nontwin relatives have shown only very small differences on psychiatric measures (e.g., Kendler, Martin, Heath, & Eaves, 1995), suggesting the current results will likely generalize to the general population.
Conclusion
We found that rumination is heritable in adulthood and that the genetic influences on rumination largely overlap with those on depression. These results support continued investigation into both the genetic and environmental influences on the development of rumination and suggest that a comprehensive understanding of the etiology of rumination and its association with depression will need to take into account both types of influences.
Table A1.
Twin Correlations for Men (Upper Panel) and Women (Lower Panel)
| RRS-B 1 | RRS-R 1 | RRQ-R 1 | MDD 1 | CES-D 1 | RRS-B 2 | RRS-R 2 | RRQ-R 2 | MDD 2 | CES-D 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | |
| RRS-B 1 | .33 | .25 | ||||||||||||||||||
| RRS-R 1 | .62 | .55 | .46 | .44 | ||||||||||||||||
| RRQ-R 1 | .77 | .61 | .63 | .46 | .61 | .39 | ||||||||||||||
| MDD 1 | .36 | −.11 | .29 | .23 | .41 | .50 | -- | -- | ||||||||||||
| CES-D 1 | .59 | .14 | .48 | .27 | .51 | .43 | .22 | .67 | .08 | .06 | ||||||||||
| RRS-B 2 | .46 | .16 | .24 | −.03 | .35 | .01 | .28 | −.33 | .29 | −.01 | .45 | .28 | ||||||||
| RRS-R 2 | .48 | .11 | .53 | .20 | .37 | .17 | .32 | −.19 | .41 | −.04 | .72 | .43 | .57 | .43 | ||||||
| RRQ-R 2 | .40 | .14 | .26 | −.05 | .45 | −.07 | .15 | −.43 | .31 | −.21 | .74 | .62 | .66 | .46 | .67 | .44 | ||||
| MDD 2 | .55 | −.28 | .43 | −.35 | .61 | −.08 | .69 | .48 | .53 | −.52 | .37 | .33 | .56 | .01 | .54 | .75 | ||||
| CES-D 2 | .40 | .09 | .26 | −.02 | .38 | −.19 | .19 | −.23 | .48 | −.04 | .63 | .42 | .53 | .23 | .61 | .33 | .47 | −.43 | .07 | .05 |
|
| ||||||||||||||||||||
| RRS-B 1 | .40 | .48 | ||||||||||||||||||
| RRS-R 1 | .59 | .62 | .44 | .57 | ||||||||||||||||
| RRQ-R 1 | .72 | .73 | .40 | .58 | .52 | .64 | ||||||||||||||
| MDD 1 | .31 | .56 | .46 | .53 | .25 | .55 | -- | -- | ||||||||||||
| CES-D 1 | .34 | .61 | .25 | .42 | .36 | .70 | .43 | .55 | .06 | .07 | ||||||||||
| RRS-B 2 | .25 | .04 | .20 | .10 | .37 | .15 | .24 | .10 | .20 | .13 | .40 | .35 | ||||||||
| RRS-R 2 | .13 | .02 | .21 | .09 | .23 | .00 | .13 | −.11 | .07 | −.05 | .56 | .39 | .49 | .48 | ||||||
| RRQ-R 2 | .22 | .23 | .25 | .15 | .31 | .22 | .38 | .23 | .24 | .16 | .74 | .55 | .50 | .20 | .57 | .50 | ||||
| MDD 2 | .08 | −.18 | .03 | .01 | .22 | −.05 | .25 | .36 | .19 | .16 | .27 | .54 | .41 | .28 | .37 | .57 | ||||
| CES-D 2 | .25 | .01 | .13 | −.04 | .30 | −.01 | .33 | −.04 | .31 | .19 | .53 | .57 | .32 | .23 | .54 | .37 | .44 | .20 | .08 | .06 |
Note. Off-diagonal cells are correlations and diagonal cells are variances. RRS-B=Ruminative Responses Scale-Brooding; RRS-R=Ruminative Responses Scale-Reflection; RRQ-R=Rumination-Reflection Questionnaire-Rumination; CES-D=Center for Epidemiological Studies-Depression; MDD=Major Depressive Disorder diagnosis (lifetime); 1=measure for twin 1; 2=measure for twin 2. CES-D was log transformed for analyses. Bold indicates statistical significance (p<.05).
Table A2.
Univariate ADE Results for Measures of Rumination and Depression
| Measure | χ2 | df | TLI | RMSEA | A | D | E | |||
|---|---|---|---|---|---|---|---|---|---|---|
| M | W | M | W | M | W | |||||
| RRS-B | 16.07 | 12 | .94 | .060 | .58(.48) | .00(.85) | .26(1.11) | .50(.10) | .78(.06)* | .87(.06)* |
| RRS-R | 11.85 | 12 | 1.00 | .000 | .60(.40) | .35(.59) | .37(.67) | .32(.71) | .71(.06)* | .88(.06)* |
| RRQ-RU | 15.78 | 12 | .95 | .060 | .00(.48) | .58(.07) | .61(.07) | .00(.67) | .79(.06)* | .82(.05)* |
| RLVa | 95.83 | 76 | .98 | .060 | .39(.92) | .53(.49) | .52(.70) | .32(.84) | .76(.06)* | .79(.07)* |
| CES-D | 18.31 | 12 | .92 | .080 | .00(.53) | .55(.08) | .66(.07) | .00(.81) | .75(.06)* | .83(.05)* |
| MDDb | 9.8 | 6 | .73 | .060 | .67* | .00 | .72* | |||
Note. A, D, and E values are standardized path coefficients (standard errors in parentheses). Squaring these values will provide the variances for the A, D, and E influences. M=Men; W=Women; RRS-B=Ruminative Responses Scale-Brooding; RRS-R=Ruminative Responses Scale-Reflection; RRQ-Ru=Rumination-Reflection Questionnaire-Rumination; CES-D=Center for Epidemiological Studies-Depression; MDD=Major Depressive Disorder diagnosis (lifetime).
p<.05.
Statistically significant gender difference (p<.05); for the RLV, gender differences were found only in the measure specific loadings.
Analyses include gender as a covariate. When using the WLSMV estimator, Mplus does not provide standard errors for standardized parameters when a covariate is included in the model.
Table A3.
Genetic/ Environmental Correlations and Proportion of Phenotypic Correlation Due to Genetic and Environmental Influences (Men/Women)
| Measures | G/E Correlation | % of Phenotypic Correlation | ||
|---|---|---|---|---|
| rA | rE | rA | rE | |
| RRS-B & CES-Da | .64(.15)*/.80(.15)* | .37(.09)*/.41(.08)* | 53*/42* | 47*/58* |
| RRS-R & CES-Da | .50(.14)*/.29(.24) | .30(.10)*/.32(.08)* | 56*/24 | 44*/76* |
| RRQ-Ru & CES-D | .58(.15)*/.82(.13)* | .44(.09)*/.34(.08)* | 44*/54* | 56*/46* |
| RLV & CES-Da | .71(.13)*/.77(.12)* | .48(.09)*/.49(.08)* | 50*/45* | 50*/55* |
| RRS-B & MDDb | .62* | .22 | 64* | 36 |
| RRS-R & MDDb | .40* | .44* | 38* | 62* |
| RRQ-Ru & MDDb | .76* | .29* | 64* | 36* |
| RLV & MDDb | .68* | .43* | 59* | 41* |
Note. Standard errors in parentheses. RRS-B=Ruminative Responses Scale-Brooding; RRS-R=Ruminative Responses Scale-Reflection; RRQ-Ru=Rumination-Reflection Questionnaire-Rumination; RLV=Rumination Latent Variable; CES-D=Center for Epidemiological Studies-Depression; MDD=Major Depressive Disorder diagnosis (lifetime); rA=genetic correlation; rE=nonshared environmental correlation.
p<.05.
Statistically significant gender difference in original model (p<.05).
Analyses include gender as a covariate. When using the WLSMV estimator, Mplus does not provide standard errors for standardized parameters when a covariate is included in the model.
Acknowledgments
This research was supported by the National Institutes of Health under grants MH063207, HD010333, HD007289, and DA011015 and by the National Alliance for Research on Schizophrenia and Depression.
Footnotes
The lack of significant dominant (D) genetic influences does not necessarily mean that there are no non-additive genetic influences on these constructs, but rather, may reflect the low power of the twin design to distinguish them from additive (A) genetic influences (Martin, Eaves, Kearsey, & Davies, 1978). Thus, we consider A to reflect broad-sense heritability.
There was a significant gender difference in the magnitude of measure-specific genetic influences on the RRS-R in the RLV model and a marginal gender difference in genetic influences in the RRS-R univariate model. This gender difference may be unique to the construct of reflection, as it was not detected in the other measures of rumination (RRS-B, RRQ-Ru, RLV).
Preliminary results from this study were presented at the Annual Meeting of the Behavior Genetics Association in Edinburgh, UK in June 2012. The abstract of the presentation is published in Behavior Genetics (volume 42, issue 6, 2012).
References
- Aldao A, Nolen-Hoeksema S. Specificity of cognitive emotion regulation strategies: A transdiagnostic examination. Behaviour Research and Therapy. 2011;48:974–983. doi: 10.1016/j.brat.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Smith JM, Gibb BE, Neeren AM. Role of parenting and maltreatment histories in unipolar and bipolar mood disorders: Mediation by cognitive vulnerability to depression. Clinical Child and Family Psychology Review. 2006;9:23–64. doi: 10.1007/s10567-006-0002-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Single sample cross-validation indexes for covariance structures. Multivariate Behavioral Research. 1989;24:445–455. doi: 10.1207/s15327906mbr2404_4. [DOI] [PubMed] [Google Scholar]
- Chen J, Li X. Genetic and environmental influences on adolescent rumination and its association with depressive symptoms. Journal of Abnormal Child Psychology. 2013 doi: 10.1007/s10802-013-9757-5. [DOI] [PubMed] [Google Scholar]
- Compton WM, Cottler LB. The Diagnostic Interview Schedule (DIS) In: Hilsenroth MJ, Segal DL, editors. Comprehensive handbook of psychological assessment, Volume 2: Personality assessment. Hoboken, NJ: Wiley; 2004. pp. 153–162. [Google Scholar]
- Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Beevers CG, McGeary JE. Toward an integration of cognitive and genetic models of risk for depression. Cognition & Emotion. 2013;27:193–216. doi: 10.1080/02699931.2012.712950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Harkness KL, Alavi N, Monroe SM, Slavich GM, Gotlib IH, Bagby RM. Gender differences in life events prior to onset of major depressive disorder: The moderating effect of age. Journal of Abnormal Psychology. 2010;119:791–803. doi: 10.1037/a0020629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt LM, Armstrong JM, Essex MJ. Early family context and development of adolescent ruminative style: Moderation by temperament. Cognition and Emotion. 2012;26:916–926. doi: 10.1080/02699931.2011.621932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Johnson DP, Rhee SH, Whisman MA, Corley RP, Hewitt JK. Genetic and environmental influences on negative life events from late childhood to adolescence. Child Development. 2013 doi: 10.1111/cdev.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DP, Whisman MA. Gender differences in rumination: A meta-analysis. Personality and Individual Differences. 2013;55:367–374. doi: 10.1016/j.paid.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: Toward an integrated etiologic model. American Journal of Psychiatry. 1993;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Martin NG, Heath AC, Eaves LJ. Self-report psychiatric symptoms in twins and their nontwin relatives: Are twins different? American Journal of Medical Genetics. 1995;60:588–591. doi: 10.1002/ajmg.1320600622. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The epidemiology of depression among women. In: Keyes CLM, Goodman SH, editors. Women and depression: A handbook for the social, behavioral, and biomedical sciences. New York: Cambridge University Press; 2006. pp. 22–37. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kofink D, Boks MP, Timmers H, Kas MJ. Epigenetic dynamics in psychiatric disorders: Environmental programming of neurodevelopmental processes. Neuroscience & Biobehavioral Reviews. 2013 doi: 10.1016/j.neubiorev.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Kearsey MJ, Davies P. The power of the classical twin study. Heredity. 1978;40:97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- Moore MN, Salk RH, Van Hullie CA, Abramson LY, Hyde JS, Lemery-Chalfant K, Goldsmith HH. Genetic and environmental influences on rumination, distraction, and depressed mood in adolescence. Clinical Psychological Science. 2013 doi: 10.1177/2167702612472884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulds ML, Kandris E, Williams AD, Lang T, Yap C, Hoffmeister K. An investigation of the relationship between cognitive reactivity and rumination. Behavior Therapy. 2008;39:65–71. doi: 10.1016/j.beth.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles, CA: Muthén & Muthén; 1998–2010. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. New York: Kluwer Academic/Plenum; 1992. [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta Earthquake. Journal of Personality and Social Psychology. 1991;61:115–121. doi: 10.1037/0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Watkins ER. A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science. 2011;6:589–609. doi: 10.1177/1745691611419672. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Plomin R, Kovacs Y. Generalist genes and learning disabilities. Psychological Bulletin. 2005;131:592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Reichborn-Kjennerud T, Czajkowski N, Røysamb E, Ørstavik RE, Neale MC, Torgersen S, Kendler KS. Major depression and dimensional representations of DSM-IV personality disorders: A population-based twin study. Psychological Medicine. 2010;40:1475–1484. doi: 10.1017/S0033291709991954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorrado Twin Registry: An update. Twin Research and Human Genetics. 2013;16:351–357. doi: 10.1017/thg.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke K. The Diagnostic Interview Schedule for DSM-IV (DIS-IV) St. Louis, MO: Washington University School of Medicine; 2000. [Google Scholar]
- Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/BF02296192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Moore PM, Thase ME. Rumination: One construct, many features in healthy individuals, depressed individuals, and individuals with lupus. Cognitive Therapy and Research. 2004;28:645–668. doi: 10.1023/B:COTR.0000045570.62733.9f. [DOI] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annual Review of Clinical Psychology. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Spasojević J, Alloy LB. Who becomes a depressive ruminator? Developmental antecedents of ruminative response style. Journal of Cognitive Psychotherapy. 2002;16:405–419. doi: 10.1891/088983902780935713. [DOI] [Google Scholar]
- Sullivan PF, Daly MJ, O’Donovan M. Genetic architetures of psychiatric disorders: The emerging picture and its implications. Nature Reviews Genetics. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tenesa A, Haley CS. The heritability of human disease: Estimation, uses and abuses. Nature Reviews Genetics. 2013;14:139–149. doi: 10.1038/nrg3377. [DOI] [PubMed] [Google Scholar]
- Trapnell PD, Campbell JD. Private self-consciousness and the five-factor model of personality: Distinguishing rumination from reflection. Journal of Personality and Social Psychology. 1999;76:284–304. doi: 10.1037/0022-3514.76.2.284. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27:247–259. doi: 10.1023/A:1023910315561. [DOI] [Google Scholar]
- Whitmer AJ, Gotlib IH. An attentional scope model of rumination. Psychological Bulletin. 2013;139:1036–1061. doi: 10.1037/a0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]