Abstract
Objective
To assess hospital and geographic variability in 30-day mortality after surgery for CRC and examine the extent to which sociodemographic, area-level, clinical, tumor, treatment, and hospital characteristics were associated with increased likelihood of 30-day mortality in a population-based sample of older CRC patients.
Data Sources/Study Setting
Linked Surveillance Epidemiology End Results (SEER) and Medicare data from 47,459 CRC patients aged 66 years or older who underwent surgical resection between 2000 and 2005, resided in 13,182 census tracts, and were treated in 1,447 hospitals.
Study Design
An observational study using multilevel logistic regression to identify hospital- and patient-level predictors of and variability in 30-day mortality.
Data Collection/Extraction Methods
We extracted sociodemographic, clinical, tumor, treatment, hospital, and geographic characteristics from Medicare claims, SEER, and census data.
Principal Findings
Of 47,459 CRC patients, 6.6 percent died within 30 days following surgery. Adjusted variability in 30-day mortality existed across residential census tracts (predicted mortality range: 2.7–12.3 percent) and hospitals (predicted mortality range: 2.5–10.5 percent). Higher risk of death within 30 days was observed for CRC patients age 85+ (12.7 percent), census-tract poverty rate >20 percent (8.0 percent), two or more comorbid conditions (8.8 percent), stage IV at diagnosis (15.1 percent), undifferentiated tumors (11.6 percent), and emergency surgery (12.8 percent).
Conclusions
Substantial, but similar variability was observed across census tracts and hospitals in 30-day mortality following surgery for CRC in patients 66 years and older. Risk of 30-day mortality is driven not only by patient and hospital characteristics but also by larger social and economic factors that characterize geographic areas.
Keywords: Colorectal cancer, neighborhood, multilevel, poverty
In the current health care reform environment of increasing transparency and accountability, postoperative mortality rates to assess hospital quality have gained increasing attention and importance. For example, the Centers for Medicare & Medicaid Services (CMS) and the Agency for Healthcare Research and Quality publicly report as a measure of quality of care the 30-day mortality rates for acute myocardial infarction, heart failure, pneumonia, and selected medical conditions on their website (http://www.hospitalcompare.hhs.gov). In addition, CMS plans to use 30-day mortality measures in its Hospital Inpatient Value-Based Purchasing program (Centers for Medicare & Medicaid Services 2011). Variability in 30-day mortality has been frequently used as a quality measure for cancer and noncancer care (Institute of Medicine 1999). Quality of cancer care may be improved by reducing variation in underuse of effective and necessary care; variation that indicates misuse of preference-sensitive care (i.e., care that offers equivalent options to be chosen among by the patient); and variation that indicates overuse of supply-sensitive care (i.e., care influenced by medical capacity; Wennberg 2010). Examining and reducing variability in medical care has been an important policy consideration for almost 30 years (Wennberg 1999; Tanenbaum 2013).
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States, accounting for an estimated 103,170 new cases and 51,690 deaths in 2012 (American Cancer Society 2012). Elderly patients with CRC are more likely to die than younger patients, especially during the 30-day postoperative period (Fazio et al. 2004; Davila et al. 2005; Dekker et al. 2011; Morris et al. 2011; Panis et al. 2011). Variation in mortality rates among subgroups of CRC patients is especially pronounced in the first month after surgery (Moller et al. 2011). Studies examining variation in 30-day mortality in CRC have predominantly focused on hospital volume (Meyerhardt et al. 2003; Schrag et al. 2003; Iversen et al. 2007). Less attention has been paid to other potentially important sociodemographic factors, including residential location and information regarding hospital characteristics. Area characteristics of the patient's residential location could influence 30-day mortality through various mechanisms such as health-system factors, lifestyle factors, tumor biology, and comorbidity (Polite, Dignam, and Olopade 2006). Identification of other factors contributing to variation in 30-day mortality rates following CRC surgery may help to identify best practices to reduce postoperative mortality among elderly patients at the hospital level. Interventions may also focus on geographic areas since they are out of the control of hospitals and may help explain apparent differences in outcomes among hospitals. Therefore, we sought to describe variation in 30-day mortality across hospitals and geographic areas while adjusting for the known effects of patient sociodemographic, clinical, tumor, treatment, and hospital characteristics associated with an increased likelihood of 30-day all-cause mortality after CRC surgery. We hypothesized that these variables would help explain the hospital and geographic variability in 30-day all-cause mortality in CRC patients.
Materials and Methods
Data Sources
We obtained data from an existing linkage of the 2000–2005 National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program data with 1999–2005 Medicare claims files from CMS. Linked SEER-Medicare data provide a rich source of information on Medicare patients included in SEER, a nationally representative collection of population-based cancer registries (Warren et al. 2002). Ninety-four percent of cancer patients reported to SEER aged 65 years or older has been successfully linked with Medicare data (Warren et al. 2002). This study included data from the following SEER registries (San Francisco, San Jose, Los Angeles, great California area, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, rural Georgia, Kentucky, Louisiana, and New Jersey) representing approximately 14 percent of the U.S. population. The study was reviewed by the Institutional Review Board at Washington University and determined to be exempt from oversight.
Study Population
We conducted a retrospective cohort study with all-cause mortality within 30 days following surgery (yes, no) as the outcome of interest. Deaths occurring prior to and after discharge were included. We selected all patients 66 years of age or older with a first primary in situ or invasive colon or rectal cancer diagnosis from 2000 through 2005 and who had both Medicare Part A and Part B coverage and were in fee-for-service Medicare during this period. We included only patients at least 66 years of age to allow for 1 year of complete claims data prior to diagnosis to determine comorbidity. We excluded patients who had only autopsy or death certificate records of their cancer diagnosis in SEER or who were members of a Health Maintenance Organization because these patients lack complete claims data or follow-up data on vital status. A total of 89,301 CRC patients aged 66 or older were identified and 41,842 were excluded for various reasons (i.e., having HMO coverage n = 22,561, not having Medicare A & B n = 5,535, death certificate or autopsy only n = 1,396, no surgery identified n = 14,119, missing data on covariates n = 2,500) leaving 47,459 CRC patients available for analysis. Patients may have been excluded for more than one reason.
Study Variables
We used Medicare data to determine vital status within 30 days of surgery, because SEER data provide only month and year of death. Treatments for CRC included definitive surgery, chemotherapy, or radiotherapy. Treatments were measured by searching inpatient, outpatient, and carrier claims using previously identified Healthcare Common Procedure Coding System and/or International Classification of Diseases (version 9) codes (Warren et al. 2008). The date on which the most extensive surgery was performed was used as the date of definitive surgery, hereafter referred to only as “surgery.”
Covariates
We selected variables associated with hospital and census-tract variation and variables associated with 30-day mortality based on previous studies and a conceptual model that includes various types of characteristics affecting quality of care (Donabedian 1988; Hodgson, Fuchs, and Ayanian 2001; Polite, Dignam, and Olopade 2006). This multilevel conceptual model consists of individual-, hospital-, provider-, and area-level characteristics predicting 30-day mortality among CRC patients.
Patient sociodemographic characteristics included sex, race/ethnicity, comorbidity, eligibility for both Medicare and Medicaid (dual eligibility), age group, and year of diagnosis. To measure comorbidity, we searched inpatient or carrier claims for multiple chronic conditions occurring 1–12 months prior to diagnosis using the Klabunde adaptation of the Charlson comorbidity index (National Cancer Institute 2011). It uses a minimum of two claims 30 days apart to validate the comorbidity occurrence. We further classified comorbidity as none, one, or two or more. We limited the patients in our analysis to those aged 66 years and older to ensure all patients had full year look-back period. Dual eligibility was defined as eligibility for Medicaid coverage for at least 1 month during the year before diagnosis.
Area-level characteristics included percentage of the population living in poverty in the patient's census tract. Each patient's residential address at time of diagnosis was matched to its respective census tract as defined for the 2000 U.S. Census.
Hospital characteristics, where the patient's surgery took place, included number of hospital beds, number of CRC surgeries performed, and whether it was a teaching hospital or not, which were obtained from the Healthcare Cost Report and the Provider of Service files from CMS. The hospital's surgery volume was calculated using the number of CRC surgeries performed during the study period.
Tumor characteristics included American Joint Commission on Cancer (AJCC) stage, tumor grade, tumor location, and histology. The location of the tumor was classified as proximal colon (cecum, ascending); transverse colon (hepatic flexure, transverse colon, splenic flexure); distal colon (descending and sigmoid colon); or rectosigmoid junction or rectum.
Treatment characteristics within 30 days of the index surgery included emergency surgery and type of surgery (Warren et al. 2008).
Statistical Analysis
Univariate associations between 30-day mortality and each covariate were tested using Chi-square tests. We also examined intercorrelations among the predictor variables. A multi-level, cross-classified logistic model for a discrete response variable was used to describe the variation across hospitals and census tracts to account for nesting of CRC patients within hospitals and within census tracts of their residence (Snijders and Bosker 1999). This nesting structure allows patients from the same census tract to be treated at different hospitals and allows for the fact that different hospitals could treat patients from the same tracts. In essence, this structure does not impose any relationship between hospitals and tracts. Adjusted odds ratios and their 95 percent confidence intervals were calculated based on all variables that were entered into the multivariable model. Model fit was based on the Deviance Information Criterion (DIC), with lower values indicating better fit.
The median odds ratio (MOR) and interquartile odds ratio (IOR) were calculated based on this multi-level model to facilitate interpretation of the variability among census tracts and hospitals on a scale that is directly comparable with the odds ratios that are used for the other variables in the study (Merlo et al. 2006). The MOR and IOR are based on the random effects variance component (V) from the logistic regression model: 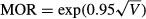 and
and 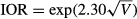 . The MOR can be interpreted as the median value of the ratio of predicted odds of 30-day mortality for two patients randomly selected from different census tracts (or hospitals) but with equivalent covariates. If the MOR is equal to 1, it indicates no variation in 30-day mortality across census tracts or hospitals. The IOR reflects the difference in likelihood of 30-day mortality between 25 percent of all patients from census tracts or hospitals with the highest risk in 30-day mortality and 25 percent of all patients from census tracts or hospitals with the lowest risk in 30-day mortality. We obtained the standard errors of the census-tract-level and hospital-level variances to compute the 95 percent credible interval for the MORs and IORs using Markov Chain Monte Carlo methods in the Bayesian multilevel models.
. The MOR can be interpreted as the median value of the ratio of predicted odds of 30-day mortality for two patients randomly selected from different census tracts (or hospitals) but with equivalent covariates. If the MOR is equal to 1, it indicates no variation in 30-day mortality across census tracts or hospitals. The IOR reflects the difference in likelihood of 30-day mortality between 25 percent of all patients from census tracts or hospitals with the highest risk in 30-day mortality and 25 percent of all patients from census tracts or hospitals with the lowest risk in 30-day mortality. We obtained the standard errors of the census-tract-level and hospital-level variances to compute the 95 percent credible interval for the MORs and IORs using Markov Chain Monte Carlo methods in the Bayesian multilevel models.
In addition, we calculated census-tract-level predicted values for 30-day mortality to describe the geographic and hospital variability. These predicted values for 30-day mortality were computed based on the multivariable model by averaging the patient-level predicted probabilities for all patients who resided in that census tract. Only census tracts with at least 20 patients were used in order to increase precision of the estimates. Similarly, we calculated hospital-level predicted values for 30-day mortality to describe the variability by randomly selecting 10 hospitals from hospitals with the following patient volume: 20–49, 50–99, 100 or more patients. Hospital-level predicted values for 30-day mortality were computed based on the multivariable model by averaging the patient-level predicted probabilities for all patients who were treated at that hospital.
Data were managed and analyzed in SAS (version 9.1, SAS Institute Inc., Cary, NC). The Bayesian analysis for the cross-classified model was performed using WinBUGS (version 1.4.3). After 5,000 burn-in iterations, 5,000 additional iterations were kept for parameter estimates.
Results
Overall, 47,459 patients underwent CRC surgery from 2000 through 2005 and were included in the analysis. Patients resided in 13,182 different census tracts and were treated at 1,447 different hospitals. Of these 47,459 patients, 3,126 (6.6 percent) died within 30 days following surgery; 63.4 percent died from any cause during their hospitalization for CRC surgery and 59.7 percent died of CRC. Table 1 shows that most CRC patients were white (85.8 percent), not participating in Medicaid (83.5 percent), younger than 85 (81.4 percent), and living in census tracts where <10 percent of the population lived below the federal poverty rate (57.9 percent). Little difference existed in the mean census-tract poverty rate between the SEER study areas (13.4 percent) and the overall findings reported for the United States (13.5 percent). Table 1 summarizes the characteristics of CRC patients who died within 30 days after surgery.
Table 1.
Sociodemographic, Hospital, Clinical, Tumor, and Treatment Variables Associated with Thirty-Day Mortality in Colorectal Cancer Patients Following Surgery, 2000–2005 (Unadjusted)
| No. CRC Patients | No. Deaths | 30-day Mortality, % (95% CI) | |
|---|---|---|---|
| Sociodemographic characteristic | |||
| Sex* | |||
| Male | 20,350 | 1,405 | 6.9 (6.6–7.3) |
| Female | 27,101 | 1,721 | 6.4 (6.1–6.6) |
| Race*† | |||
| White | 40,718 | 2,702 | 6.6 (6.4–6.9) |
| African American | 3,644 | 270 | 7.4 (6.6–8.3) |
| Hispanic | 725 | 42 | 5.8 (4.1–7.5) |
| Other | 796 | 35 | 4.4 (3.0–5.8) |
| Asian | 1,398 | 62 | 4.4 (3.4–5.5) |
| Comorbidity* | |||
| 0 | 20,500 | 1,079 | 5.3 (5.0–5.6) |
| 1 | 13,841 | 890 | 6.4 (6.0–6.8) |
| 2+ | 13,110 | 1,157 | 8.8 (8.3–9.3) |
| Medicaid (dual eligibility)* | |||
| Yes | 7,809 | 630 | 8.1 (7.5–8.7) |
| No | 39,642 | 2,496 | 6.3 (6.1–6.5) |
| Age group* | |||
| 66–74 | 17,170 | 684 | 4.0 (3.7–4.3) |
| 75–84 | 21,462 | 1,321 | 6.2 (5.8–6.5) |
| 85+ | 8,819 | 1,121 | 12.7 (12.0–13.4) |
| Year of diagnosis | |||
| 2000 | 8,022 | 585 | 7.3 (6.7–7.9) |
| 2001 | 7,972 | 531 | 6.7 (6.1–7.2) |
| 2002 | 8,035 | 493 | 6.1 (5.6–6.7) |
| 2003 | 8,169 | 548 | 6.7 (6.2–7.3) |
| 2004 | 7,899 | 500 | 6.3 (5.8–6.9) |
| 2005 | 7,354 | 469 | 6.4 (5.8–6.9) |
| Area-level characteristics | |||
| Poverty rate* | |||
| <10% | 27,478 | 1,620 | 5.9 (5.6–6.2) |
| 10–19% | 12,048 | 874 | 7.3 (6.8–7.7) |
| 20+% | 7,677 | 615 | 8.0 (7.4–8.6) |
| Hospital characteristics | |||
| Hospital volume (beds)* | |||
| 1–199 | 12,003 | 890 | 7.4 (7.0–7.9) |
| 200–349 | 13,471 | 905 | 6.7 (6.3–7.1) |
| 350–499 | 11,199 | 711 | 6.4 (5.9–6.8) |
| 500+ | 10,763 | 619 | 5.8 (5.3–6.2) |
| Surgeon case load* | |||
| <21 | 11,070 | 830 | 7.5 (7.0–8.0) |
| 21–38 | 10,352 | 702 | 6.8 (6.3–7.3) |
| 39–69 | 10,126 | 628 | 6.2 (5.7–6.7) |
| 70+ | 10,312 | 494 | 4.8 (4.4–5.2) |
| Unknown | 5,581 | 472 | 8.5 (7.7–9.2) |
| Teaching hospital* | |||
| Yes | 23,696 | 1,426 | 6.0 (5.7–6.3) |
| No | 16,706 | 1,185 | 7.1 (6.7–7.5) |
| Unknown | 7,049 | 515 | 7.3 (6.7–7.9) |
| Tumor characteristics | |||
| AJCC stage* | |||
| 0/I | 10,911 | 339 | 3.1 (2.8–3.4) |
| II | 14,929 | 870 | 5.8 (5.5–6.2) |
| III | 12,542 | 662 | 5.3 (4.9–5.7) |
| IV | 6,468 | 976 | 15.1 (14.2–16.0) |
| Unknown | 2,601 | 279 | 10.7 (9.5–11.9) |
| Tumor grade/differentiation* | |||
| Well | 3,916 | 200 | 5.1 (4.4–5.8) |
| Moderate | 30,397 | 1,680 | 5.5 (5.3–5.8) |
| Poor | 9,307 | 809 | 8.7 (8.1–9.3) |
| Undifferentiated | 507 | 59 | 11.6 (8.9–14.4) |
| Unknown | 3,324 | 378 | 11.4 (10.3–12.5) |
| Tumor location* | |||
| Proximal colon | 18,334 | 1,151 | 6.3 (5.9–6.6) |
| Transverse colon | 7,039 | 563 | 8.0 (7.4–8.6) |
| Distal colon | 12,734 | 929 | 7.3 (6.8–7.8) |
| Rectal | 9,344 | 483 | 5.2 (4.7–5.6) |
| Tumor histology* | |||
| Mucinous adenocarcinoma | 40,434 | 2,525 | 6.2 (6.0–6.5) |
| Other adenocarcinoma | 5,967 | 418 | 7.0 (6.4–7.7) |
| Nonadenocarcinoma | 1,050 | 183 | 17.4 (15.1–19.7) |
| Treatment characteristics | |||
| Emergency surgery* | |||
| Yes | 11,845 | 1,518 | 12.8 (12.2–13.4) |
| No | 35,606 | 1,608 | 4.5 (4.3–4.7) |
| Type of surgery* | |||
| Local tumor excision | 962 | 50 | 5.2 (3.8–6.6) |
| Partial colectomy | 18,690 | 1,049 | 5.6 (5.3–5.9) |
| Subtotal colectomy or hemicolectomy | 22,603 | 1,363 | 6.0 (5.7–6.3) |
| Total (procto)colectomy | 1,782 | 105 | 5.9 (4.8–7.0) |
| Colectomy NOS | 955 | 91 | 9.5 (7.7–11.4) |
| Other surgery | 187 | 16 | 8.6 (4.6–12.6) |
| Unknown surgery | 2,227 | 452 | 19.9 (18.3–21.5) |
p < .01; CI, confidence interval.
Native American not included for confidentiality reasons.
Statistically significant variability in 30-day mortality was present across census tracts and hospitals in unadjusted analysis (Table 2). For patients with CRC, variability across census-tracts and hospitals was similar in magnitude; the MOR was about 1.4, which corresponds to the median value of the relative odds of 30-day mortality between two randomly chosen census tracts for CRC patients. Variability in 30-day mortality was similar across census tracts and hospitals in unadjusted analysis.
Table 2.
Unadjusted Variability in Thirty-Day Mortality across Patient Census Tracts and Hospitals among Colorectal Cancer Patients Following Surgery
| Census-Tract Variability |
Hospital Variability |
|||
|---|---|---|---|---|
| Parameter | 95% CI | Parameter | 95% CI | |
| CRC | ||||
| Variance | 0.14 | 0.08–0.21 | 0.13 | 0.09–0.17 |
| MOR | 1.44 | 1.32–1.55 | 1.41 | 1.34–1.49 |
| IOR | 2.40 | 1.94–2.85 | 2.31 | 2.02–2.59 |
| DIC | 22,734 | |||
CI, credible interval; IOR, interquartile odds ratio; MOR, median odds ratio.
In multivariable analysis (Table 3), several characteristics were independently associated with higher odds of 30-day mortality, including sociodemographics (being male, having at least one comorbid condition, age ≥75), area-level characteristics (poverty rate >10 percent), hospital characteristics (nonteaching hospital), tumor characteristics (advanced stage at diagnosis, poor or undifferentiated tumors, colon vs rectal cancer), and treatment characteristics (emergency surgery). Characteristics associated with lower odds of death included being other race, diagnosis during 2005, and higher surgeon case load. Characteristics not associated with odds of death included being African American and Medicaid enrollment. Variability across census tracts and hospitals remained evident in the multivariable model and was similar to the unadjusted model based on the estimates of the variance and IOR, suggesting that none of the measured variables were able to account for the observed variation across census tracts or hospitals (Table 4), although the fit of the multivariable model was substantially better based on the reduction in the DIC. Thus, while the fixed effect of area-level poverty was associated with 30-day mortality, this variable was unable to explain the geographic variation across census tracts or hospitals. Figure1 shows that the observed 30-day mortality rate for 30 census tracts with at least 20 CRC patients ranged from 0.0 percent to 15.0 percent (predicted rate range: 3.5–10.9 percent). For census tracts 1 through 16, the observed mortality rate was lower than predicted, suggesting that CRC patients in these census tracts fared better than expected adjusting for all variables in the multivariable model. In contrast, the observed mortality rate for CRC patients in census tract 28 and 30 was higher than predicted, suggesting that patients in these census tracts fared worse than expected.
Table 3.
Adjusted Odds Ratio (95% Confidence Interval) for Characteristics Associated with Thirty-Day Mortality Following Colorectal Cancer Surgery, 2000–2005
| Adjusted OR (95% CI) | |
|---|---|
| Sociodemographic characteristics | |
| Male sex (vs. female) | 1.31 (1.21–1.42) |
| Race (vs. white) | |
| African American | 0.89 (0.77–1.04) |
| Other | 0.67 (0.56–0.82) |
| Comorbidity (vs. 0) | |
| 1 | 1.17 (1.06–1.29) |
| 2+ | 1.60 (1.45–1.75) |
| Medicaid (yes vs. no) | 1.06 (0.95–1.18) |
| Age group (vs. age 66–74) | |
| 75–84 | 1.65 (1.50–1.82) |
| 85+ | 3.53 (3.16–3.94) |
| Year at diagnosis (vs. 2000) | |
| 2001 | 0.95 (0.83–1.09) |
| 2002 | 0.87 (0.76–1.00) |
| 2003 | 0.97 (0.85–1.11) |
| 2004 | 0.88 (0.77–1.01) |
| 2005 | 0.86 (0.75–0.99) |
| Area-level characteristics | |
| Poverty rate (vs. <10%) | |
| 10–19% | 1.23 (1.12–1.36) |
| 20+% | 1.30 (1.15–1.47) |
| Hospital characteristics | |
| Hospital volume (beds) versus 1–199 beds | |
| 200–349 | 0.91 (0.81–1.04) |
| 350–499 | 0.94 (0.81–1.09) |
| 500+ | 0.86 (0.72–1.02) |
| Surgeon case load (vs. 70+) | |
| <21 | 1.29 (1.13–1.47) |
| 21–38 | 1.23 (1.07–1.41) |
| 39–69 | 1.14 (0.99–1.31) |
| Unknown | 1.19 (1.02–1.39) |
| Teaching hospital (vs. Yes) | |
| No | 1.15 (1.02–1.30) |
| Unknown | 1.22 (1.05–1.42) |
| Tumor characteristics | |
| AJCC stage (vs. 0/I) | |
| II | 1.60 (1.40–1.83) |
| III | 1.46 (1.27–1.69) |
| IV | 4.12 (3.57–4.75) |
| Unknown | 1.65 (1.36–2.01) |
| Tumor grade (vs. well differentiated) | |
| Moderate differentiation | 0.96 (0.82–1.12) |
| Poor differentiation | 1.35 (1.14–1.60) |
| Undifferentiated | 1.75 (1.25–2.45) |
| Unknown | 1.26 (1.03–1.55) |
| Tumor location (vs. rectal) | |
| Proximal colon | 1.41 (1.25–1.60) |
| Transverse colon | 1.57 (1.36–1.82) |
| Distal colon | 1.28 (1.11–1.46) |
| Tumor histology (vs. mucinous adenocarcinoma) | |
| Nonadenocarcinoma/unknown | 1.63 (1.31–2.01) |
| Other CRC | 1.00 (0.88–1.12) |
| Treatment characteristics | |
| Emergency surgery (vs. No) | 2.35 (2.17–2.55) |
Table 4.
Adjusted Variability in Thirty-Day Mortality across Patient Census Tracts and Hospitals among Colorectal Cancer Patients Following Surgery
| Census-Tract Variability |
Hospital Variability |
|||
|---|---|---|---|---|
| Parameter | 95% CI | Parameter | 95% CI | |
| CRC | ||||
| Variance | 0.18 | 0.11–0.25 | 0.12 | 0.08–0.15 |
| MOR | 1.50 | 1.38–1.62 | 1.38 | 1.31–1.45 |
| IOR | 2.68 | 2.17–3.19 | 2.18 | 1.92–2.44 |
| DIC | 20,173 | |||
Adjusted for sociodemographic, hospital, tumor, and treatment characteristics. CI, credible interval; IOR, interquartile odds ratio; MOR, median odds ratio.
Figure 1.
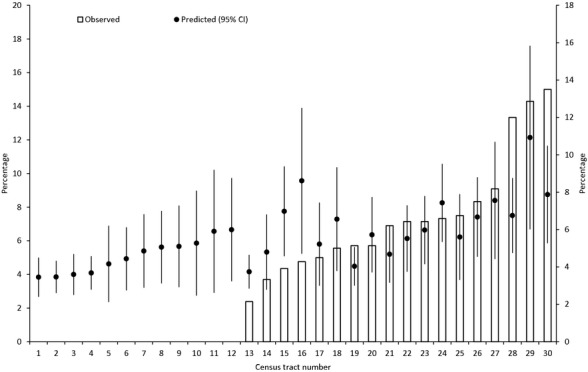
Observed and Predicted Variability in Thirty-Day Mortality Based on SEER-Medicare Data from 2000 to 2005 across the Thirty Census Tracts with at Least Twenty CRC Patients Based on the Multivariable Model Note. Predicted 30-day mortality, based on the multilevel logistic regression model adjusted for sociodemographic, census-tract, hospital, clinical, tumor, and treatment characteristics, are plotted as circles above the encrypted census tract identifiers along the horizontal axis. Error bars indicate 95 percent confidence intervals. Census-tract-level predicted values were computed by averaging the patient-level predicted probabilities for all patients who resided in that census tract. Census tracts 1 through 12 had zero observed deaths; thus, there is no bar associated with these census tracts.
The observed 30-day mortality rate for 30 hospitals with at least 20 CRC patients ranged from 0.0 percent to 12.9 percent (Figure2). For hospitals 1 through 10, the observed mortality rate was lower than the predicted rate based on the multivariable model (range: 3.8–9.3 percent), suggesting that CRC patients in these hospitals fared better than expected adjusting for all variables in the multivariable model. In contrast, the observed mortality rate for CRC patients in hospitals 20, 22, 23, and 27 through 30 was higher than predicted, suggesting that patients in these hospitals fared worse than expected.
Figure 2.
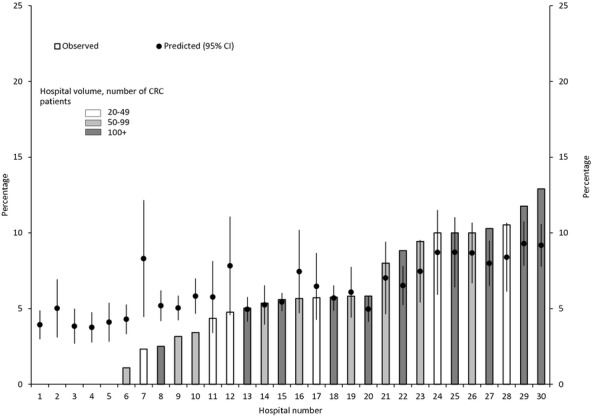
Observed and Predicted Variability in Thirty-Day Mortality Based on SEER-Medicare Data from 2000 to 2005 across a Random Sample of Thirty Hospitals with at Least Twenty CRC Patients Based on the Multilevel Model Note. Predicted 30-day mortality, based on the multilevel logistic regression model adjusted for sociodemographic, hospital, clinical, tumor, and treatment characteristics, are plotted as circles above the encrypted hospital identifiers along the horizontal axis. Error bars indicate 95 percent confidence intervals. Hospital-level predicted values were computed by averaging the patient-level predicted probabilities for all patients who were treated at that hospital. Hospitals 1 through 5 had zero observed deaths; thus, there is no bar associated with any of these hospitals.
Discussion
Our findings demonstrate an overall 30-day mortality rate of 6.6 percent that is slightly higher than some studies (Schrag et al. 2000; Dekker et al. 2011), lower than others (Tekkis et al. 2003), and similar to other studies (Morris et al. 2011; Panis et al. 2011). Our findings demonstrate that the location of patient residence plays an important role in predicting 30-day mortality following CRC surgery. We observed that the variability across patient census tracts was at least as large as across hospitals and that census-tract-level poverty rate was associated with higher risk of death independent of hospital and other characteristics included in the multivariable model. Thus, regardless of the facility in which subjects were treated or the experience of the surgeon, and independent tumor and patient characteristics, CRC patients had an increased risk of death if they lived in areas with worse economic conditions. While some studies have shown the adverse effects of living in economically deprived areas on CRC survival (Hole and McArdle 2002), ours is the first population-based study in the United States that showed this to be the case for 30-day mortality after CRC surgery. This finding suggests that where patients live affects their short-term mortality risk. CRC patients in some census tracts had higher mortality than expected while other census tracts had lower than expected 30-day mortality. Because none of the variables, including census-tract poverty, included in the model was able to entirely explain the geographic variability in the independent effect of census-tract poverty rate on 30-day mortality, intervening upon variables included in the model would not be expected to reduce the geographic variability in 30-day mortality.
It is not known why living in an economically deprived area would increase the 30-day mortality risk even after controlling for hospital and clinical factors. Identifying the causes of this geographic variability and finding ways to minimize this risk could increase our ability to improve outcomes following CRC surgery. One potential mechanism could be that patients living in economically deprived areas are more likely to experience environmental stress resulting in systemic inflammation, to be obese, to have low levels of vitamin D, or to smoke, all of which have been shown to affect long-term survival following CRC diagnosis (McMillan, Canna, and McArdle 2003; Dignam et al. 2006; Freedman et al. 2007; Roxburgh et al. 2011). Unfortunately, information regarding these exposures was not available in the SEER-Medicare data.
An alternative mechanism could be that those living in economically deprived areas have less access to social or medical services such as nearby primary care facilities, timely transportation to necessary follow-up appointments or for urgent medical conditions, or basic home-health or social services that could support immobile or low-functioning patients. Previous studies also have shown that patients with lower preoperative physical status measured using the American Society of Anesthesiologists (ASA) Score (Owens, Felts, and Spitznagel 1978) had increased 30-day mortality risk (Longo et al. 2000; Cohen et al. 2009; Al-Refaie et al. 2011), but ASA scores were unavailable in the SEER-Medicare data. However, we included comorbidity in our models, which may relate to 30-day mortality in a similar fashion, because comorbidity and physical status are correlated (Smith et al. 2008; Deshpande et al. 2011). Thus, while a unifying explanation is yet to emerge, our findings emphasize the importance of identifying the observed variability in 30-day mortality across geographic areas in order to intervene upon modifiable risk factors that can optimize patient outcomes.
Our findings also show that 30-day mortality varied across hospitals, regardless of patient sociodemographic, area-level characteristics, clinical factors, tumor characteristics, type of treatment received, and hospital characteristics. CRC patients in some hospitals fared worse than expected while others fared better. Similar to other studies (Meyerhardt et al. 2003; Billingsley et al. 2007; Iversen et al. 2007), we observed that patients treated at hospitals with higher patient volumes or at teaching hospitals had lower risk of death. While patients treated at these hospitals had decreased mortality, neither the hospital characteristics nor the surgeon case load explained the variation as described by the MOR. Billingsley (Billingsley et al. 2007) suggested the availability of sophisticated clinical services that facilitate the timely management of medical or surgical complications explained why high-volume hospitals have better outcomes following CRC surgery. Previous studies have suggested that “failure to rescue,” that is, mortality among patients with major complications, may be an important mechanism underlying hospital mortality associated with surgery (Silber et al. 1992; Ghaferi and Dimick 2012) and may help explain remaining variability among hospitals. Multilevel observational studies, such as ours, may be used to monitor such variation across levels such as hospitals and geographic regions. Such methods may serve as important future methods for quality measurement of cancer care.
Our results should be interpreted with the understanding that our sample included only fee-for-service Medicare-insured patients, limiting generalizability to younger patients or to uninsured or privately insured patients. Because hospital size and location are closely related, we were unable to ascertain the independent effect of urban–rural hospital location. Strengths of our study include integrating a large number of patient, tumor, clinical, hospital, and treatment factors into one analytic model that simultaneously estimated both hospital and geographic variability using a multilevel cross-classified model. Additionally, unlike previous studies that only looked at the fixed effects of hospital characteristics, our study used a random term (median odds ratio) to describe the variability across hospitals. This approach is starting to gain popularity because, unlike fixed effects, it can quantify the extent of the variability in health outcomes across different “levels” (e.g., hospitals, surgeons, or neighborhoods; Lian et al. 2011; McCahill et al. 2012). However, our analysis does not take into account the spatial relationship between adjacent census tracts. Future research may use such methods to identify where 30-day mortality was concentrated spatially using geographically weighted regression methods.
In conclusion, census-tract and hospital variation was observed in 30-day mortality following CRC surgery in patients 66 years of age and older that was not explained by patient sociodemographic, clinical, tumor, treatment, or hospital characteristics. Risk of 30-day mortality is driven by these individual-level characteristics and by both hospital-level and area-level social, economic, and organizational factors that distinguish different hospitals and geographic areas.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study was supported by grants from the National Cancer Institute at the National Institutes of Health (grant number CA112159); and the Health Behavior, Communication and Outreach Core; the Core is supported in part by the National Cancer Institute Cancer Center Support Grant (grant number P30 CA91842) to the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, Missouri. NOD was supported in part through grants HL-38180, DK-56260, and Digestive Disease Research Core Center DK-52574.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
References
- Al-Refaie WB, Parsons HM, Habermann EB, Kwaan M, Spencer MP, Henderson WG. Rothenberger DA. “Operative Outcomes Beyond 30-day Mortality: Colorectal Cancer Surgery in Oldest Old”. Annals of Surgery. 2011;253(5):947–52. doi: 10.1097/SLA.0b013e318216f56e. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts & Figures, 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- Billingsley KG, Morris AM, Dominitz JA, Matthews B, Dobie S, Barlow W, Wright GE. Baldwin LM. “Surgeon and Hospital Characteristics as Predictors of Major Adverse Outcomes Following Colon Cancer Surgery: Understanding the Volume-Outcome Relationship”. Archives of Surgery. 2007;142(1):23–31. doi: 10.1001/archsurg.142.1.23. discussion 32. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. “Medicare Program; Hospital Inpatient Value-Based Purchasing Program. Final Rule”. Federal Register. 2011;76(88):26490–547. [PubMed] [Google Scholar]
- Cohen ME, Bilimoria KY, Ko CY. Hall BL. “Development of an American College of Surgeons National Surgery Quality Improvement Program: Morbidity and Mortality Risk Calculator for Colorectal Surgery”. Journal of the American College of Surgeons. 2009;208(6):1009–16. doi: 10.1016/j.jamcollsurg.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Davila J, Rabeneck L, Berger D. El-Serag H. “Postoperative 30-day Mortality Following Surgical Resection for Colorectal Cancer in Veterans: Changes in the Right Direction”. Digestive Diseases and Sciences. 2005;50(9):1722–8. doi: 10.1007/s10620-005-2925-x. [DOI] [PubMed] [Google Scholar]
- Dekker J, van den Broek C, Bastiaannet E, van de Geest L, Tollenaar R. Liefers G. “Importance of the First Postoperative Year in the Prognosis of Elderly Colorectal Cancer Patients”. Annals of Surgical Oncology. 2011;18(6):1533–9. doi: 10.1245/s10434-011-1671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande AD, Sefko JA, Jeffe DB. Schootman M. “The Association between Chronic Disease Burden and Quality of Life among Breast Cancer Survivors in Missouri”. Breast Cancer Research. 2011;129(3):877–86. doi: 10.1007/s10549-011-1525-z. PMCID: PMC3250926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O'Connell MJ. Wolmark N. “Body Mass Index and Outcomes in Patients Who Receive Adjuvant Chemotherapy for Colon Cancer”. Journal of the National Cancer Institute. 2006;98(22):1647–54. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- Donabedian A. “The Quality of Care: How Can It Be Assessed?”. Journal of the American Medical Association. 1988;260(12):1743–8. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- Fazio VW, Tekkis PP, Remzi F. Lavery IC. “Assessment of Operative Risk in Colorectal Cancer Surgery: The Cleveland Clinic Foundation Colorectal Cancer Model”. Diseases of the Colon and Rectum. 2004;47(12):2015–24. doi: 10.1007/s10350-004-0704-y. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Looker AC, Chang S-C. Graubard BI. “Prospective Study of Serum Vitamin D and Cancer Mortality in the United States”. Journal of the National Cancer Institute. 2007;99(21):1594–602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- Ghaferi AA. Dimick JB. “Variation in Mortality after High-Risk Cancer Surgery: Failure to Rescue”. Surgical Oncology Clinics of North America. 2012;21(3):389–95. doi: 10.1016/j.soc.2012.03.006. vii. [DOI] [PubMed] [Google Scholar]
- Hodgson DC, Fuchs CS. Ayanian JZ. “Impact of Patient and Provider Characteristics on the Treatment and Outcomes of Colorectal Cancer”. Journal of the National Cancer Institute. 2001;93(7):501–15. doi: 10.1093/jnci/93.7.501. [DOI] [PubMed] [Google Scholar]
- Hole D. McArdle C. “Impact of Socioeconomic Deprivation on Outcome after Surgery for Colorectal Cancer”. British Journal of Surgery. 2002;89(5):586–90. doi: 10.1046/j.1365-2168.2002.02073.x. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Ensuring Quality Cancer Care. Washington, DC: National Academy Press; 1999. [Google Scholar]
- Iversen LH, Harling H, Laurberg S. Wille-Jorgensen P. “Influence of Caseload and Surgical Speciality on Outcome Following Surgery for Colorectal Cancer: A Review of Evidence. Part 1: Short-Term Outcome”. Colorectal Disease. 2007;9(1):28–37. doi: 10.1111/j.1463-1318.2006.01100.x. [DOI] [PubMed] [Google Scholar]
- Lian M, Schootman M, Doubeni CA, Park Y, Major JM, Torres Stone RA, Laiyemo AO, Hollenbeck AR, Graubard BI. Schatzkin A. “Geographic Variation in Colorectal Cancer Survival and the Role of Small-Area Socioeconomic Deprivation: A Multilevel Survival Analysis of the NIH-AARP Diet and Health Study Cohort”. American Journal of Epidemiology. 2011;174(7):828–38. doi: 10.1093/aje/kwr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo W, Virgo K, Johnson F, Oprian C, Vernava A, Wade T, Phelan M, Henderson W, Daley J. Khuri S. “Risk Factors for Morbidity and Mortality after Colectomy for Colon Cancer”. Diseases of the Colon & Rectum. 2000;43(1):83–91. doi: 10.1007/BF02237249. [DOI] [PubMed] [Google Scholar]
- McCahill LE, Single RM, Aiello Bowles EJ, Feigelson HS, James TA, Barney T, Engel JM. Onitilo AA. “Variability in Reexcision Following Breast Conservation Surgery”. The Journal of the American Medical Association. 2012;307(5):467–75. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- McMillan DC, Canna K. McArdle CS. “Systemic Inflammatory Response Predicts Survival Following Curative Resection of Colorectal Cancer”. British Journal of Surgery. 2003;90(2):215–9. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Rastam L. Larsen K. “A Brief Conceptual Tutorial of Multilevel Analysis in Social Epidemiology: Using Measures of Clustering in Multilevel Logistic Regression to Investigate Contextual Phenomena”. Journal of Epidemiology and Community Health. 2006;60(4):290–7. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt JA, Catalano PJ, Schrag D, Ayanian JZ, Haller DG, Mayer RJ, Macdonald JS, Benson AB., 3rd Fuchs CS. “Association of Hospital Procedure Volume and Outcomes in Patients with Colon Cancer at High Risk for Recurrence”. Annals of Internal Medicine. 2003;139(8):649–57. doi: 10.7326/0003-4819-139-8-200310210-00008. [DOI] [PubMed] [Google Scholar]
- Moller H, Sandin F, Robinson D, Bray F, Klint Ãs, Linklater KM, Lambert PC, PÃ¥hlman L, Holmberg L. Morris E. “Colorectal Cancer Survival in Socioeconomic Groups in England: Variation is Mainly in the Short Term after Diagnosis”. European Journal of Cancer. 2011;48(1):46–53. doi: 10.1016/j.ejca.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Taylor EF, Thomas JD, Quirke P, Finan PJ, Coleman MP, Rachet B. Forman D. “Thirty-Day Postoperative Mortality after Colorectal Cancer Surgery in England”. Gut. 2011;60(6):806–13. doi: 10.1136/gut.2010.232181. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. 2011. “SEER-Medicare: Calculation of Comorbidity Weights” [accessed on July 17, 2011, 2013]. Available at http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
- Owens WD, Felts JA. Spitznagel EL., Jr “ASA Physical Status Classifications: A Study of Consistency of Ratings”. Anesthesiology. 1978;49(4):239–43. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- Panis Y, Maggiori L, Caranhac G, Bretagnol F. Vicaut E. “Mortality after Colorectal Cancer Surgery: A French Survey of More Than 84,000 Patients”. Annals of Surgery. 2011;254(5):738–43. doi: 10.1097/SLA.0b013e31823604ac. discussion 43–4. [DOI] [PubMed] [Google Scholar]
- Polite BN, Dignam JJ. Olopade OI. “Colorectal Cancer Model of Health Disparities: Understanding Mortality Differences in Minority Populations”. Journal of Clinical Oncology. 2006;24(14):2179–87. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- Roxburgh C, Platt J, Leitch E, Kinsella J, Horgan P. McMillan D. “Relationship between Preoperative Comorbidity, Systemic Inflammatory Response, and Survival in Patients Undergoing Curative Resection for Colorectal Cancer”. Annals of Surgical Oncology. 2011;18(4):997–1005. doi: 10.1245/s10434-010-1410-8. [DOI] [PubMed] [Google Scholar]
- Schrag D, Cramer LD, Bach PB, Cohen AM, Warren JL. Begg CB. “Influence of Hospital Procedure Volume on Outcomes Following Surgery for Colon Cancer”. Journal of the American Medical Association. 2000;284(23):3028–35. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- Schrag D, Panageas KS, Riedel E, Hsieh L, Bach PB, Guillem JG. Begg CB. “Surgeon Volume Compared to Hospital Volume as a Predictor of Outcome Following Primary Colon Cancer Resection”. Journal of Surgical Oncology. 2003;83(2):68–78. doi: 10.1002/jso.10244. [DOI] [PubMed] [Google Scholar]
- Silber JH, Williams SV, Krakauer H. Schwartz JS. “Hospital and Patient Characteristics Associated with Death after Surgery: A Study of Adverse Occurrence and Failure to Rescue”. Medical Care. 1992;30(7):615–29. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- Smith AW, Reeve BB, Bellizzi KM, Harlan LC, Klabunde CN, Amsellem M, Bierman AS. Hays RD. “Cancer, Comorbidities, and Health-Related Quality of Life of Older Adults”. Health Care Financing Review. 2008;29(4):41–56. [PMC free article] [PubMed] [Google Scholar]
- Snijders TAB. Bosker RJ. Multilevel Analysis. An Introduction to Basic and Advanced Multilevel Modeling. London: Sage Publications; 1999. [Google Scholar]
- Tanenbaum SJ. “Reducing Variation in Health Care: The Rhetorical Politics of a Policy Idea”. Journal of Health Politics, Policy and Law. 2013;38(1):5–26. doi: 10.1215/03616878-1898774. [DOI] [PubMed] [Google Scholar]
- Tekkis PP, Poloniecki JD, Thompson MR. Stamatakis JD. “Operative Mortality in Colorectal Cancer: Prospective National Study”. British Medical Journal. 2003;327(7425):1196–201. doi: 10.1136/bmj.327.7425.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JL, Klabunde CN, Schrag D, Bach PB. Riley GF. “Overview of the SEER-Medicare Data: Content, Research Applications, and Generalizability to the United States Elderly Population”. Medical Care. 2002;40(8 Suppl):3–18. doi: 10.1097/01.MLR.0000020942.47004.03. IV–. [DOI] [PubMed] [Google Scholar]
- Warren JL, Yabroff KR, Meekins A, Topor M, Lamont EB. Brown ML. “Evaluation of Trends in the Cost of Initial Cancer Treatment”. Journal of the National Cancer Institute. 2008;100(12):888–97. doi: 10.1093/jnci/djn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennberg J. “Understanding Geographic Variations in Health Care Delivery”. New England Journal of Medicine. 1999;340:52–3. doi: 10.1056/NEJM199901073400111. [DOI] [PubMed] [Google Scholar]
- Wennberg DE. Tracking Medicine: A Researcher's Quest to Understand Health Care. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.


