Abstract
Objective
To examine racial/ethnic disparities in quality of schizophrenia care and assess the size of observed disparities across states and over time.
Data Sources
Medicaid claims data from CA, FL, NY, and NC.
Study Design
Observational repeated cross-sectional panel cohort study of white, black, and Latino fee-for-service adult beneficiaries with schizophrenia. Main outcome was the relationship of race/ethnicity and year with a composite measure of quality of schizophrenia care derived from 14 evidence-based quality indicators.
Principal Findings
Quality was assessed for 325,373 twelve-month person-episodes between 2002 and 2008, corresponding to 123,496 Medicaid beneficiaries. In 2002, quality was lowest for blacks in all states. With the exception of FL, quality was lower for Latinos than whites. In CA, blacks had about 43 percent of the individual indicators met compared to 58 percent for whites. Quality improved annually for all groups in CA, NY, and NC. While in CA the improvement was slightly larger for Latinos, in FL quality improved for blacks but declined for Latinos and whites.
Conclusions
Quality of schizophrenia care is poor and racial/ethnic disparities exist among Medicaid beneficiaries from four states. The size of the disparities varied across the states, and most of the initial disparities were unchanged by 2008.
Keywords: Racial/ethnic disparities, schizophrenia, quality of care, trends, Medicaid
It has been a decade since the Institute of Medicine published its report Unequal Treatment on racial/ethnic disparities in health in the United States (Smedley, Stith, and Nelson 2003). Substantial effort has been devoted to documenting racial/ethnic disparities and understanding their causes. Disparities among people with schizophrenia further burden a severely ill and disabled population whose quality of care is undermined by underuse of evidence-based practices and overuse of low-value or unsupported practices (U.S. Department of Health and Human Services 2003; Horvitz-Lennon et al. 2009a).
Racial/ethnic disparities in schizophrenia care have been documented, but little research exists on Latino-white disparities (Vega et al. 2007). Furthermore, most studies have focused on one or a small number of interventions (Barrio et al. 2003; Kreyenbuhl et al. 2003; Leslie and Rosenheck 2004), and although some evidence exists on longitudinal trends (e.g., Busch et al. 2009) it pertains to earlier time periods. Last, although there is evidence of variations in racial/ethnic disparities in health care across regions (Fisher, Goodman, and Chandra 2008), we are not aware of studies that have investigated geographic variations in disparities in schizophrenia care. These knowledge gaps limit our ability to guide policy and improve clinical practice and outcomes (Zaslavsky and Ayanian 2005).
We examined whether a comprehensive measure of quality of mental health care differed for black, Latino, and white Medicaid beneficiaries in four large states that contribute about one-third of all Medicaid beneficiaries. We also examined whether overall quality and disparities in quality changed over time, and whether disparities in quality of care differed among the states. We focused on Medicaid beneficiaries because of the dominant role played by Medicaid as health care payer for people with schizophrenia in the United States (Frank and Glied 2006). We hypothesized that (1) racial/ethnic disparities in quality of care exist in all study states; (2) the size of the disparity varies across the study states; and (3) disparities have not improved meaningfully over time.
Method
Data Sources and Study Population
Our data sources were datasets for the Medicaid programs in CA, FL, NY, and NC for calendar years 2002–2009 assembled as Medicaid Analytic eXtract (MAX) data. Specific MAX files used in this study were Personal Summary, the source for eligibility and ICD-9 diagnoses; Inpatient; Pharmacy; and Other Services, the source for data on use of outpatient services. We selected these states because of the racial/ethnic diversity and size of their Medicaid population. The relatively low rates of managed care penetration during the study period permitted a focus on fee-for-service (FFS) beneficiaries for whom complete data are available in MAX. Because states differ in the manner they capture provision of services, especially psychosocial services, we analyzed data separately for each state.
We identified continuously enrolled FFS beneficiaries aged 18–64 who had ≥2 outpatient claims with a primary or secondary diagnosis of schizophrenia (ICD-9 codes 295.xx) recorded on two different service dates during a 12-month period, or who had ≥1 inpatient admission for schizophrenia. We defined continuous enrollment as having ≥9 months of enrollment per year and enrollment gaps of <2 months. We created 12-month person-episodes of care received as of the date of the first claim with a schizophrenia diagnosis. Data from 2009 were used to provide complete follow-up for 2008 person-episodes. Individuals could contribute person-episodes in each of the 7 years of the study period. We excluded data for individuals with dual Medicaid-Medicare coverage because we could not observe all their care.
The study was approved by the RAND IRB.
Measures
Outcome Variable
Our outcome variable was a composite measure of overall quality of care constructed with 14 pharmacological, psychosocial, and appropriateness indicators. Sources for these process indicators included the schizophrenia PORT study (Lehman, Steinwachs, and The Co-Investigators of the PORT Project 1998; Buchanan et al. 2010); the American Psychiatric Association clinical practice guidelines (Lehman et al. 2004); the Healthcare Effectiveness Data and Information Set (HEDIS); and published studies on quality of schizophrenia care (e.g., Dickey et al. 2003; Busch, Frank, and Lehman 2004; Gilmer et al. 2007; Essock et al. 2009; Olfson, Marcus, and Wan 2009; Olfson, Marcus, and Doshi 2010).
Pharmacological indicators were as follows: any use of antipsychotic drugs, and conditional on antipsychotic use, any use of long-acting injectable antipsychotics, two indicators of antipsychotic drug adherence (full adherence, defined as ≥80 percent medication possession ratio (MPR), and low adherence, defined as ≤50 percent MPR), two indicators of clozapine use (any clozapine use, and adequate duration and dose of clozapine treatment among users, defined as ≥90 days of clozapine use with a daily dose of ≥300 mg as of the 29th day of the trial), and nonclozapine antipsychotic polypharmacy (simultaneous use of ≥2 nonclozapine antipsychotics for >90 days). Psychosocial indicators were as follows: any receipt of psychosocial services (at least once), and routine receipt of psychotherapy (at least once every quarter). Appropriateness indicators were as follows: two indicators of continuity of care (follow-up care within 7 and 30 days of discharge from the first observed inpatient admission for schizophrenia), receipt of routine psychiatric services (any outpatient psychiatric service at least once every quarter), heavy use of Emergency Department (ED) services (≥4 ED visits with a primary diagnosis of schizophrenia), and heavy use of inpatient services for schizophrenia (≥30 inpatient days or ≥3 inpatient admissions with a primary diagnosis of schizophrenia).
The composite measure excluded three indicators initially considered for inclusion. These were adequate duration of clozapine treatment (90 or more days of clozapine use); above-range dose among maintenance users of first-generation antipsychotic (FGA) drugs (PORT-discordant dosing during a 3-month or longer period following the end of acute-phase treatment); and use intensity among users of psychosocial services (number of visits). We excluded the clozapine indicator because a more informative measure of clozapine treatment adequacy was included in the composite measure. We excluded the other two indicators because of small numbers.
Population Characteristics
We characterized the cohort using demographic and diagnostic information available in the MAX files. Variables were gender, age (continuous), and seven health status variables constructed during the 6-month period of uninterrupted FFS enrollment preceding the first schizophrenia claim: ever Supplemental Security Income (SSI) as a basis for Medicaid eligibility, an indicator of disability; vascular-metabolic comorbidity (e.g., diabetes); other chronic medical comorbidity (e.g., AIDS); psychiatric comorbidity; substance use disorder comorbidity; mean inpatient days for schizophrenia; and ≥2 ED visits for schizophrenia.
Explanatory Variables
Race/ethnicity. MAX data classifies race/ethnicity as white; black; Hispanic; American Indian/Alaskan Native; Asian; Native Hawaiian/Other Pacific Islander; Hispanic and one or more Races; Multiple Race; and Unknown. We first grouped the Hispanic groups together and renamed this group as Latino. Next, among persons observed in ≥2 twelve-month cohorts, we identified those classified as Multiple Race or Unknown and those whose classification changed over time. We reclassified their race/ethnicity to the category most frequently observed over all available cohorts, first reclassifying the Multiple Race and Unknown categories. We used the following algorithm to break ties: If ever Latino, then Latino; else, if ever black or African-American, then black; else, if ever American Indian, Asian, or Native Hawaiian, then other; else, non-Latino white. Latinos were placed at the top of this hierarchy because several states report Unknown race for individuals with Latino ethnicity (Borck et al. 2012). Because reclassification of Multiple Race and Unknown was only possible for individuals appearing in ≥2 cohorts, we were unable to reclassify race/ethnicity for 7.3, 9.5, 9.6, and 2.5 percent of beneficiaries in CA, FL, NY, and NC, respectively, all of whom were excluded from analyses. Individuals of American Indian/Alaskan Native, Asian, and Native Hawaiian/Other Pacific Islander race/ethnicity were excluded from analyses due to small numbers.
Time. We assigned each person-episode to the calendar year when the schizophrenia diagnosis was first observed. We coded year as a count ranging from 0 to 6, where 0 was used for episodes occurring in 2002. We treated time as a continuous variable.
Statistical Analyses
Creation of Composite Schizophrenia Quality Measure
Our unit of observation was the person-episode. Within each state, we created a composite quality score for each episode based on the indicators using a two-parameter probit item response theory (IRT) model. An IRT model permits construction of a composite measure that is conceptually similar to that produced by a factor analysis model (Hays, Morales, and Reise 2000). We used IRT because unlike factor analysis, IRT makes use of discrete quality indicators and permits the relationship of each quality indicator with the composite to vary (see Notes, Online Appendix). We transformed the direction of some indicators to ensure that larger values of the composite measure consistently corresponded to better quality of care. For each state, we included person-episodes across all years and race/ethnicity groups. We estimated IRT models using the SAS procedure “nlmixed.”
We constructed item characteristic curves (Kearns, Khosla, and Kostenuik 2008) to assess the relationships between the composite and the probabilities of meeting the binary indicators. We expected indicators more discriminating of underlying quality of schizophrenia care to change more rapidly from a low to a high probability of meeting the indicator across the latent scale than less discriminating indicators. Indicators having IRT slope parameters larger than one were considered discriminating. To aid in interpretation of the composite score, we determined the expected percentage of the process indicators met associated with a range of values of the composite score. We also calculated the total number of indicators met for episodes falling in the lowest and highest deciles of the scores. Finally, we assessed the criterion validity of our composite using two indicators that were not included in the composite measure: adequate duration of clozapine treatment and use intensity among users of psychosocial services. The mean composite score for those not having adequate clozapine was subtracted from the mean score among those having adequate clozapine; a Spearman rank correlation coefficient was calculated between the composite score and the number of psychosocial services used.
Because the composite measure is an estimate obtained from the IRT model, the error associated with it is likely to vary across individuals. We therefore divided the estimated composite by its associated standard error, creating a standardized composite for use in subsequent regression models.
Relationship of Quality with Race/Ethnicity and Time
Using the standardized composite measure as a response variable, we estimated linear regression models that included race/ethnicity, year, and the interaction of race/ethnicity with year as covariates. Positive values of the estimated coefficients indicate better quality of care, with the intercept representing average quality in 2002 for whites. Effects were interpreted using as a reference norm the average score for whites and distance, measured in standard deviations, from whites. Models included the main effects and all pairwise interaction terms. Variables that were not statistically significant at the 0.05 level were eliminated unless they were terms related to significant higher order terms. Thus, main effects were retained if their interactions were statistically significant. We estimated the regression models separately for each state using the SAS procedure “glm.”
Results
Study Population
We observed the care received by 123,496 Medicaid beneficiaries in the four study states for a total of 325,373 person-episodes during the period 2002–2008: 139,065 episodes (29 percent black, 18 percent Latino, 53 percent white) for 51,071 beneficiaries in CA; 25,146 episodes (31 percent black, 28 percent Latino, 41 percent white) for 13,622 beneficiaries in FL; 120,704 episodes (40 percent black, 22 percent Latino, 38 percent white) for 44,925 beneficiaries in NY; and 40,458 episodes (60 percent black, 1 percent Latino, 39 percent white) for 13,878 beneficiaries in NC. Females comprised 43–53 percent of the samples across the states, with average age in the early 40's (Table 1). Between 90 and 99 percent of the samples were ever SSI-eligible during the 6-month period preceding their entry into the cohort, and rates of medical and psychiatric comorbidity documented during that relatively short time window ranged between one quarter and one half of the samples. Rates of diagnosed substance use disorders ranged between 7 and 18 percent. Use of acute care services for schizophrenia varied across the states.
Table 1.
Cohort Characteristics, by State (2002–2008): Demographic and Health Status Variables
| State | California | Florida | New York | North Carolina |
|---|---|---|---|---|
| Number person-episodes | 139,065 | 25,146 | 120,704 | 40,458 |
| % Black, Latino, white | 29, 18, 53 | 31, 28, 41 | 40, 22, 38 | 60,1, 39 |
| % Female | 43 | 50 | 45 | 53 |
| Mean age ± SD | 42.9 ± 11.49 | 42.4 ± 11.97 | 43.2 ± 11.24 | 41.4 ± 12.20 |
| % Ever supplemental security income* | 99 | 97 | 90 | 95 |
| % Vascular-metabolic comorbidities* | 43 | 36 | 48 | 50 |
| % Other chronic medical comorbidities* | 32 | 29 | 29 | 26 |
| % Psychiatric comorbidities* | 33 | 29 | 40 | 40 |
| % Substance use disorders* | 13 | 7 | 18 | 15 |
| Mean inpatient (IP) days ± SD* | 1.2 ± 6.68 | 1.5 ± 8.38 | 2.6 ± 12.96 | 0.8 ± 4.27 |
| % ≥2 emergency department (ED) visits* | 1.11 | 0.06 | 0.19 | 0.78 |
These summary statistics were calculated only for person-episodes having 6 months of uninterrupted FFS enrollment preceding the first schizophrenia claim (CA = 107,714; FL = 16,412; NY = 88,700; NC = 32,102).
Principal Findings
Quality of Schizophrenia Care
Individual Measures (selected results). Across the four states and over the entire study period, 66–70 percent of person-episodes had full antipsychotic drug adherence, and 55–71 percent received routine psychiatric care. Low antipsychotic drug adherence was not insubstantial (14–19 percent); clozapine use was low (5–7 percent); above-range FGA dosing during maintenance treatment was frequent (31–52 percent); and heavy inpatient use was not rare, observed in 3–10 percent of the person-episodes (Table A.1., Online Appendix).
Composite Measure. The indicators most discriminating of quality in all states' IRT models were full adherence to antipsychotic drugs, and at least 50 percent (e.g., not low) adherence to antipsychotic drugs. Clozapine use and adequate duration and dose of clozapine treatment were also among the most discriminating indicators of quality in CA (Table A.2. and Figure A.1., Online Appendix). The interpretation of the correspondence between changes in the composite and changes in the individual process indicators varies across the indicators, and also across the states. For example, increasing the composite by a third of a standard deviation increases the probability of adequate duration and dose of clozapine treatment by 4 percentage points in CA, less than 1 point in FL, and 7 points in NC and NY.
All state-specific distributions of the standardized quality scores were skewed toward poor quality of schizophrenia care (Figure1). For example, the mean composite score in NC was −0.55 (corresponding to having 52 percent of the process indicators met) yet 50 percent of the patient-episodes had scores higher than 0.28 (having close to 70 percent met). The range of composite scores was largest in CA. Across the states, a composite score of −5 corresponded to having between 12 and 19 percent of the indicators met, while a score of 5 corresponded to having between 92 and 99 percent of the indicators met.
Figure 1.
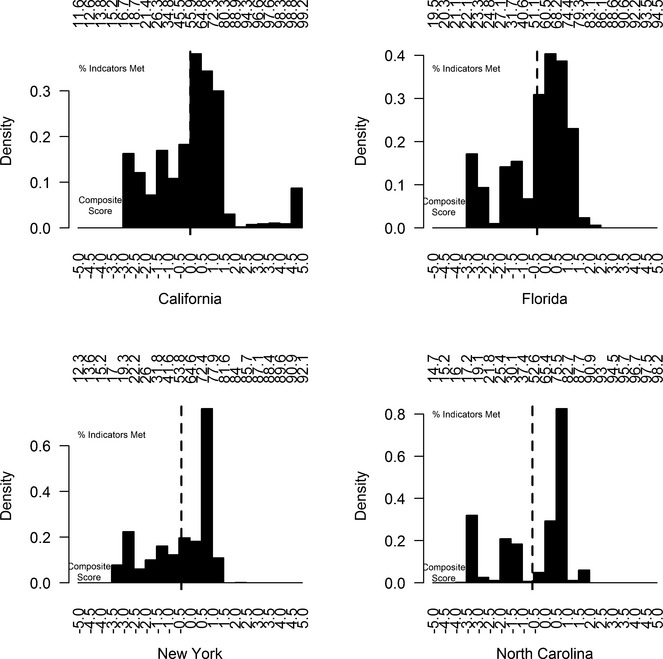
Distribution of Standardized Composite Score, by State Note. y-axis displays the relative frequency of composite scores. Lower x-axis denotes the composite scale while upper x-axis expresses the composite as the expected percentage of process indicators met. Solid vertical line indicates state mean.
The average number of indicators met was lower for episodes in the lowest compared to the highest decile of the composite score distribution—for example, in CA, the average (SD) numbers of indicators met were 5.7 (0.79) and 9.2 (1.28), in the lowest and highest deciles, respectively (Figure A.2., Online Appendix). In addition, because the composite score correlated with the states' performance on the two indicators not considered in its construction, we confirmed the criterion validity of the composite measure. The difference in the composite score (95 percent confidence interval) between those receiving adequate clozapine treatment and those who did not was positive and ranged from 1.45 (1.25, 1.64) in FL to 2.10 (2.02, 2.18) in CA. As anticipated, the Spearman rank correlation coefficients between the composite score and the number of psychosocial services was positive, ranging from 0.24 (0.23, 0.25) in NC to 0.40 (0.39, 0.40) in CA.
Racial/Ethnic Disparities in Quality of Care
Disparities Findings Based on Individual Measures. An examination of racial/ethnic groups' performance on the individual measures over the entire study period indicated that regardless of state and with few exceptions, quality of care was worst for blacks. Latinos' quality of care tended to be better than blacks' but lower than that of whites. An exception was FL, where Latinos outperformed whites in most psychosocial and appropriateness indicators (Table 2).
Table 2.
Performance on Indicators of Quality of Schizophrenia Care, by State and Race/Ethnicity (2002–2008)
| Indicator |
California (N = 139,065) |
Florida (N = 25,146) |
New York (N = 120,704) |
North Carolina (N = 40,458) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race | Black | Latino | White | Black | Latino | White | Black | Latino | White | Black | Latino | White |
| Number of person-episodes | 39,917 | 24,976 | 74,172 | 7,907 | 7,007 | 10,232 | 48,652 | 26,664 | 45,388 | 24,122 | 528 | 15,808 |
| Any antipsychotic use | 91 (100) | 94 (100) | 93 (100) | 95 (100) | 96 (100) | 95 (100) | 91 (100) | 91 (100) | 92 (100) | 91 (100) | 94 (100) | 95 (100) |
| Any clozapine use | 4.0 (91) | 6.8 (94) | 9.2 (93) | 2.8 (95) | 3.3 (96) | 8.6 (95) | 4.7 (91) | 4.8 (91) | 12 (92) | 4.2 (91) | 13 (94) | 8.3 (95) |
| Adequate clozapine treatment | ||||||||||||
| Duration only* | 85 (4) | 84 (7) | 89 (9) | 79 (3) | 84 (3) | 86 (9) | 83 (5) | 84 (5) | 91 (12) | 89 (4) | 89 (13) | 87 (8) |
| Duration and dose | 72 (4) | 67 (7) | 74 (9) | 63 (3) | 65 (3) | 69 (9) | 64 (5) | 65 (5) | 71 (12) | 73 (4) | 45 (13) | 64 (8) |
| Antipsychotic polypharmacy | 21 (91) | 23 (94) | 25 (93) | 21 (95) | 20 (96) | 23 (95) | 18 (91) | 17 (91) | 21 (92) | 18 (91) | 23 (94) | 21 (95) |
| Any use, long acting injectable antipsychotic | 12 (91) | 13 (94) | 12 (93) | 25 (95) | 10 (96) | 15 (95) | 15 (91) | 9 (91) | 13 (92) | 29 (91) | 16 (94) | 14 (95) |
| Full antipsychotic adherence (≥.8) | 60 (86) | 69 (91) | 76 (91) | 60 (91) | 71 (94) | 76 (94) | 63 (86) | 66 (88) | 77 (90) | 60 (86) | 65 (90) | 74 (92) |
| Number of person-episodes | 39,917 | 24,976 | 74,172 | 7,907 | 7,007 | 10,232 | 48,652 | 26,664 | 45,388 | 24,122 | 528 | 15,808 |
| Low antipsychotic adherence (≤.5) | 22 (86) | 14 (91) | 11 (91) | 21 (91) | 12 (94) | 11 (94) | 20 (86) | 16 (88) | 11 (90) | 24 (86) | 17 (90) | 13 (92) |
| Above-range dose, first-generation antipsychotic* | 42 (1.9) | 39 (1.9) | 42 (1.7) | 57 (4.6) | 57 (2.5) | 45 (3.6) | 34 (2.3) | 27 (1.6) | 30 (2.3) | 45 (1.7) | 60 (1) | 30 (1.4) |
| Any receipt psychosocial services | 86 (100) | 88 (100) | 88 (100) | 63 (100) | 62 (100) | 66 (100) | 49 (100) | 43 (100) | 47 (100) | 78 (100) | 83 (100) | 81 (100) |
| Psychosocial visits among users* | 34 [50.9] | 30 [44.7] | 32 [46.8] | 51 [69.7[ | 19 [40.3] | 38 [60.9] | 75 [80.1] | 62 [76.2] | 67 [74.9] | 58 [74.6] | 61 [69.0] | 54 [72.2] |
| Routine receipt of psychotherapy | 22 (100) | 24 (100) | 23 (100) | 8.4 (100) | 16 (100) | 13 (100) | 2.5 (100) | 4.1 (100) | 4.4 (100) | 13 (100) | 18 (100) | 17 (100) |
| Routine psychiatric care | 52 (100) | 54 (100) | 58 (100) | 50 (100) | 65 (100) | 55 (100) | 65 (100) | 72 (100) | 74 (100) | 70 (100) | 75 (100) | 73 (100) |
| ≥4 Emergency Department visits | 1.4 (100) | 0. 8 (100) | 1.1 (100) | 0.09 (100) | 0.03 (100) | 0.07 (100) | 0.1 (100) | 0.07 (100) | 0.1 (100) | 0.6 (100) | 1.0 (100) | 0.3 (100) |
| ≥3 Inpatient admissions or ≥30 inpatient days | 7.2 (100) | 5.8 (100) | 5.2 (100) | 11 (100) | 6.6 (100) | 10 (100) | 12 (100) | 8.7 (100) | 8.5 (100) | 2.5 (100) | 1.1 (100) | 3.0 (100) |
| Number of person-episodes | 39,917 | 24,976 | 74,172 | 7,907 | 7,007 | 10,232 | 48,652 | 26,664 | 45,388 | 24,122 | 528 | 15,808 |
| Timely follow-up post discharge (7 days) | 2.5 (24) | 3.1 (22) | 2.8 (19) | 20 (34) | 26 (20) | 20 (30) | 4.7 (28) | 5.6 (22) | 6.6 (22) | 35 (15) | 42 (17) | 39 (16) |
| Timely follow-up post discharge (30 days) | 5.9 (24) | 7.2 (22) | 7.5 (19) | 32 (34) | 50 (20) | 36 (30) | 8.7 (28) | 11 (22) | 12 (22) | 62 (15) | 73 (17) | 69 (16) |
Note. Entries are percentages for dichotomous variables; means [SD] for continuous variables. Number in parentheses is percent of person-episodes eligible for indicator.
Indicator not included in item response theory model.
Main Disparities Findings Based on the Composite Measure. Overall quality of schizophrenia care was lowest for blacks in all states in 2002 (Table 3). Across the states, quality of care for blacks was between a third (NC: −0.484/1.51) and two-fifths (FL: −0.541/1.35) of a standard deviation lower than whites'. For example, in CA, blacks had about 43 percent of the indicators met compared to 58 percent for whites; in NY, the respective figures were 48 percent versus 61 percent; and in NC, 40 percent versus 55 percent (Figure2). Latinos received poorer quality of care compared to whites in all states except in FL. In CA, quality of care for Latinos was on average 0.17 standard deviations lower than whites', a difference that corresponds to about 5 percentage points of indicators met.
Table 3.
Linear Regression Estimates, by State (2002–2008)
| State | California | Florida | New York | North Carolina |
|---|---|---|---|---|
| Number of patient-episodes | 139,065 | 25,146 | 120,704 | 40,458 |
| Unadjusted average standardized composite score [SD] | 0.0047 [1.65] | −0.34 [1.35] | −0.39 [1.36] | −0.55 [1.51] |
| Parameter | Estimate | SE | Estimate | SE | Estimate | SE | Estimate | SE |
|---|---|---|---|---|---|---|---|---|
| Intercept (White) | 0.0822 | 0.0090 | −0.188 | 0.0194 | −0.188 | 0.0082 | −0.318 | 0.0099 |
| Black | −0.613 | 0.0101 | −0.541 | 0.0301 | −0.458 | 0.0088 | −0.484 | 0.0128 |
| Latino | −0.286 | 0.0209 | 0.0185 | 0.0303 | −0.285 | 0.0104 | −0.223 | 0.0555 |
| Year | 0.0488 | 0.0024 | −0.000708 | 0.0076 | 0.0176 | 0.0021 | 0.0182 | 0.0037 |
| Year by race interactions | ||||||||
| Year × Black | 0.0364 | 0.0112 | ||||||
| Year × Latino | 0.0133 | 0.0056 | −0.0340 | 0.0141 | ||||
| R2 (%) | 2.9 | 2.5 | 2.3 | 2.5 | ||||
Note. Only statistically significant (p < .05) predictors were retained unless a higher order term was significant.
Figure 2.
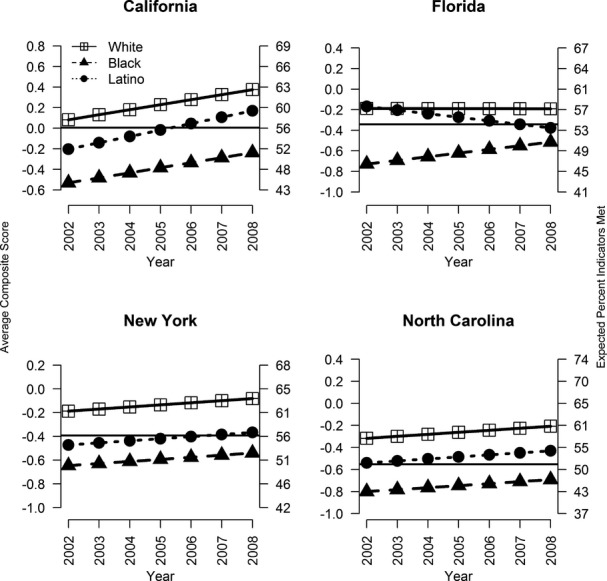
Expected Average Standardized Composite Score, by Year and Race/Ethnicity Group Note. Point estimates obtained from linear regression models. Solid black horizontal line represents average for state. Left-most y-axis provides composite scale while right-most y-axis provides the expected percentage of process indicators met.
Longitudinal changes in quality by race/ethnicity also differed markedly by state (Figure2). Quality of care improved annually for the three racial/ethnic groups in CA, NY, and NC, although in NY the rate of improvement was small. In CA, the annual increase in quality was 0.049 points for whites and blacks, and slightly larger for Latinos (0.062), an increase that corresponds to a change in indicators met of about 1.0 percentage point per year. As a result, the Latino-white disparity narrowed between 2002 and 2008, but it was not eliminated. In FL, quality of care improved for blacks while it declined for Latinos and, to a lesser extent, also for whites. Thus, between 2002 and 2008, the initial black-white disparity narrowed but a new Latino-white disparity emerged, as evidenced by Latinos meeting 53 percent of indicators in 2008 compared to 57 percent for whites. The variance in quality of care explained by race and time was small, ranging between a low of 2.3 percent (NY) and a high of 2.9 percent (CA).
Discussion
Using a composite measure of quality of schizophrenia care encompassing a broad spectrum of treatment practices, we found evidence of poor overall quality of care and of racial/ethnic disparities in quality, larger for blacks than Latinos, among Medicaid beneficiaries residing in CA, FL, NY, and NC. However, in FL and at the beginning of the study period in 2002, quality of care was lowest for blacks, yet it was not different between Latinos and whites. The size of the minority-white disparities varied across the study states, in 2002 and over time. Most of the minority-white disparities observed in 2002 remained unchanged over the 7-year study period, with the exceptions of the narrowing of the black-white disparity in FL and the narrowing of the Latino-white disparity in CA. In FL, a new Latino-white disparity emerged during the period. Notably, we found that race/ethnicity, while important, explains only a small portion of the variation in quality within the states.
The Affordable Care Act required that DHHS establish an Adult Medicaid Quality Measurement Program to fund development, testing, and validation of evidence-based measures to track quality of care for adults enrolled in Medicaid. The current measure set released January 2013 includes 26 measures with only 1 targeted to schizophrenia (Centers for Medicare and Medicaid Services 2013) in spite of the important role played by Medicaid in financing schizophrenia care (Frank and Glied 2006) and the substantial impact of schizophrenia care on the program's budget (Banthin and Miller 2006). Improving quality of care and health outcomes for schizophrenia requires attention to a broad array of processes of care, a task that is facilitated by the use of composite quality measures constructed with modern psychometric and statistical modeling techniques. Although other studies have assessed quality of schizophrenia care using several process-of-care indicators (e.g., Watkins et al. 2011) as well as racial/ethnic disparities in schizophrenia care (e.g., Busch et al. 2009), to our knowledge, ours is the first study in the mental health field that has used a composite measure to comprehensively assess quality of care and to assess whether quality varies by race/ethnicity and whether quality and disparities have changed over time. Moreover, we are not aware of other studies that have assessed whether disparities in quality of care for psychiatric populations vary across state Medicaid programs. While the use of composite quality measures has been hailed as a means to “provide a more complete understanding of the quality of the U.S. health care system” (Agency for Healthcare Research and Quality 2013), not many studies have used composite measures to assess quality and equity of care for publicly insured FFS beneficiaries with non–mental health conditions. Bynum et al. (2010) assessed quality of diabetes care among FFS Medicare beneficiaries with diabetes using a composite measure that represented the average rates of three HEDIS indicators pooled across the years 2003–2005 and found that blacks received 70 percent of recommended care while non-blacks received 76.9 percent. Although our studies are not comparable due to the different populations, conditions, and approaches used to construct the composite, the order of magnitude of the disparity is not too dissimilar from ours, but the overall level of quality of care is much lower in our population (e.g., in CA in 2002, blacks had about 43 percent of the measures met compared to 58 percent for whites).
Our finding that disparities in quality vary both across and within states is consistent with evidence for other health conditions (Fisher, Goodman, and Chandra 2008). The effect of geography on disparities in care among Medicaid beneficiaries is likely mediated by the characteristics of the communities where minority populations live, for example, the states' public policy environment (Alegria, Perez, and Williams 2003; Zuvekas and Taliaferro 2003; Smedley 2008); characteristics of the health care system, including availability of safety net providers (Zuvekas and Taliaferro 2003; Trivedi et al. 2005a); and socio-economic characteristics of the geographic region (Zuvekas and Taliaferro 2003).
These factors probably vary in their importance across states. Policies by the state Medicaid agency and the State Mental Health Agency overseeing publicly funded services are the main drivers of the states' health policy environment for this population. Per capita Medicaid spending on disabled beneficiaries varies widely across our study states, with NY spending more than twice than FL in 2008, for example (Centers for Medicare and Medicaid Services 2011). The four states ranked in similar order in terms of per capita expenditures by the state mental health agency (2004), with NY's at the top (and no. 3 nationally) and FL's at the bottom (and no. 49 nationally) (). Crude spending metrics, however, may not accurately reflect the effectiveness of the states' quality and equity improvement efforts. For example, by 2007, only NC had taken steps toward implementing an optional Medicaid benefit that covers interpreter services, a service that may have little association with per capita spending but may be enormously important in alleviating disparities (Youdelman 2007).
Our study states also differ in the characteristics of their health care systems, including accessibility, availability, and quality of individual and institutional providers. Accessibility to services may be lower in states like CA, where the percentage of minorities in rural communities is over 25 percent, than in states like NY, where share of minorities in rural communities is four times lower (US Census Bureau 2013). Poverty, crime, and other social ills may affect quality of care (Krieger et al. 2005), as documented for people with schizophrenia (Kuno and Rothbard 2005). If minorities are over-represented in socially disadvantaged communities, this effect will also impact equity of care, as demonstrated by a study that found that a state-level Latino-white disparity in access to a pharmacological innovation by FL Medicaid beneficiaries with schizophrenia was driven by the geographic concentration of Latinos in a low-adoption area (Horvitz-Lennon, Alegría, and Normand 2012). Some of these factors may underlie our finding that quality of care varied substantially within state Medicaid programs even after accounting for the variation associated with patients' race/ethnicity.
The lack of significant improvement in racial/ethnic disparities in quality of schizophrenia care between 2002 and 2008, a period of growing awareness and policy interest in health care disparities (e.g., U.S. Department of Health and Human Services 2001) is concerning. The evidence on whether disparities are narrowing over time is decidedly mixed, both for physical health care (Trivedi et al. 2005b; Brady et al. 2007) and other mental health care (Cook, McGuire, and Miranda 2007; Stockdale et al. 2008). Prior evidence on temporal trends in schizophrenia care is limited and rater dated (e.g., Busch et al. 2009). A recent study found that disparities in utilization and spending among Medicaid beneficiaries with schizophrenia were either modestly reduced or unchanged during the period 1994–2006 (Horvitz-Lennon et al. 2009b).
Our study has some limitations. First, because we relied on administrative claims data, our findings may have been unduly influenced by the varying completeness of the documentation. Although the lower specificity and greater variability across states in the coding of psychosocial services limited our characterization of quality of psychosocial care and our ability to compare states' quality of care, this potential limitation does not affect our ability to compare states on disparities and temporal trends in both quality and disparities. Second, our results have limited generalizability beyond our study states. However, that one-third of all Medicaid beneficiaries in the United States live in those states enhances the significance of our findings. Third, we are unable to directly validate the impact of our composite measure on those outcomes. However, most indicators selected for inclusion in our composite measure describe processes of care with strong empirical evidence of effectiveness or safety or lack thereof. With regard to pharmacological indicators: antipsychotic drugs reduce disturbing psychotic symptoms (e.g., Buchanan et al. 2010); clozapine is uniquely effective in reducing treatment-resistant and suicidal presentations (e.g., McEvoy et al. 2006; Buchanan et al. 2010); poor antipsychotic adherence leads to symptomatic decompensation and higher risk of hospitalization (e.g., Leucht and Heres 2006; Novick et al. 2010); nonclozapine antipsychotic polypharmacy lacks evidence of effectiveness and can be harmful (e.g., Waddington, Youssef, and Kinsella 1998; Kreyenbuhl et al. 2007; Correll et al. 2008); and although the evidence is less consistent largely due to methodological reasons, long-acting injectable antipsychotics are associated with reduced relapse rates (e.g., Leucht et al. 2011; Tiihonen et al. 2011). With regard to psychosocial indicators, psychosocial interventions such as assertive community treatment (ACT) and supported employment improve functioning and, in the case of ACT, also reduce risk of hospitalization (e.g., Dixon et al. 2010), and psychotherapy (namely, cognitive behavioral therapy) reduces disturbing psychotic symptoms and improves functioning (e.g., Wykes et al. 2008; Dixon et al. 2010). With regard to our appropriateness indicators, prompt follow-up care following discharge (continuity of care) reduces risk of relapse and hospitalization (e.g., Cuffel, Held, and Goldman 2002; Lin and Lee 2008). Although there is no direct empirical evidence on the process-outcome link for routine psychiatric care, the American Psychiatric Association (APA) calls for regular visits to assess treatment response, tolerability, and adherence (APA 2006). The same may be said about heavy use of ED and inpatient services: although use of these services can be warranted and beneficial, their heavy use may indicate inadequate access to or quality of schizophrenia care (e.g., Catalano et al. 2003; Gilmer et al. 2010). Additionally, several of these indicators have been endorsed by accrediting bodies and professional organizations (NCQA, HEDIS, Joint Commission, APA).
Our findings have important implications for efforts to improve quality while also reducing disparities among vulnerable populations. A critical question in the disparities field is whether improvements in quality will lead to improvements in equity and eradication of health care disparities. Prior research has been equivocal in this regard. Quality improvement intervention trials in depression care have yielded mixed findings (Miranda et al. 2003; Bao et al. 2011). The only study that to our knowledge has evaluated the disparities effects of evidence-based care among people with schizophrenia and other severe mental illnesses found a reduction in disparities for blacks but not for Latinos (e.g., Horvitz-Lennon et al. 2011). Quality improvement efforts need to be designed to target all racial/ethnic groups equally, thus effecting progress in both quality and equity of care. Further research is needed to understand county-level policy and other contextual factors associated with quality and equity, and to identify clinical, systems, and policy interventions that may lead to gains in both dimensions. Evaluating the predictive validity of our composite measure is also an important area for future research.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was supported by R01 MH087488 from the National Institute of Mental Health. The authors are grateful to two anonymous reviewers for their comments on an earlier draft of this article. Authors report no financial relationships with commercial interests.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Appendix SA2: Total Number of Measures Met, Stratified by Groups Formed by Lowest and Highest Deciles of Composite Quality Scores.
References
- Agency for Healthcare Research and Quality. National Healthcare Disparities Report 2012. Rockville, MD: USDHHS; 2013. [Google Scholar]
- Alegria M, Perez DJ. Williams S. “The Role of Public Policies in Reducing Mental Health Status Disparities for People of Color”. Health Affairs. 2003;22(5):51–64. doi: 10.1377/hlthaff.22.5.51. [DOI] [PubMed] [Google Scholar]
- APA. “Practice Guideline for Psychiatric Evaluation of Adults, Second Edition. American Psychiatric Association”. American Journal of Psychiatry. 2006;163(6 Suppl):3–36. [PubMed] [Google Scholar]
- Banthin JS. Miller GE. “Trends in Prescription Drug Expenditures by Medicaid Enrollees”. Medical Care. 2006;44(5):I-27–35. doi: 10.1097/01.mlr.0000208132.36055.84. [DOI] [PubMed] [Google Scholar]
- Bao Y, Alexopoulos GS, Casalino LP, Ten Have TR, Donohue JM, Post EP, Schackman BR. Bruce ML. “Collaborative Depression Care Management and Disparities in Depression Treatment and Outcomes”. Archives of General Psychiatry. 2011;68(6):627–36. doi: 10.1001/archgenpsychiatry.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio C, Yamada AM, Hough RL, Hawthorne W, Garcia P. Jeste DV. “Ethnic Disparities in Use of Public Mental Health Case Management Services among Patients with Schizophrenia”. Psychiatric Services. 2003;54(9):1264–70. doi: 10.1176/appi.ps.54.9.1264. [DOI] [PubMed] [Google Scholar]
- Borck R, Dodd AH, Zlatinov A, Verghese S, Malsberger R. Petroski C. The Medicaid Analytic Extract 2008 Chartbook. Centers for Medicare and Medicaid Services; 2012. [accessed on November 29, 2012]. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/MedicaidDataSourcesGenInfo/MAX_Chartbooks.html. [Google Scholar]
- Brady J, Ho K, Kelley E. Clancy CM. “AHRQ's National Healthcare Quality and Disparities Reports: An Ever-Expanding Road Map for Improvement”. Health Services Research. 2007;42(3 Pt 1):xi–xxi. doi: 10.1111/j.1475-6773.2007.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, Himelhoch S, Fang B, Peterson E, Aquino PR. Keller W. “The 2009 Schizophrenia Port Psychopharmacological Treatment Recommendations and Summary Statements”. Schizophrenia Bulletin. 2010;36(1):71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch AB, Frank RG. Lehman AF. “The Effect of a Managed Behavioral Health Carve-Out on Quality of Care for Medicaid Patients Diagnosed as Having Schizophrenia”. Archives of General Psychiatry. 2004;61(5):442–8. doi: 10.1001/archpsyc.61.5.442. [DOI] [PubMed] [Google Scholar]
- Busch AB, Lehman AF, Goldman H. Frank RG. “Changes over Time and Disparities in Schizophrenia Treatment Quality”. Medical Care. 2009;47(2):199–207. doi: 10.1097/MLR.0b013e31818475b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynum JP, Fisher ES, Song Y, Skinner J. Chandra A. “Measuring Racial Disparities in the Quality of Ambulatory Diabetes Care”. Medical Care. 2010;48(12):1057–63. doi: 10.1097/MLR.0b013e3181f37fcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano R, McConnell W, Forster P, McFarland B. Thornton D. “Psychiatric Emergency Services and the System of Care”. Psychiatric Services. 2003;54(3):351–5. doi: 10.1176/appi.ps.54.3.351. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. 2011. “Research, Statistics, Data and Systems: Medicare and Medicaid Statistical Supplement 2011 Edition” [accessed on July 1, 2013]. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareMedicaidStatSupp/2011.html.
- Centers for Medicare & Medicaid Services. 2013. “Initial Core Set of Health Care Quality Measures for Adults Enrolled in Medicaid (Medicaid Adult Core Set). Technical Specifications and Resource Manual for Federal Fiscal Year 2013” [accessed June 20, 2013]. Available at http://www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Quality-of-Care/Adult-Health-Care-Quality-Measures.html. [PubMed]
- Cook BL, McGuire T. Miranda J. “Measuring Trends in Mental Health Care Disparities, 2000-2004”. Psychiatric Services. 2007;58(12):1533–40. doi: 10.1176/ps.2007.58.12.1533. [DOI] [PubMed] [Google Scholar]
- Correll CU, Rummel-Kluge C, Corves C, Kane JM. Leucht S. “Antipsychotic Combinations vs Monotherapy in Schizophrenia: A Meta-Analysis of Randomized Controlled Trials”. Schizophrenia Bulletin. 2008;35(2):443–57. doi: 10.1093/schbul/sbn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffel BJ, Held M. Goldman W. “Predictive Models and the Effectiveness of Strategies for Improving Outpatient Follow-up under Managed Care”. Psychiatric Services. 2002;53(11):1438–43. doi: 10.1176/appi.ps.53.11.1438. [DOI] [PubMed] [Google Scholar]
- Dickey B, Normand S-LT, Hermann RC, Eisen SV, Cortes DE, Cleary PD. Ware N. “Guideline Recommendations for Treatment of Schizophrenia: The Impact of Managed Care”. Archives of General Psychiatry. 2003;60(4):340–8. doi: 10.1001/archpsyc.60.4.340. [DOI] [PubMed] [Google Scholar]
- Dixon LB, Dickerson F, Bellack AS, Bennett M, Dickinson D, Goldberg RW, Lehman A, Tenhula WN, Calmes C, Pasillas RM, Peer J, Kreyenbuhl J T. Schizophrenia Patient Outcomes Research. “The 2009 Schizophrenia Port Psychosocial Treatment Recommendations and Summary Statements”. Schizophrenia Bulletin. 2010;36(1):48–70. doi: 10.1093/schbul/sbp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essock SM, Covell NH, Leckman-Westin E, Lieberman JA, Sederer LI, Kealey E. Finnerty MT. “Identifying Clinically Questionable Psychotropic Prescribing Practices for Medicaid Recipients in New York State”. Psychiatric Services. 2009;60(12):1595–602. doi: 10.1176/ps.2009.60.12.1595. [DOI] [PubMed] [Google Scholar]
- Fisher ES, Goodman DC. Chandra A. “Regional and Racial Variation in Health Care among Medicare Beneficiaries. A Brief Report of the Dartmouth Atlas Project”. Aligning Forces for Quality. 2008 [accessed on December 18, 2008]. Available at http://www.rwjf.org/qualityequality/product.jsp?id=36729. [PubMed] [Google Scholar]
- Frank RG. Glied SA. Better but Not Well: Mental Health Policy in the U.S. Since 1950. Baltimore, MA: Johns Hopkins University Press; 2006. [Google Scholar]
- Gilmer TP, Dolder CR, Folsom DP, Mastin W. Jeste DV. “Antipsychotic Polypharmacy Trends among Medi-Cal Beneficiaries with Schizophrenia in San Diego County, 1999-2004”. Psychiatric Services. 2007;58(7):1007–10. doi: 10.1176/ps.2007.58.7.1007. [DOI] [PubMed] [Google Scholar]
- Gilmer TP, Stefancic A, Ettner SL, Manning WG. Tsemberis S. “Effect of Full-Service Partnerships on Homelessness, Use and Costs of Mental Health Services, and Quality of Life among Adults with Serious Mental Illness”. Archives of General Psychiatry. 2010;67(6):645–52. doi: 10.1001/archgenpsychiatry.2010.56. [DOI] [PubMed] [Google Scholar]
- Hays RD, Morales LS. Reise SP. “Item Response Theory and Health Outcomes Measurement in the 21st Century”. Medical Care. 2000;38(9 Suppl):1128–42. doi: 10.1097/00005650-200009002-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz-Lennon M, Alegría M. Normand S-LT. “The Effect of Race-Ethnicity and Geography on Adoption of Innovations in the Treatment of Schizophrenia”. Psychiatric Services. 2012;63(12):1171–7. doi: 10.1176/appi.ps.201100408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz-Lennon M, Donohue JM, Domino ME. Normand SL. “Improving Quality and Diffusing Best Practices: The Case of Schizophrenia”. Health Affairs. 2009a;28(3):701–12. doi: 10.1377/hlthaff.28.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz-Lennon M, McGuire TG, Alegria M. Frank RG. “Racial and Ethnic Disparities in the Treatment of a Medicaid Population with Schizophrenia”. Health Services Research. 2009b;44(6):2106–22. doi: 10.1111/j.1475-6773.2009.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz-Lennon M, Zhou D, Normand SL, Alegria M. Thompson WK. “Racial and Ethnic Service Use Disparities among Homeless Adults with Severe Mental Illnesses Receiving Act”. Psychiatric Services. 2011;62(6):598–604. doi: 10.1176/appi.ps.62.6.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns AE, Khosla S. Kostenuik PJ. “Receptor Activator of Nuclear Factor Kappab Ligand and Osteoprotegerin Regulation of Bone Remodeling in Health and Disease”. Endocrine Reviews. 2008;29(2):155–92. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyenbuhl J, Zito JM, Buchanan RW, Soeken KL. Lehman AF. “Racial Disparity in the Pharmacological Management of Schizophrenia”. Schizophrenia Bulletin. 2003;29(2):183–94. doi: 10.1093/oxfordjournals.schbul.a006996. [DOI] [PubMed] [Google Scholar]
- Kreyenbuhl JA, Valenstein M, McCarthy JF, Ganoczy D. Blow FC. “Long-Term Antipsychotic Polypharmacy in the Va Health System: Patient Characteristics and Treatment Patterns”. Psychiatric Services. 2007;58(4):489–95. doi: 10.1176/appi.ps.58.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Rehkopf DH. Subramanian SV. “Painting a Truer Picture of Us Socioeconomic and Racial/Ethnic Health Inequalities: The Public Health Disparities Geocoding Project”. American Journal of Public Health. 2005;95(2):312–23. doi: 10.2105/AJPH.2003.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno E. Rothbard AB. “The Effect of Income and Race on Quality of Psychiatric Care in Community Mental Health Centers”. Community Mental Health Journal. 2005;41(5):613–22. doi: 10.1007/s10597-005-6365-z. [DOI] [PubMed] [Google Scholar]
- Lehman AF, Steinwachs DM The Co-Investigators of the PORT Project. “Translating Research into Practice: The Schizophrenia Patient Outcomes Research Team (Port) Treatment Recommendations”. Schizophrenia Bulletin. 1998;24(1):1–10. doi: 10.1093/oxfordjournals.schbul.a033302. [DOI] [PubMed] [Google Scholar]
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO. Kreyenbuhl J. “Practice Guideline for the Treatment of Patients with Schizophrenia, Second Edition”. American Journal of Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- Leslie DL. Rosenheck RA. “Adherence of Schizophrenia Pharmacotherapy to Published Treatment Recommendations: Patient, Facility, and Provider Predictors”. Schizophrenia Bulletin. 2004;30(3):649–58. doi: 10.1093/oxfordjournals.schbul.a007112. [DOI] [PubMed] [Google Scholar]
- Leucht S. Heres S. “Epidemiology, Clinical Consequences, and Psychosocial Treatment of Nonadherence in Schizophrenia”. Journal of Clinical Psychiatry. 2006;67(suppl 5):3–8. [PubMed] [Google Scholar]
- Leucht C, Heres S, Kane JM, Kissling W, Davis JM. Leucht S. “Oral versus Depot Antipsychotic Drugs for Schizophrenia—a Critical Systematic Review and Meta-Analysis of Randomised Long-Term Trials”. Schizophrenia Research. 2011;127(1–3):83–92. doi: 10.1016/j.schres.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Lin HC. Lee HC. “The Association between Timely Outpatient Visits and the Likelihood of Rehospitalization for Schizophrenia Patients”. American Journal of Orthopsychiatry. 2008;78(4):494–7. doi: 10.1037/a0014515. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RSE, Davis CE, Severe J, Hsiao JK for the CATIE Investigators. “Effectiveness of Clozapine versus Olanzapine, Quetiapine, and Risperidone in Patients with Chronic Schizophrenia Who Did Not Respond to Prior Atypical Antipsychotic Treatment”. American Journal of Psychiatry. 2006;163(4):600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Miranda J, Duan N, Sherbourne C, Schoenbaum M, Lagomasino I, Jackson-Triche M. Wells KB. “Improving Care for Minorities: Can Quality Improvement Interventions Improve Care and Outcomes for Depressed Minorities? Results of a Randomized, Controlled Trial”. Health Services Research. 2003;38(2):613–30. doi: 10.1111/1475-6773.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Association of State Mental Health Program Directors Research Institute Inc. “State Profile Data and Reports” [accessed on July 1, 2013]. Available at http://www.nri-inc.org/projects/profiles/data_search.cfm.
- Novick D, Haro JM, Suarez D, Perez V, Dittmann RW. Haddad PM. “Predictors and Clinical Consequences of Non-Adherence with Antipsychotic Medication in the Outpatient Treatment of Schizophrenia”. Psychiatry Research. 2010;176(2–3):109–13. doi: 10.1016/j.psychres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC. Doshi JA. “Continuity of Care after Inpatient Discharge of Patients with Schizophrenia in the Medicaid Program: A Retrospective Longitudinal Cohort Analysis”. Journal of Clinical Psychiatry. 2010;71(7):831–8. doi: 10.4088/JCP.10m05969yel. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC. Wan GJ. “Treatment Patterns for Schizoaffective Disorder and Schizophrenia among Medicaid Patients”. Psychiatric Services. 2009;60(2):210–6. doi: 10.1176/ps.2009.60.2.210. [DOI] [PubMed] [Google Scholar]
- Smedley BD. “Moving beyond Access: Achieving Equity in State Health Care Reform”. Health Affairs. 2008;27(2):447–55. doi: 10.1377/hlthaff.27.2.447. [DOI] [PubMed] [Google Scholar]
- Smedley BD, Stith AY. Nelson AR. In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Smedley BD, Stith AY, Nelson AR, editors; Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- Stockdale SE, Lagomasino IT, Siddique J, McGuire T. Miranda J. “Racial and Ethnic Disparities in Detection and Treatment of Depression and Anxiety among Psychiatric and Primary Health Care Visits, 1995-2005”. Medical Care. 2008;46(7):668–77. doi: 10.1097/MLR.0b013e3181789496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX. Korhonen P. “A Nationwide Cohort Study of Oral and Depot Antipsychotics after First Hospitalization for Schizophrenia”. American Journal of Psychiatry. 2011;168(6):603–9. doi: 10.1176/appi.ajp.2011.10081224. [DOI] [PubMed] [Google Scholar]
- Trivedi AN, Gibbs B, Nsiah-Jefferson L, Ayanian JZ. Prothrow-Stith D. “Creating a State Minority Health Policy Report Card”. Health Affairs. 2005a;24(2):388–96. doi: 10.1377/hlthaff.24.2.388. [DOI] [PubMed] [Google Scholar]
- Trivedi AN, Zaslavsky AM, Schneider EC. Ayanian JZ. “Trends in the Quality of Care and Racial Disparities in Medicare Managed Care”. New England Journal of Medicine. 2005b;353(7):692–700. doi: 10.1056/NEJMsa051207. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2013. “American Community Survey” [accessed on June 18, 2013]. Available at http://factfinder2.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t.
- U.S. Department of Health and Human Services. Mental Health: Culture, Race, and Ethnicity—A Supplement to Mental Health: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services; 2001. [Google Scholar]
- U.S. Department of Health and Human Services. New Freedom Commission on Mental Health: Achieving the Promise: Transforming Mental Health Care in America. Rockville, MD: U.S. Department of Health and Human Services; 2003. [Google Scholar]
- Vega WA, Karno M, Alegria M, Alvidrez J, Bernal G, Escamilla M, Escobar J, Guarnaccia P, Jenkins J, Kopelowicz A, Lagomasino IT, Lewis-Fernandez R, Marin H, Lopez S. Loue S. “Research Issues for Improving Treatment of U.S. Hispanics with Persistent Mental Disorders”. Psychiatric Services. 2007;58(3):385–94. doi: 10.1176/ps.2007.58.3.385. [DOI] [PubMed] [Google Scholar]
- Waddington JL, Youssef HA. Kinsella A. “Mortality in Schizophrenia. Antipsychotic Polypharmacy and Absence of Adjunctive Anticholinergics over the Course of a 10-Year Prospective Study”. British Journal of Psychiatry. 1998;173:325–9. doi: 10.1192/bjp.173.4.325. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Pincus HA, Paddock S, Smith B, Woodroffe A, Farmer C, Sorbero ME, Horvitz-Lennon M, Mannle T, Hepner KA, Solomon J. Call C. “Care for Veterans with Mental and Substance Use Disorders: Good Performance, but Room to Improve on Many Measures”. Health Affairs. 2011;30(11):2194–203. doi: 10.1377/hlthaff.2011.0509. [DOI] [PubMed] [Google Scholar]
- Wykes T, Steel C, Everitt B. Tarrier N. “Cognitive Behavior Therapy for Schizophrenia: Effect Sizes, Clinical Models, and Methodological Rigor”. Schizophrenia Bulletin. 2008;34(3):523–37. doi: 10.1093/schbul/sbm114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdelman M. “Medicaid and Schip Funding for Language Services”. Research Brief. 2007 “.” [accessed on June 4, 2013]. Available at http://www.naph.org. [Google Scholar]
- Zaslavsky AM. Ayanian JZ. “Integrating Research on Racial and Ethnic Disparities in Health Care over Place and Time. [Editorial]”. Medical Care. 2005;43(4):303–7. doi: 10.1097/01.mlr.0000159975.43573.8d. [DOI] [PubMed] [Google Scholar]
- Zuvekas SH. Taliaferro GS. “Pathways to Access: Health Insurance, the Health Care Delivery System, and Racial/Ethnic Disparities, 1996-1999”. Health Affairs. 2003;22(2):139–53. doi: 10.1377/hlthaff.22.2.139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: Total Number of Measures Met, Stratified by Groups Formed by Lowest and Highest Deciles of Composite Quality Scores.


