Abstract
Acute lung injury and acute respiratory distress syndrome are accompanied by thrombin activation and fibrin deposition that enhances lung inflammation, activates endothelial cells and disrupts lung paracellular permeability. Heparin possesses anti-inflammatory properties but its clinical use is limited by hemorrhage and heparin induced thrombocytopenia. We studied the effects of heparin and low anticoagulant 2-O, 3-O desulfated heparin (ODSH) on thrombin-induced increases in paracellular permeability of cultured human pulmonary endothelial cells (EC). Pretreatment with heparin or ODSH blocked thrombin-induced decrease in the EC transendothelial electrical resistance (TER), attenuated thrombin-stimulated paracellular gap formation and actin cytoskeletal rearrangement. Our data demonstrated that heparin and ODSH had inhibitory effects on thrombin-induced RhoA activation and intracellular calcium elevation. Thrombin-stimulated phosphorylation of the cytoskeletal regulatory proteins, myosin light chain and ezrin/radixin/moesin, were also reduced. In these effects, low anticoagulant ODSH was more potent than heparin. Heparin or ODSH alone produced decreases in the EC TER that were abolished by siRNA-mediated depletion of the thrombin receptor, PAR-1. We also demonstrated that, in contrast to heparin, ODSH did not possess thrombin-binding activity. Results suggest that heparin and low anticoagulant ODSH, can interfere with thrombin-activated signaling.
Keywords: Heparin; thrombin; protease activated receptor; 2-O, 3-O desulfated heparin (ODSH); endothelium; acute lung injury
1. INTRODUCTION
Acute lung injury (ALI) and its more severe form, acute respiratory distress syndrome (ARDS), produce serious respiratory failure with 30–50% mortality (Rubenfeld et al., 2005). Treatment of ALI/ARDS relies on supportive care, mechanical ventilation and control of the initiating cause. ALI/ARDS are characterized by non-cardiogenic pulmonary edema, increased endothelial permeability from injury and inflammation (Villar, 2011), and intra-alveolar and intravascular fibrin deposition. Fibrin deposits enhance inflammatory responses by increasing vascular permeability and activating endothelial cells (ECs) to produce pro-inflammatory cytokines (Idell et al., 1989)(Ware et al., 2005). The distribution of fibrin in lungs of patients with ALI/ARDS suggests that coagulation pathways are pathologically activated (Idell et al., 1989)(Ware et al., 2005). The serine protease thrombin is frequently activated in ALI/ARDS and is postulated to disrupt EC junctional integrity and increase lung paracellular permeability through activation of protease activated receptors (PARs) (Vadász et al., 2005)(Vogel et al., 2000), with formation of small gaps between ECs and disrupted function of the endothelial monolayer (van Nieuw Amerongen et al., 2008). There are four known protease activated receptors, PAR 1–4. PAR-1, PAR-2, PAR-3, are expressed on human umbilical vascular endothelial cells (HUVEC) (O’Brien et al., 2000)(Popović et al., 2010). PAR-4 has been reported in bovine endothelial cells but not in human pulmonary ECs (Momota et al., 2006). PAR-2 is activated by tryptase. PAR-1-induced endothelial barrier permeability is well established (McLaughlin et al., 2005)(Bogatcheva et al., 2002). PAR-3’s role in endothelial cells is not well established but it has been reported to transduce signaling alone in epithelial cells (Seminario-Vidal et al., 2009). Thrombin inhibitors such as antithrombin III, hirudin and heparin have shown some promise experimentally in ameliorating EC barrier disruption and EC gap formation (Cadroy et al., 1996)(Gori et al., 1999)(Bao et al., 2012)(Schmidt et al., 1996). Thrombin activation of the PARs is unique as the process involves the proteolytic cleavage of a tethered ligand at the amino- N terminal extracellular domain of the receptor. This proteolytic cleavage unmasks a new amino-terminal sequence (SFLLRNPNDKYEPF… in human PAR-1) that serves as a new tethered ligand which binds intramolecularly to the body of the receptor and stimulates signal transduction of the pathway (Chung et al., 2002)(Macfarlane et al., 2001).
The anticoagulant heparin is a highly anionic macromolecule that interacts via heparin-binding sites with a number of proteins, endowing it with a number of properties (Casu et al., 2010), including effects on vascular permeability. We therefore tested the effect of fully anticoagulant heparin and its low anticoagulant 2-O, 3-O desulfated derivative (ODSH) in cultured human pulmonary artery endothelial cells (HPAEC) and found that heparin and ODSH attenuated thrombin-induced barrier EC disruption. In the absence of thrombin, heparin and ODSH induced modest barrier disruption involving PAR-1. Heparin or ODSH pretreatment significantly attenuated thrombin-induced EC barrier disruption. Moreover, ODSH pretreatment significantly diminished thrombin effect in human lung microvascular endothelial cells (HLMVEC), including thrombin induced cleavage of its receptor PAR 1, activation of the RhoA/ROCK pathway and stimulation of Ca2+-dependent signaling. These results suggest that heparin and its derivative, ODSH, are potentially beneficial in a cellular model of ALI/ARDS from direct interference with thrombin-induced vascular permeability in a non-coagulant manner.
2. MATERIALS AND METHODS
2.1. Materials
All chemicals or reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted. ODSH was from ParinGenix, Inc. (Weston, FL, USA). Antibodies were from Cell Signaling (Danvers, MA, USA).
2.2. Cell culture
Human pulmonary artery endothelial cells (HPAEC) or human lung microvascular endothelial cells (HLMVEC) from Lonza Group Ltd. (Walkersville, MD, USA) were cultured in complete EBM-2 medium (Lonza), maintained at 37°C in humidified atmosphere of 5% CO2-95% air, and used at passages 3–6.
2.3. Measurement of TER
The barrier properties of the cell monolayers were characterized using an electrical cell-substrate impedance instrument (ECIS) (Applied BioPhysics, Troy, NY, USA) as previously described (Birukova et al., 2004b) (Kim et al., 2012). Cells were grown to confluence in 8 well gold plated arrays (Applied BioPhysics, Troy, NY, USA). Before each experiment the cells were incubated in serum-free medium for 2 h. The total resistance across the monolayers was composed of the resistance generated between the ventral cell surface and the electrode as well as by the resistance between cells. Initial resistance at the onset of our multiple experiments was 1300 to 1700 in array wells, then all wells normalized to1. A 4,000-Hz AC signal with a1-V amplitude was applied to the EC monolayer through a 1-M-Ω resistor, creating an approximate constant-current source (1 µA). After a baseline measurement, cells were treated with various concentrations of thrombin, heparin, ODSH, and changes in TER in response to the stimuli were real-time recorded. In the heparin or ODSH/thrombin experiments, the cells were pretreated with heparin or ODSH for 40 min followed by thrombin and the changes in TER were real-time recorded in real-time.
2.4. Immunocytochemistry
The cells grown on coverslips were pretreated with heparin or ODSH (50 µg/ml, 40 min) then challenged with thrombin (20 nM, 15 min). After treatments, cells plated on coverslips were washed with PBS, fixed with 3.7% paraformaldehyde, and permeabilized with 0.25% Triton X-100 in 0.1% Tween 20 in PBS, pH 7.4 (PBST). After blocking with 5% normal goat serum in PBST, coverslips were incubated with Alexa 488-Phalloidin (Molecular Probes, Eugene, OR, USA) to stain F-actin or Alexa 594 to stain VE-cadherin, then mounted on slide glasses with ProLong Gold Antifade (Molecular Probes, Eugene, OR, USA). Immunostained EC were observed and photographed using a × 630 magnification on a Zeiss Axio Observer video imaging system.
2.5. Cell surface PAR-1 immunofluorescence staining
HPMVEC were grown to confluence on glass cover slips in 12 well plates. The cells were washed three times with serum-free medium and kept for 2 h at 37°C in medium containing 1% FBS before agonist treatment. After thrombin, heparin, ODSH treatments, cells were washed three times with HBSS and fixed with 1% paraformaldehyde for 15 min at room temperature (RT). Cells were then blocked with 5% BSA at 4°C for 60 min prior to incubation with anti-PAR-1 rabbit polyclonal IgG (1:40) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The anti-PAR-1 antibodies were detected after incubation of the cells with rhodamine-labeled goat anti-mouse antibodies (1:400) for 30 min at 4°C. Cell surface fluorescence was visualized and photographed using Zeiss Axio Observer imaging system.
2.6. Western blotting
After treatments, the cells were washed with PBS, lysed in Laemmli buffer with protease/phosphatase inhibitors and boiled for 5 min and cleared by centrifugation. Next the protein extracts were resolved by SDS-PAGE. Proteins were transferred to nitrocellulose membranes and subsequently blocked with 5% nonfat dry milk in PBST, then incubated at 4° C overnight with respective primary antibodies of interest. After washing three times for 5 min with PBST, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at RT for 1 h, followed by three washes for 5 min with PBST. Immunoreactive proteins were visualized with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA).
2.7. Particles size measurement by dynamic laser light scattering
The particle size of the molecular complexes was analyzed using the Microtac Nanotrac ULTRA dynamic light scattering instrument. This instrument is designed to detect particle size distributions in low concentration and is sensitive to size ranges below 10 nm. Thrombin was diluted in PBS (final concentration 3.3 µM) and protein size was measured. After that, a single dose of ODSH (final concentration 0.7 µg/ml) or heparin (final concentration 0.34 µg/ml) was added into the thrombin solution, incubated for 5 min at RT and resulted complex sizes were measured. Measurements were repeated at least two times in two separate experiments.
2.8. siRNA-mediated PAR-1 depletion
Cells were transfected with PAR-1-specific siRNA (final concentration 20 nM) using siPortAmine (Ambion, Austin, TX, USA) according to manufacturer’s instructions. Non-specific siRNA control siRNA-A (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used for control transfection.
2.9. RT-PCR
cDNA was synthesized with M-MLV reverse transcriptase from total RNA extracted with Trizol (Invitrogen, Carlsbad, CA, USA). Specific primers for human PAR-1 (5’ primer: GGGTCTGAATTGTGTCGCTTCG; 3’ primer: GCTGCTGACACAGACACAGAGG), human PAR-3 (5’ primer: GGAAGGCTGGACAGGAGCCACG; 3’ primer: CCACACCAGTCCACATGTTACC), human GAPDH (5’ primer: GCAATGCCTCCTGCACCACC; 3’ primer: CCCAGCGTCAAAGGTGGAGG) were used for PCR.
2.10. RhoA activity
HPAEC monolayers were pretreated with heparin (50 µg/ml) or ODSH (50 µg/ml) for 40 min before challenging with thrombin (20 nM). The cells were lysed 5 minutes after thrombin addition and used for RhoA activation G-LISA assay. RhoA activity was measured in the cell lysates using G-LISA RhoA Activation Assay colorimetric kit according to the manufacturer’s protocol (Cytoskeleton, Denver, CO, USA).
2.11. Measurement of intracellular calcium
Measurement of intracellular calcium concentration was performed using the green fluorescing calcium indicator dye FluoForte™ (Enzo Life Sciences, Farmingdale, NY, USA). Briefly, HPAEC were grown to confluence in 96-well plates, preloaded with Fluo-Forte (5µg/ml in Hank’s buffer with 20 mM HEPES containing 10 × diluted original solution of dye efflux inhibitor) for 1 hour at RT; all reagents were from the FluoForte™ Calcium Assay kit (Enzo Life Sciences, Farmingdale, NY, USA). Basal fluorescence of quiescent cells was monitored for 5 min using Titertek Fluoroscan II plate reader. Cells were then treated with heparin (50 µg/ml), ODSH (50 µg/ml) or BAPTA-AM (TOCRIS, Bristol, UK) (10 µM), or not treated for 40 min before thrombin (20 nM) was added. Changing fluorescence was recorded for 160 seconds after thrombin addition and a time response curve of intracellular calcium signal was recorded via real time monitoring of fluorescence intensity at excitation 490 nm and emission at 525 nm on the Titertek Fluoroscan II plate reader.
2.12. Statistical analysis
Data is expressed as mean ± standard error of the mean (SE). Statistical analysis of TER over time was performed using analysis of variance (ANOVA) with determination of significant differences at select time points of observation using Dunnett’s procedure. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Heparin and its low anticoagulant derivative, ODSH, affect the endothelial monolayer integrity
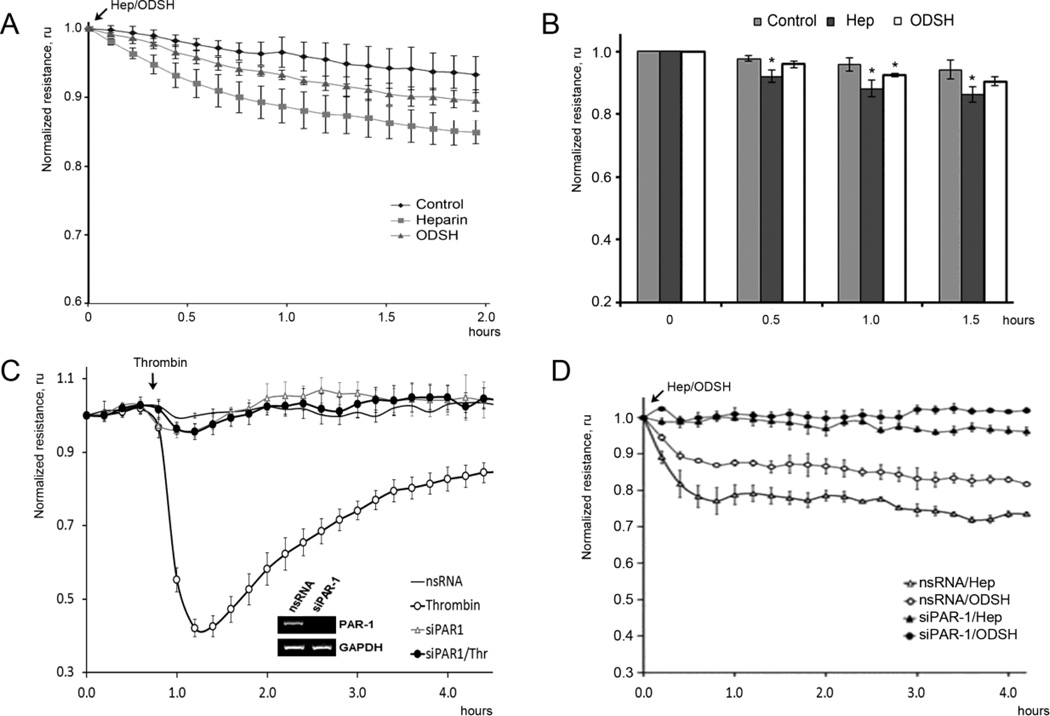
To examine direct effects of heparin and ODSH on endothelial barrier function, HPAEC grown in ECIS arrays were treated with either heparin or ODSH (50 µg/ml for each) and integrity of the EC monolayers was assessed in real-time transendothelial resistance (TER) measurement (Figure 1). Data presented in Figure 1A demonstrated that treatment of the EC with heparin or ODSH had a slight negative effect on the monolayer integrity slightly decreasing TER (~10–15%). This effect was more evident for heparin-treated cells, whereas ODSH-induced disturbance of the barrier function was less and not always statistically significant. To study whether the effects of heparin or ODSH on the EC monolayer TER can be dependent on thrombin receptors, we inhibited the receptor expression in the EC by specific siRNA and evaluated the effect in the receptor-depleted EC. First, we checked specificity and efficiency of respective siRNA by RT-PCR and ECIS approach (Figure 1 C). Thrombin treatment of the siRNA-transfected HPAEC allowed determination of a major thrombin receptor in the EC. TER loss was PAR-1-dependent as siRNA-directed depletion demonstrated (Figure 1 C). Using siRNA, we also demonstrated that PAR-1 is the main thrombin receptor in HLMVEC (data not shown). To examine whether the effect of heparin on monolayer integrity may also be PAR-1-dependent, we treated PAR-1-depleted EC with heparin or ODSH and observed changes in the barrier function using TER assay (Figure 1 D). Results demonstrated that heparin and ODSH might regulate endothelial permeability via the thrombin receptor directly: PAR-1 depletion abolished a negative effect of heparin derivatives on HPAEC barrier (Figure 1 D).
Figure 1. Effect of heparin and ODSH on the EC TER.
Panels A, B. Heparin or ODSH increase the permeability of HPAEC. The cells were treated with heparin (50 µg/ml) or ODSH (50 µg/ml) and transendothelial resistance (TER) was recorded in real time in ECIS assay. Panel C. Depletion of PAR-1 with specific siRNA attenuated thrombin-induced barrier disruption in HPAEC. The cells grown in ECIS arrays were transfected with specific siRNA for PAR-1 or non-silencing (ns) RNA as described in Materials and Methods. 48 hours later, the cells were treated with thrombin (20 nM) and changes in the monolayer integrity were analyzed by ECIS assay. Depletion of PAR-1 was confirmed by RT-PCR. Panel D. Depletion of PAR-1 with specific siRNA attenuated heparin- or ODSH-induced TER decrease. The involvement of PAR-1 in heparin- or ODSH-induced TER decrease was examined in the same conditions used in panel A. TER values were normalized with the initial resistance and expressed as the mean of three or four individual experiments. Arrows indicate time points when reagents were added. Data is expressed as a mean of four individual experiments ± SE, and statistical significances compare to control were evaluated using Student’s t-test; * (p < 0.05).
3.2. Heparin and ODSH antagonize thrombin-induced endothelial cell hyperpermeability
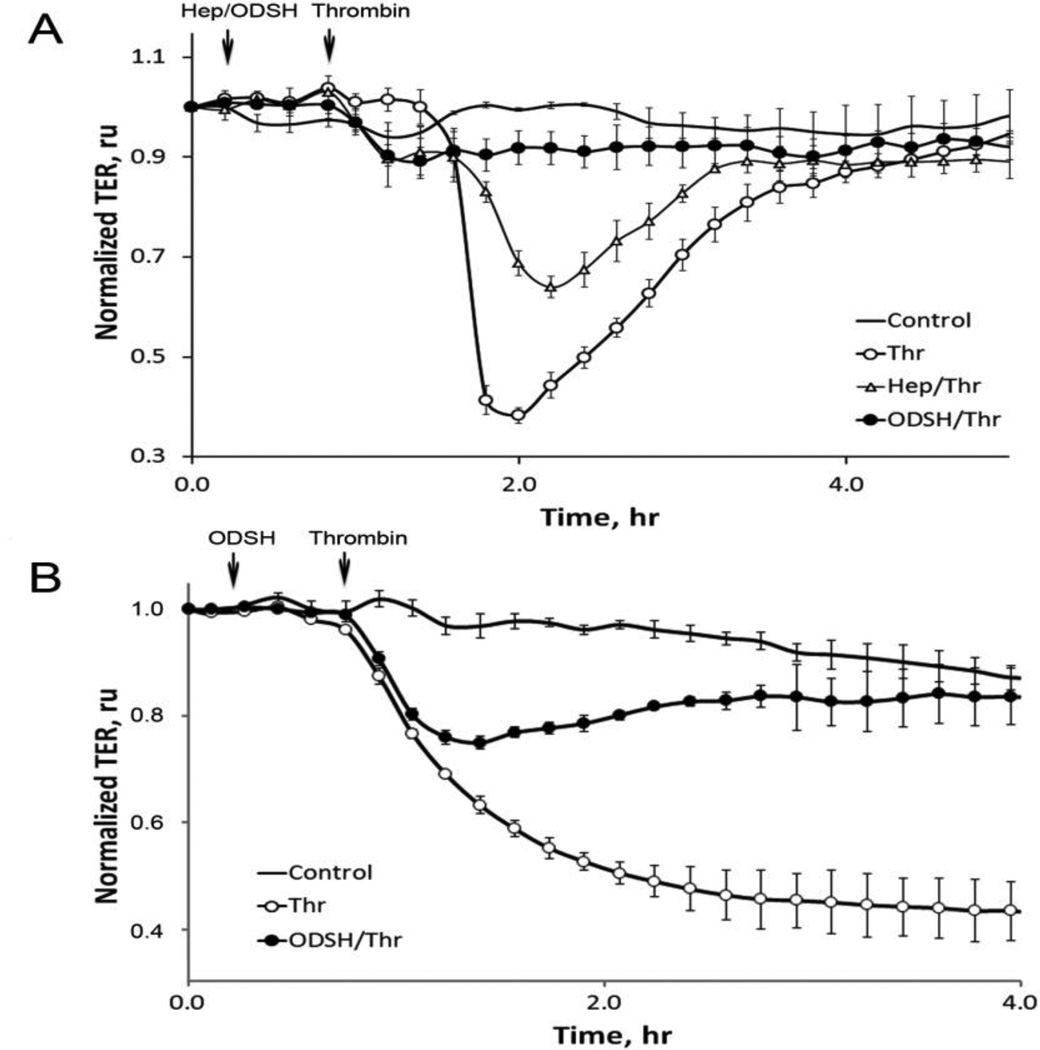
To test our hypothesis about a novel function of the heparins in thrombin signaling, we next studied a possible protective role of heparins in HPAEC (Figure 2 A) and HLMVEC (Figure 2 B) using ECIS approach. To examine the effect of heparin and ODSH on endothelial barrier function in thrombin-treated EC monolayers, cells cultured in ECIS arrays (as described in Materials and Methods (2.3) were pretreated with heparin or ODSH and challenged with thrombin. Changes in TER were recorded over time. Thrombin dose for the HPAEC used in this study was based on prior studies demonstrating its disruption of the endothelial barrier (Smurova et al., 2004)(Tar et al., 2006). As expected, thrombin alone produced an abrupt, substantial decrease in TER that gradually recovered by 4 hours (Figure 2 A). Heparin and ODSH antagonized the effect of thrombin on the EC permeability, and importantly, ODSH maintained the barrier integrity close to the normal state (Figure 2 A). Initially, we tested several heparin and ODSH doses within 25–100 µg/ml range and found no increased effect when concentrations were greater than 50 µg/ml (data not shown). Therefore, 50 µg/ml concentration was selected for both heparins in our experiments with HPAEC.
Figure 2. Effect of heparin or ODSH on thrombin-induced endothelial permeability.
Panel A. Heparin or ODSH prevents thrombin-induced TER loss in HPAEC. The cells grown in ECIS arrays were pretreated with heparin or ODSH for 40 min and then challenged with 20 nM thrombin as described in Materials and Methods. Arrows indicate time points when the effectors were added. TER values were normalized with the initial resistance and expressed as mean ± standard error (SE), relative units (ru) of four individual experiments. Panel B. ODSH prevents thrombin-induced TER loss in HLMVEC. The same experiment as in Panel A but another EC (HLMVEC) was used. Thrombin concentration – 0.5 nM.
Using the same concentration of ODSH, we were able to demonstrate the barrier-protective effect in thrombin-challenged HLMVEC (Figure 2 B). We found that these cells were more sensitive to thrombin than HPAEC. However, pre-treatment with ODSH dramatically protected them in TER assay.
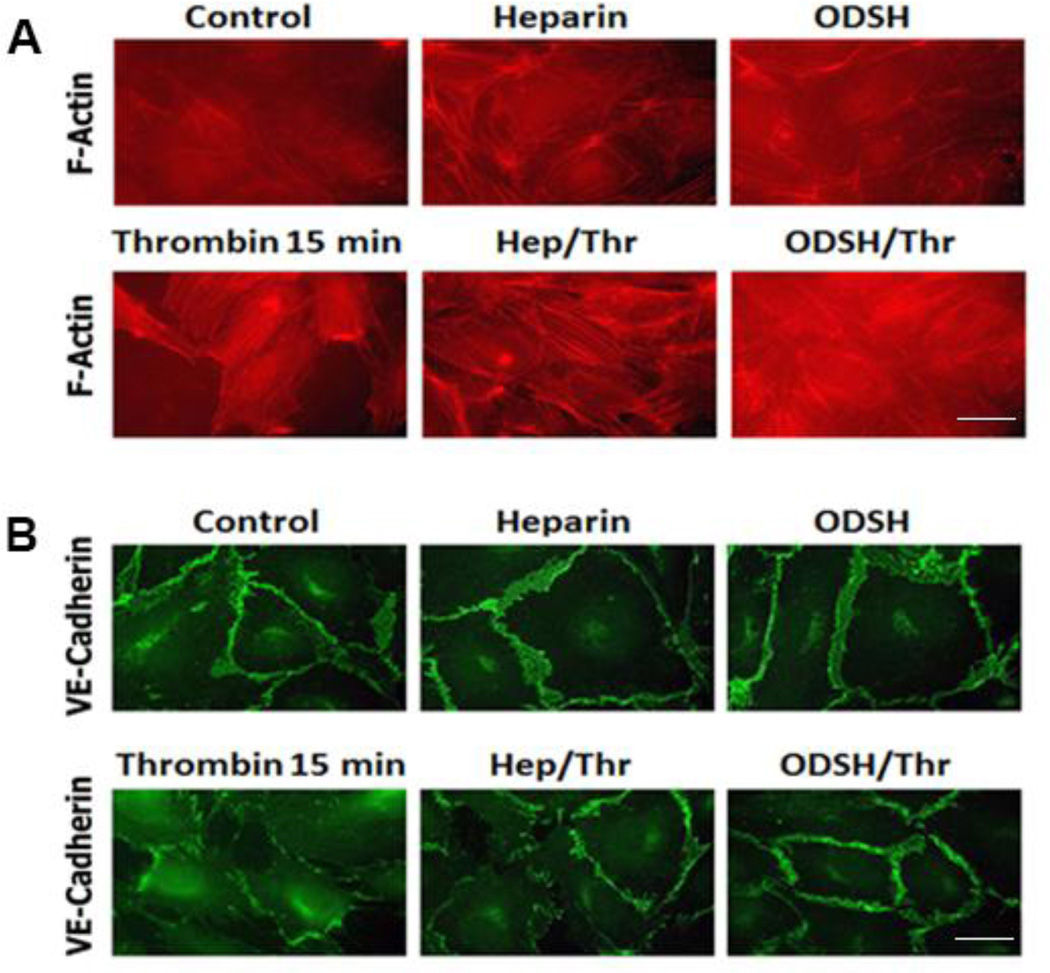
The barrier-disruptive action of thrombin results in the EC cytoskeleton remodeling and actin stress fiber formation as well as loss of cell-cell contacts (Birukova et al., 2004a). To visualize protective effects of heparin or ODSH in thrombin-challenged EC, we studied the EC cytoskeleton and cell-cell contact changes in the cells pretreated with either heparin or ODSH (50 µg/ml each for 40 min) and challenged with thrombin (20 nM, 15 min) or treated with thrombin alone (Figure 3 A and B). Actin polymerization was assessed using Alexa 488-phalloidin staining. Immunostaining of cell-cell adherens junctions (AJ) in the EC was performed as described in Materials and Methods using VE-cadherin-specific antibodies. We found that both heparin and ODSH inhibited thrombin-induced intercellular gap formation. F-actin stress fibers characteristic for thrombin-treated cells were less evident in heparin-pretreated EC and virtually absent in ODSH-pretreated cells (Figure 3 A). VE-cadherin immunostaining revealed a striking difference in the EC pretreated with heparin or ODSH (Figure 3 B). The pretreatments greatly preserved AJ and they appeared to be intact, whereas thrombin treatment resulted in almost complete disassembly of VE-cadherin-positive cell-cell contacts (Figure 3 B).
Figure 3. Heparin or ODSH pretreatment prevents thrombin-induced actin cytoskeleton rearrangement and prevents thrombin-induced cell-cell contact loss in HPAEC.
Panel A. The cells grown on coverslips were pretreated with heparin or ODSH (50 µg/ml, 40 min) and challenged with thrombin (20 nM, 15 min). F-actin visualization by Alexa-488-phalloidin staining was performed as described in Materials and Methods. Panel B. The cells grown on coverslips were pretreated with heparin or ODSH (50 µg/ml, 40 min) then challenged with thrombin (20 nM, 15 min). Visualization of adherens junctions by VE-cadherin immunostaining was performed as described in Materials and Methods. Scale bars represent 20microns.
3.3. The barrier-protective effect of heparin derivatives is not dependent on thrombin binding
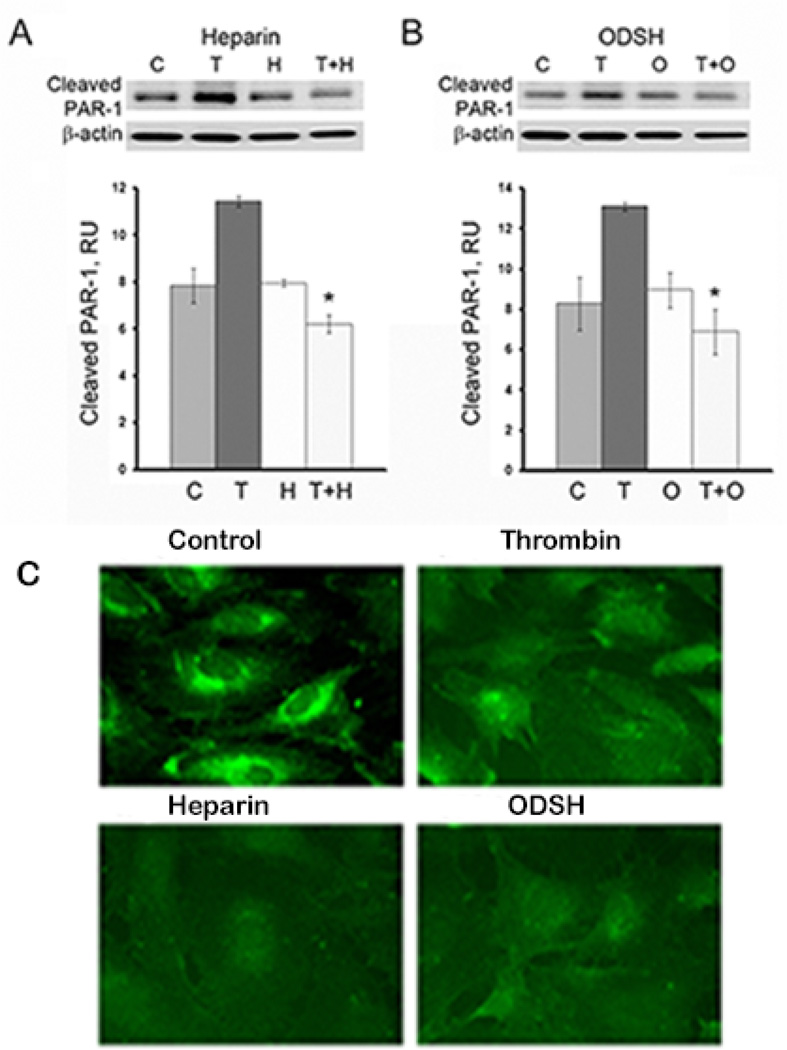
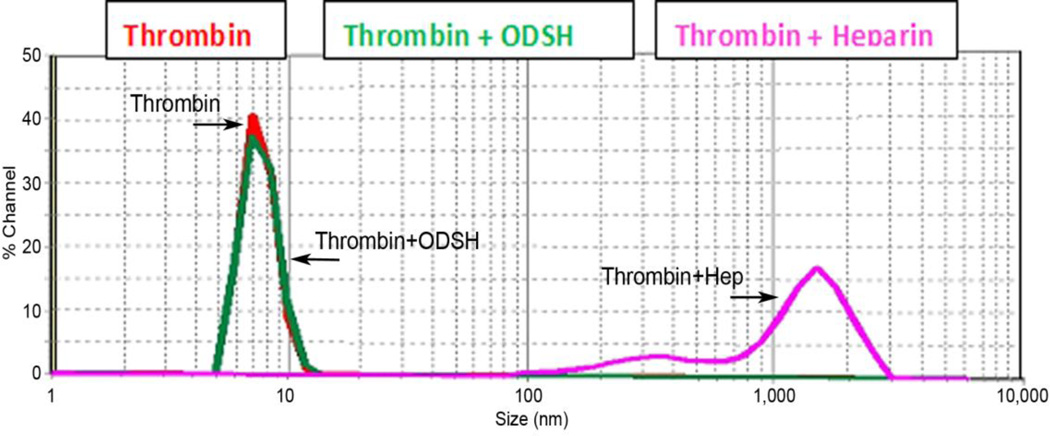
Interaction of heparin and thrombin is well-established, and the mechanism of their binding has been described (Li et al., 1976). However, we found that heparin derivatives may also be involved in thrombin receptor signaling without binding to thrombin. First, we confirmed that heparin treatment of the EC targeted PAR-1 receptor. We hypothesized that direct interaction with the heparins could protect PAR-1 from thrombin cleavage. To show such protection, we used the antibody designed to recognize proteolytically-activated PAR-1. It is well known that thrombin cleaves PAR-1 to expose new NH2-terminus, S(42)FLLRN. Using antibody specific for cleaved (S42) PAR-1, we can detect thrombin-activated PAR-1 by Western blotting and compare the levels of thrombin-activated receptor in presence or absence of heparin or ODSH. To exclude a possibility of heparin- or ODSH-thrombin interactions in cell culture medium, we incubated the cells with heparin or ODSH to allow possible binding to the receptor, then changed to heparin- or ODSH-free medium and challenged with thrombin (“wash-out” technique). HLMVEC were pretreated with heparin or ODSH (50 µg/ml, 40 min), then challenged with thrombin (8 nM, 10 min), and analyzed by Western blotting (Figure 4 A). As expected, compared to control cells or to cells pretreated with heparin or ODSH alone, levels of cleaved PAR-1 were markedly increased in the presence of thrombin. Results (Figure 4 A and B) demonstrated that ODSH and heparin can protect PAR-1 receptor from thrombin cleavage and, importantly, this protection is perhaps a result of direct interaction between heparins and PAR-1. Western blotting analysis (Figure 4 A and B) demonstrated that, in cells pretreated with either heparin or ODSH prior thrombin challenge, levels of proteolytically-activated PAR-1 was markedly lower than those in cells without heparin or ODSH pretreatment. Decreased levels of proteolytically-activated PAR-1 correlated well with protective effect on TER response (Figure 2 A and B), actin cytoskeleton morphology (Figure 3 A) and preservation of cell-cell contacts (Figure 3 B) in the EC pretreated with either heparin or ODSH prior thrombin challenge. Interestingly, cell-surface immunostaining of PAR-1 revealed heparin and ODSH-induced internalization of the receptor similar to that induced by thrombin (Figure 4 B). These data suggest PAR-1-heparin or -ODSH interactions. To explore potential interaction of thrombin with heparin or ODSH directly we employed dynamic laser light scattering (Figure 5) to detect thrombin-heparin or -ODSH complexes in solution as described in Materials and Methods. Using this method, the size of purified recombinant human thrombin was found around 7 nm (Figure 5, red line). Addition of ODSH (green line) did not alter either mean thrombin size or distribution, suggesting lack of interaction between ODSH and thrombin. In contrast, heparin addition (pink line) resulted in formation of highly-aggregated complexes with particle sizes around 200 nm and 1 micron (Figure 5). Thus, our data demonstrate that unmodified heparin can bind to thrombin in solution which leads to thrombin-heparin aggregation, whereas ODSH has no affinity for thrombin. Taken together, our results strongly suggest that heparin and ODSH exert barrier-protective function in thrombin-challenged endothelium and such protection may depend on direct interaction between heparins and thrombin receptors
Figure 4. Heparin and ODSH protect PAR-1 from cleavage by thrombin.
Panels A, B. HPAEC were pre-treated with heparin (A) or ODSH (B) (50 µg/ml, 40 min), washed with fresh serum-free cell culture medium to remove unbound heparins, then challenged with thrombin (8 nM, 10 min) and analyzed by Western blotting using antibody specifically recognizing thrombin-cleaved PAR-1. Statistical analyses of the experimental data expressed in relative units are presented as a mean of three individual experiments ± SE. *P < 0.01 versus cells treated with thrombin alone. Panel C. Heparin and ODSH affect cell surface expression of PAR-1. Exposure to thrombin, heparin or ODSH alters cell surface expression of PAR-1 as revealed by immunofluorescence. Cells were incubated with thrombin (20 ng/ml) or heparin (50 µg/ml) or ODSH (50 µg/ml) for 30 min prior to immunostaining with anti-PAR-1 antibody. Images of control, thrombin-, heparin- and ODSH-treated EC are shown. Results are representative of a minimum of 3 individual experiments.
Figure 5. ODSH has low binding affinity for thrombin.
The particle size of the macromolecular complexes was analyzed using the Microtac Nanotrac ULTRA dynamic light scattering instrument. The instrument detects particle size distributions in low concentration and is sensitive to size ranges below 10 nm. Thrombin was diluted in 300 µl of PBS and particle size was measured (red line indicated by arrow). After that, a single dose of ODSH (5 µl/ml, 50 mg/ml) (green line indicated by arrow) or heparin (5 µl/ml, 25 mg/ml) (pink line indicated by arrow) was added into the thrombin solution, incubated for 5 min at RT and resulted complex sizes were measured. Measurements were repeated at least two times in two separate experiments.
3.4. Heparin and ODSH negatively regulate thrombin-induced signaling pathways
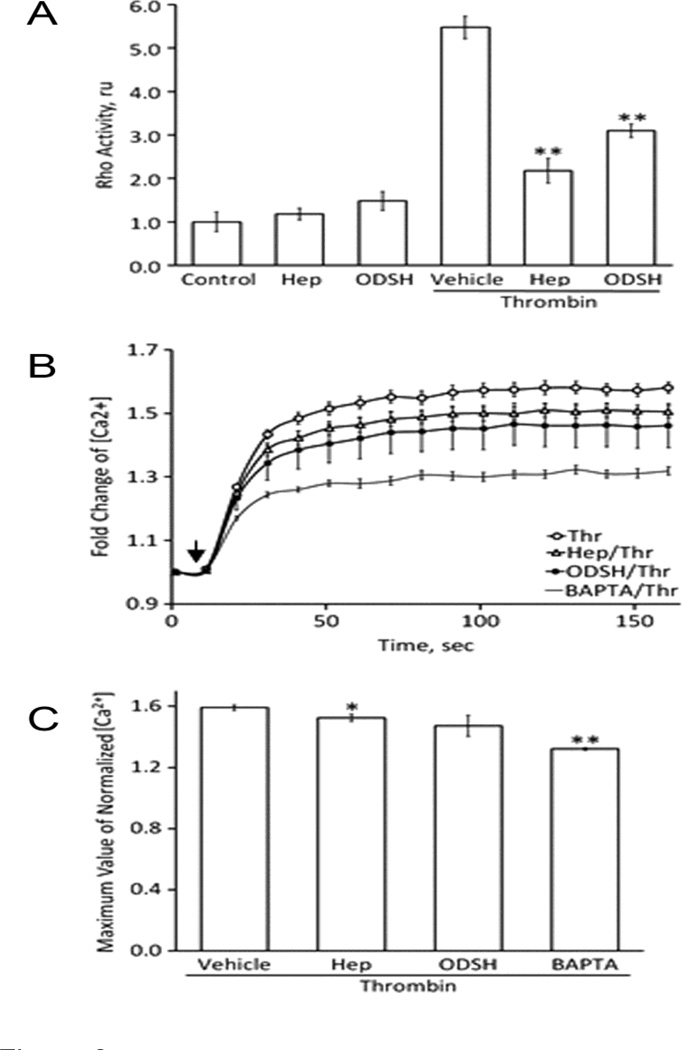
In our next experiments we studied molecular mechanisms of the cellular protection provided by heparin and ODSH against thrombin-induced permeability. It is well known that thrombin-induced cytoskeleton rearrangement is mediated mainly by: (1) activation of RhoA/Rho-kinase cascade, and (2) elevation of intracellular calcium and, therefore, stimulation of Ca2+-dependent enzymes, such as myosin light chain kinase (MLCK) and protein kinase C isoforms (van Nieuw Amerongen et al., 2008)(Hoang et al., 2004). We therefore tested the effects of the heparins on both signaling pathways (Figure 6). HPAEC were pretreated as described in Materials and Methods, challenged with thrombin for 5 min, and changes in RhoA activity and intracellular calcium levels were assessed. As expected, thrombin produced substantial elevations in RhoA activity (more than 5-fold) (Figure 6 A) and intracellular calcium (more than 1.5-fold) (Figure 6 B) in EC monolayers. However, pretreatment with either heparin or ODSH attenuated these thrombin-induced changes (Figure 6 B). Data obtained allowed the inference that heparin- or ODSH-dependent protection of PAR-1 from proteolytic activation dramatically impaired upstream events of thrombin-activated signaling cascades.
Figure 6. Thrombin-induced increases in RhoA activity and intracellular calcium can be attenuated by pretreatment with heparin or ODSH.
Panel A. HPAEC monolayers were pretreated with heparin (50 µg/ml) or ODSH (50 µg/ml) for 40 min before challenging with thrombin (20 nM). The cells were harvested 5 minutes after thrombin addition and used for RhoA activation G-LISA assay. RhoA activation induced by thrombin was significantly inhibited in the heparin- or ODSH-pretreated EC. **P<0.001 (Hep- or ODSH-pretreatment vs. thrombin treatment alone). Panel B. The increase of intracellular calcium in the response of thrombin treatment (20 nM) was recorded for 160 seconds after thrombin addition (indicated as arrow). BAPTA-AM (10 µM), cell-permeable calcium chelator. Panel C. Statistical analysis of the results shown in Panel B. Data is expressed as a mean of six individual experiments ± SE. *P<0.05 and **P<0.01 (Hep- or ODSH-pretreatment vs. thrombin alone treatment.
3.5. Heparin and ODSH attenuate thrombin-induced phosphorylation of the cytoskeletal regulatory proteins
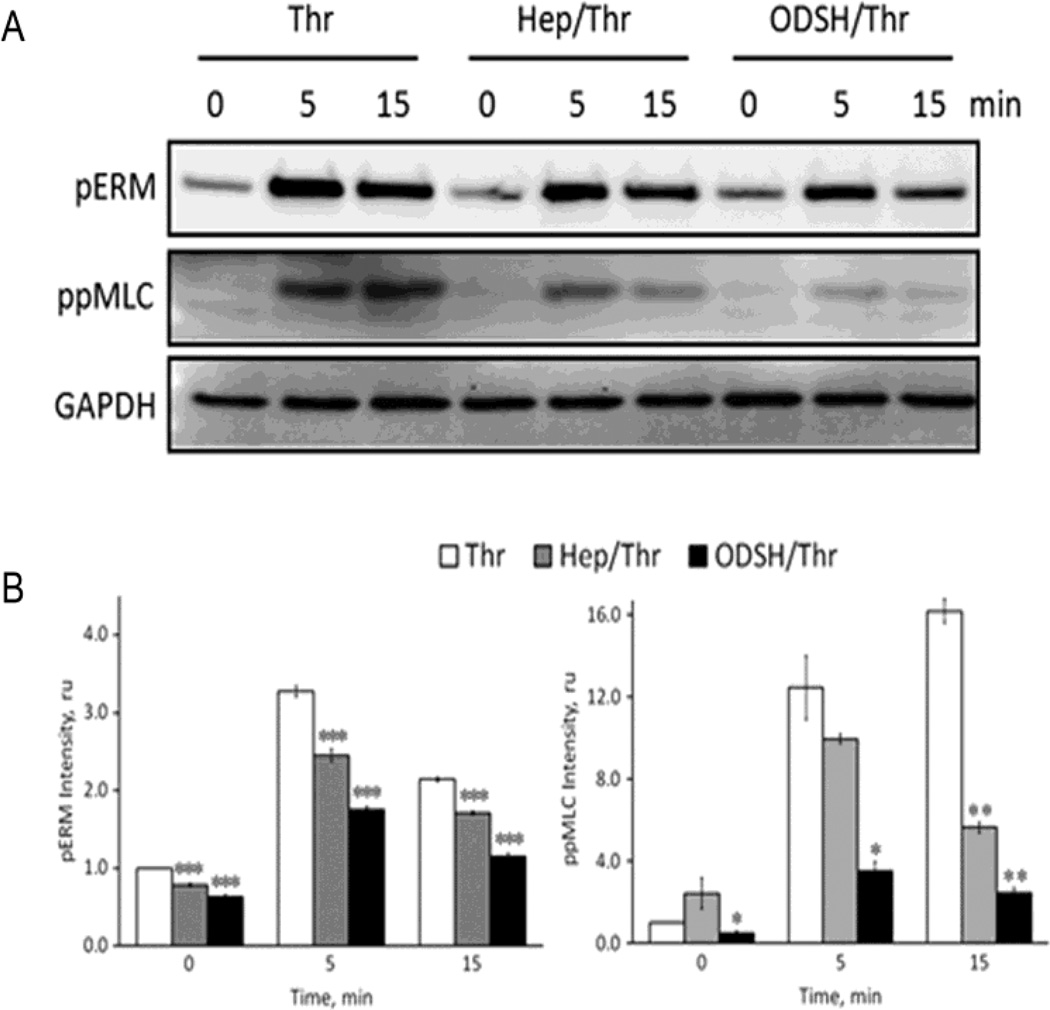
Impairment of thrombin-induced RhoA activation and elevation of intracellular Ca2+ may affect modification status of downstream protein targets involved in the cytoskeleton remodeling and intercellular gap formation. In the EC, phosphorylation of myosin light chain (MLC) regulatory subunit at Thr18 and Ser19 is a key upstream point of regulation for actin-myosin contraction. MLC phosphorylation level is tightly regulated to maintain cell integrity, and two enzymes, Ca2+-dependent MLC kinase (MLCK) and myosin light chain phosphatase (MLCP), are involved in this regulation (Murphy et al., 2001)(Sheehan and Sadler, 1994). In addition, MLC can be phosphorylated by Rhokinase directly (Di Ciano-Oliveira et al., 2003) and Rho-kinase can also increase MLC phosphorylation via inhibition of MLCP activity by phosphorylation of MLCP regulatory subunit, MYPT1 (Csortos et al., 2007) (Somlyo and Somlyo, 2003) (Ruiz-Loredo et al., 2011). Moreover, inhibition of MLCP can lead to increase ERM (ezrin-radixin-moesin) protein phosphorylation resulting in cytoskeletal rearrangement and permeability changes (Kim et al., 2012). Therefore, to evaluate the effect of heparins on thrombin-induced changes in balance between MLCK/Rho-kinase (pro-barrier) and MLCP (anti-barrier) activities, HPAEC were pretreated with the heparins for 40 min., challenged with thrombin (20 nM, 5 or 15 min) and cell homogenates were subjected to Western blotting with di-phospho-MLC or phospho-ERM antibodies. Data shown in Figure 7 strongly suggest that pretreatment with either heparin or ODSH decreased thrombin-induced phosphorylation of MLC and ERM. Importantly, this effect was more pronounced when the cells were pretreated with ODSH. Thus, these results demonstrate that ODSH alleviates thrombin-induced hyperpermeability in the EC through regulating the activity balance between MLCK/Rho-kinase and MLCP, and, therefore, phosphorylation/dephosphorylation status of cytoskeletal regulatory proteins.
Figure 7. Heparin or ODSH pretreatment down-regulates thrombin-induced cell signaling cascades.
Panel A. HPAEC monolayers were pretreated with heparin (50 µg/ml) or ODSH (50 µg/ml) for 40 min before challenging with thrombin (20 nM). The cells were harvested at indicated time points and analyzed by Western blotting for ezrin/radixin/moesin phosphorylation (pERM) and MLC di-phosphorylation (ppMLC). GAPDH immunostaining was used as a loading control. Panel B. Statistical analysis of the results shown in panel A. Experimental data is expressed in relative units and presented as a mean of four individual experiments ± SE. *P < 0.05 and **P < 0.01, and ***p<0.001 versus cells treated with thrombin alone.
4. DISCUSSION
In this study, we demonstrate that pretreatment with heparin and its low anticoagulant 2-O, 3-O desulfated derivative, ODSH, attenuate thrombin-induced increases in pulmonary EC transendothelial permeability (Figures 1, 2 and 3). The effect of ODSH was superior to heparin, despite its lower degree of sulfation and reduced anticoagulant activity, indicating that this activity of heparin may be independent of its other functions. Further, we were able to link this protective effect of heparin or ODSH to morphological changes in the EC cytoskeletal structure. Indeed, our immunocytochemistry data demonstrated that pretreatment with heparin or ODSH inhibited thrombin-stimulated intercellular gap formation, prevented a formation of characteristic actin stress fibers and induced the characteristic sub-membrane F-actin rim that is a signature of barrier protective agents. In addition, heparin or ODSH pretreatment significantly preserved the EC cell-cell contacts (Figure 3 A and B).
In vitro studies on thrombin-induced endothelial permeability have identified at least four independent signaling pathways that may contribute to barrier dysfunction (McLaughlin et al., 2005). In our present study, we used two major thrombin-dependent signaling pathways initiated by RhoA activation and intracellular Ca2+ elevation, as a functional test to examine molecular mechanisms of possible protective effects of heparin or ODSH in the EC (McLaughlin et al., 2005)(van Nieuw Amerongen et al., 2008). In thrombin-challenged cells pretreated with heparin or ODSH, RhoA activation was found significantly attenuated (Figure 6 A). This was consistent with immunocytochemical findings (Figure 3) and changes in intracellular Ca2+ (Figure 6 B). Consistent with thrombin-induced changes in TER, intercellular gap formation and actin cytoskeleton rearrangement, heparins also reduced thrombin-induced increases in phosphorylation of ERM and MLC closely involved in the regulation of the cytoskeletal structures and critical for the barrier-disruptive effect of thrombin (Figure 7). The heparin derivative, ODSH, was particularly effective in this regard.
There are two potential explanations for findings in respect to heparin and ODSH effects on PAR-1. First, heparin, a highly negatively charged molecule, is known to bind to many substances including thrombin (Carter et al., 2005)(Loke et al., 2012)(Li et al., 1976)(Forster and Mulloy, 2006). Early work has also demonstrated that heparin binds to a specific site on antithrombin III (AT), producing an altered conformation of the protein, and the new conformation has a higher affinity for thrombin. The thrombin-AT ll complex is formed then releases heparin (Li et al., 1976). Thrombin exhibits two anion–binding exosites (Sheehan and Sadler, 1994) (Bode et al., 1992). Exosite I can bind substrates such as fibrinogen and exosite II can bind heparin (Fenton et al., 1988) (Wu et al., 1991) and the thrombin receptor (Liu et al., 1991). Proteins that bind heparin express heparin binding motifs, which are linear heparin binding motifs with conserved sequences of basic (B) and hydropathic (X) amino acids in specific patterns (Green et al., 2013)(Cardin et al., 1991)(Sobel et al., 1992)(Sheehan and Sadler, 1994). The anion-binding on exo-site II contains a structural motif (-B-X-B-B-) that resembles sequences found in other heparin-binding peptides and proteins (Cardin et al., 1991) (De Cristofaro and De Candia, 2003). We identified a similar motif (25RARR28) in the N-terminal part of PAR-1, and hypothesized that the heparins may directly bind at this site to PAR-1. Furthermore, we suppose that this binding may interfere with thrombin-mediated cleavage of PAR-1 since the cleavage site (Ser42) is located in the vicinity to hypothetical heparin-binding site and can be masked by heparin molecule. A second possible explanation to our findings is that heparins might modulate PAR-1 cleavage by binding to other molecules on EC surface. On human platelets it has been previously shown that the glycoprotein (Gp) Ibα can positively modulate the PAR-1 cleavage by thrombin and increase the velocity of PAR-1 cleavage by thrombin. Direct measurement of the hydrolysis of PAR-1 on intact platelets demonstrated that inhibition by heparin of thrombin binding to GpIbα inhibited PAR-1 cleavage on human platelets(De Candia et al., 2001). While we do not have direct evidence (De Candia et al., 1999), our results suggest an important role for heparin and ODSH as an inhibitor of thrombin-induced, PAR-mediated EC barrier disruption, through a direct or indirect action on PAR-1. Further studies are required to evaluate the above mentioned hypothesis.
Some studies demonstrate that binding to heparin might not affect thrombin activity. For example, in early work of Li et al 1977, heparin did not prevent an association of an active site probe for serine proteases or proflavine with thrombin. The occurrence of heparin binding to thrombin is an independent event from the finding that it is capable of binding to other proteins and receptors (Forster and Mulloy, 2006) (Weitz and Weitz, 2010), which may include the PAR receptors. Particle size characterization data we have obtained using laser light scattering is consistent with known heparinthrombin binding and demonstrates that thrombin and heparin form large complexes (Figure 5). However, ODSH, a heparin derivative with 2-O, 3-O desulfation and decreased negative charge displayed no change of the thrombin curve indicating that ODSH does not bind to thrombin in these experimental conditions (Figure 5).
We have shown that heparin and ODSH both decreased PAR-1 cell-surface expression as determined by immunofluorescence microscopy. Cell-surface PAR-1 disappeared when exposed to heparin or ODSH alone in comparable fashion to exposure of thrombin alone (Figure 4 B). PAR-1 internalization demonstrated by immunocytochemistry after exposing the cells to thrombin, heparin or ODSH compared to the control is consistent with previously known internalization of PAR-1 when exposed to thrombin (Ellis et al., 1999). Our data suggest that protective functions of heparin derivatives may occur on the cell surface and involve heparin or ODSH ligand activity via thrombin receptor interaction (Figure 4 B). To further address the possible interaction of thrombin by either heparin or ODSH, we removed unbound heparin/ODSH after the pretreatments and prior to the addition of thrombin. The continued inhibition of PAR-1 activation was compared to experiments performed with thrombin alone (Figure 4). This experiment suggests that heparin and ODSH inhibit thrombin effects on vascular permeability not through direct heparin/thrombin binding but perhaps in an interaction of the heparins with the PAR-1 receptor.
Contractility of the actin cytoskeleton is regulated through actomyosin interaction induced by phosphorylation of the regulatory light chain of myosin ll (MLC) (Popović et al., 2010)(Satpathy et al., 2004). In our studies, attenuation of thrombin-induced increase in MLC phosphorylation at Ser19 and Thr18 produced by heparins (Figure 7), suggest that they regulate signaling upstream of MLCK and MLCP most likely PAR-1-mediated (Satpathy et al., 2004) to alleviate thrombin-induced hyperpermeability in human pulmonary EC. Taken together, our data suggest that the heparin and ODSH can interfere with thrombin-activated PAR1-mediated signaling in the EC possibly via interaction of heparin and ODSH with thrombin receptors. However, these interactions have to be confirmed in future experiments. While we do not have direct evidence of heparin or ODSH interaction with PAR-1, our results suggest an important role for heparin and ODSH as antagonists to thrombin-induced PAR-mediated EC barrier disruption.
5. Conclusion
These studies suggest that heparin may be a potential therapy for ALI/ARDS. However, clinical use of heparin as an anti-inflammatory drug might prove limited because of risks of heparin-induced thrombocytopenia and bleeding complications at therapeutic doses (Bernard et al., 2001)(Doutremepuich et al., 1996)(Warkentin and Kelton, 2001). Low anticoagulant ODSH was developed to separate the anticoagulant and the anti-inflammatory properties of heparin (Fryer et al., 1997). We have demonstrated that ODSH significantly attenuates activation of PAR-1 by thrombin thereby preventing adverse effects of thrombin on human lung EC permeability. Collectively, our findings suggest that heparin and ODSH may attenuate EC barrier disruption in diseases such as ALI/ARDS. Furthermore, ODSH treatment may present a safer alternative to unfractionated heparin and offer potential as a valuable and translatable therapy for human ALI/ARDS.
ACKNOWLEDGMENTS
We gratefully acknowledge assistance from Dr. Narayanam V. Rao, University of Utah, with thrombin binding studies and for careful editorial review by Dr. Bruce Davis, Georgia Regents University.
Funding support provided by NIH grant HL101902 (Dr. Verin and Dr. Black), GHSU CVDI Intramural Grant and American Heart Association 11SDG7670035 (Dr. Zemskov) and by funds generously supplied to Drs. Gonzales and Kennedy by Dr. Michael Madaio from the Department of Internal Medicine, Georgia Regents University. Dr. Gonzales was supported by the NIH T32 fellowship grant. None of the sponsors had a role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Dr. Kennedy Is the Scientific founder of ParinGenix, Inc. and technology inventor and consultant to ParinGenix, Inc.. He maintains founder’s stock in the company. He is the inventor of 2-O, 3-O desulfated heparin. He maintains no other conflict of interest.
All other authors declare no conflict of interest.
Contributor Information
Joyce N. Gonzales, Email: jgonzales@gru.edu.
Kyung-mi Kim, Email: i60p68@hotmail.com.
Marina A. Zemskova, Email: mzemskova@gru.edu.
Ruslan Rafikov, Email: rrafikov@gru.edu.
Brenten Heeke, Email: bheeke@gru.edu.
Matthew N. Varn, Email: mvarn@gru.edu.
Stephen Black, Email: sblack@gru.edu.
Thomas P. Kennedy, Email: tkennedy@gru.edu.
Alexander D. Verin, Email: averin@gru.edu.
Evgeny A. Zemskov, Email: ezemskov@gru.edu.
References
- Bao Y, Geng Y, Jing H. Effect of hirudin on the levels of acute lung injury rat tumor necrosis factor-α and matrix metalloproteinase-12. Mol. Med. Rep. 2012;5:873–875. doi: 10.3892/mmr.2011.739. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JGN, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB. J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004a;18:1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc. Res. 2004b;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Bode W, Turk D, Karshikov A. The refined 1.9-A X-ray crystal structure of D-Phe-Pro-Arg chloromethylketone-inhibited human alpha-thrombin: structure analysis, overall structure, electrostatic properties, detailed active-site geometry, and structure-function relationships. Protein Sci. Publ. Protein Soc. 1992;1:426–471. doi: 10.1002/pro.5560010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogatcheva NV, Garcia JGN, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochem. Biokhimiia. 2002;67:75–84. doi: 10.1023/a:1013904231324. [DOI] [PubMed] [Google Scholar]
- Cadroy Y, Gaspin D, Dupouy D, Lormeau JC, Boneu B, Sié P. Heparin reverses the procoagulant properties of stimulated endothelial cells. Thromb. Haemost. 1996;75:190–195. [PubMed] [Google Scholar]
- Cardin AD, Demeter DA, Weintraub HJ, Jackson RL. Molecular design and modeling of protein-heparin interactions. Methods Enzymol. 1991;203:556–583. doi: 10.1016/0076-6879(91)03030-k. [DOI] [PubMed] [Google Scholar]
- Carter WJ, Cama E, Huntington JA. Crystal structure of thrombin bound to heparin. J. Biol. Chem. 2005;280:2745–2749. doi: 10.1074/jbc.M411606200. [DOI] [PubMed] [Google Scholar]
- Casu B, Naggi A, Torri G. Heparin-derived heparan sulfate mimics to modulate heparan sulfate-protein interaction in inflammation and cancer. Matrix Biol. J. Int. Soc. Matrix Biol. 2010;29:442–452. doi: 10.1016/j.matbio.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AWY, Jurasz P, Hollenberg MD, Radomski MW. Mechanisms of action of proteinase-activated receptor agonists on human platelets. Br. J. Pharmacol. 2002;135:1123–1132. doi: 10.1038/sj.bjp.0704559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csortos C, Kolosova I, Verin AD. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the PPP family. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L843–L854. doi: 10.1152/ajplung.00120.2007. [DOI] [PubMed] [Google Scholar]
- De Candia E, De Cristofaro R, Landolfi R. Thrombin-induced platelet activation is inhibited by high- and low-molecular-weight heparin. Circulation. 1999;99:3308–3314. doi: 10.1161/01.cir.99.25.3308. [DOI] [PubMed] [Google Scholar]
- De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J. Biol. Chem. 2001;276:4692–4698. doi: 10.1074/jbc.M008160200. [DOI] [PubMed] [Google Scholar]
- De Cristofaro R, De Candia E. Thrombin domains: structure, function and interaction with platelet receptors. J. Thromb. Thrombolysis. 2003;15:151–163. doi: 10.1023/B:THRO.0000011370.80989.7b. [DOI] [PubMed] [Google Scholar]
- Di Ciano-Oliveira C, Sirokmány G, Szászi K, Arthur WT, Masszi A, Peterson M, Rotstein OD, Kapus A. Hyperosmotic stress activates Rho: differential involvement in Rho kinase-dependent MLC phosphorylation and NKCC activation. Am. J. Physiol. Cell Physiol. 2003;285:C555–C566. doi: 10.1152/ajpcell.00086.2003. [DOI] [PubMed] [Google Scholar]
- Doutremepuich C, Azougagh Oualane F, Doutremepuich F, Fareed J. New class of heparin derivatives with a potent antithrombotic effect and a very limited hemorrhagic activity. Thromb. Res. 1996;83:217–228. doi: 10.1016/0049-3848(96)00130-2. [DOI] [PubMed] [Google Scholar]
- Ellis CA, Tiruppathi C, Sandoval R, Niles WD, Malik AB. Time course of recovery of endothelial cell surface thrombin receptor (PAR-1) expression. Am. J. Physiol. 1999;276:C38–C45. doi: 10.1152/ajpcell.1999.276.1.C38. [DOI] [PubMed] [Google Scholar]
- Fenton JW, 2nd, Olson TA, Zabinski MP, Wilner GD. Anion-binding exosite of human alpha-thrombin and fibrin(ogen) recognition. Biochemistry (Mosc.) 1988;27:7106–7112. doi: 10.1021/bi00418a066. [DOI] [PubMed] [Google Scholar]
- Forster M, Mulloy B. Computational approaches to the identification of heparinbinding sites on the surfaces of proteins. Biochem. Soc. Trans. 2006;34:431–434. doi: 10.1042/BST0340431. [DOI] [PubMed] [Google Scholar]
- Fryer A, Huang YC, Rao G, Jacoby D, Mancilla E, Whorton R, Piantadosi CA, Kennedy T, Hoidal J. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J. Pharmacol. Exp. Ther. 1997;282:208–219. [PubMed] [Google Scholar]
- Gori AM, Pepe G, Attanasio M, Falciani M, Abbate R, Prisco D, Fedi S, Giusti B, Brunelli T, Giusti B, Brunelli T, Comeglio P, Gensini GF, Neri Serneri GG. Tissue factor reduction and tissue factor pathway inhibitor release after heparin administration. Thromb. Haemost. 1999;81:589–593. [PubMed] [Google Scholar]
- Green JV, Orsborn KI, Zhang M, Tan QK-G, Greis KD, Porollo A, Andes DR, Long JL, Hostetter MK. Heparin Binding Motifs and Biofilm Formation by Candida albicans. J. Infect. Dis. 2013 doi: 10.1093/infdis/jit391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1874–1879. doi: 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idell S, James KK, Gillies C, Fair DS, Thrall RS. Abnormalities of pathways of fibrin turnover in lung lavage of rats with oleic acid and bleomycininduced lung injury support alveolar fibrin deposition. Am. J. Pathol. 1989;135:387–399. [PMC free article] [PubMed] [Google Scholar]
- Kim K-M, Csortos C, Czikora I, Fulton D, Umapathy NS, Olah G, Verin AD. Molecular characterization of myosin phosphatase in endothelium. J. Cell. Physiol. 2012;227:1701–1708. doi: 10.1002/jcp.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li EH, Fenton JW, 2nd, Feinman RD. The role of heparin in the thrombinantithrombin III reaction. Arch. Biochem. Biophys. 1976;175:153–159. doi: 10.1016/0003-9861(76)90494-x. [DOI] [PubMed] [Google Scholar]
- Liu LW, Vu TK, Esmon CT, Coughlin SR. The region of the thrombin receptor resembling hirudin binds to thrombin and alters enzyme specificity. J. Biol. Chem. 1991;266:16977–16980. [PubMed] [Google Scholar]
- Loke C, Ali SS, Johari V. Pharmacology of anticoagulants. Dis.--Mon. DM. 2012;58:424–430. doi: 10.1016/j.disamonth.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinaseactivated receptors. Pharmacol. Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J. Biol. Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- Momota F, Hirano K, Hirano M, Nishimura J, Kanaide H. Involvement of Gi/o in the PAR-4-induced NO production in endothelial cells. Biochem. Biophys. Res. Commun. 2006;342:365–371. doi: 10.1016/j.bbrc.2006.01.165. [DOI] [PubMed] [Google Scholar]
- Murphy JT, Duffy SL, Hybki DL, Kamm K. Thrombin-mediated permeability of human microvascular pulmonary endothelial cells is calcium dependent. J. Trauma. 2001;50:213–222. doi: 10.1097/00005373-200102000-00005. [DOI] [PubMed] [Google Scholar]
- O’Brien PJ, Prevost N, Molino M, Hollinger MK, Woolkalis MJ, Woulfe DS, Brass LF. Thrombin responses in human endothelial cells. Contributions from receptors other than PAR1 include the transactivation of PAR2 by thrombin-cleaved PAR1. J. Biol. Chem. 2000;275:13502–13509. doi: 10.1074/jbc.275.18.13502. [DOI] [PubMed] [Google Scholar]
- Popović M, Paskas S, Zivković M, Burysek L, Laumonnier Y. Human cytomegalovirus increases HUVEC sensitivity to thrombin and modulates expression of thrombin receptors. J. Thromb. Thrombolysis. 2010;30:164–171. doi: 10.1007/s11239-010-0447-7. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- Ruiz-Loredo AY, López E, López-Colomé AM. Thrombin promotes actin stress fiber formation in RPE through Rho/ROCK-mediated MLC phosphorylation. J. Cell. Physiol. 2011;226:414–423. doi: 10.1002/jcp.22347. [DOI] [PubMed] [Google Scholar]
- Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombininduced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp. Eye Res. 2004;79:477–486. doi: 10.1016/j.exer.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Davis P, La Pointe H, Monkman S, Coates G, deSa D. Thrombin inhibitors reduce intrapulmonary accumulation of fibrinogen and procoagulant activity of bronchoalveolar lavage fluid during acute lung injury induced by pulmonary overdistention in newborn piglets. Pediatr. Res. 1996;39:798–804. doi: 10.1203/00006450-199605000-00009. [DOI] [PubMed] [Google Scholar]
- Seminario-Vidal L, Kreda S, ones L, O’Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J. Biol. Chem. 2009;284:20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JP, Sadler JE. Molecular mapping of the heparin-binding exosite of thrombin. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5518–5522. doi: 10.1073/pnas.91.12.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smurova KM, Biriukova AA, Garcia JG, Vorob’ev IA, Alieva IB, Verin AD. [Reorganization of microtubule system in pulmonary endothelial cells in response to thrombin treatment] Tsitologiia. 2004;46:695–703. [PubMed] [Google Scholar]
- Sobel M, Soler DF, Kermode JC, Harris RB. Localization and characterization of a heparin binding domain peptide of human von Willebrand factor. J. Biol. Chem. 1992;267:8857–8862. [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Tar K, Csortos C, Czikora I, Olah G, Ma S-F, Wadgaonkar R, Gergely P, Garcia JGN, Verin AD. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J. Cell. Biochem. 2006;98:931–953. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- Vadász I, Morty RE, Olschewski A, Königshoff M, Kohstall MG, Ghofrani HA, Grimminger F, Seeger W. Thrombin impairs alveolar fluid clearance by promoting endocytosis of Na+,K+-ATPase. Am. J. Respir. Cell Mol. Biol. 2005;33:343–354. doi: 10.1165/rcmb.2004-0407OC. [DOI] [PubMed] [Google Scholar]
- Van Nieuw Amerongen GP, Musters RJP, Eringa EC, Sipkema P, van Hinsbergh VWM. Thrombin-induced endothelial barrier disruption in intact microvessels: role of RhoA/Rho kinase-myosin phosphatase axis. Am. J. Physiol. Cell Physiol. 2008;294:C1234–C1241. doi: 10.1152/ajpcell.00551.2007. [DOI] [PubMed] [Google Scholar]
- Villar J. What is the acute respiratory distress syndrome? Respir. Care. 2011;56:1539–1545. doi: 10.4187/respcare.01395. [DOI] [PubMed] [Google Scholar]
- Vogel SM, Gao X, Mehta D, Ye RD, John TA, Andrade-Gordon P, Tiruppathi C, Malik AB. Abrogation of thrombin-induced increase in pulmonary microvascular permeability in PAR-1 knockout mice. Physiol. Genomics. 2000;4:137–145. doi: 10.1152/physiolgenomics.2000.4.2.137. [DOI] [PubMed] [Google Scholar]
- Ware LB, Bastarache JA, Wang L. Coagulation and fibrinolysis in human acute lung injury--new therapeutic targets? Keio J. Med. 2005;54:142–149. doi: 10.2302/kjm.54.142. [DOI] [PubMed] [Google Scholar]
- Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N. Engl. J. Med. 2001;344:1286–1292. doi: 10.1056/NEJM200104263441704. [DOI] [PubMed] [Google Scholar]
- Weitz DS, Weitz JI. Update on heparin: what do we need to know? J. Thromb. Thrombolysis. 2010;29:199–207. doi: 10.1007/s11239-009-0411-6. [DOI] [PubMed] [Google Scholar]
- Wu QY, Sheehan JP, Tsiang M, Lentz SR, Birktoft JJ, Sadler JE. Single amino acid substitutions dissociate fibrinogen-clotting and thrombomodulin-binding activities of human thrombin. Proc. Natl. Acad. Sci. U. S. A. 1991;88:6775–6779. doi: 10.1073/pnas.88.15.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]