Introduction
Recurrent joint bleeding, leading to the development of joint disease and arthropathy, are the hallmarks of haemophilia. Prevention of bleeding through prophylaxis, rather than the on-demand treatment of bleeding events when they occur, is considered the gold standard of treatment for severe haemophilia. The original aim of prophylaxis was to avoid arthropathy by changing the bleeding phenotype from a severe to moderate form. While the use of prophylaxis has undoubtedly improved patients’ outcome and quality of life (QoL) by preventing bleeding and joint disease progression, challenges remain, and there is a growing awareness that prophylaxis may also benefit vulnerable patients with mild/moderate haemophilia.
The ultimate treatment goal for haemophilia is the absence of joint bleeds, and ensuing joint disease and arthropathy, for all. Members of the Zürich Haemophilia Forum convened for their tenth meeting in November 2012 to discuss current and future treatments for haemophilia and the aims to mitigate disease severity and prevent arthropathy. In particular, discussions regarding the concept of an optimal trough level - what this should be and how to achieve it (now and in the future) for haemophilia patients without inhibitors - are summarised in this report.
Prophylaxis for patients with haemophilia A and B and the “optimal trough level” What is the optimal trough level for prophylaxis?
Current opinion on optimising haemophilia care is centred on the idea of the need to maintain factor activity above a trough of 1% baseline factor activity level (Figure 1). This recommendation is based on limited papers1,2, but supported by the finding that increased time with a factor VIII (FVIII) activity below 1% is associated with an increased rate of breakthrough bleeding3. Only recently have data emerged that suggest a baseline greater than 1% might be preferable4,5.
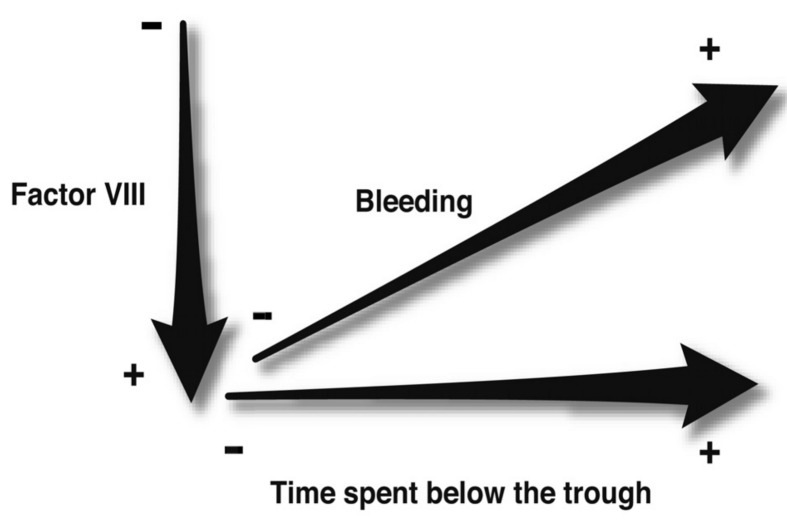
Figure 1.
Schematic illustrating two main concepts considered when deciding on prophylactic regimens for patients with haemophilia.
Data on the occurrence of joints bleeds suggest that haemophilia A patients experience fewer joint bleed per year when their factor activity is increased, and/ or the time spent below a certain trough level is decreased. Traditionally, factor activity at 1% above baseline was considered the target trough level to prevent joint bleeding, but more recent findings suggest that patients will benefit from regimens that increase the trough level to greater than 1% factor activity.
In his plenary address at the 2012 International Congress of the World Federation of Haemophilia, Mark Skinner suggested that the haemophilia community should aim for a baseline replacement factor activity level of 15%, and the absence of joint bleeds, for all6. This proposal was based on data including those from an analysis of self-reported joint bleeding in 433 patients with moderate or mild haemophilia A treated on demand, which found that no joint bleeds would be expected in patients with baseline factor activity greater or equal to 15%4. In addition, the model showed that there was an 18% reduction in bleeding frequency for every 1% increase in residual FVIII activity4. In this context, it has also been observed that the number of joint bleeds decreases to approximately zero for patients with more than 12% FVIII activity5, although the physical activity of the patients was not known in this study5. However, as vigorous physical activity is only transiently associated with a moderate increased bleeding risk7, replacement coagulation factor concentration is more likely to have a larger absolute effect on bleeding risk than physical activity.
Collectively the data suggest that raising trough levels may be an appropriate goal for prophylaxis. The minimum coagulation factor level required to maintain haemostasis is likely to be different for each patient. However, it is important to note that this goal may not be possible for all patients worldwide, for example, those in countries with limited resources, for whom the cost implications may deny them access to any form of prophylaxis, or enable increased dosing or treatment frequency to increase the trough.
While controversy persists regarding the optimal trough level, we suggest that most patients could benefit from maintaining a trough above 1% baseline factor activity level.
The importance of also considering other types of “troughs”
While traditionally prophylaxis and the concept of an optimal trough have been viewed in terms of replacement coagulation factor activity levels, other measures are also important to consider. For example, maintaining a trough of 15% activity would have little clinical value if individuals still experience breakthrough bleeding and worsening joint disease despite this increased trough. In addition, for patients with existing joint disease, it may not be feasible to halt joint disease progression, and the aim of prophylaxis in such cases may be to maintain mobility and QoL. Hence, for these patients, it is also important to assess QoL, and to modify the prophylaxis regimen accordingly if QoL measures decrease.
As it is well known that bleeding phenotype may not necessarily be related to factor activity, it has been suggested that global coagulation assays (thromboelastography, the thrombin generation test) may be useful for predicting bleeding phenotype8. Thromboelastography uses whole blood to determine the characteristics of clot formation, and its potential predictive utility was explored in a study of 47 children with moderate or severe haemophilia8. Thromboelastography was able to discriminate between the different populations of haemophilia patients, with higher maximum thrombin/fibrin generation reported in patients with a milder bleeding phenotype than in those patients who had a more severe bleeding tendency8. While the thrombin generation test has been accepted as a valuable research tool to evaluate haemostatic capacity, the lack of standardisation and large inter-laboratory variance have hindered evaluation of this technique for clinical practice. However, more recently, the use of a standardised thrombin generation test protocol has been reported to reduce inter- and intra-assay variability to within acceptable levels for clinical use9. Global coagulation assays such as thromboelastography and the thrombin generation test may therefore be useful tools for identifying patients with a severe bleeding tendency who require prophylaxis (including those who may have been classified as having mild haemophilia on the basis of their factor activity level), and for monitoring the efficacy of such treatment.
Hence, coagulation factor activity level should not be the only “trough” parameter examined; other tools and measures should be used in combination with factor activity level to assess treatment efficacy and to optimise the outcome of individual patients. However, trough levels are just one aspect of patients’ care, and since there are no long-term follow-up or outcome studies, it is not known whether current practice is improving care. Careful, regular monitoring of joint status is also required in order to enable early intervention to prevent arthropathy. In this respect, despite the lack of standardisation, we suggest that global coagulation assays, such as thromboelastography and the thrombin generation test, may be useful tools for monitoring the efficacy of prophylaxis.
Current ways to achieve and maintain an optimum trough level in patients with haemophilia A or B
Four main dosing strategies are used for prophylaxis, the advantages and disadvantages of which have been reviewed recently10, and as such, will not be reviewed here. Regardless of the regimen used, patients’ outcomes have undoubtedly been improved by the use of prophylaxis in haemophilia A or B. However, challenges remain. Breakthrough bleeding still occurs in many patients on prophylaxis11,12, and as such, the currently utilised regimens do not fulfil Mark Skinner’s suggestion that the aim of treatment should be the absence of joint bleeds for all6. In addition, the finding that some patients develop joint damage in the absence of overt joint bleeding13 may indicate the occurrence of subclinical bleeding not prevented by current prophylactic strategies. In this respect, data from a long-term observational magnetic resonance imaging study identified early joint disease in the absence of joint bleeding in patients on prophylaxis14. Poor adherence to treatment is also a concern and a major contributing factor to failure of prophylaxis. For these reasons, the haemophilia community is looking at ways to improve prophylaxis, and the development of more convenient, less time-consuming regimens may result in increased treatment adherence and improved outcome for patients.
If the new treatment aim for prophylaxis is to increase the trough, for currently available products, existing prophylactic regimens are likely to require adjustment. One way the trough may be increased is to consider prophylaxis with daily dosing. A recent randomised, crossover, pilot study evaluated the feasibility of daily dosing, treatment efficacy and whether treatment cost was reduced15. While a 30% reduction in cost was observed, some patients experienced an increase in breakthrough bleeding. The authors concluded that daily dosing was feasible and efficacious for some patients15. Of note, the daily dose chosen for each patient in this study was based on maintaining the trough above that achieved with their previous prophylactic regimen. However, it is possible that greater efficacy would have been observed if higher troughs had been a treatment goal. Regardless, the results of this study suggest that daily dosing may be considered. Daily dosing may lead to improved treatment outcome by increasing the trough, or decreasing the time spent below the trough.
If daily dosing is not feasible, another way to increase the trough for prophylaxis with current products is simply to increase the dose, which may lead to fewer breakthrough bleeds and improved outcomes. In this respect, it has been reported that the occurrence of joint bleeds was reduced in haemophilia A or B patients (without inhibitors) treated with high-dose (median: 0.3 per year) versus intermediate-dose regimens (median: 3.3 per year)16. In addition, the proportion of patients without arthropathy (as measured by the Pettersson score) was higher for those treated with high-dose (69%) versus intermediate-dose regimens (32%), although QoL measures were similar. The development of new products that could achieve increased troughs without increased dose would avoid concerns of the potential increased risk of thrombotic events.
Recently, the Prophylaxis Study Group demonstrated similar efficacy for two prophylaxis regimens, both of which resulted in a significant reduction of bleeding episodes compared with on-demand treatment17. One regimen was standard prophylaxis (20–40 IU/kg) and the other, pharmacokinetic-tailored prophylaxis (20–80 IU/kg every third day), both intended to maintain FVIII activity above a trough of 1%. Based on the results of this study it was concluded that pharmacokinetic-tailored prophylaxis offers an alternative to the standard prophylactic regimen, with similar costs but with fewer infusions17.
The future: new molecules with prolonged half-life - what is the treatment goal, higher troughs or fewer injections?
For some patients, the possibility of prophylaxis with fewer injections may be the treatment aim, which could result in increased treatment adherence (and therefore improved prophylaxis and fewer breakthrough bleeds); prophylaxis with fewer injections may also encourage patients to switch from on-demand treatment to prophylaxis. However, for others, it may be more important to increase trough levels, for example, to provide increased coverage for vigorous physical activity or to prevent any spontaneous breakthrough bleeding. Increasing the trough may also help to prevent subclinical bleeding and ensuing joint disease that is thought to occur in more vulnerable patients18.
Table I provides a summary of published manuscripts detailing the data available from clinical trials on half-life prolongation, extended time to target factor level and increased troughs (if any) for molecules in development with prolonged half-lives. For haemophilia A, information is included on a recombinant fusion protein linking FVIII to the Fc domain of human IgG1 (rFVIII Fc), a rFVIII molecule with site-directed glycoPEGylation (N8-GP), and a sucrose-formulated rFVIII (rFVIII-FS) combined with PEGylated liposomes (BAY 79-4980); the prolongations of half-life for N8-GP and rFVIII Fc were very similar (between ~1.5 and 1.7-fold), while the half-life of BAY 79-4980 did not differ from that of rFVIII-FS. For haemophilia B and recombinant factor IX (rFIX), the molecules reported include a glycoPEGylated rFIX (NP-GP), and recombinant fusion proteins linking FIX with either the Fc domain of human IgG1 (rFIXFc) or albumin (rFIX-FP); half-life prolongation was achieved for all of these rFIX molecules, and was more promising than that observed for the FVIII molecules (ranging between 3-fold and 5-fold improvement).
Table I.
Summary of published findings from clinical trials of new replacement factor molecules in development designed with prolonged half-lives.
| Molecule | Study | Half-life prolongation | Time to X% factor activity | Trough levels |
|---|---|---|---|---|
| Recombinant fusion protein linking FVIII to the Fc domain of human IgG1 (rFVIII Fc) | Phase I, single-dose escalation trial (25 or 65 IU/kg)19 | 25 IU/kg: 1.54-fold increase vs rFVIII; 65 IU/kg: 1.70-fold increase vs rFVIII | Time to 1%: at 25 IU/kg, 1.53-fold longer with rFVIII Fc than rFVIII; at 65 IU/kg, 1.68-fold longer with rFVIII Fc than rFVIII | NA |
| Recombinant FVIII with site-directed glycoPEGylation (N8-GP) | Phase I, single-dose escalation trial (25 U/kg, 50 U/kg and 75 U/kg)24 | 1.6-fold increase vs patients’ previous FVIII products | Time to 1%: 3.9 days (25 U/kg), 6.5 days (50 U/kg) and 5.5 days (75 U/kg) | NA |
| Sucrose-formulated rFVIII (rFVIII-FS) combined with PEGylated liposomes (BAY 79-4980) | Phase I, crossover study (rFVIII-FS infusions at 35 IU/kg with high-[22 mg/kg] or low-dose [13 mg/kg] BAY 79-4980)25 | No statistically significant differences observed between BAY 79-4980 and rFVIII-FS | NA | NA |
| GlycoPEGylated rFIX (N9-GP) | Phase I, single-dose escalation trial (25, 50 and 100 U/kg)26 | 5-fold increase vs patients’ previous FIX products | Time to 1%, 22.5 days; time to 3%, 16.2 days (post hoc estimation based on dose normalisation to 50 U/kg) | NA |
| Recombinant fusion protein linking FIX with the Fc domain of human IgG1 (rFIXFc) | Phase I/IIa single-dose escalation trial (1, 5, 12.5, 25, 50 and 100 IU/kg)27 | 3-fold increase vs that reported for current FIX products | Time to 1%: 7.34, 10.1±1.58, 12.3±2.49 days for doses of 25, 50 and 100 IU/kg, respectively. Time to 3%: 3.81, 6.28±1.11, 8.53±1.58 days for doses of 25, 50 and 100 IU/kg, respectively |
Day 7, 25 IU/kg, 1.1%, 50 IU/kg, 2.47%, 100 IU/kg, 4.65% |
| Recombinant fusion protein linking FIX with the Fc domain of human IgG1 (rFIXFc) | Phase III, non-randomised, open-label trial of prophylaxis with 50 or 100 IU/kg28 | 2.42-fold increased vs rFIX | Time to 1%: 11.2 days vs 5.1 days for rFIX. Time to 3%: 5.8 days vs 2.8 days for rFIX |
NA |
| Recombinant fusion protein linking FIX with albumin (rIX-FP) | Phase I, single-dose escalation trial (25 and 50 IU/kg)(PROLONG-9FP)29,30 | 5-fold increase vs rFIX | Trough levels maintained above 5% after 7 days for 25 IU/kg dose, and after 14 days for 50 IU/kg dose | 25 IU/kg: day 7, 7.4%, day 14, 2.5%;50 IU/kg: day 7, 13.4%, day 14, 5.5% |
| Recombinant fusion protein linking FIX with albumin (rIX-FP) | Phase I/II, safety and efficacy trial of on-demand or prophylaxis therapy (25 IU/kg)31 | PK data are under analysis but authors state that preliminary results suggest an improvement in half-life | Time to 5%: crossed much sooner with 50 IU/kg rFIX or pd-FIX (~48 h) than with 25 IU/kg rIX-FP (~156 h) | 25 IU/kg rIX-FP: day 7, 4.4% |
NA: not available; pd: plasma-derived; PEG: polyethylene glycol; PK: pharmacokinetic.
Of note, clinical trials for new molecules with prolonged half-life often appear to concentrate on the aim of achieving similar or better efficacy with fewer injections (e.g. by prolonging the time to reach a target factor level). For example, for one FVIII molecule in development, a rFVIII Fc fusion protein, the time to 1% FVIII activity above baseline was approximately 1.53–1.68 longer than that with rFVIII following a 25 IU/kg or 65 IU/kg single dose in patients with haemophilia A19. Based on these findings, a phase III trial has been designed to identify effective prolonged prophylactic regimens for rFVIII Fc. For another FVIII molecule, BAY 79-4980, an rFVIII reconstituted with PEGylated liposomes with prolonged action, the aim of the BAY 79-4980 LIPLONG study was to compare the efficacy and safety of once-weekly prophylaxis with 35 IU/kg BAY 79-4980 to that with thrice-weekly (25 IU/kg) sucrose-formulated recombinant factor VIII (rFVIII-FS)20. The study was prematurely discontinued as it failed to demonstrate non-inferiority to rFVIII-FS (e.g. mean annualised bleeding rate: BAY 79-4980, 15; rFVIII-FS, 5.8). However, improved treatment outcome might have been observed for BAY 79-4980 if increasing the trough, rather than decreasing dosing frequency, had been the treatment aim.
For haemophilia A and FVIII, the prolongation of product half-life achieved to date (Table I) remains challenging21, and may not be sufficient to allow prophylaxis with less frequent injections. However, improved outcome for patients with haemophilia A may be achieved if instead the treatment goal is to provide higher troughs, which may be a useful question for further study for the FVIII molecules with prolonged half-lives under development.
For haemophilia B and FIX, the new products currently undergoing clinical trials promise both to increase trough levels and allow prophylaxis with fewer injections, which may result in long-term cost savings by postponing or completely avoiding the need for joint replacement. For example, a population pharmacokinetic model, derived using data from an early single-dose pharmacokinetic study of N9-GP (a glycoPEGylated rFIX with a prolonged half-life), was developed in order to explore dosing and treatment strategies for prophylaxis or on-demand therapy with N9-GP.22 The resulting simulations predicted reduced dosing frequency and consumption for N9-GP versus rFIX or plasma-derived FIX concentrates.
While the trough was the topic of this review and a potential focus of attention for clinical trials of new molecules, is the trough the only pharmacokinetic variable that should be considered? For example, the total amount of coagulation factor (area under the curve) a patient receives, or recurrent high peaks, may be more important than the trough in determining treatment efficacy23. The results of ongoing clinical studies for new molecules with prolonged half-life will be valuable for determining the roles of the trough versus that of the peak in preventing bleeding.
Lastly, while we are awaiting the results of ongoing clinical trials, we believe that long-lasting recombinant proteins may enable less frequent dosing or offer alternative dosing regimens which may be tailored to meet the need(s) of individual patients. The results of these trials will also provide data on the efficacy of the new therapies and the incidence of breakthrough bleeding, thereby providing guidance to clinicians on the dosing and frequency of infusions to prevent such bleeds; the data will also help to reassure patients that improved treatment outcome will be possible with the new coagulation factors in development.
Conclusions
The development of new factor concentrates with prolonged half-lives promises improved prophylaxis for haemophilia A or B patients (without inhibitors) and may enable treatment that prevents spontaneous breakthrough bleeding (and subclinical bleeds) in all. Whether the aim for future regimens should be prophylaxis with fewer injections, or to increase the trough, remains a matter of debate and may be best decided on a patient-by-patient basis, depending on their individual clinical profiles and the treatment aims.
Acknowledgements
Novo Nordisk provided financial support for the Tenth Zürich Haemophilia Forum and for medical writing assistance, provided by Sharon Eastwood of PAREXEL, in compliance with international guidelines for good publication practice.
Footnotes
Conflict of interests disclosure
Victor Jiménez-Yuste has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Baxter, Bayer, CSL Behring, Grifols, Novo Nordisk, Octapharma and Pfizer. Thierry Lambert has acted as a consultant and advisory board member for Baxter, Bayer, CSL Behring, Novo Nordisk and Pfizer. Silva ZupančićŠalek has received speaker's fees from Baxter, Novo Nordisk and Pfizer. Günter Auerswald, Gary Benson, Eduardo Remor, and Massimo Morfini have no conflicts of interest to declare.
References
- 1.Ahlberg A. Haemophilia in Sweden. VII. Incidence, treatment and prophylaxis of arthropathy and other musculo-skeletal manifestations of haemophilia A and B. Acta Orthop Scand Suppl. 1965;(Suppl 77):3–132. doi: 10.3109/ort.1965.36.suppl-77.01. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson IM, Berntorp E, Lofqvist T, Pettersson H. Twenty-five years’ experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Collins PW, Blanchette VS, Fischer K, et al. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7:413–20. doi: 10.1111/j.1538-7836.2008.03270.x. [DOI] [PubMed] [Google Scholar]
- 4.den Uijl IE, Fischer K, Van Der Bom JG, et al. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17:41–4. doi: 10.1111/j.1365-2516.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 5.den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17:849–53. doi: 10.1111/j.1365-2516.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 6.Skinner MW. WFH: closing the global gap-achieving optimal care. Haemophilia. 2012;18(Suppl 4):1–12. doi: 10.1111/j.1365-2516.2012.02822.x. [DOI] [PubMed] [Google Scholar]
- 7.Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308:1452–9. doi: 10.1001/jama.2012.12727. [DOI] [PubMed] [Google Scholar]
- 8.Chitlur M, Warrier I, Rajpurkar M, et al. Thromboelastography in children with coagulation factor deficiencies. Br J Haematol. 2008;142:250–6. doi: 10.1111/j.1365-2141.2008.07063.x. [DOI] [PubMed] [Google Scholar]
- 9.Dargaud Y, Wolberg AS, Luddington R, et al. Evaluation of a standardized protocol for thrombin generation measurement using the calibrated automated thrombogram: an international multicentre study. Thromb Res. 2012;130:929–34. doi: 10.1016/j.thromres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379:1447–56. doi: 10.1016/S0140-6736(11)61139-2. [DOI] [PubMed] [Google Scholar]
- 11.Dodd C, Watts RG. A comparison of traditional vs. Canadian tailored prophylaxis dosing of prophylactic factor infusions in children with haemophilia A and B in a single hemophilia treatment center. Haemophilia. 2012;18:561–7. doi: 10.1111/j.1365-2516.2011.02741.x. [DOI] [PubMed] [Google Scholar]
- 12.Fischer K, Steen CK, Petrini P, et al. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood. 2013;122:1129–36. doi: 10.1182/blood-2012-12-470898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 14.Olivieri M, Kurnik K, Pfluger T, Bidlingmaier C. Identification and long-term observation of early joint damage by magnetic resonance imaging in clinically asymptomatic joints in patients with haemophilia A or B despite prophylaxis. Haemophilia. 2012;18:369–74. doi: 10.1111/j.1365-2516.2011.02682.x. [DOI] [PubMed] [Google Scholar]
- 15.Lindvall K, Astermark J, Bjorkman S, et al. Daily dosing prophylaxis for haemophilia: a randomized crossover pilot study evaluating feasibility and efficacy. Haemophilia. 2012;18:855–9. doi: 10.1111/j.1365-2516.2012.02879.x. [DOI] [PubMed] [Google Scholar]
- 16.Fischer K, Astermark J, Van Der Bom JG, et al. Prophylactic treatment for severe haemophilia: comparison of an intermediate-dose to a high-dose regimen. Haemophilia. 2002;8:753–60. doi: 10.1046/j.1365-2516.2002.00694.x. [DOI] [PubMed] [Google Scholar]
- 17.Valentino LA, Mamonov V, Hellmann A, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10:359–67. doi: 10.1111/j.1538-7836.2011.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valentino LA. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. J Thromb Haemost. 2010;8:1895–902. doi: 10.1111/j.1538-7836.2010.03962.x. [DOI] [PubMed] [Google Scholar]
- 19.Powell JS, Josephson NC, Quon D, et al. Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood. 2012;119:3031–7. doi: 10.1182/blood-2011-09-382846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell J, Martinowitz U, Windyga J, et al. Efficacy and safety of prophylaxis with once-weekly BAY 79-4980 compared with thrice-weekly rFVIII-FS in haemophilia A patients. A randomised, active-controlled, double-blind study. Thromb Haemost. 2012;108:913–22. doi: 10.1160/TH12-03-0188. [DOI] [PubMed] [Google Scholar]
- 21.Peyvandi F, Garagiola I, Seregni S. Future of coagulation factor replacement therapy. J Thromb Haemost. 2013;11(Suppl 1):84–98. doi: 10.1111/jth.12270. [DOI] [PubMed] [Google Scholar]
- 22.Collins PW, Moss J, Knobe K, et al. Population pharmacokinetic modeling for dose setting of nonacog beta pegol (N9-GP), a glycoPEGylated recombinant factor IX. J Thromb Haemost. 2012;10:2305–12. doi: 10.1111/jth.12000. [DOI] [PubMed] [Google Scholar]
- 23.Ljung R, Auerswald G, Benson G, et al. Novel coagulation factor concentrates: issues relating to their clinical implementation and pharmacokinetic assessment for optimal prophylaxis in haemophilia patients. Haemophilia. 2013;19:481–6. doi: 10.1111/hae.12094. [DOI] [PubMed] [Google Scholar]
- 24.Tiede A, Brand B, Fischer R, et al. Enhancing the pharmacokinetic properties of recombinant factor VIII: first-in-human trial of glycoPEGylated recombinant factor VIII in patients with hemophilia A. J Thromb Haemost. 2013;11:670–8. doi: 10.1111/jth.12161. [DOI] [PubMed] [Google Scholar]
- 25.Powell JS, Nugent DJ, Harrison JA, et al. Safety and pharmacokinetics of a recombinant factor VIII with pegylated liposomes in severe hemophilia A. J Thromb Haemost. 2008;6:277–83. doi: 10.1111/j.1538-7836.2008.02856.x. [DOI] [PubMed] [Google Scholar]
- 26.Negrier C, Knobe K, Tiede A, et al. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2011;118:2695–701. doi: 10.1182/blood-2011-02-335596. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro AD, Ragni MV, Valentino LA, et al. Recombinant factor IX-Fc fusion protein (rFIXFc) demonstrates safety and prolonged activity in a phase 1/2a study in hemophilia B patients. Blood. 2012;119:666–72. doi: 10.1182/blood-2011-07-367003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369:2313–23. doi: 10.1056/NEJMoa1305074. [DOI] [PubMed] [Google Scholar]
- 29.Santagostino E. PROLONG-9FP clinical development program - phase I results of recombinant fusion protein linking coagulation factor IX with recombinant albumin (rIX-FP) Thromb Res. 2013;131(Suppl 2):S7–10. doi: 10.1016/S0049-3848(13)70151-8. [DOI] [PubMed] [Google Scholar]
- 30.Santagostino E, Negrier C, Klamroth R, et al. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patients. Blood. 2012;120:2405–11. doi: 10.1182/blood-2012-05-429688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinowitz U, Lubetsky A. Phase I/II, open-label, multicenter, safety, efficacy and PK study of a recombinant coagulation factor IX albumin fusion protein (rIX-FP) in subjects with hemophilia B. Thromb Res. 2013;131(Suppl 2):S11–14. doi: 10.1016/S0049-3848(13)70152-X. [DOI] [PubMed] [Google Scholar]