Abstract
Background
Allogeneic hematopoietic-cell transplantation (HCT), although curative for some high-risk diseases, is a complex and costly procedure. The costs of transplantation among children have not been described previously.
Procedure
We compared the costs of HCT within the first 100-days among children who received myeloablative HCT from either a matched related donor (MRD, N=27), matched unrelated donor (MUD, N=28) or unrelated umbilical cord blood (UCB, N=91). We also conducted analyses to describe predictors of higher costs of transplantation.
Results
The 100-day probabilities of overall survival were 96%, 96% and 87% for MRD, MUD and UCB, respectively. The median cost per day survived (excluding costs of graft acquisition) was $3,403 (interquartile-range [IQR], 2,838-3,819) for MRD, $3,833 (IQR, 3,402-4,134) for MUD and $3,964 (IQR, 3,351-4,952) for UCB recipients. The costs of MUD and UCB HCT remained similar when costs of graft acquisition were considered within total costs of transplantation. In multivariate analysis adjusting for important patient, disease and transplant related characteristics, factors associated with higher costs within the first 100-days were HCT using MUD (relative-risk [RR] 1.2 [95% confidence-intervals, 1.0-1.3]) or UCB (RR 1.2 [1.1-1.3]), Lansky score <90 at transplant (RR 1.3 [1.2-1.4]), graft failure (RR 1.6 [1.5-1.7]), need for dialysis (RR 1.7 [1.6-1.8]), need for mechanical ventilation (RR 1.4 [1.3-1.5]) and occurrence of hepatic veno-occlusive disease (RR 1.3 [1.1-1.6]).
Conclusions
Within the first 100-days, the absolute costs of MUD and UCB HCT are similar, while MRD HCT is less costly. These costs are primarily driven by severe post-transplant complications and graft failure.
Keywords: Hematopoietic-cell transplantation, Pediatric, Allogeneic, Costs, Complications
Introduction
Hematopoietic-cell transplantation (HCT) is potentially curative for many high-risk hematologic and non-hematologic disorders in children. However, it is a complex, resource intense and costly procedure. Besides the costs of graft procurement, patient evaluations, preparative therapy, an “uncomplicated” transplant and its associated care, additional significant expense can occur due to treatment related complications such as infections, organ failure, graft failure and graft-versus-host disease (GVHD). Although many studies have investigated the costs of allogeneic transplantation in adults,[1-8] these costs have not been well described for pediatric HCT recipients. Esperou et al included children as young as 8 years of age in a study of costs of allogeneic transplantation, but their study cohort was predominantly composed of adult patients (median age was 39 years).[9] There exists considerable variation in transplant procedures and supportive care practices among adult and pediatric transplant physicians and centers,[10,11] which could translate into significant differences in transplantation costs between children and adults. A better understanding of the costs of pediatric allogeneic transplantation is of importance from the health care resource utilization perspective and identification of specific predictors of expensive care can assist with the development of strategies for effective cost containment. We conducted a retrospective cohort study in a contemporary group of pediatric allogeneic HCT recipients to describe the costs of transplantation in this population, exclusive of the costs associated with provider-based care. We also compared the costs of transplant for recipients of matched related donors (MRD), matched unrelated donors (MUD) and unrelated umbilical cord blood (UCB) grafts and explored various risk factors for their association with increased costs of allogeneic transplantation.
Methods
Patients
The study cohort consisted of consecutive patients who received a myeloablative allogeneic HCT between 2004 and 2006 and were ≤18 years of age at the time of transplantation. Transplant related and outcome data were retrieved from the University of Minnesota Blood and Marrow Transplant Program Database, which prospectively collects these data on all patients transplanted at our institution. Additional data for this study were abstracted from patient medical records. Patients were treated on clinical protocols approved by our institutional review board.
All patients received a transplant according to predetermined eligibility and treatment criteria outlined in specific transplant protocols and supportive care guidelines. HLA-compatible related donors were utilized if available; otherwise they received UCB or grafts from. Our UCB selection criteria have been previously published and allow the use of two UCB units to optimize cell dose, if necessary.[12]
Patients were classified as having standard or high risk disease. Standard risk disease included acute leukemia in first complete remission, chronic myeloid leukemia in first chronic phase, myelodysplastic syndrome (refractory anemia only), and nonmalignant hematologic disorders; all other diagnoses were categorized as high risk disease.
Conditioning regimen and supportive care
Conditioning regimens used at our institution have been described previously.[13-16] Briefly, patients undergoing myeloablative HCT for hematologic malignancies received a regimen consisting of fractionated total body irradiation, cyclophosphamide and fludarabine or a regimen consisting of busulfan, cyclophosphamide with or without melphalan. Patients with Fanconi's anemia received a regimen that included cyclophosphamide, fludarabine and anti-thymocyte globulin with or without busulfan while patients with other non-malignant congenital bone marrow failure disorders received busulfan, fludarabine or clofarabine, anti-thymocyte globulin and total lymphoid irradiation. Our GVHD prophylaxis and treatment regimens have also been described previously.[17,18]
Allogeneic HCT recipients were admitted to a dedicated inpatient transplant unit for initiating conditioning therapy and were discharged from the hospital after engraftment (absolute neutrophil count (ANC) more than 0.5 × 109/L for three days), had adequate oral intake, had transfusion or other infusion requirements that could be met as an outpatient and had no complications requiring continued hospitalization. Frequency of outpatient followup was based on patient overall clinical condition and need for ongoing support (e.g. transfusions, antibiotic infusions). All patients received antibacterial, antiviral, and antifungal prophylaxis and blood product and nutritional support per institutional guidelines.
All patients are followed within our transplant program and institution from the time of pre-transplant evaluation until at least 100 days post-transplant. Patients are required to stay within a 30 minute driving distance from our transplant center and accommodation is arranged for patients who do not live locally. All hospitalizations within the first 100 days are exclusively in a dedicated inpatient transplant unit that has resources for management of severe post-HCT complications (e.g. mechanical ventilation, dialysis, pressor support). All outpatient clinic and infusion visits within the first 100 days occur in our dedicated transplant outpatient facility. Hence the institutional accounting department captures all relevant medical costs for the first 100 days except costs for outpatient prescription drugs including drugs administered through home-care services. Transplant related care in this early post-transplant period was coordinated exclusively by our group of transplant physicians and mid-level providers.
Cost data
Data regarding inpatient costs, days of hospitalization and number of outpatient clinic visits were obtained from the institutional accounting department for all transplant related costs prior to day 0 (from day -30) and until day 100 post-transplantation. Costs (inpatient and outpatient) were determined by each hospital department's item and procedure specific costs and then summed from the itemized listing of each patient's accounting record through day 100. Besides total cost of care (direct and indirect costs), specific categories of costs were also available. These categories included costs for ‘graft acquisition’, ‘laboratory services’, ‘radiological investigations’, ‘pharmacy services’, ‘room and board’, ‘blood components’ and ‘other services’. Examples of ‘other service’ costs include costs for occupational therapy, physical therapy and vascular access and operating room costs. We excluded costs for ‘physician services’. Also, we could not account for outpatient prescription drug costs, home infusion costs and did not include patient related non-medical costs (e.g. out-of-pocket costs, transportation and accommodation) in our analysis.
We report costs for initial graft acquisition separate from total costs of transplantation. However, graft acquisition costs for a second graft infusion for graft failure or donor lymphocyte infusion for relapse within the first 100 days were included in cost-analyses and were combined with the ‘other’ category.
Since our data consisted of the actual dollar amount for cost incurred and given the relatively contemporary nature of our cohort, we did not adjust for inflation in our cost-analyses.
Statistical methods
The primary objective of this study was to compare medical costs among recipients of MRD, MUD and UCB transplantation. We also wanted to explore factors that were associated with increased costs of transplantation. To allow comparison among different transplant categories, especially because of the variation in patient selection, risks for transplant-related complications and overall mortality, costs are presented as cost per day survived (in dollars).
Data are described as proportions or as median with range or interquartile range (lowest quartile-highest quartile). Comparison of patient, disease and transplant characteristics was performed using chi-square, Fisher's exact or Wilcoxon's rank sum test, as appropriate. The Kaplan-Meier method was used to estimate overall survival. Multivariate Cox regression analysis was performed for overall survival after including the following variables: transplant donor type (main effect variable), age at HCT, Lansky performance score at HCT, disease risk, cytomegalovirus (CMV) serological status, acute GVHD (grade 2-4), graft failure, dialysis, mechanical ventilation and hepatic veno-occlusive disease. Event times were measured from date of transplantation to date of death or last contact.
Analysis of variance (ANOVA) method was used to compare costs among different transplant types and was adjusted for the following variables: age at transplantation, Lansky performance score at HCT, disease risk, CMV status, acute GVHD, graft failure, dialysis, mechanical ventilation, hepatic veno-occlusive disease and duration of hospital stay (days of initial and any subsequent hospitalizations) in the first 100 days. HLA-match status correlated with transplant type and was not included as a separate variable. There were no significant interactions between transplant type and other predictor variables included in the ANOVA models.
All p-values reported are two sided. Analyses were performed using the SAS 9.1 software (Cary, North Carolina, USA).
Results
Patient characteristics and outcomes
Patient, disease and transplant characteristics of our cohort are described in Table I. Recipients of UCB received HLA-mismatched grafts more frequently than MRD and MUD recipients. UCB recipients also had the longest duration of hospitalization within the first 100 days. Acute GVHD occurred less commonly in MRD recipients, while a greater proportion of UCB recipients needed mechanical ventilation for respiratory failure. The rates of dialysis and hepatic veno-occlusive disease were similar among the three donor types. The duration of hospitalization varied by graft source; the median duration of inpatient stay in the first 100 days was 36 days for MRD, 47 days for MUD and 57 days for UCB recipients.
Table I. Patient, disease and transplant characteristics.
| Variable | Related donor | Unrelated donor | Umbilical cord blood | P-value |
|---|---|---|---|---|
| N | 27 | 28 | 91 | |
| Median age (range), years | 10 (0.7-18) | 9 (0.3-17) | 7 (02-18) | 0.55 |
| Male gender | 23 (85%) | 18 (64%) | 52 (57%) | 0.03 |
| Lansky score at transplant | 0.76 | |||
| 90-100 | 23 (85%) | 25 (89%) | 72 (79%) | |
| ≤80 | 3 (11%) | 2 (7%) | 12 (13%) | |
| Missing | 1 (4%) | 1 (4%) | 7 (8%) | |
| Diagnosis | 0.01 | |||
| Metabolic disorders | 7 (26%) | 4 (14%) | 31 (34%) | |
| Acute myeloid leukemia | 8 (30%) | 1 (4%) | 25 (27%) | |
| Acute lymphoblastic leukemia | 6 (22%) | 0 | 19 (21%) | |
| Chronic myeloid leukemia | 0 | 5 (18%) | 4 (4%) | |
| Fanconi's anemia | 3 (11%) | 14 (50%) | 11 (12%) | |
| Aplastic anemia | 2 (7%) | 4 (14%) | 0 | |
| Lymphoma | 1 (4%) | 0 | 1 (1%) | |
| Disease risk | 0.05 | |||
| Standard | 20 (74%) | 26 (93%) | 64 (70%) | |
| High | 7 (26%) | 2 (7%) | 27 (30%) | |
| Previous transplant | 0 | 1 (4%) | 5 (5%) | 0.45 |
| CMV seropositive (donor or recipient) | 14 (52%) | 15 (54%) | 47 (52%) | 0.98 |
| Graft source | <0.01 | |||
| Bone marrow | 25 (92%) | 27 (96%) | 0 | |
| Peripheral blood | 1 (4%) | 1 (4%) | 0 | |
| UCB | 1 (4%) | 0 | 91 (100%) | |
| Single UCB | 1 | - | 57 | |
| Double UCB | 0 | - | 34 | |
| HLA match a | <0.01 | |||
| 6/6 | 22 (81%) | 22 (79%) | 18 (20%) | |
| 5/6 | 5 (19%) | 6 (21%) | 48 (53%) | |
| 4/6 | 0 | 0 | 25 (27%) | |
| Median time to ANC engraftment (range), days | 19 (10-39) | 14 (9-29) | 20 (11-43) | 0.01 |
| Median time to platelet engraftment (range), days b | 33 (19-100) | 36 (18-98) | 55 (17-100) | <0.01 |
| Graft failure | 0 | 2 (7%) | 4 (4%) | 0.41 |
| Second graft infusion | - | 1 | 1 | |
| Median duration of hospital stay (interquartile range), days b | 36 (32-49) | 47 (38-56) | 57 (44-80) | <0.01 |
| Major complications | ||||
| Acute graft-versus-host disease (grade 2-4) | 4 (15%) | 11 (39%) | 40 (44%) | 0.02 |
| Dialysis | 2 (7%) | 1 (4%) | 10 (11%) | 0.46 |
| Mechanical ventilation | 2 (7%) | 4 (14%) | 25 (27%) | 0.05 |
| Hepatic veno-occlusive disease | 3 (11%) | 0 | 7 (8%) | 0.23 |
KPS – Karnofsky performance status; CMV – cytomegalovirus; HLA – human leukocyte antigen; ANC – absolute neutrophil count
Worst match for recipients of double UCB transplant
Followup was censored at day 100
Probability of overall survival at 100 days was 96% (95% CI, 89-100%) for MRD recipients, 96% (89-100%) for MUD recipients and 87% (80-94%) for recipients of UCB. On multivariate regression analysis, donor type had no impact on survival; compared to MRD, the RR for overall mortality was 1.7 (95% CI, 0.4-7.3) for MUD and 2.5 (0.7-8.2) for UCB recipients (P=0.31). Dialysis (RR 3.6 [1.6-8.1], P=0.003) and mechanical ventilation (RR 4.7 [2.3-9.6], P<0.001) were independent predictors for adverse 100-day survival. Age at transplant, Lansky score at transplant, diagnosis, disease risk, CMV serological status, acute GVHD and hepatic veno-occlusive disease did not impact overall survival.
Costs of transplantation
Costs of graft acquisition varied by donor source and were $9,566 (interquartile range [IQR], 7,175-11,209) for MRD, $58,243 (IQR, 47,917-61,364) for MUD and $46,669 (IQR, 45,488-68,830) for UCB, respectively.
For the whole cohort, the median cost per day survived (excluding graft acquisition costs) was $3,816 (IQR, 3,297-4,544). Costs of transplantation also varied by donor source; the median cost per day survived was $3,403 (IQR, 2,838-3,819) for MRD recipients, $3,833 (IQR, 3,402-4,134) for MUD recipients and $3,964 (IQR, 3,351-4,952) for recipients of UCB (P<0.01). MRD HCT was less expensive than MUD and UCB HCT; however, the costs of MUD and UCB HCT were similar (Figure 1). The costs of MUD and UCB HCT were also comparable when costs of graft acquisition were considered within total costs of transplantation (median cost per day survived $4,986 for MUD vs. $5,171 for UCB, P=0.54).
Figure 1.
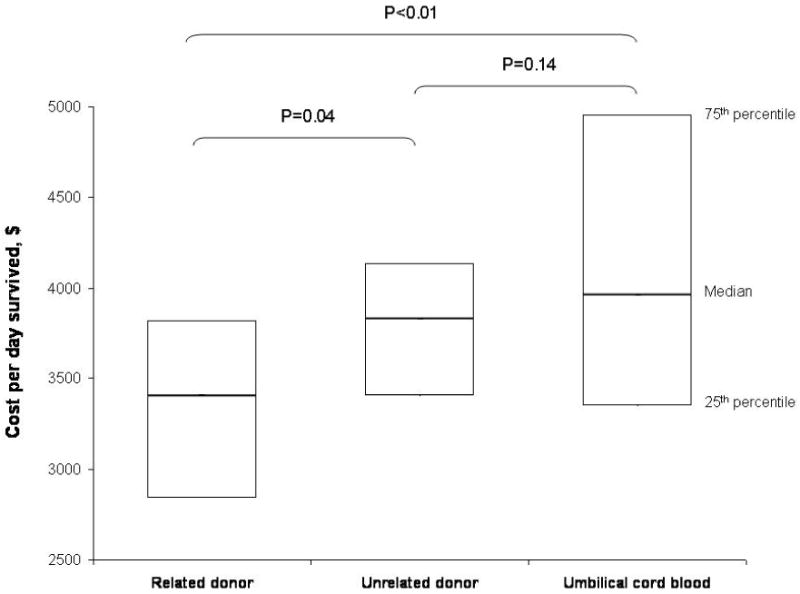
Costs of pediatric allogeneic transplantation by donor source. The boxes represent median and interquartile range of cost per day survived for each donor type. P-values based on Wilcoxon rank sum test and have not been adjusted for multiple comparisons.
The major categories of cost by donor source are summarized in Figure 2. In general, costs for room and board and pharmacy costs were the major contributor of costs. Also, the contribution of each category to total costs was similar for the three donor types.
Figure 2.
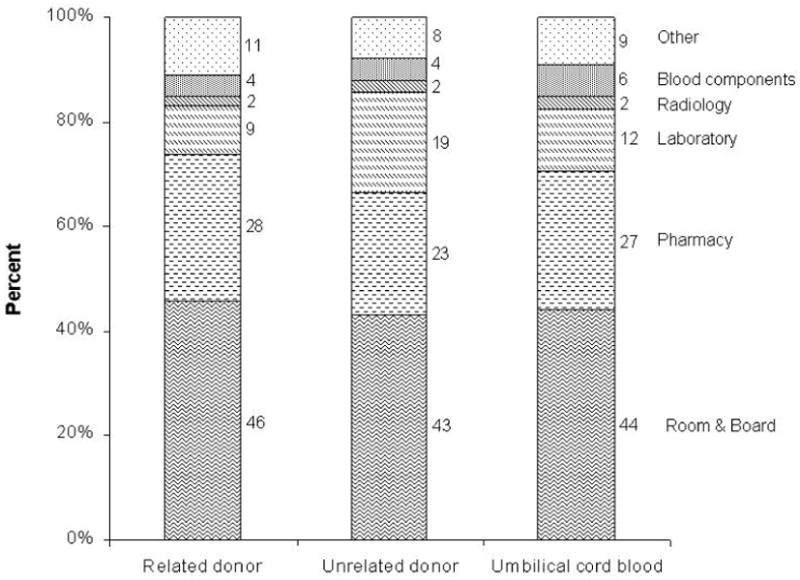
Categories of costs by donor source. The contribution of each category to total cost is represented as percent. Costs of graft acquisition were excluded from this figure.
In multivariate analysis adjusting for important variables that could impact costs, we observed a significant difference in the costs of transplantation between the three donor types (Table II). Compared to MRD transplants, the cost per day survived was 20% higher for both recipients of MUD and UCB. Other independent predictors of higher costs included Lansky score at transplant, occurrence of graft failure post-transplant, need for dialysis, need for mechanical ventilation and occurrence of hepatic veno-occlusive disease (Figure 3).
Table II. Multivariate analysis for predictors of costs of allogeneic transplantation in children; actual costs for predictors are also described.
| Variablesa,b | Multivariate analysis | Cost per day survived, $ b | ||
|---|---|---|---|---|
|
| ||||
| Relative-risk (95% CI) | P-value | Median | Interquartile range | |
| Donor type | ||||
| Related donor | 1.0 | 0.02 | 3,403 | 2,838-3,819 |
| Unrelated donor | 1.2 (1.0-1.3) | 0.05 | 3,833 | 3,402-4,134 |
| Umbilical cord blood | 1.2 (1.1-1.3) | 0.006 | 3,964 | 3,351-4,952 |
| Lansky score at transplant | ||||
| 90-100 | 1.0 | 3,660 | 3,243-4,339 | |
| ≤80 | 1.3 (1.2-1.4) | 0.03 | 4,488 | 3,954-6,043 |
| Graft failure | ||||
| No | 1.0 | 3,768 | 3,285-4,443 | |
| Yes | 1.6 (1.5-1.7) | <0.001 | 6,547 | 4,731-8,108 |
| Dialysis | ||||
| No | 1.0 | 3,665 | 3,251-4,234 | |
| Yes | 1.7 (1.6-1.8) | <0.001 | 6,637 | 5,117-7,750 |
| Mechanical ventilation | ||||
| No | 1.0 | 3,557 | 3,115-4,028 | |
| Yes | 1.4 (1.3-1.5) | <0.001 | 5,820 | 4,391-7,392 |
| Hepatic veno-occlusive disease | ||||
| No | 1.0 | 3,803 | 3,266-4,510 | |
| Yes | 1.3 (1.1-1.6) | 0.03 | 4,092 | 3,371-5,699 |
Other variables considered in the model included age at transplantation, disease risk, CMV status, acute graft-versus-host disease and total duration of hospital stay in the first 100 days.
Excluding costs of graft acquisition
Figure 3.
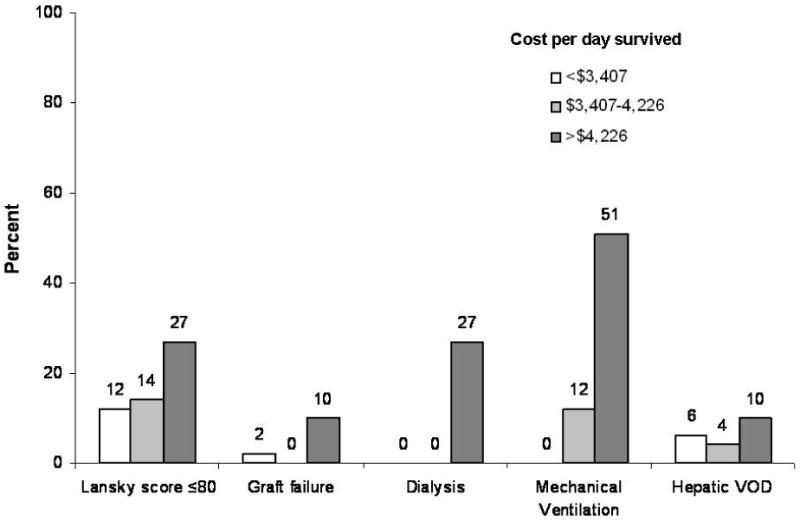
Predictors of transplant costs (by tertiles of cost per day survived). Poor Lansky score, graft failure, dialysis, mechanical ventilation and hepatic veno-occlusive disease were associated with increased costs on multivariate analysis. Within each risk factor category, column heights represent proportion of patients within the low, intermediate and high cost tertiles.
We also looked at categories of costs among patients with the least expensive and most expensive care (Figure 4). Again, room and board and pharmacy costs were the two major contributors to total costs. The contribution of each category to total costs was similar for patients with cost of care in the lowest, middle and highest tertiles.
Figure 4.
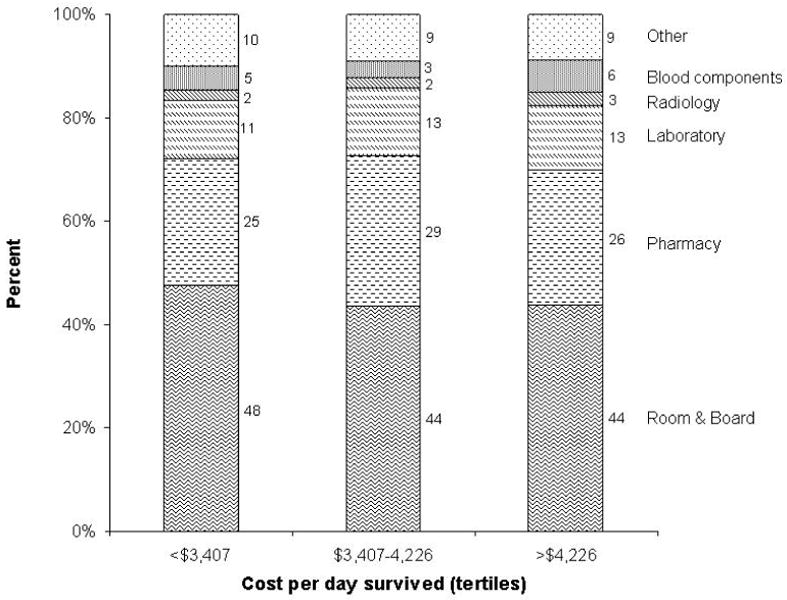
Categories of costs by tertiles of cost per day survived. The contribution of each category to total cost is represented as percent. Costs of graft acquisition were excluded from this figure.
Discussion
In our contemporary cohort of pediatric allogeneic HCT recipients, we observed MRD HCT to be less expensive than MUD or UCB HCT. However, MUD and UCB transplantation had comparable costs, even after considering costs of graft acquisition. The costs of transplantation were primarily driven by severe post-transplant complications (dialysis, mechanical ventilation and hepatic veno-occlusive disease, and for unrelated donor recipients, graft failure).
Both pediatric and adult allogeneic HCT are costly procedures; however, the costs of transplantation among children tend to be higher compared to adult recipients. In our previous analysis of costs of adult allogeneic transplantation,[5] the median cost per day survived for 130 patients who received a myeloablative HCT (MRD 67 and UCB 63) was $1,424 (IQR, 977-5,023) within the first 100 days post-transplant. In comparison, the median cost per day survived in our present analysis for children who were transplanted during the same time period was $3,816 (IQR, 3,297-4,544). The incidence of major early post-transplant complications was similar among pediatric and adult recipients: dialysis 9% vs. 15% (P=0.13), mechanical ventilation 21% vs. 24% (P=0.60), hepatic veno-occlusive disease 7% vs. 5% (P=0.42) and acute GVHD 38% vs. 48% (P=0.09). Graft failure occurred more frequently among adult recipients (11% vs. 4% in pediatric recipients, P=0.03). There was an important difference in the duration of hospitalization between adult and pediatric recipients. The median duration of inpatient stay in the first 100 days was 44 days (IQR, 35-60) for adults and 51 days (IQR, 40-71) for children (P<0.01). Since charges for room and board and inpatient pharmacy and laboratory services were the major contributors to total costs of transplantation, the longer hospital stay could account for the higher costs of pediatric transplantation. It was not within the scope of this study to address the reasons for longer hospitalization in children despite comparable rates of major complications. Variability in transplant practices between pediatric and adult transplant physicians could be an important cause for these cost differences. Lee et al have documented significant practice variation among pediatric and adult transplant physicians in their approach to transplantation for hematologic disorders, choice of graft source, and management strategies for acute and chronic GVHD.[11]
It is intuitive that occurrence of major complications would increase costs of transplantation. This has been well described in cost-analyses of adult allogeneic HCT.[4-7,9] In this study, we again observed complications such as graft failure, renal failure needing dialysis, pulmonary failure needing mechanical ventilation and hepatic veno-occlusive disease to be the primary drivers of HCT costs among children. Although major advances in post-transplant supportive care have occurred over the past four decades, better strategies to prevent and treat these complications are still needed in order to reduce costs of allogeneic transplantation. Poor Lansky score at the time of HCT was also associated with higher costs independent of the occurrence of other complications. Patients with poor performance status may have received more resources to prevent post-transplant complications. Alternatively, management of their comorbidities (e.g. pre-existing fungal infection) may have contributed to their total costs.
We observed the costs of MUD and UCB HCT in children to be comparable. UCB is increasingly being used as an alternative donor source for patients without a matched sibling donor. There is emerging data that UCB HCT is associated with lower risks of chronic GVHD.[19,20] Whether this would translate into lower long-term costs for UCB versus MUD needs to be investigated. A formal cost-effectiveness analysis that takes into account risks for transplant-related mortality, non-engraftment, GVHD, relapse and overall mortality among UCB and MUD HCT is also warranted.
Several limitations have to be considered in the interpretation of our analysis. Our results may not be generalizable since considerable practice variation exists among transplant centers and transplant physicians. Within our center, transplant conditioning and GVHD prophylaxis and management regimens were dictated by specific protocols and supportive care was based on established guidelines, limiting the impact of individual physician practice variation on costs. Also, we captured costs within the first 100 days following transplantation and did not consider costs of long term care or management of chronic GVHD and its complications. Other studies, although limited to adult HCT recipients, have shown that the costs of transplantation are largely concentrated within the first 100 days.[5,6] We could not account for costs of outpatient prescription drugs and home-care services, nor for the costs of physician services or those of other providers. However, these costs would have only made a small contribution to the total costs of transplantation.
In conclusion, allogeneic HCT in children is a costly procedure. In the first 100 days after transplantation, the costs of MUD and UCB HCT are similar, while MRD HCT is less costly. Severe complications and graft failure are the major contributors to total costs in the early post-transplant period. Strategies to decrease the risk of severe complications would reduce the overall costs of transplantation.
Acknowledgments
We gratefully acknowledge Donna Anderson, Transplant Finance Director and Cindy Nygaard, Senior Analyst, with the Department of Transplant Financial Management, University of Minnesota Medical Center, Fairview, for providing us with the cost data for this study.
References
- 1.Bennett C, Waters T, Stinson T, et al. Valuing clinical strategies early in development: a cost analysis of allogeneic peripheral blood stem cell transplantation. Bone marrow transplantation. 1999;24(5):555–560. doi: 10.1038/sj.bmt.1701945. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, Anasetti C, Kuntz KM, et al. The costs and cost-effectiveness of unrelated donor bone marrow transplantation for chronic phase chronic myelogenous leukemia. Blood. 1998;92(11):4047–4052. [PubMed] [Google Scholar]
- 3.Lee SJ, Weller E, Alyea EP, et al. Efficacy and costs of granulocyte colony-stimulating factor in allogeneic T-cell depleted bone marrow transplantation. Blood. 1998;92(8):2725–2729. [PubMed] [Google Scholar]
- 4.Lee SJ, Klar N, Weeks JC, et al. Predicting costs of stem-cell transplantation. J Clin Oncol. 2000;18(1):64–71. doi: 10.1200/JCO.2000.18.1.64. [DOI] [PubMed] [Google Scholar]
- 5.Majhail NS, Mothukuri JM, Brunstein CG, et al. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15(5):564–573. doi: 10.1016/j.bbmt.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Saito AM, Cutler C, Zahrieh D, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14(2):197–207. doi: 10.1016/j.bbmt.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito AM, Zahrieh D, Cutler C, et al. Lower costs associated with hematopoietic cell transplantation using reduced intensity vs high-dose regimens for hematological malignancy. Bone marrow transplantation. 2007;40(3):209–217. doi: 10.1038/sj.bmt.1705733. [DOI] [PubMed] [Google Scholar]
- 8.van Agthoven M, Groot MT, Verdonck LF, et al. Cost analysis of HLA-identical sibling and voluntary unrelated allogeneic bone marrow and peripheral blood stem cell transplantation in adults with acute myelocytic leukaemia or acute lymphoblastic leukaemia. Bone marrow transplantation. 2002;30(4):243–251. doi: 10.1038/sj.bmt.1703641. [DOI] [PubMed] [Google Scholar]
- 9.Esperou H, Brunot A, Roudot-Thoraval F, et al. Predicting the costs of allogeneic sibling stem-cell transplantation: results from a prospective, multicenter, French study. Transplantation. 2004;77(12):1854–1858. doi: 10.1097/01.tp.0000129409.84087.62. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Astigarraga CC, Eapen M, et al. Variation in supportive care practices in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(11):1231–1238. doi: 10.1016/j.bbmt.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SJ, Joffe S, Artz AS, et al. Individual physician practice variation in hematopoietic cell transplantation. J Clin Oncol. 2008;26(13):2162–2170. doi: 10.1200/JCO.2007.15.0169. [DOI] [PubMed] [Google Scholar]
- 12.Majhail NS, Brunstein CG, Wagner JE. Double umbilical cord blood transplantation. Current opinion in immunology. 2006;18(5):571–575. doi: 10.1016/j.coi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Harker-Murray PD, Thomas AJ, Wagner JE, et al. Allogeneic hematopoietic cell transplantation in children with relapsed acute lymphoblastic leukemia isolated to the central nervous system. Biol Blood Marrow Transplant. 2008;14(6):685–692. doi: 10.1016/j.bbmt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 14.MacMillan ML, Auerbach AD, Davies SM, et al. Haematopoietic cell transplantation in patients with Fanconi anaemia using alternate donors: results of a total body irradiation dose escalation trial. British journal of haematology. 2000;109(1):121–129. doi: 10.1046/j.1365-2141.2000.01955.x. [DOI] [PubMed] [Google Scholar]
- 15.Tan PL, Wagner JE, Auerbach AD, et al. Successful engraftment without radiation after fludarabine-based regimen in Fanconi anemia patients undergoing genotypically identical donor hematopoietic cell transplantation. Pediatric blood & cancer. 2006;46(5):630–636. doi: 10.1002/pbc.20538. [DOI] [PubMed] [Google Scholar]
- 16.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 17.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 19.Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14(3):282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora M, Nagaraj S, Wagner JE, et al. Chronic graft-versus-host disease (cGVHD) following unrelated donor hematopoietic stem cell transplantation (HSCT): higher response rate in recipients of unrelated donor (URD) umbilical cord blood (UCB) Biol Blood Marrow Transplant. 2007;13(10):1145–1152. doi: 10.1016/j.bbmt.2007.06.004. [DOI] [PubMed] [Google Scholar]


