Abstract
Associations between CHRNA5-A3-B4 variants and smoking behaviors exist, however the association with smoking abstinence is less understood, particularly among African Americans. In 1295 African Americans enrolled in two clinical trials, we investigated the association between CHRNA5-A3-B4 and smoking abstinence. Rs2056527[A] was associated with lower abstinence with active pharmacotherapy (during-treatment: OR=0.42&P<0.001; end of treatment (EOT): OR=0.55&P=0.004), or with nicotine gum alone (during-treatment: OR=0.31&P<0.001; EOT: OR=0.51&P=0.02), but not significantly with bupropion, although similar directions and magnitudes were observed (during-treatment: OR=0.54&P=0.05; EOT: OR=0.59&P=0.08). Additionally, rs588765[T] was associated with abstinence with gum during treatment (OR=2.31&P<0.01). Rs16969968 occurred at a low frequency and was not consistently associated with abstinence. CHRNA5-A3-B4 variants were not associated with tobacco consumption and adjustments for smoking behaviors did not alter the associations with smoking abstinence. Together, our data suggest that in African Americans CHRNA5-A3-B4 variants are not associated with baseline smoking, but can influence smoking abstinence during active pharmacotherapy.
Keywords: African Americans, CHRNA5-A3-B4, Smoking cessation, Nicotine, Bupropion, Tobacco Consumption
Introduction
Tobacco smoking is the largest preventable cause of premature death in the United States. There are currently 45 millions smokers in the U.S, and only 3% of them are able to quit smoking each year (1). Twin studies have estimated that the heritability of smoking cessation is roughly 50%, suggesting that genetic factors play an important role in determining smoking cessation outcomes (2). A number of genes have been significantly associated with smoking cessation outcomes in Caucasians (3-5). However, relatively few studies have focused on African American smokers despite their comparatively higher risks of smoking-related morbidity and mortality (6).
Nicotine exerts its pharmacologic effects by acting on nicotinic cholinergic receptors in the brain. In Caucasians, multiple independent SNPs (single-nucleotide polymorphisms) in the CHRNA5-A3-B4 gene cluster, which encodes for the α5, α3 and β4 subunits of the nicotinic acetylcholine receptor (nAChR), have been associated with smoking quantity (7-13) and smoking cessation outcomes (14-18). These loci included rs16969968 and correlated SNPs (sometimes referred as ‘Bin A’ or ‘Locus 1′), rs588765 and correlated SNPs (sometimes referred as ‘Bin B’ or ‘Locus 3′), and rs578776 and correlated SNPs (sometimes referred as ‘Bin C’ or ‘Locus 2′) (11, 19). However, it is not clear whether the influence of CHRNA5-A3-B4 gene variants on smoking cessation is a general effect on smoking cessation (i.e. would alter cessation in placebo treatment), is independent of the specific type of pharmacological treatment (ie would alters all active treatments) or alters cessation for specific pharmacological treatments (i.e nicotine patch). For example, one study suggested that the association between CHRNA5-A3-B4 variants (rs16969968 [Bin A] and rs680244 [Bin B]) and smoking cessation is primarily observed among smokers treated with placebo, and was not observed among those who received active pharmacological therapy (17). In contrast, another study reported significant associations between CHRNA5-A3-B4 variant (rs1051730 which is in high linkage disequilibrium with rs16969968) and smoking cessation outcomes in smokers who were receiving nicotine replacement therapy with little effect observed in the placebo arm (15). A recent study also observed a treatment by genotype interaction on smoking cessation outcomes (i.e. the genotype effects are in opposite directions for placebo vs. nicotine replacement therapy) (20).
In African Americans, the associations between CHRNA5-A3-B4 gene variants and smoking behaviors are not as well understood. While some studies reported positive associations between rs16969968 and smoking behaviors in African American smokers (11), other studies were not able to replicate this finding (21, 22). The low allele frequency of rs16969968 (0% to 8% in African Americans compared to 38% to 40% in Caucasians) could contribute to this discrepancy. However, a very large genome-wide meta-analysis in African American smokers (N=32,389) identified a significant association between rs2036527 within CHRNA5-A3-B4 and self-reported cigarettes per day (CPD) but no significant associations between rs16969968 or rs1051730 and CPD (21). We are not aware of any studies that have investigated the association between CHRNA5-A3-B4 gene variants and smoking cessation in African American smokers.
Here we used two placebo controlled clinical trials to compare the influence of CHRNA5-A3-B4 variants on smoking cessation outcomes in African Americans treated with placebo and active pharmacological treatments. We hypothesized that there would be a significant association between CHRNA5-A3-B4 gene variants and smoking cessation outcomes in the active pharmacological treatment arms as previously reported by Munafò and colleagues (15). Since rs16969968 occurs at a relatively low allele frequency in African Americans, we focused initially on rs2036527, which has previously been significantly associated with CPD and lung cancer risk in African American (21, 23); we then extended the investigation to rs16969968 (‘Bin A’), rs588765 (‘Bin B’) and rs578776 (‘Bin C’).
Results
Descriptive data of the participants
Among the 1295 participants (6, 24), 1143 DNA samples were extracted from blood and genotyped (609 in Study 1 and 534 in Study 2). All four of the genotyped SNPs were in Hardy-Weinberg equilibrium (all P>0.05, Supplementary Table S1). Rs2036527 was selected for its role in influencing smoking behaviors in African Americans (21) while Rs16969968 (‘Bin A’), rs588765 (‘Bin B’), and rs578776 (‘Bin C’) were selected to represent three different haplotype bins which were previously associated with smoking behaviors (11). Rs2036527 had a minor allele frequency of 22.3%, Rs16969968 had a minor allele frequency of 5.7%, Rs588765 had a minor allele frequency of 31.7%, and Rs578776 had a minor allele frequency of 47.9%. These four SNPs were in low linkage disequilibrium (all r2 =0.02-0.39) suggesting that they were independent of each other (Supplementary Table S1). None of the four genotyped SNPs were significantly associated with baseline demographics, CPD, % smoking mentholated cigarettes, baseline plasma cotinine levels, baseline total nicotine equivalents levels, levels of nicotine dependence or treatment group assignment (Table 1 & Supplementary Table S2).
Table 1. CHRNA5-A3-B4 variants are not significantly associated with baseline smoking behaviors.
| Study 1 | Study 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Rs2036527 | GG | GA | AA | P-Values | GG | GA | AA | P-Values |
| n | 372 | 210 | 26 | 311 | 198 | 25 | ||
| Baseline cigarettes per day | 7.4 (7.1-7.7) | 7.7 (7.3-8.2) | 7.4 (6.2-8.6) | 0.51 | 8.0 (7.8-8.3) | 7.9 (7.5-8.3) | 7.4 (6.5-8.3) | 0.33 |
| Plasma Cotinine (ng/mL) | 238 (223-254) | 257 (236-279) | 262 (193-330) | 0.25 | 239 (225-253) | 239 (220-259) | 214 (167-260) | 0.66 |
| Urinary total nicotine equivalent1 (nmol/ mg Cre) | N/A | 62.7 (54.2-71.1) | 50.7 (45.5-55.9) | 60.5 (43.3-77.6) | 0.24 | |||
| FTND | 2.8 (2.7-3.0) | 3.0 (2.7-3.2) | 3.5 (2.8-4.3) | 0.13 | 3.2 (3.0-3.4) | 3.1 (2.9-3.4) | 3.4 (2.6-4.1) | 0.82 |
| Rs16969968 | GG | GA | AA | P-Values | GG | GA | AA | P-Values |
| n | 544 | 62 | 3 | 475 | 56 | 3 | ||
| Baseline cigarettes per day | 7.6 (7.3-7.8) | 7.3 (6.4-8.2) | 7.7 (1.4-13.9) | 0.84 | 7.9 (7.7-8.2) | 8.1 (7.5-8.8) | 7.3 (1.1-13.6) | 0.63 |
| Plasma Cotinine (ng/mL) | 245 (232-258) | 253 (210-297) | 230 (135-325) | 0.92 | 239 (227-251) | 225 (192-258) | 288 (-32-608) | 0.59 |
| Urinary total nicotine equivalent1 (nmol/ mg Cre) | N/A | 59 (53-65) | 51 (41-62) | 68 (-19.4-155) | 0.71 | |||
| FTND | 2.92 (2.77-3.07) | 2.94 (2.45-3.41) | 4.0 (1.5-6.5) | 0.59 | 3.2 (3.0-3.3) | 2.9 (2.4-3.3) | 4.0 (1.5-6.5) | 0.56 |
| Rs588765 | CC | CT | TT | P-values | CC | CT | TT | P-values |
| n | 293 | 252 | 63 | 246 | 229 | 58 | ||
| Baseline cigarettes per day | 7.3 (6.9-7.6) | 7.8 (7.4-8.2) | 7.63 (6.8-8.5) | 0.16 | 8.0 (7.7-8.3) | 8.0 (7.7-8.3) | 7.6 (7.0-8.2) | 0.55 |
| Plasma Cotinine (ng/mL) | 251 (232-269) | 242 (223-26) | 243 (201-286) | 0.68 | 236 (220-252) | 235 (218-253) | 251 (221-282) | 0.69 |
| Urinary total nicotine equivalent1 (nmol/ mg Cre) | N/A | 61 (51-71) | 57 (50-63) | 55 (47-64) | 0.73 | |||
| FTND | 2.9 (2.7-3.1) | 3.0 (2.8-3.2) | 2.9 (2.4-3.3) | 0.68 | 3.2 (3.0-3.4) | 3.2 (2.9-3.4) | 3.1 (2.6-3.6) | 0.10 |
| Rs578776 | AA | GA | GG | P-values | AA | GA | GG | P-values |
| n | 173 | 303 | 133 | 141 | 245 | 148 | ||
| Baseline cigarettes per day | 7.3 (6.8-7.8) | 7.5 (7.1-7.9) | 7.9 (7.3-8.4) | 0.35 | 7.8 (7.3-8.2) | 8.1 (7.8-8.4) | 7.8 (7.4-8.2) | 0.93 |
| Plasma Cotinine (ng/mL) | 243 (219- 266) | 240 (223-257) | 263 (236-291) | 0.35 | 248 (226-270) | 221 (206-237) | 255 (232-277) | 0.02 |
| Urinary total nicotine equivalent1 (nmol/ mg Cre) | N/A | 54 (48-60) | 57 (51-63.4) | 64 (49-79) | 0.38 | |||
| FTND | 2.8 (2.6-3.1) | 2.9 (2.7-3.1) | 3.2 (2.9-3.5) | 0.25 | 3.1 (2.9-3.4) | 3.2 (3.0-3.4) | 3.2 (2.9-3.4) | 0.97 |
Data presented as Mean (95% Confident Interval). P-values below 0.0125 were considered statistically significant due to multiple comparison adjustments.
The urinary total nicotine equivalents were only available for a subset of individuals in Study 2.
FTND=Fagerstrom Test for Nicotine Dependence
Associations of CHRNA5-A3-B5 variants with smoking abstinence
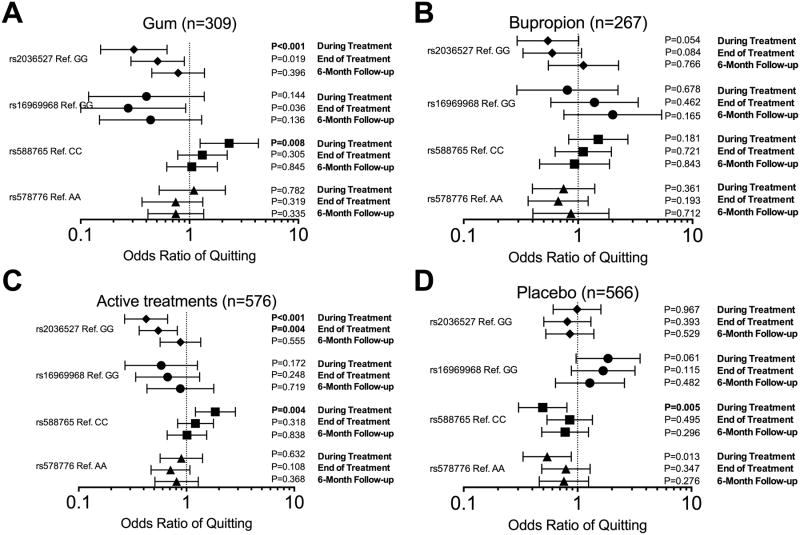
Fig. 1 summarizes the association between the four CHRNA5-A3-B4 variants and verified smoking abstinence at the different time points assessed among the participants who received the active nicotine gum treatment (Study 1, Fig.1A), the active bupropion treatment (Study 2, Fig.1B), any active pharmacological treatment (Study 1 and 2, Fig.1C), or the placebo treatment (Study 1 and 2, Fig.1D). For additive models please see Supplementary Fig.S1). Some notable impacts of the variants on abstinence are highlighted below.
Figure 1.
The associations (dominant model) between CHRNA5-A3-B5 variants and smoking abstinence in African American smokers. A) Participants who received active nicotine gum treatment (Study 1). B) Participants who received active bupropion treatment (Study 2). C) Participants who received active pharmacological treatments (combined analysis). D) Participants who received placebo (combined analysis). P-values below 0.0125 were considered statistically significant due to multiple comparison adjustments. Statistically significant values are bolded.
Association between rs2036527 and smoking abstinence
Overall, we found that the ‘A’ allele of rs2036527 was associated with lower abstinence rates during, and at the end of, treatment in participants who received active pharmacotherapy, but not in those who received placebo (see Fig.2C for statistical comparisons). Specifically, among the participants who received the active nicotine gum treatment (Study 1) those with the ‘A’ allele of rs2036527 had a similar magnitude of reduced abstinence throughout treatment which diminished during follow-up (during treatment OR=0.31 and end of treatment OR=0.51, Fig.2A); adjusting for age, sex, baseline CPD, menthol status and type of counseling sessions did not meaningfully alter this (during treatment OR=0.29 and end of treatment OR=0.48, Fig.2A). Likewise, adjusting for CYP2A6 genotype, the main nicotine-metabolizing enzyme, did not alter the odds ratio between rs2036527 and smoking abstinence (during treatment OR=0.31 and end of treatment OR=0.51). In the second study, a similar direction and magnitude of the rs2036527 effect was observed in those receiving the active bupropion treatment, which was not statistically significant (during treatment OR=0.54 and end of treatment OR=0.59, Fig.2B). Adjusting for age, sex, baseline CPD, and menthol status did not meaningfully alter the magnitude of these odds ratios (during treatment OR=0.56 and end of treatment OR=0.61, Fig.2B) nor did adjusting for CYP2B6 genotype, the main bupropion-metabolizing enzyme (during treatment OR=0.53 and end of treatment OR=0.59).
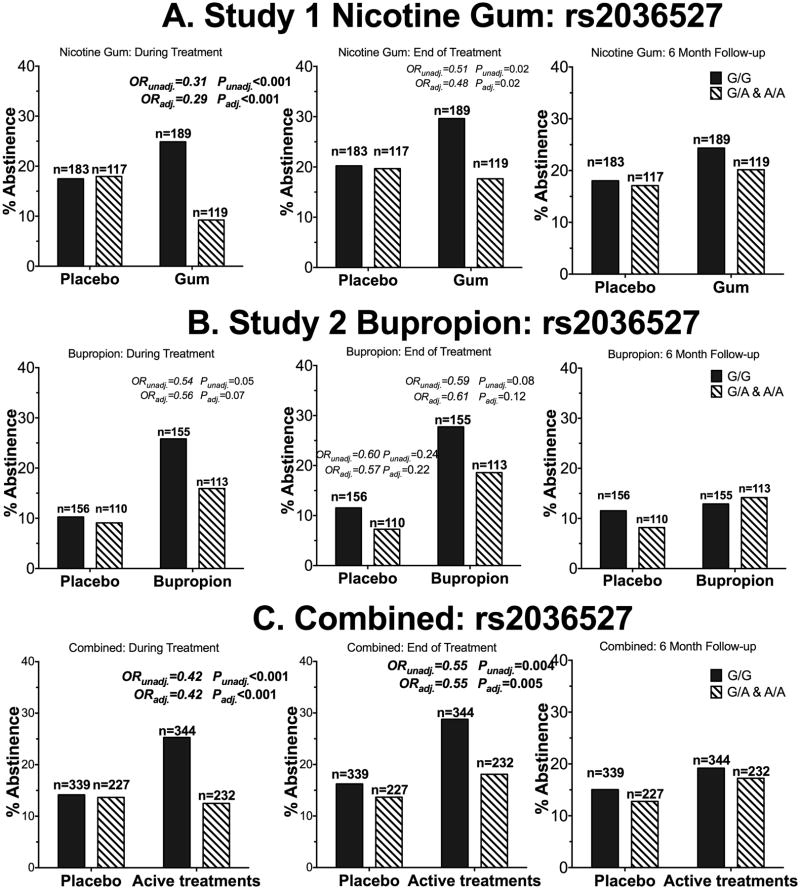
Figure 2.
The ‘A’ allele of rs2036527 was associated with lower smoking cessation rates in African American smokers receiving active pharmacological treatment. A) Participants in who received nicotine gum or placebo (Study 1). B) Participants who received active bupropion or placebo (Study 2). C) Participants who received active pharmacological treatments (combined analysis). ORunadj. = unadjusted odds ratio of quitting for the GA&AA genotype group compared to the GG genotype. ORadj.= odds ratio of quitting for the GA&AA genotype group compared to the GG genotype after adjusting for age, sex, baseline CPD, menthol status and type of counseling session. There was also a significant treatment (combined active vs. combined placebo) by rs2036527 genotype interaction during treatment (OR=0.43, P=0.01). The OR and P-values are shown for all comparisons with ORs smaller than 0.7 or greater than 1.4. P-values below 0.0125 were considered statistically significant due to multiple comparison adjustments. Statistically significant values are bolded. N represents the number of participants.
As suggested by analyses of each study alone, among the participants who received any active pharmacological treatments the ‘A’ allele of rs2036527 was associated with lower smoking abstinence rates (during treatment OR=0.42 and end of treatment OR=0.55, Fig.2C); adjusting for age, sex, baseline CPD, menthol status, type of counseling sessions and type of pharmacological treatment did not alter the association between rs2036527 and smoking abstinence (during treatment OR=0.42 and end of treatment OR=0.55, Fig.2C). Rs2036527 was not associated with smoking abstinence at 6-month follow up. The number needed to treat (NTT) was 8.9 for the GG group during active treatment (active treatment had no benefit in the GA/AA group). At the end of treatment, NTT were 9.3 and 22.8 for the GG and the GA/AA group, respectively. No statistically significant association between rs2036527 and smoking abstinence was observed at any time-point in the placebo arm, including after adjustment for age, sex, baseline CPD, menthol status, and type of counseling sessions (Fig.1D&2C).
The association of rs588765 and smoking abstinence
Overall, and in contrast to rs2036527, the ‘T’ allele of rs588765 was associated with short term smoking abstinence (only during treatment) in participants who received either active pharmacotherapy or placebo, with a differing direction of effect for active versus placebo treatment (see Fig.3C for statistical comparisons). Among the participants who received the active nicotine gum treatment (Study 1), those with the ‘T’ allele of rs588765 had higher abstinence rates during treatment (OR=2.31, Fig.1A&3A). Adjusting for age, sex, baseline CPD, menthol status and type of counseling sessions did not alter the association between rs588765 and smoking abstinence (OR=2.39, Fig.3A) nor did adjusting for CYP2A6 genotype (OR=2.34). In the second study, while the directions of effect were similar to nicotine gum, the magnitude was smaller and we did not observe any significant association between rs588765 and smoking abstinence in those receiving active bupropion or placebo treatment (Fig.1B&3B).
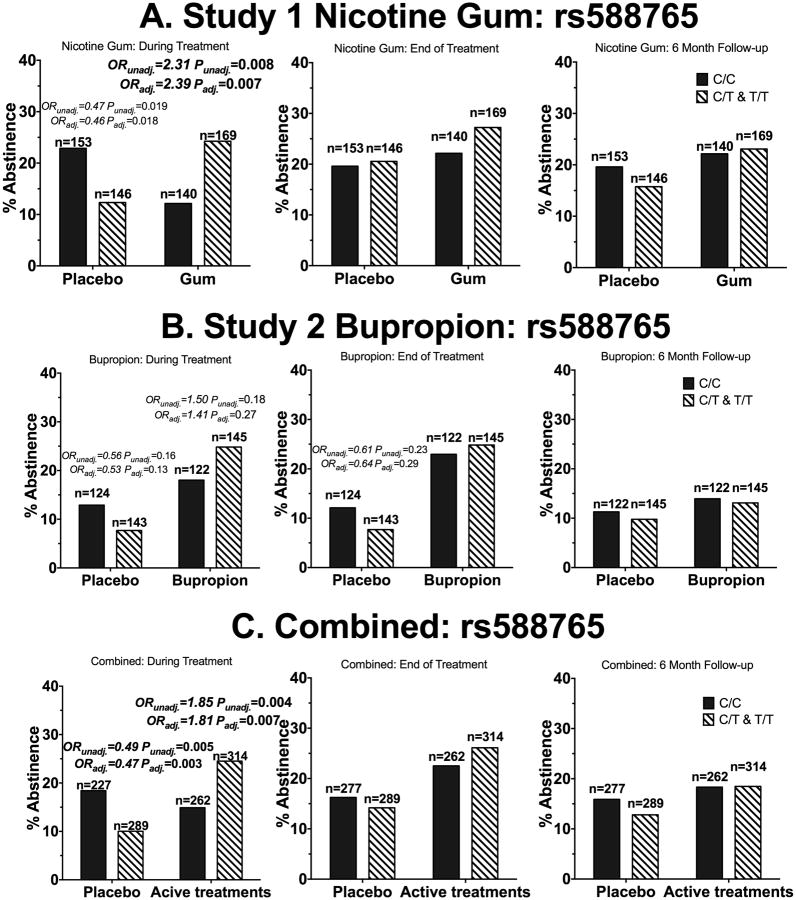
Figure 3.
The ‘T’ allele of rs588765 was associated with smoking abstinence in African American smokers receiving active pharmacological treatment. A) Participants in who received nicotine gum or placebo (Study 1). B) Participants who received active bupropion or placebo (Study 2). C) Participants who received active pharmacological treatments (combined analysis). ORunadj. = unadjusted odds ratio of quitting for the CT&TT genotype group compared to the CC genotype. ORadj.= odds ratio of quitting for the CT&TT genotype group compared to the CC genotype after adjusting for age, sex, baseline CPD, menthol status and type of counseling sessions. There was also a significant treatment (combined active vs. combined placebo) by rs588765 genotype interaction during treatment (OR=3.75, P<0.001). The OR and P-values are shown for all comparisons with ORs smaller than 0.7 or greater than 1.4. P-values below 0.0125 were considered statistically significant due to multiple comparison adjustments. Statistically significant values are bolded. N represents the number of participants.
When analyzed together to investigate the role of active pharmacological treatment, the ‘T’ allele of rs588765 was associated with increased smoking abstinence during treatment (OR=1.85, Fig.1C&3C). Adjusting for age, sex, baseline CPD, menthol status, type of counseling sessions and type of pharmacological treatment did not meaningfully alter the association between rs588765 and smoking abstinence (OR=1.81, Fig.3C). Among the participants who received the placebo treatment (Fig.1D&3C), the ‘T’ allele of rs588765 was associated with reduced abstinence during treatment (OR=0.49, Fig.1D&3C); adjusting for age, sex, baseline CPD, menthol status, and type of counseling sessions did not meaningfully alter the odds ratio between rs588765 and smoking abstinence during treatment (OR=0.47, Fig.3C). There was also a significant treatment (combined active vs. combined placebo) by rs588765 genotype interaction during treatment (OR=3.75, P<0.001). Rs588765 was not associated with smoking abstinence at end of treatment or at 6-month follow up.
The association of rs16969968 or rs578776 with smoking abstinence
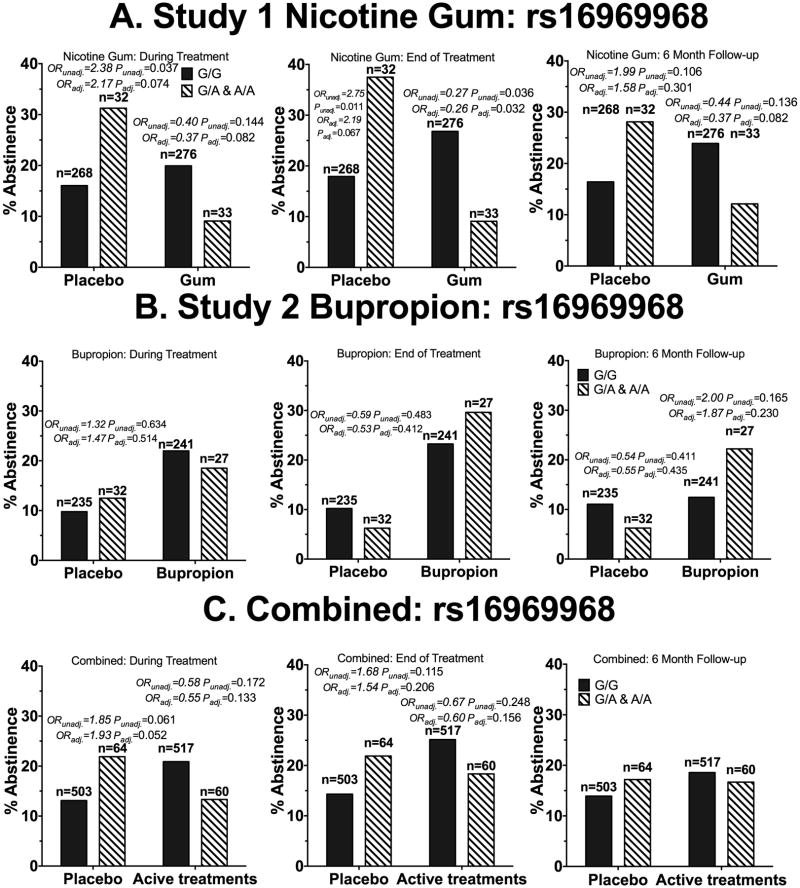
In these two clinical trials of African American smokers, while some trends were apparent, there was no consistent association between rs16969968 or rs578776 and smoking abstinence with active or placebo treatment, at any time point, with or without baseline demographic adjustments (Fig. 1&4).
Figure 4.
Rs16969968 was not consistently associated with smoking abstinence in African American smokers receiving active pharmacological treatment. A) Participants in who received nicotine gum or placebo (Study 1). B) Participants who received active bupropion or placebo (Study 2). C) Participants who received active pharmacological treatments (combined analysis). ORunadj. = unadjusted odds ratio of quitting for the GA&AA genotype group compared to the GG genotype. ORadj.= odds ratio of quitting for the GA&AA genotype group compared to the GG genotype after adjusting for age, sex, baseline CPD, menthol status and type of counseling sessions. The OR and P-values are shown for all comparisons with ORs smaller than 0.7 or greater than 1.4. P-values below 0.0125 were considered statistically significant due to multiple comparison adjustments. Statistically significant values are bolded. N represents the number of participants.
Discussion
We reported novel findings of an association between a CHRNA5-A3-B4 gene variant and smoking abstinence in African Americans. We identified a consistent association between the ‘A’ allele of rs2036527 and lower rates of smoking abstinence during active pharmacotherapy treatment in African American light smokers.
CHRNA5-A3-B4 and smoking behaviors
Gene variants in CHRNA5-A3-B4, particularly rs16969968 and correlated SNPs, have been consistently associated with heaviness of smoking and the levels of nicotine dependence in Caucasians (9-11, 25). In contrast to a recent meta-analysis in African American smokers (21), in the current study we did not observe any significant association between CHRNA5-A3-B4 variants, particularly rs2036527, and baseline smoking behaviors, including self-reported CPD, plasma cotinine levels or urinary total nicotine equivalents. This was unexpected since our study, although smaller in size compared to the meta-analysis, had objective biomarker data. Since it is well known that self-reported CPD does not account for the depth of inhalation and is a relatively weak marker of tobacco exposure (26), we expected the accuracy of biomarker data to compensate for the smaller sample size (N=1142). One reason for the lack of association between tobacco exposure and rs2036527 in our study may be that the previous meta-analyses included a substantial number of former smokers whereas our studies included only current smokers (21). It was previously observed that the influence of CHRNA5-A3-B4 gene variants on self-reported CPD is stronger in the former smokers compared to the current smokers (see Table S7 of Amos et al. study (8)). It is also possible that the lower number of cigarettes smoked per day by light smokers in the current study (mean=7.7) in contrast to the previous meta-analysis (mean ranged from 11.5-15.7) mitigated the expression of the underlining biological impact of the CHRNA5-A3-B4 gene variants on smoking level. However, it is important to note that CPD is a poor marker of tobacco consumption, susceptible to reporting and recalling biases and insensitive to smoking topography. The average cotinine levels, a more objective marker of tobacco consumption, in our study (mean=240 ng/mL, standard deviation=138) were comparable to previously observed in Caucasian and American African heavy smokers (mean≈170 ng/mL) (26). Previous studies in the CHRNA5-A3-B4 field have suggested objective biomarkers are more sensitive than CPD (7). We have previously shown that CHRNA5-A3-B4 variants are significantly associated with cotinine levels in as few as 163 smokers (12). Therefore, we believe that the accuracy of biomarkers (versus self-report CPD) compensates for the smaller size of our study.
CHRNA5-A3-B4 and smoking abstinence
In Caucasians, a number of studies have examined the association between CHRNA5-A3-B4 gene variants and clinical trial outcomes for quitting smoking. These studies have varied in whether the predominant effect of CHRNA5-A3-B4 gene variants was in the placebo arm, a specific treatment arm, or in all active treatment arms more generally (15, 17, 20). Our observations regarding rs2036527 and smoking abstinence in African American smokers are in agreement with a previous report by Munafò and colleagues in Caucasians with rs1051730 (15). Both studies reported significant associations between CHRNA5-A3-B4 gene variants and abstinence 1) primarily in the active pharmacological therapy groups, with little effect observable within the placebo arm, and 2) only during treatment (no association remains at follow up, after active treatment had stopped). Of note, in Caucasians rs2036527 is in high linkage disequilibrium with rs1051730 (r2=0.90). Therefore, rs2036527 may be a better pharmacogenetic marker for smoking cessation than rs16969968/rs1051730 as it predicts similar relapse risks in both Caucasians and African Americans.
The α5 nAChR subunit mediates an inhibitory effect of nicotine on brain reward systems (27). Mice with lower α5 nAChR function exhibited increased nicotine intake (27). Since rs2036527 was previously associated with increased nicotine intake in African Americans (21), it is likely that rs2036527 (or SNPs in high linkage disequilibrium) results in lower α5 nAChR function. Both nicotine gum and bupropion could have inhibitory effects on nicotine reward since both of them can bind to the nAChR (28, 29). The inhibitory effects of nicotine gum and bupropion may be weaker in individuals with rs2036527 (due to their lower α5 function), reducing the efficacy of these treatments.
Our findings with rs588765 and smoking abstinence in African American smokers are very similar to previous findings in Caucasian smokers (20). Both studies observed that the ‘T’ allele of rs588765 was associated with lower abstinence in the placebo group and higher abstinence when receiving nicotine replacement therapy (with significant interactions). However, the association between rs588765 and smoking abstinence was still observed at 6-month follow up in Caucasians, but not in African American smokers. This could be partially due to the relatively low smoking abstinence rates in African American smokers at 6-month follow up compared with the Caucasian smokers.
We did not observe any consistent association between rs16969968 and smoking abstinence in this study. It is also worth noting that the direction of the association between rs16969968 and smoking abstinence in African Americans was in the opposite direction compared to the direction of effect observed in Caucasians (20). The ‘A’ allele of rs16969968 was associated lower abstinence with nicotine gum treatment and higher abstinence with placebo treatment in our study of African American smokers. In contrast, the ‘A’ allele of rs16969968 was associated with higher abstinence with nicotine replacement therapy and lower abstinence with placebo treatment in Caucasians (20). It is possible that different linkage disequilibrium/haplotype structures between African Americans and Caucasians contributes to this discrepancy. Overall, our smoking abstinence data and the relatively low prevalence of rs16969968 in African American would suggest that rs16969968 has very little pharmacogenetic importance in African American smokers.
In contrast to the study published by Chen and colleagues (17), we did not observe any long lasting association between CHRNA5-A3-B4 gene variants and smoking abstinence rates in participants who received the placebo treatment. This discrepancy was unlikely to be the results of limited power since the current present investigation had a much bigger placebo group compared to the study by Chen and colleagues (566 in our combined studies versus 132 in the Chen et al., study (17)). It is possible the effects of the ‘tag’ SNPs are different among different ethnic groups due to the distinct haplotype structures.
Interestingly, adjusting for CPD had little influence on the association between the CHRNA5-A3-B4 gene variants and abstinence in our study and in a number of previous studies which evaluated the association between CHRNA5-A3-B4 gene variants and smoking abstinence (15, 20). This suggests that the influence of CHRNA5-A3-B4 gene variants on smoking abstinence is not related to their modest effect size on altering tobacco consumption. In addition, the impact of rs2036527 remained unaltered when we controlled for CYP2A6 and/or CYP2B6 genotype, suggesting the impact of rs2036527 on cessation was independent of variation in nicotine or bupropion metabolism.
Lastly, our findings suggested that roughly 60% of African Americans (i.e. individuals with rs2036527[G/G] genotype) would benefit greatly from active smoking cessation treatments, whereas the 40% with the rs2036527[A] variant may not. This demonstrates the potential value of pharmacogenetic approaches in improving smoking cessation outcomes among African American smokers (30, 31).
Strength and Limitations
A major strength of our study is that the smoking status (and baseline level of smoking) was biochemically verified. Our selection of African American light smokers can be viewed as both a strength and limitation. African Americans smokers experience disproportionately higher risks of smoking-related morbidity and mortality compared to Caucasians (32). However, very little is known about the efficacy of standard smoking cessation treatments in this population, particularly among light smokers who make up more than 50% of the African American smokers. However, the inclusion of light smokers may limit the generalizability of our findings to other groups. In addition, the placebo arm in both trials had relatively low smoking abstinence rates, the low number of abstinence events could limit the statistical power to evaluate these associations. The low number of abstinent individuals also limited our ability to evaluate the effects of multiple SNPs in the same model. Another limitation was that we did not have reliable (biochemically verified) time of relapse data in this study, which could provide some time course information about the effect of rs2036527 on smoking cessation.
Population stratification is an important issue in genetic association studies. A limitation of this study was that we did not have ancestry informative markers to statistically control for population stratification however allele frequencies were similar to previously published studies (rs2036527 was 22% here and in the large meta–analysis)(21). In addition, as improving smoking cessation treatments for Africa Americans is our goal, we believe that the limitations due to admixture are balanced against the need to improve smoking cessation in this population. Eliminating individuals who are modestly admixed, but would still self-identify as African Americans, could greatly reduce the clinical applicability of our findings.
Overall, our findings indicate that gene variants in CHRNA5-A3-B4 affect smoking abstinence in African Americans during the pharmacological treatment phase, but not during follow-up, even after adjusting for baseline smoking behaviors. Greater smoking abstinence was observed with active pharmacological treatments in those with the rs2036527[G/G] genotype while those with a rs2036527[A] allele had cessation rates no greater than the placebo treatment and may require alternative approaches to treatment. Further studies should focus on understanding the mechanism(s) underlying this association in order to optimize the efficacy of smoking cessation treatments.
Materials and Methods
Study descriptions
We evaluated the association between the CHRNA5-A3-B4 variants and smoking cessation outcomes in two independent smoking cessation trials. Both trials were conducted in African American light smokers (≤10 CPD) at the same community health center and the enrollment criteria were similar. These trials were focused on light smokers because 1) more than 50% of African American smokers are light smokers, and 2) little is known about the pharmacology and pharmacogenetics of smoking cessation treatments in this population.
Nicotine gum study (Study 1)
This study was a randomized double-blind, placebo-controlled trial to evaluate the efficacy of nicotine gum (2 mg) in African American light smokers (6). Eligible participants self-identified as “African American” or “Black”, were at least 18 years old, had smoked 10 or fewer CPD for at least 6 months prior to enrollment, and smoked on at least 25 of last 30 days. This study consisted of four treatment arms (N=∼190 each): 1) nicotine gum with health education (HE) counseling; 2) nicotine gum with motivational interviewing (MI) counseling; 3) placebo with HE and 4) placebo with MI. The nicotine gum treatment lasted 8 weeks, and six counseling sessions were provided to each participant. The participants were followed for a total of 26 weeks (6 months).
Bupropion study (Study 2)
This study was a randomized double-blind, placebo-controlled trial to evaluate the efficacy of bupropion (300 mg per day, total N=540) in African American light smokers (24). Eligibility criteria were similar to Study 1 and included exclusion based on contraindication of bupropion use. Bupropion use lasted 7 weeks and HE counseling was similar to Study 1. The participants were followed for a total of 26 weeks (6 month). Both studies were approved by the University of Kansas Human Subject Committee, the University of Toronto Ethics Review Office, and the University of California San Francisco Human Research Protection Program.
Both studies assessed age, sex, menthol use, baseline CPD, baseline plasma cotinine, Fagerstrom Test for Nicotine Dependence (FTND). Study 2 also assessed urinary total nicotine equivalents, which is the total urinary level of nicotine and 8 of its metabolites. The analytical procedures were described previously (33). Together, the 9 analytes account for about 90% of nicotine dose (34), and creatinine adjusted spot urinary TNE correlates with daily tobacco consumption (35).
Smoking abstinence
Biochemically verified abstinence was assessed during treatment (Week 1 for Study 1 and week 3 for the Study 2), at end of treatment (Week 8 for Study 1 and week 7 for Study 2) and at 6-month follow-up (6 month for both Study 1 and Study 2). In Study 1, abstinence was verified by exhaled CO levels of lower than 10 ppm. In Study 2, abstinence was verified by having salivary cotinine levels less than 15 ng/ml. All statistical analyses were performed on an intent-to-treatment basis, and subjects lost during follow-up were considered smokers.
Genotyping
CHRNA5-A3-B4 SNPs were genotyped using ABI Viia 7 real time PCR machine (Applied Biosystems, Foster City, CA). The genotyping reaction was performed with 5 μL TaqMan GTXpress master mix and 5 μL of water containing 10 ng of DNA and 0.25 μL of 40× Taqman SNP genotyping assay (see Supplementary Table S1 for the specific probes used for each SNP). The allele discrimination data were analyzed by Viia 7 software version 1.2. All genotyping results included in our analyses had call quality values above 0.985.
Statistical Analyses
The comparisons of baseline demographic variables were performed by Mann Whitney tests or Chi2 tests. Evaluation of the association between the dichotomized SNPs (rs2036527[GG=0 and GA/AA=1] rs16969968[GG=0 and AG/AA=1] rs588765[CC=0 and CT/TT=1] and rs578776[AA=0 and AG/GG=1].) and smoking abstinence were conducted using logistic regression. Modeling the influence of the variants as ‘additive’ resulted in very similar statistical results compared to modeling the influence of the variants as dichotomized valuable (results shown in Supplementary Fig.S1). Logistic regressions were used to evaluate the association between rs2036527 or rs588765 and smoking abstinence after adjusting for age, sex, baseline CPD, menthol status, the type of counseling sessions (Study 1) and other CHRNA5-A3-B4 SNPs. Adjusting for other CHRNA5-A3-B4 variants (such as rs16969968) did not alter the associations between rs2036527 and rs588765 with smoking abstinence (Supplementary Fig. S2, S3&S4). Adjusting for CYP2A6 (coded as normal activity>intermediate activity>slow activity) and CYP2B6 (coded as normal activity>intermediate activity>slow activity) also did not alter the association between rs2036527 and rs588765 with smoking abstinence. A detailed description of the specific CYP2A6 and CYP2B6 alleles assessed was reported previously, and included CYP2A6*2, *4, *9, *17, *20, *23, *24, *25, *26, *27, *31, &*35 and CYP2B6*4, *6, *9, *18, &*22 (30, 31). The P-values were not adjusted for multiple comparisons. Because there were 4 independent SNPs, we considered P-values below 0.0125 (i.e. 0.05/4) to be statistically significant. The number needed to treat represents the average number of patients needed to treat with the active versus placebo pharmacotherapy to prevent one patient from smoking relapse. Statistical analyses were performed using Stata 12 (StataCorp, College Station, TX).
Supplementary Material
Study Highlights.
What is the current knowledge of the topic?
Smoking is a major cause of preventable death globally. Smoking cessation at any age has substantial health benefits.
What question did this study address?
While the association between variants in CHRNA5-A3-B4 (a nicotinic receptor gene cluster) and smoking has been explored previously, the association between CHRNA5-A3-B4 variants and smoking cessation is poorly understood, particularly among African Americans.
What this study adds to our knowledge?
In African American smokers, a common variant in CHRNA5-A3-B4 is associated with significantly lower smoking cessation rates during active pharmacological treatment.
How this might change clinical pharmacology and therapeutics?
This study suggests that roughly 60% of African Americans would benefit greatly from either of these two active smoking cessation treatments, whereas the other 40% may not. This illustrates the potential value of pharmacogenetic approaches in improving smoking cessation outcomes among African American smokers.
Acknowledgments
We acknowledge the support of NIH grant CA091912 to fund this study, National Center for Minority Health Disparities grant 1P60MD003422 (JSA), the Endowed Chair in Addiction for the Department of Psychiatry (RFT), CIHR grant MOP86471 (RFT), Ontario Graduate Scholarship (AZZ), CAMH and the CAMH foundation (RFT), NIH PGRN grants DA020830 (RFT, NB), DA017441 (SPD) and DA012353 (NB), the Canada Foundation for Innovation (#20289 and #16014) and the Ontario Ministry of Research and Innovation (RFT). The authors would like to thank Kelly Li for her technical assistance.
Funding: NIH grants CA091912, DA020830 and CIHR grant MOP86471
Footnotes
Conflict of Interest: NLB serves as a consultant to several pharmaceutical companies that market smoking cessation medications and has been a paid expert witness in litigation against tobacco companies. SPD is a scientific advisor to Genophen. RFT has participated in one-day advisory meetings for Novartis and McNeil. Associate Editor RFT was not involved in the review or decision process for this paper.
Author Contributions: Tyndale, Zhu, Cox, David, Ahluwalia, and Benowitz wrote the anuscript
Tyndale, Zhu, Cox, Ahluwalia, and Benowitz designed the research
Zhu, Zhou, Cox, Ahluwalia, and Benowitz performed the research
Tyndale and Zhu analyzed the data
References
- 1.Benowitz NL. Nicotine addiction. N Engl J Med. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xian H, et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob Res. 2003;5:245–54. [PubMed] [Google Scholar]
- 3.Gold AB, Wileyto EP, Lori A, Conti D, Cubells JF, Lerman C. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology. 2012;37:1683–8. doi: 10.1038/npp.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchison KE, et al. CHRNA4 and tobacco dependence: from gene regulation to treatment outcome. Arch Gen Psychiatry. 2007;64:1078–86. doi: 10.1001/archpsyc.64.9.1078. [DOI] [PubMed] [Google Scholar]
- 5.Lerman C, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology. 2006;31:231–42. doi: 10.1038/sj.npp.1300861. [DOI] [PubMed] [Google Scholar]
- 6.Ahluwalia JS, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction. 2006;101:883–91. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 7.Munafo MR, et al. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. J Natl Cancer Inst. 2012;104:740–8. doi: 10.1093/jnci/djs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amos CI, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–22. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thorgeirsson TE, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–42. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thorgeirsson TE, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–53. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LS, et al. Smoking and genetic risk variation across populations of European, Asian, and African American ancestry--a meta-analysis of chromosome 15q25. Genet Epidemiol. 2012;36:340–51. doi: 10.1002/gepi.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu AZ, Renner CC, Hatsukami DK, Benowitz NL, Tyndale RF. CHRNA5-A3-B4 genetic variants alter nicotine intake and interact with tobacco use to influence body weight in Alaska Native tobacco users. Addiction. 2013;108:1818–28. doi: 10.1111/add.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103:1342–6. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freathy RM, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–7. doi: 10.1093/hmg/ddp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munafo MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 genotype and short-term smoking cessation. Nicotine Tob Res. 2011;13:982–8. doi: 10.1093/ntr/ntr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarginson JE, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2011;156B:275–84. doi: 10.1002/ajmg.b.31155. [DOI] [PubMed] [Google Scholar]
- 17.Chen LS, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. The American journal of psychiatry. 2012;169:735–42. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King DP, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology. 2012;37:641–50. doi: 10.1038/npp.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saccone NL, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergen AW, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23:94–103. doi: 10.1097/FPC.0b013e32835cdabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David SP, et al. Genome-wide meta-analyses of smoking behaviors in African Americans. Transl Psychiatry. 2012;2:e119. doi: 10.1038/tp.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li MD, Xu Q, Lou XY, Payne TJ, Niu T, Ma JZ. Association and interaction analysis of variants in CHRNA5/CHRNA3/CHRNB4 gene cluster with nicotine dependence in African and European Americans. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2010;153B:745–56. doi: 10.1002/ajmg.b.31043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amos CI, et al. Nicotinic acetylcholine receptor region on chromosome 15q25 and lung cancer risk among African Americans: a case-control study. J Natl Cancer Inst. 2010;102:1199–205. doi: 10.1093/jnci/djq232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox LS, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. J Natl Cancer Inst. 2012;104:290–8. doi: 10.1093/jnci/djr513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JZ, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–40. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob Res. 2011;13:772–83. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berrettini WH, Doyle GA. The CHRNA5-A3-B4 gene cluster in nicotine addiction. Mol Psychiatry. 2012;17:856–66. doi: 10.1038/mp.2011.122. [DOI] [PubMed] [Google Scholar]
- 29.Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–7. [PubMed] [Google Scholar]
- 30.Zhu AZ, et al. CYP2B6 and Bupropion's Smoking-Cessation Pharmacology: The Role of Hydroxybupropion. Clin Pharmacol Ther. 2012;92:771–7. doi: 10.1038/clpt.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho MK, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85:635–43. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haiman CA, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354:333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 33.Zhu AZ, et al. Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco-specific nitrosamine exposure and lower tobacco-specific nitrosamine bioactivation. Carcinogenesis. 2013;34:93–101. doi: 10.1093/carcin/bgs306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benowitz NL, Jacob P, 3rd, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J Pharmacol Exp Ther. 1994;268:296–303. [PubMed] [Google Scholar]
- 35.Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P., 3rd Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19:3013–9. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.