Abstract
Background
There is a range of factors that predict the development of Alzheimer’s disease (AD) dementia among patients with amnestic Mild Cognitive Impairment (MCI).
Objectives
To identify the neuropsychological, genetic and functional brain imaging data that best predict conversion to AD dementia in patients with amnestic MCI.
Methods
From an initial group of 42 amnestic MCI patients assessed with neurological, neuropsychological and brain SPECT, 39 (25 converters, 14 non-converters) were followed for 4 years, and 36 had APOE ε4 genotyping. Baseline neuropsychological data and brain SPECT data were used to predict which of the MCI patients would develop dementia by the end of the 4 years of observation.
Results
The MCI patients who had converted to AD dementia had poorer performance on long-term visual memory and Semantic Fluency tests. The MCI subjects who developed dementia were more likely to carry at least one copy of the APOE ε4 allele (Hazard Risk = 4.22). There was lower brain perfusion in converters than non-converters, mainly in postcentral gyrus. An additional analysis of the SPECT data found differences between the MCI subjects and controls in the posterior cingulate gyrus and the basal forebrain. When the brain imaging and neuropsychological test data were combined in the same Cox regression model, only the neuropsychological test data were significantly associated with time to dementia.
Conclusion
Although the presence of reduced brain perfusion in postcentral gyrus and basal forebrain indicated an at-risk condition, it was the extent of memory impairment that was linked to the speed of decline from MCI to AD.
Keywords: Visual memory, cerebral perfusion, brain SPECT, four-year follow-up, prospective, longitudinal, Mild Cognitive Impairment, Alzheimer’s disease
INTRODUCTION
The dementia associated with Alzheimer's disease (AD) is commonly diagnosed among individuals older than 65 years [1], however, the underlying neuropathological changes begin many years before clinical onset [2, 3]. The identification of the preclinical state has led to the desire to develop intervention models that can delay or prevent the onset of the clinical dementia [4]. Studies that examine risk factors for the development of clinical dementia from an asymptomatic state reveal the complexity of the problem [4]. However, the risk factors associated with the development of dementia from the prodromal syndrome of Mild Cognitive Impairment (MCI) are somewhat more clear [5–8], and include age, the APOE ε4 allele and memory function (among others).
With the increase of interest in these prodromal syndromes, there is also an increase of interest in the use of biomarker data, often from expensive or invasive tests including Magnetic Resonance Imaging, Single Photon Emission Computed Tomography (SPECT), Positron Emmission Tomography, and the analysis of cerebral spinal fluid for the presence of beta amyloid. By contrast, more cost-effective measures of brain function, specifically neuropsychological tests, are also able to predict risk of developing dementia from MCI [9], and are sufficiently sensitive that the combination with biomarker data does not carry a significant increase in prediction quality [5–6, 10].
MCI typically (but not always) includes evidence of memory loss, and the memory loss most commonly exists in the presence of deficits in performance in other cognitive domains [8, 11–21]. Those patients with MCI with multiple cognitive deficits are more likely to develop clinical dementia than those who have an isolated memory deficit [8, 16, 22–27]. Nevertheless, the best neuropsychological predictors of the development of dementia from MCI appear to be related to the extent of the memory loss (Delayed recall of Logical Memory WMS-R [26, 28, 29], Delayed recall of a list of words [6, 30–32]; Recognition task of a list of words [7, 33]; Delayed recall of the Rey’s Complex Figure [31, 32]). Others have noted that impairments in executive functions (i.e., Trail Making B [6, 32]), language (i.e., Semantic Fluency [6, 26, 34]), or even a summary measure of mental state (i.e., MMSE [6, 35], Clock test [26]) are better predictors of dementia than memory function alone. However, it should be noted that even among the longitudinal studies, follow-up is generally limited to two years or fewer and most of the conversions from MCI to dementia occur relatively early in the longitudinal follow-up suggesting that these patients were on the cusp of clinical dementia (for discussion, see [36]).
Brain functional imaging studies with SPECT have found that those patients who develop dementia from MCI showed reduced cerebral blood flow in a variety of brain regions including the medial temporal lobe, the temporal/parietal cortex, the posterior cingulate gyrus, the precuneus, and the prefrontal cortex [35, 37–43]. This pattern of altered perfusion is similar to that seen in mild AD [7, 38, 44–47].
We have previously shown that patients with MCI who developed dementia within two years had poorer performance on measures of verbal recognition memory than those who did not [7]. However, we did not find differences in brain perfusion, as measured by SPECT, between the converters and non-converters. In the present study, we added an additional approach to the analysis of the brain perfusion data by determining the extent to which perfusion in two cerebral regions of interest (posterior cingulate and basal forebrain) contributed to prediction of dementia after a longer, 4-year follow-up period. The purpose of the present study was to describe the neuropsychological and brain SPECT imaging data, along with APOE genotype, in predicting the development of dementia among 42 patients with the amnestic form of MCI.
METHODS
Subjects
Forty-two patients with the amnestic form of MCI were originally enrolled in the study [18]; all of the patients were older than 64 years and were functionally literate at the time of the baseline assessment. None had significant depressive symptoms or a DSM-IV Axis-I psychiatric disorder (except for dementia), neurological disease (other than dementia), or a focal structural lesion on CT imaging. None of the patients had a history of alcohol or other substance abuse, severe visual abnormalities or significant aphasia at the time of study enrollment.
At the time of the baseline assessment and annually thereafter, each participant completed a neurological and neuropsychological assessment. Each of the patients was classified according to current research-level criteria for MCI and dementia (See [7] for details). Over the course of the four years of observation, one of the patients died, one refused to continue, and one was lost to follow-up, leaving 39 for the analysis of dementia incidence. All of them had a baseline SPECT, and 36 had APOEε4 genotyping. Data regarding the demographical and APOE status of the subjects are shown in Table 1.
Table 1.
Demographical and genetic characteristics of the MCI subjects.
| Converters | Non converters | Statistics | Effect size3 | |
|---|---|---|---|---|
| 25 | 14 | |||
| Sex ((%(N)) Male) | 46.2 (6) | 53.8 (7) | 2.731 | 0.26 |
| Education (%(N) ≤ Elementary school) | 61.9 (13) | 38.1 (8) | 0.961 | 0.05 |
| Age in years (mean (SD)) | 77.6 (4.1) | 74.6 (4.0) | 2.182 | 0.75* |
| APOE ε4 allele (%(N) Carrier) | 73.9 (17) | 26.1 (6) | 4.391 | 0.35* |
SD: Standard deviation;
χ2;
t-test.
Cohen’s d for continuous variables, and Cramer’s V for categorical data are reported.
p< 0.05
At baseline, a part from the 42 MCI patients, 42 control subjects and 42 patients with mild AD were assessed. The study inclusion and exclusion criteria were detailed elsewhere [18]. Briefly, the control subjects were volunteers who had no neurological or psychiatric symptoms, no evidence (by history) of functional impairment due to declining cognition, and who had normal performance on the neuropsychological battery. The AD patients all met the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) criteria for Probable AD, with a Clinical Dementia Rating (CDR) score of 1, and they were all taking acetylcholinesterase inhibitors (AChEIs). In the present study, Control and AD groups data were only used for the second SPECT analysis.
Neuropsychological assessment
The neuropsychological battery has been described in detail previously (see previous studies [18, 48] for details) and it includes measures sensitive to orientation, attention, verbal and visual long-term memory, language, visual gnosis, praxis and executive functions. The tests were: Temporal, Spatial and Personal Orientation; Digit span forwards and backwards, Block Design and Similarities subtests of Wechsler Adult Intelligence Scale-Third Edition (WAIS-III); The Word List Learning test from the Wechsler Memory Scale-Third Edition (WMS-III), including a recognition task; the Long-term Visual Memory subtest of The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), including a recognition task; Verbal comprehension (2 simple, 2 semi-complex and 2 complex commands); an abbreviated 15 item confrontation naming test from the Boston Naming Test; the Poppelreuter test; Luria’s Clock test; Ideomotor and Imitation praxis; the Automatic Inhibition subtest of the Syndrom Kurtz Test (SKT); Phonetic Verbal Fluency (words beginning with ‘P’ during one minute); Semantic Verbal Fluency (‘animals’ during one minute), and the Spanish version of the Clock Test.
APOE genotyping
The APOE ε4 allele was identified with commercial kits for APOE rs429358 (SNP112) and rs7412 (SNP158) from Roche Diagnostics (Germany). The APOE alleles were amplified using LightCycler ApoE Mutation Detection Kit (Roche diagnostics, Germany) and were detected using real-time PCR technology (LightcyclerR 480 System, Roche Diagnostics, Germany) following the manufacturer's instructions. Different compound heterozygotes for APOE SNPs were verified in an independent research laboratory in order to check the quality of the results.
Brain SPECT procedure
Within three weeks of the baseline visit, a brain SPECT was performed using procedures previously described [18]. A voxel-based analysis was performed on the SPECT images using Statistical Parametric Mapping (SPM8), running on Matlab 7.9 (Mathworks Inc, Sherborn, MA). Images were initially converted from DICOM to Analyze format using MRIcro (http://www.mccauslandcenter.sc.edu/mricro/index.html), and transferred to SPM8.
The SPECT images were normalized to the Montreal Neurological Institute template and smoothed with a 3D Gaussian kernel (8-mm FWHM). T-test comparisons of SPECT images were performed between converter and non-converter groups. The analysis was carried on a voxel-by-voxel basis, generating Statistical Parametric Maps of group-related differences in brain perfusion (T-map). Results meeting a height threshold p< 0.01 (uncorrected) [49] and an extent threshold of 30 voxels were considered significant. The eigenvariates of the clusters that differed between converter and non-converter groups were extracted and added to the SPSS database.
In addition to identifying directly the brain regions associated with developing dementia (i.e., MCI converters vs MCI non-converters), we also identified regions from the analysis of the SPECT images that were associated with MCI and AD in the posterior cingulate gyrus and basal forebrain (i.e., Controls vs MCI/AD). Cluster-level eigenvariates were extracted, and the values for the MCI patients were also added to the SPSS database (see Figure 2 and Table 4). Finally, the baseline SPECT images were modeled in a multiple regression model with performances on visual recall as a covariate within SPM8. Results meeting a height threshold of p< 0.01 (uncorrected) and k= 100 were considered statistically significant.
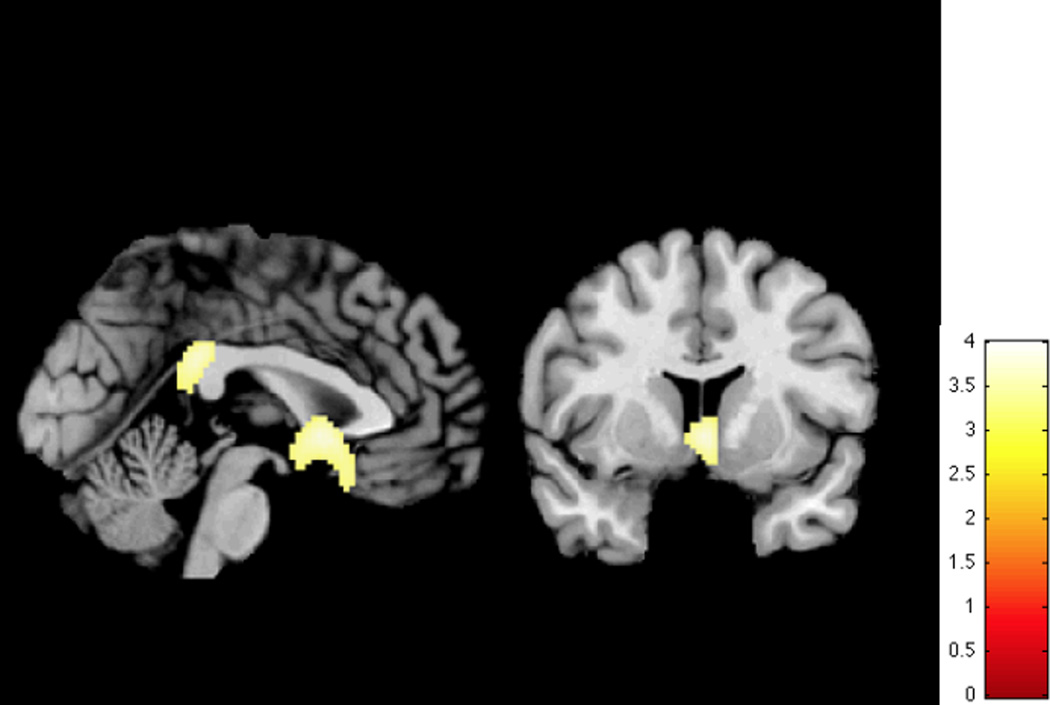
Figure 2.
Regional perfusion defects (Controls > MCI > AD)
The colored scale represents statistical values (T-map), showing statistically significant lower brain perfusion in the MCI and AD groups compared to the Control group.
Table 4.
Cerebral Perfusion in Regions Defined by Between-Group Analysis
| Normal Controls | MCI | AD | Effect size | |
|---|---|---|---|---|
| Posterior Cingulate | 76.9 (5.4) | 72.5 (6.9) | 69.1 (7.2) | 0.181 |
| Basal Forebrain | 67.7 (5.7) | 63.1 (6.9) | 59.9 (8.1) | 0.161 |
eta-squared effect size
Statistical analysis
Statistical analysis of the clinical variables was performed using SPSS (version 20.0; SPSS Inc., Chicago, Ill). T-tests with Bonferroni’s correction for multiple comparisons, and χ2 were carried out to compare baseline sociodemographic, clinical, neuropsychological, SPECT and genetic data as a function of group status at the end of the study. Correlation analyses were performed between neuropsychological data, and between SPECT and neuropsychological data. Adjusted effect sizes for those parameters associated with conversion were calculated. A Cox Regression analysis of time to develop dementia was carried out with the variables that showed statistically significant differences in the unadjusted analysis.
RESULTS
As detailed in [18]), at baseline, the 42 MCI patients, the 42 Controls and the 42 mild AD patients had equivalent ages and educational level, and gender distribution. 25 of the 39 MCI patients (64.1%) developed AD dementia (see Table 1); the scores of the participants on the baseline neuropsychological tests are detailed in Table 2.
Table 2.
Comparison of basal neuropsychological performances between converters and non-converters MCI group subjects.
| Converters X̅ (SD) |
Non converters X̅ (SD) |
t (1,37) | Cohen’s d | |
|---|---|---|---|---|
| MMSE | 25.2 (2.0) | 26.8 (1.6) | 2.48 | 0.88 |
| Temporal Orientation | 3.6 (1.4) | 4.1 (0.9) | 1.14 | 0.37 |
| Memory | ||||
| Total Learning WMS-III | 18.6 (5.5) | 19.9 (3.9) | 0.79 | 0.26 |
| Delayed Recall WMS-III | 1.2 (1.4) | 1.6 (1.4) | 0.78 | 0.25 |
| Recognition WMS-III | 9.2 (2.7) | 8.0 (3.2) | −1.21 | −0.40 |
| Visual mem. RBANS | 2.2 (1.9) | 4.8 (2.5) | 3.68 | 1.21* |
| Visual recog. RBANS | 6.9 (1.8) | 6.4 (2.7) | −0.77 | −0.25 |
| Digit span Forward | 5.0 (1.0) | 5.1 (1.4) | 0.18 | 0.06 |
| Digit span Backward | 3.3 (0.8) | 3.9 (0.9) | 2.03 | 0.67 |
| Praxis | ||||
| Ideomotor | 4.0 (0.2) | 4.0 (0.0) | 0.74 | 0.24 |
| Construction | 3.2 (0.9) | 3.2 (1.2) | 0.16 | 0.05 |
| Imitation | 3.4 (0.9) | 3.4 (0.9) | 0.10 | 0.03 |
| Language | ||||
| 15-item BNT | 13.5 (1.6) | 13.4 (1.8) | −0.16 | −0.05 |
| Visuoperception | ||||
| Poppelreuter (correct) | 9.3 (0.9) | 9.1 (1.1) | −0.42 | −0.14 |
| 15-OT (correct) | 11.1 (2.1) | 11.6 (1.9) | 0.66 | 0.22 |
| Luria’s Clock test | 2.8 (1.0) | 2.7 (0.8) | −0.28 | −0.09 |
| Executive functions | ||||
| SKT seconds | 33.0 (9.0) | 29.8 (6.1) | −1.18 | −0.39 |
| Phonetic Fluency | 11.3 (4.4) | 11.4 (5.0) | 0.05 | 0.02 |
| Semantic Fluency | 10.9 (2.9) | 13.7 (3.1) | 2.79 | 0.92 |
| Abstract Reasoning | 9.4 (3.1) | 9.4 (2.3) | −0.01 | −0.01 |
MMSE: Mini-Mental State Examination; 15-OT: The 15-Objects test; S.E.: Standard error; WMS-III: Wechsler Memory Scale-III; 15-item BNT: 15 items abreviated Boston Naming Test; SKT: Automatic Inhibition subtest of the Syndrom Kurtz Test (number of errors).
Statistically significant after Bonferroni’s correction (p≤ 0.002).
The MCI subjects who converted to AD dementia during the 4-years of follow-up differed from non-converters on the RBANS long-term visual memory scores. Although the performance on the measure of Semantic Fluency and the MMSE scores differed between groups, they did not reach significance after Bonferroni’s correction (p≤ 0.002) (see Table 2).
In addition to the memory impairment, to have language (χ2= 0.36, p= 0.55), visuoperceptive (χ2= 0.098, p= 0.75), praxis (χ2= 2.34, p= 0.126) or executive (χ2= 0.70, p= 0.792) functioning mildly affected was not associated with an higher risk of conversion to AD dementia. Moreover, a greater number of impaired functions was not related to an increased risk of dementia conversion (t= 0.34, p= 0.737).
In the whole MCI group, the visual retention scores significantly correlated with other measures of memory (Temporal Orientation: r= 0.45, p= 0.003; Verbal Retention: r= 0.54, p< 0.0001; and Semantic Fluency: r= 0.44, p= 0.003). Verbal Retention was significantly related to another measure of memory (Visual Retention: r= 0.54, p< 0.0001). Semantic Fluency significantly correlated with memory (Visual Retention: r= 0.44, p= 0.003).
As shown in Table 1, 73.9% of the patients who developed dementia had at least one copy of the APOE ε4 allele compared with 26.1% in non-converters. Although the Logistic Regression analysis showed that after adjusting by age and education, ε4 was not significantly associated with conversion to dementia, the presence of at least one APOE ε4 allele increases by 4 times the risk of conversion to dementia (Hazard Risk, HR= 4.22, CI 95% 0.89–19.84, p= 0.068).
Baseline Differences in Perfusion
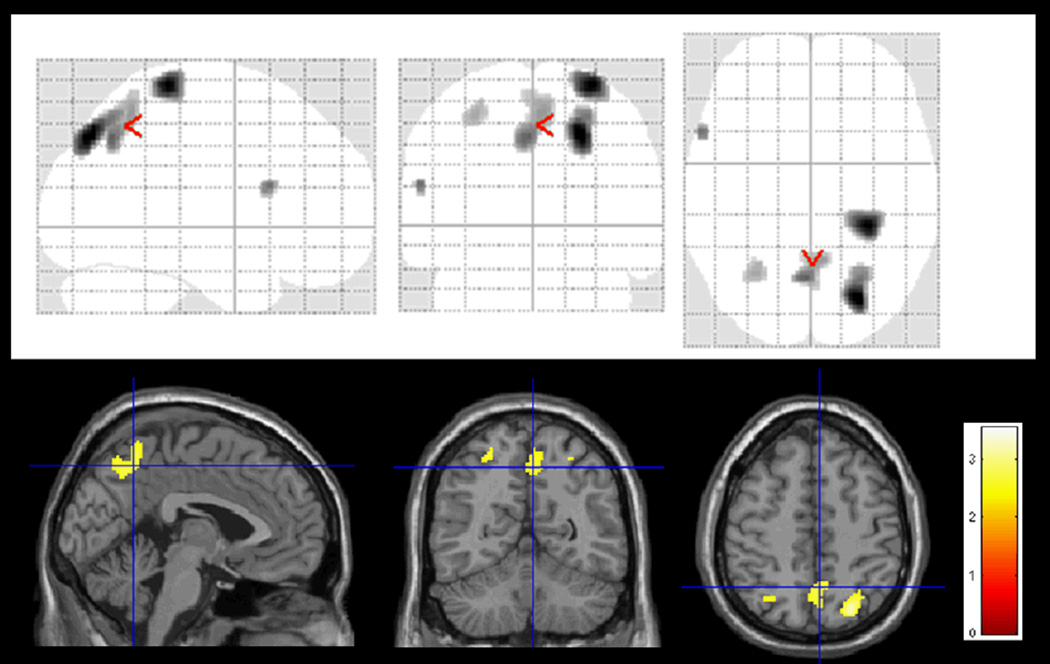
The MCI converter group showed statistically significant lower perfusion at baseline than the non-converter one in the right superior parieto-occipital lobe, right postcentral gyrus, left inferior frontal cortex, left superior parietal lobe, and bilateral precuneus (see Table 3 and Figure 1 for details).
Table 3.
Brain areas showing statistically significant differences between those MCI who converted to AD and those who did not.
| Region | Cluster size (num.voxels) |
T- Score | Z score |
p uncorrected (voxel) |
|---|---|---|---|---|
| Right Superior Occipital | 356 | 3.54 | 3.26 | 0.001 |
| Right Superior Parietal | 2.91 | 2.75 | 0.003 | |
| Right Postcentral gyrus | 327 | 3.52 | 3.25 | 0.001 |
| Left Precuneus | 342 | 3.01 | 2.83 | 0.002 |
| Right Precuneus | 2.75 | 2.60 | 0.005 | |
| Right Precuneus | 2.59 | 2.47 | 0.007 | |
| Left Inferior Frontal (pars triangularis) | 31 | 2.92 | 2.75 | 0.003 |
| Left Superior Parietal | 83 | 2.68 | 2.54 | 0.005 |
Figure 1.
Basal low blood flow on the right superior parieto-occipital lobe, right postcentral gyrus, left inferior frontal cortex, left superior parietal lobe, and bilateral precuneus associated with conversion to AD in amnestic MCI subjects.
At the top, T-map was represented onto a glass brain in three orthogonal planes, called MIP (maximum intensity projection). At the bottom, T-map was superimposed on the canonical single-subject MR image of SPM8, for a better anatomical location.
The colored scale represents statistical values (T-map), showing statistically significant lower brain perfusion in the converter MCI group than in the non converter MCI group (p< 0.01, extent threshold 30 voxels).
The analysis of the baseline SPECT images also revealed two critical brain regions that were sensitive to the presence of MCI and AD. The regions of the basal forebrain and the posterior cingulate gyrus had perfusion levels that were significantly lower in the MCI and AD patients compared to Controls (See Table 4 and Figure 2). The differences in the perfusion values in the posterior cingulate (F(2, 89)= 9.93, p< 0.001, η2 = 0.18) and basal forebrain regions (F(2, 89)= 8.70, p< 0.001, η2 = 0.16) were significant as a function of diagnostic group. On average, perfusion in the normal Controls exceeded that of the MCI group which exceeded that of the AD group (LSD Tests, p< 0.05) (Figure 2).
Prediction of Developing AD from MCI
A Logistic Regression analysis using a forward conditional variable selection (Wald criteria) was carried using the neuropsychological variables that showed statistically significant differences between the two groups of MCI patients in the unadjusted analyses (before Bonferroni correction): RBANS long-term visual memory, Semantic Fluency and MMSE. The presence of APOE ε4 and age (at baseline) were forced into the model in the first step. Only performance on the RBANS long-term visual memory test showed a statistically significant effect (Wald= 4.44, p= 0.035, HR= 0.62; CI 95% 0.39–0.97).
A second Logistic Regression analysis using a forward conditional variable selection (Wald criteria) was performed with the eigenvariates of the clusters that differed between converter and non-converter groups. Only the first eigenvariate (right postcentral gyrus) showed a statistically significant effect (Wald= 5.72, p= 0.017, HR= 0.89; CI 95% 0.82 – 0.98).
Finally, a Logistic Regression analysis with neuropsychological and SPECT significant variables was carried out, showing that only the first eigenvariate (right postcentral gyrus) showed a statistically significant effect (Wald= 5.72, p=0.017, HR= 0.89; CI 95% 0.82 – 0.98). Moreover, a Cox Proportional Hazard model was performed to measure the associations between the various predictor variables and time to develop dementia, showing that only the RBANS long-term visual memory showed a statistically significant effect (Wald= 10.39, p= 0.001, HR= 0.72; CI 95% 0.59 – 0.88).
In the complementary analysis of the SPECT data, eigenvariates from the basal forebrain and posterior cingulate gyrus were extracted. A Cox Proportional Hazard model was performed to measure the associations between the various predictor variables and time to develop dementia. In the first step of the model, only age was significantly associated with time to dementia (See Table 5). In the second step, the RBANS Memory score and Semantic Fluency were significantly associated with time to dementia, and sex was now a significant predictor. Finally, in the third model, using data from the 36 MCI patients with APOE genotyping, we found that once age, sex and RBANS memory performance were entered, APOE and Semantic Fluency were not associated with time to dementia. Neither the perfusion of the basal forebrain nor the posterior cingulate gyrus were significantly linked to dementia in the adjusted model.
Table 5.
Results of Cox Regression Models of Time to Develop Dementia from MCI
| Model 1 | Model 2 | Model 31 | |
|---|---|---|---|
| Age | 1.13 (1.02–1.25)2 | 1.21 (1.08–1.35)2 | 1.47 (1.18–1.84)2 |
| Education | 1.80 (0.70–4.67) | 0.93 (0.32–2.70) | 1.56 (0.45–5.47) |
| Sex | 1.05 (0.73–1.50) | 1.73 (1.09–2.76)2 | 2.96 (1.47–5.99)2 |
| Visual mem. RBANS | 0.67 (0.52–0.86)2 | 0.57 (0.38–0.82)2 | |
| Semantic Fluency | 0.85 (0.74–0.98)2 | 0.89 (0.75–1.05) | |
| APOE ε4 | 0.72 (0.22–2.32) |
N= 36;
p< 0.05
Relationship between neuropsychological and brain perfusion data
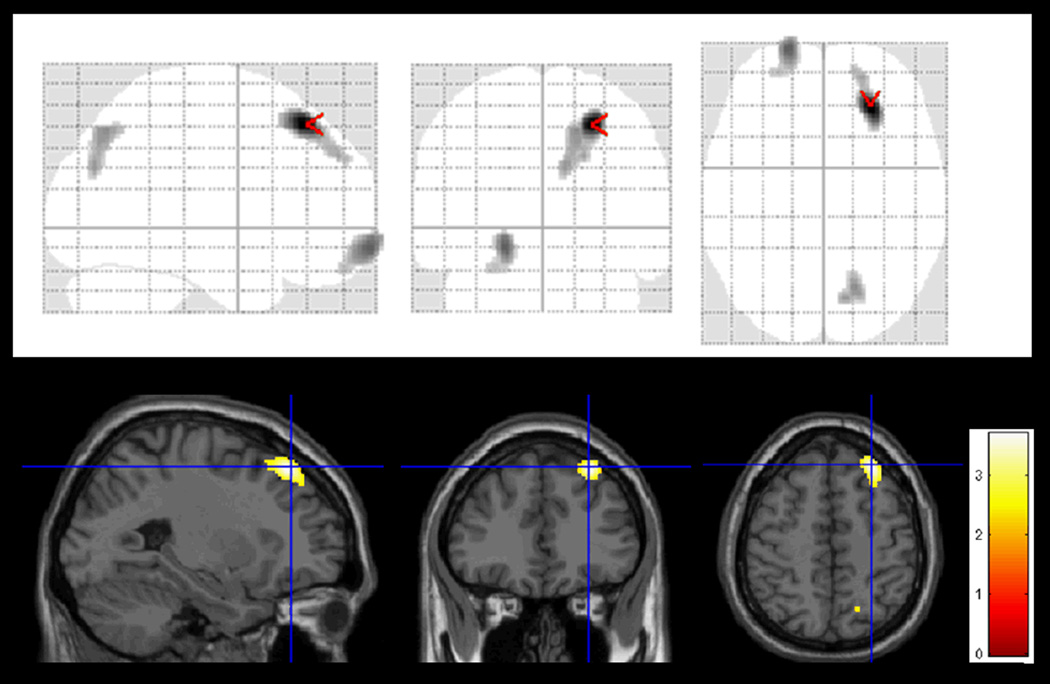
Within the MCI group, the correlations between the two regions (PC and BF) and the neuropsychological test data were small and not significant. However, the baseline SPECT images modeled in a multiple regression model with performances on visual recall as a covariate within SPM8 showed that lower performances on RBANS visual recall were statistically correlated with lower perfusions in Brodman’s Areas 7, 9, 18 and 19 (right) and 11 (left) (see Figure 3).
Figure 3.
Regions showing statistically significant correlation between baseline cerebral perfusion and performance on visual recall in all MCI subjects.
The colored significant areas (X, Y, Z) are located in Brodman’s Areas 7, 9, 18 and 19 (right) and 11 (left).
DISCUSSION
This is the first longitudinal follow-up study of amnestic MCI using a combination of neuropsychological, APOE and brain SPECT data to predict the development of dementia. We found that the measures of neuropsychological function best predicted the time to develop AD in fully adjusted models. Our findings suggest that as visual memory performance begins to failure, the decline toward AD becomes more detectable.
From the 39 subjects with MCI, 64.1% developed dementia during the 4 years of observation. The incidence rate of ~15% per year is similar to that reported in previous longitudinal studies with amnestic MCI subjects [27, 50]. All of the patients who developed dementia developed Probable AD, likely due to the fact that our original sample of MCI patients was carefully selected. In order to be included in this study, they must have had a memory impairment, and they could not have other comorbid medical conditions such as cerebral vascular disease, developmental disabilities, and the like, that could account for their cognitive deficits [8, 51]. Consistent with previous studies [27, 52], to be older and a woman seems to be related with a higher risk to develop AD dementia, and the presence of at least one APOE ε4 allele increased by 4 times the risk of conversion to dementia. In addition, the fact that nearly 75% of the individuals who developed dementia were also carrying at least one copy of the APOE ε4 allele suggests that our MCI patients were, in fact, demonstrating the first clinical signs and symptoms of their underlying AD.
According to previous studies [35, 37–43], MCI patients subjects who developed dementia had perfusion deficits at study entry mainly in the precuneus, but also in right superior parieto-occipital lobes, right postcentral gyrus, left inferior frontal cortex and left superior parietal lobe. Thus, signs of decreased blood flow can already be seen prior to clinical expression of the dementia in the MCI patient who will go on to AD within 4 years. However, at the same time, we found perfusion deficits in the basal forebrain and posterior cingulate that differentiate MCI and AD from normal controls (and from each other), but which are not significantly related to the time to develop dementia. This suggests the possibility that while perfusion defects, reflecting altered function in these brain regions, are necessary for the development of AD, they are not sufficient. Additional impaired function is needed to produce the full clinical syndrome.
Long-term visual memory performance, assessed by RBANS Memory test, has demonstrated to be useful to predict conversion to dementia in MCI subjects. Lower performances on the RBANS Memory test not only were associated with an increased risk of conversion to dementia, but also with a faster conversion. Performances on RBANS retention were significantly correlated with other measures of memory, such as temporal orientation, verbal retention on the WMS-III and semantic fluency, confirming that it is really sensitive to memory. Moreover, the SPM analysis, within the MCI group, showed that better baseline performances on visual recall were related to increased brain perfusion in dorsolateral prefrontal and visual associative areas, reinforcing that visual memory accuracy depends on executive and visuospatial abilities. Thus, our results are confirming the overlap between memory pathways and AD pathogenesis. Visual recall impairment seems to be reflecting the early clinical signs of the underlying AD pathogenesis.
Our study suffers from a small sample of MCI patients. Even though the majority developed dementia within the follow-up window, we likely lacked the statistical power to fully examine the influence of all of the available predictor variables. Our failure to find that the SPECT data did not predict dementia in the fully adjusted models may be due, in part to the sample size. Indeed, the impact of the small sample size can be seen in the instability of the Hazard Risks between models 2 and 3 (when 3 cases are lost due to missing APOE data). Finally, as has been noted previously [36] estimation of true incidence of dementia from MCI, and the identification of risk factors and risk modifiers, requires the opportunity to observe the onset of the MCI. Otherwise, we risk combining patients with long MCI histories with those who only recently developed cognitive impairments into a single group, thus reducing our sensitivity to biomarker variables.
Our findings reinforce the need of administering a complete neuropsychological test assessment for visual/verbal memory recall in the clinical setting, with the consideration of time constraints and effects of testing on the patient. Time of testing can produce both emotional and physical stress on the patient, due to performance anxiety, worry about the diagnosis and AD effects in general. Thus, this study is helpful to know which tests are the most useful to detect prodromal AD to optimize the neuropsychological battery, and to allow an early detection of individuals at risk to develop AD dementia. SPECT data and APOE ε4 genotyping may also be useful to identify those MCI patients with an underlying AD pathology and who will develop clinical dementia within 4 years.
ACKNOWLEDGEMENTS
This work was supported by the Spanish Ministry of Health (FIS PI070739 and FIS PI10/00945) from Instituto de Salud Carlos III (Madrid), by the Agència d’Avaluació de Tecnologia i Recerca Mèdiques, Departament de Salut de la Generalitat de Catalunya (Health Department of the Catalan Government) (390), by funds from Fundació ACE, Institut Català de Neurociències Aplicades, and by the University of Pittsburgh Alzheimer's Disease Research Center (AG05133).
Fundació ACE is grateful to Mrs. Trinitat Port, for her legacy to support our research. We are grateful to companions who have contributed to ensure that subjects continued participating in the study: MaJosé Castillón, Charo Romero, Carmen Quindos; and to all participants and their caregivers.
REFERENCES
- 1.Ritchie CW, Ritchie K. The PREVENT study: a prospective cohort study to identify mid-life biomarkers of late-onset Alzheimer's disease. BMJ Open. 2012;2:e001893. doi: 10.1136/bmjopen-2012-001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19:163–165. doi: 10.1097/01.wad.0000184005.22611.cc. [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K Carrillo MC, Thies B, Morrison-Bogarad M, Wgster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ewers M, Walsh C, Trojanowski JQ, Shaw LM, Petersen RC, Jack CR, Jr, Feldman HH, Bokde AL, Alexander GE, Scheltens P, Vellas B, Dubois B, Weiner M, Hampel H North American Alzheimer’s Disease Neuroimaging Initiative (ADNI) Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2012;33:1203–1214. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alegret M, Cuberas-Borrós G, Vinyes-Junqué G, Espinosa A, Valero S, Hernández I, Roca I, Ruíz A, Rosende-Roca M, Mauleón A, Becker JT, Castell-Conesa J, Tárraga L, Boada M. A two-year follow-up of cognitive deficits and brain perfusion in Mild Cognitive Impairment and mild Alzheimer’s disease. J Alzheimers Dis. 2012;30:109–120. doi: 10.3233/JAD-2012-111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa A, Alegret M, Valero S, Vinyes-Junqué G, Hernández I, Mauleón A, Rosende-Roca M, Ruiz A, López O, Tárraga L, Boada M. A Longitudinal Follow-Up of 550 Mild Cognitive Impairment Patients: Evidence for Large Conversion to Dementia Rates and Detection of Major Risk Factors Involved. J Alzheimers Dis. 2013;34:769–780. doi: 10.3233/JAD-122002. [DOI] [PubMed] [Google Scholar]
- 9.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR, Jagust W, Liu E, Morris JC, Petersen RC, Saykin AJ, Schmidt ME, Shaw L, Siuciak JA, Soares H, Toga AW, Trojanowski JQ Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimer’s & Dementia. 2012;8:S1–S68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richard E, Schmand BA, Eikelenboom P, Van Gool WA Alzheimer’s Disease Neuroimaging Initiative. MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer’s disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002541. doi:pii: e002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinosa A, Alegret M, Boada M, Vinyes G, Valero S, Martínez-Lage P, Peña-Casanova J, Becker JT, Wilson BA, Tárraga L. Ecological assessment of executive functions in Mild Cognitive Impairment and mild Alzheimer’s Disease. J Int Neuropsychol Soc. 2009;15:751–757. doi: 10.1017/S135561770999035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amieva H, Jacqmin-Gadda H, Orgogozo JM, Le Carret N, Helmer C, Letenneur L, Barberger-Gateau P, Fabrigoule C, Dartigues JF. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128:1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 13.Tales A, Haworth J, Nelson S, Snowden RM, Wilcock G. Abnormal visual search in mild cognitive impairment and Alzheimer’s disease. Neurocase. 2005;11:80–84. doi: 10.1080/13554790490896974. [DOI] [PubMed] [Google Scholar]
- 14.Tales A, Bayer AJ, Haworth J, Snowden RJ, Philips M, Wilcock G. Visual search in mild cognitive impairment: A longitudinal study. J Alzheimers Dis. 2011;24:151–160. doi: 10.3233/JAD-2010-101818. [DOI] [PubMed] [Google Scholar]
- 15.Nordlund A, Rolstad S, Hellström P, Sjögren M, Hansen S, Wallin A. The Goteborg MCI study: mild cognitive impairment is a heterogeneous condition. J Neurol Neurosurg Psychiatry. 2005;76:1485–1490. doi: 10.1136/jnnp.2004.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordlund A, Rolstad S, Göthlin M, Edman A, Hansen S,Wallin A. Cognitive profiles of incipient dementia in the Goteborg MCI Study. Dement Geriatr Cogn Disord. 2010;30:403–410. doi: 10.1159/000321352. [DOI] [PubMed] [Google Scholar]
- 17.Alegret M, Boada-Rovira M, Vinyes-Junqué G, Valero S, Espinosa A, Hernández I, Modinos G, Rosende-Roca M, Mauleón A, Becker JT, Tárraga L. Detection of visuoperceptual deficits in preclinical and mild Alzheimers disease. J Clin Exp Neuropsychol. 2009;31:860–867. doi: 10.1080/13803390802595568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alegret M, Vinyes-Junqué G, Boada M, Martínez-Lage P, Cuberas G, Espinosa A, Roca I, Hernández I, Valero S, Rosende-Roca M, Mauleón A, Becker JT, Tárraga L. Brain perfusion correlates of visuoperceptual deficits in Mild Cognitive Impairment and mild Alzheimer’s disease. J Alzheimers Dis. 2010;21:557–567. doi: 10.3233/JAD-2010-091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson DK, Storandt M, Morris JC, Calvin JE. Longitudinal study of the transition from healthy aging to Alzheimer Disease. Arch Neurol. 2009;66:1254–1259. doi: 10.1001/archneurol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levinoff EJ, Saumier D, Chertkov H. Focused attention deficits in partients with Alzheimer’s disease and mild cognitive impaiment. Brain Cogn. 2005;57:127–130. doi: 10.1016/j.bandc.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 21.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 22.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairment predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment as a diagnostic entitiy. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Do all patients with mild cognitive impairment progress to dementia? J Am Geriatr Soc. 2006;54:1008–1010. doi: 10.1111/j.1532-5415.2006.00752.x. [DOI] [PubMed] [Google Scholar]
- 25.Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, Zamora D, Goodkind M, Bell K, Stern Y, Devanand DP. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 26.Aretouli E, Okonkwo OC, Samek J, Brandt J. The fate of 0.5s: Predictors of 2-year outcome in mild cognitive impairment. J Int Neuropsychol Soc. 2011;17:277–288. doi: 10.1017/S1355617710001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aretouli E, Tsilidis KK, Brandt J. Four-year outcome of mild cognitive impairment: The contribution of executive dysfunction. Neuropsychology. 2013;27:95–106. doi: 10.1037/a0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubin EH, Storandt M, Miller JP, Kinscherf DA, Grant EA, Morris JC, Berg L. A Prospective Study of Cognitive Function and Onset of Dementia in Cognitively Healthy Elders. Arch Neurol. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- 29.Madureira S, Verdelho A, Moleiro C, Ferro JM, Erkinjuntti T, Jokinen H, Pantoni L, Fazekas F, Van der Flier W, Visser M, Waldemar G, Wallin A, Hennerici M, Inzitari D. Neuropsychological predictors of dementia in a three-year follow-up period: data from the LADIS study. Dement Geriatr Cogn Disord. 2010;29:325–334. doi: 10.1159/000278333. [DOI] [PubMed] [Google Scholar]
- 30.Tierney MC, Szalai JP, Snow WG, Fisher RH, Nores A, Nadon G, Dunn E, St George-Hyslop PH. Prediction of probable Alzheimer's disease in memory-impaired patients: A prospective longitudinal study. Neurology. 1996;46:661–665. doi: 10.1212/wnl.46.3.661. [DOI] [PubMed] [Google Scholar]
- 31.Perri R, Serra L, Carlesimo GA, Caltagirone C Early Diagnosis Group of the Italian Interdisciplinary Network on Alzheimer's Disease. Amnestic mild cognitive impairment: Difference of memory profile in subjects who converted or did not convert to Alzheimer's disease. Neuropsychology. 2007;21:549–558. doi: 10.1037/0894-4105.21.5.549. [DOI] [PubMed] [Google Scholar]
- 32.Perri R, Serra L, Carlesimo GA, Caltagirone C Early Diagnosis Group of Italian Interdisciplinary Network on Alzheimer's Disease. Preclinical dementia: an Italian multicentre study on amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:289–300. doi: 10.1159/000100871. [DOI] [PubMed] [Google Scholar]
- 33.Lonie JA, Parra-Rodriguez MA, Tierney KM, Herrmann LL, Donaghey C, O’Carroll EO, Ebmeier KP. Predicting outcome in mild cognitive impairment: 4-year follow-up study. Br J Psychiatry. 2010;197:135–140. doi: 10.1192/bjp.bp.110.077958. [DOI] [PubMed] [Google Scholar]
- 34.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64:1853–1859. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 35.Devanand DP, Van Heertum RL, Kegeles LS, Liu X, Jin ZH, Pradhaban G, Rusinek H, Pratap M, Pelton GH, Prohovnik I, Stern Y, Mann JJ, Parsey R. 99m Hexamethyl-Propylene-Aminoxime Single-Photon Emission Computed Tomography prediction of conversion from mild cognitive impairment to Alzheimer’s disease. Am J Geriatr Psychiatry. 2010;18:959–972. doi: 10.1097/JGP.0b013e3181ec8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez OL, Becker JT, Chang YF, Sweet RA, DeKosky ST, Gach MH, Carmichael OT, McDade E, Kuller LH. Incidence of mild cognitive impairment in the Pittsburgh Cardiovascular Health Study-Cognition Study. Neurology. 2012;79:1599–1606. doi: 10.1212/WNL.0b013e31826e25f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, Nakano S, Takasaki M. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–1162. [PubMed] [Google Scholar]
- 38.Matsuda H, Kitayama N, Ohnishi T, Asada T, Nakano S, Sakamoto S, Imabayashi E, Katoh A. Longitudinal evaluation of both morphologic and functional changes in the same individuals with Alzheimer’s disease. J Nucl Med. 2002;43:304–311. [PubMed] [Google Scholar]
- 39.Hirao K, Ohnishi T, Hirata Y, Yamashita F, Mori T, Moriguchi Y, Matsuda H, Nemoto K, Imabayashi E, Yamada M, Iwamoto T, Arima K, Asada T. The prediction of rapid conversion of Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage. 2005;28:1014–1021. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 40.Wolf H, Jelic V, Gertz HJ, Nordberg A, Julin P, Wahlund LO. A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand Suppl. 2003;179:52–76. doi: 10.1034/j.1600-0404.107.s179.10.x. [DOI] [PubMed] [Google Scholar]
- 41.Nobili F, De Carli F, Frisoni GB, Portet F, Verhey F, Rodriguez G, Caroli A, Touchon J, Morbelli S, Guerra UP, Dessi B, Brugnolo A, Visser PJ. SPECT predictors of cognitive decline and Alzheimer’s disease in mild cognitive impairment. J Alzheimers Dis. 2009;17:761–772. doi: 10.3233/JAD-2009-1091. [DOI] [PubMed] [Google Scholar]
- 42.Huang C, Wahlund LO, Svensson L, Winblad B, Julin P. Cingulate cortex hypoperfusion predicts Alzheimer’s disease in mild cognitive impairment. BMC Neurology. 2002;2:9–14. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson KA, Moran EK, Becker JA, Blacker D, Fischman AJ, Albert MS. Single photon emission computed tomography perfusion differences in mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78:240–247. doi: 10.1136/jnnp.2006.096800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, Albert MS. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology. 1998;50:1563–1571. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- 45.Pearlson GD, Harris GJ, Powers RE, Barta PE, Camargo EE, Chase GA, Noga JT, Tune LE. Quantitative changes in mesial temporal volume, regional cerebral blood flow, and cognition in Alzheimer's disease. Arch Gen Psychiatry. 1992;49:402–408. doi: 10.1001/archpsyc.1992.01820050066012. [DOI] [PubMed] [Google Scholar]
- 46.Encinas M, De Juan R, Marcos A, Gil P, Barabash A, Fernández C, De Hugarte C, Cabranes JA. Regional cerebral blood flow assessed with 99mTc-ECD SPET as a marker of progression of mild cognitive impairment to Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2003;30:1473–1480. doi: 10.1007/s00259-003-1277-z. [DOI] [PubMed] [Google Scholar]
- 47.Ashford JW, Shih WJ, Coupal J, Shetty R, Schneider A, Cool C, Aleem A, Kiefer VH, Mendiondo MS, Schmitt FA. Single SPECT measures of cerebral cortical perfusion reflect time-index estimation of dementia severity in Alzheimer’s disease. J Nucl Med. 2000;41:57–64. [PubMed] [Google Scholar]; Modrego PJ. Predictors of conversión to dementia of probable Alzheimer type in patients with mild cognitive impairment. Curr Alzheimer Res. 2006;3:161–170. doi: 10.2174/156720506776383103. [DOI] [PubMed] [Google Scholar]
- 48.Alegret M, Espinosa A, Vinyes-Junqué G, Valero S, Hernández I, Tárraga L, Becker JT, Boada M. Normative data of a brief neuropsychological battery for Spanish individuals older than 49. J Clin Exp Neuropsychol. 2012;34:209–219. doi: 10.1080/13803395.2011.630652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 50.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild Cognitive Impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 51.Lopez OL, Kuller LH, Becker JT, Dulberg C, Sweet RA, Gach HM, Dekosky ST. Incidence of Dementia in Mild Cogntive Impairment in the Cardiovascular Health Study Cognition Study. Arch Neurol. 2007;64:416–420. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- 52.Small GW, Scott WK, Komo S, Yamaoka LH, Farrer LA, Auerbach SH, Saunders AM, Roses AD, Haines JL, Pericak-Vance MA. No association between the HLA-A2 allele and Alzheimer disease. Neurogenetics. 1999;2:177–182. doi: 10.1007/s100480050080. [DOI] [PubMed] [Google Scholar]