Abstract
Background
The aim of this study was to investigate the effect of the combined administration of intravenous immunoglobulins and steroids as a second-line therapy in 34 children with primary immune thrombocytopenia and persistent, symptomatic bleeding.
Materials and methods
Combined therapy (intravenous immunoglobulins 0.4 g/kg daily on days 1 and 2, and methylprednisolone 20 mg/kg daily on days 1–3) was administered to 12 patients with newly diagnosed ITP who did not respond to the administration of a single therapy (either intravenous immunoglobulins or steroids) and to 22 children with persistent and chronic disease who required frequent administrations (i.e. more frequently than every 30 days) of either immunoglobulins or steroids (at the same standard dosages) in order to control active bleeding.
Results
A response (i.e. platelet count >50×109/L and remission of active bleeding) was observed in 8/12 (67%) patients with newly diagnosed ITP. The clinical presentation of responders and non-responders did not differ apparently. Patients in the chronic/persistent phase of disease had a significantly longer median period of remission from symptoms compared with the previous longest period of remission (p=0.016). The treatment was well tolerated.
Discussion
Our data suggest that the combined approach described is a well-tolerated therapeutic option for children with primary immune thrombocytopenia and persistent bleeding symptoms that can be used in both emergency and/or maintenance settings.
Keywords: primary immune thrombocytopenia, IVIG, steroids, children, ITP
Introduction
Primary immune thrombocytopenia (ITP) is the most common childhood haematological disease, characterised by low platelet counts and mucocutaneous bleeding.
ITP in children is usually benign and, in most patients, resolves spontaneously 6–18 months after diagnosis. Three different phases of the disease were recently defined by members of an International Working Group (IWG)1: “newly diagnosed ITP” which applies to the disease in all paediatric and adult patients at the time of diagnosis, including self-limited forms; “persistent ITP” which defines the disease in patients who do not achieve spontaneous remission or maintain their response after stopping treatment between 3 and 12 months after diagnosis; and “chronic ITP” which is defined by ongoing thrombocytopenia more than 12 months after the diagnosis.
Since available treatment for ITP does not affect the natural evolution of the disease, but is used in order to alleviate clinical symptoms, it is indicated in only a minority of patients who have a low platelet count associated with active bleeding2–5.
Among children who need treatment, only a few fail to achieve a platelet count increase in response to first-line treatments such as intravenous immunoglobulins or steroids. Intravenous immunoglobulins are expected to raise the platelet count in more than 80% of children; oral corticosteroids, depending on dosages, may be effective at inducing a response in up to 75% of children6. Another small group of patients is not truly unresponsive to first-line treatments but has persistent bleeding requiring frequent courses of therapy.
Both these categories of paediatric patients, which together account for approximately 2–5% of children presenting with newly diagnosed ITP7, must be treated aggressively, frequently off-label, with immunosuppressive drugs, chemotherapy agents7,8 or splenectomy9.
The combination of intravenous immunoglobulins and steroids derived from the observation that steroids administered to reduce headaches in children treated with intravenous immunoglobulins (0.8 g/kg/day for 2 days)10 resulted in greater increases in platelet counts than expected. This observation prompted us to give both drugs simultaneously to patients with newly diagnosed ITP who failed to respond to a single administration of either intravenous immunoglobulins (0.8 g/kg/day for 2 days) or steroids (methylprednisone or prednisone at standard dosages). Good preliminary results prompted us to use the same combined approach to treat patients in the persistent/chronic phase of the disease who were responsive to single therapies but required frequent administration (i.e. more frequently than every 30 days) for recurrences of active bleeding.
Materials and methods
Treatment and patients
We suggested this combined therapeutic approach to all the Haematology Units affiliated with the Italian Association of Paediatric Haematology and Oncology (AIEOP) for all ITP patients with active, persistent bleeding and platelet counts below 20×109/L despite the administration of intravenous immunoglobulins and steroids. Bone marrow analysis had previously been performed in all patients to confirm the diagnosis of ITP prior to treatment with steroids.
Exclusion criteria were hypertension, hyperglycaemia, overweight (defined as a body mass index at, or above, the 85th percentile and lower than the 95th percentile for children of the same age and sex) or obesity (defined as a body mass index at, or over, the 95th percentile for children of the same age and sex).
The proposed treatment schedule was intravenous immunoglobulins 0.4 g/kg per day, administered over 5 hours, on days +1 and +2, and methyl-prednisone 20 mg/kg per day, over 30 minutes, on days +1, +2 and +3. On days 1 (before treatment), 2, and 3, clinical evaluation, blood-pressure determination, urinalysis, and blood tests (blood cell count, glycaemia, lactate dehydrogenase, aspartate transaminase, and creatinine) were recommended.
Subsequent intervals at which to perform laboratory tests were not defined. Follow-up clinical visits and assessments of platelet counts were recommended in relation to the patients’ clinical conditions (i.e. only in the case of new bleeding manifestations). Each institution could treat its patients in accordance with their clinical conditions.
Informed written consent, approved by local research ethics committees, for the collection of blood samples and data was obtained from the parents of all patients.
An ad hoc form was used to collect and analyse data from patients who had been treated with combined therapy.
Thirty-four patients (16 males and 18 females) with ITP were enrolled and treated at 6 AIEOP centres.
Twelve patients with newly diagnosed ITP (i.e. ITP lasting <3 months at the time of combined treatment) were enrolled in the study. All these patients had active, persistent bleeding and platelet counts below 20×109/L despite intravenous immunoglobulins and steroid administration.
Twenty-two children were enrolled during the persistent or chronic phase of their disease. All these patients had previously responded to either intravenous immunoglobulins or steroids (administered at the same standard dosages) but required frequent administration (i.e. more frequently than every 30 days) for early recurrence of active bleeding. At the time of the combined approach they all had active bleeding and platelet counts below 20×109/L.
The patients’ characteristics are reported in Table I.
Table I.
Acute patients’ characteristics.
| Phase of disease | Sex | Age at diagnosis (years) | Time from diagnosis (months) | Baseline platelet count (n×109/L) | Overall response | Complete response | Relapse | Duration of response (days) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Newly diagnosed | F | 7 | 0.2 | 3 | No | X | X | X |
| 2 | Newly diagnosed | M | 12.7 | 0 | 5 | Yes | No | No | X |
| 3 | Newly diagnosed | M | 1.3 | 1.1 | 19 | Yes | No | Yes | 32 |
| 4 | Newly diagnosed | M | 11 | 1 | 17 | Yes | Yes | No | X |
| 5 | Newly diagnosed | F | 3.2 | 1 | 11 | Yes | No | Yes | 46 |
| 6 | Newly diagnosed | M | 5.8 | 2 | 3 | No | X | X | X |
| 7 | Newly diagnosed | F | 10.5 | 0 | 5 | Yes | Yes | No | X |
| 8 | Newly diagnosed | M | 10 | 2 | 8 | Yes | No | Yes | 28 |
| 9 | Newly diagnosed | M | 0.9 | 0 | 14 | No | X | X | X |
| 10 | Newly diagnosed | F | 15.4 | 1 | 4 | No | X | X | X |
| 11 | Newly diagnosed | F | 11 | 2 | 4 | Yes | No | Yes | 22 |
| 12 | Newly diagnosed | F | 12 | 2 | 6 | Yes | No | No | X |
| 13 | Persistent | M | 5.6 | 3 | 13 | Yes | Yes | Yes | 27 |
| 14 | Persistent | F | 14.6 | 4 | 6 | No | X | X | X |
| 15 | Persistent | F | 9 | 7 | 3 | Yes | No | Yes | 14.5 |
| 16 | Persistent | F | 9 | 6 | 14 | Yes | Yes | Yes | 41 |
| 17 | Persistent | M | 5 | 6 | 15 | Yes | No | Yes | 29 |
| 18 | Persistent | M | 12 | 8 | 5 | Yes | Yes | Yes | 13 |
| 19 | Persistent | F | 4 | 11 | 7 | Yes | Yes | Yes | 73 |
| 20 | Persitent | M | 1 | 5.7 | 2 | No | No | X | X |
| 21 | Chronic | F | 4.7 | 19 | 9 | Yes | Yes | Yes | 13 |
| 22 | Chronic | M | 5.8 | 26 | 4 | Yes | Yes | Yes | 77.37 |
| 23 | Chronic | M | 6.3 | 55 | 8 | Yes | Yes | Yes | 134 |
| 24 | Chronic | F | 10.5 | 21 | 8 | Yes | Yes | Yes | 65 |
| 25 | Chronic | F | 4.9 | 38 | 4 | No | X | X | X |
| 26 | Chronic | F | 8.9 | 27 | 19 | Yes | Yes | Yes | 89 |
| 27 | Chronic | M | 0.6 | 14 | 13 | Yes | No | Yes | 12 |
| 28 | Chronic | F | 1.5 | 137 | 22 | Yes | No | Yes | 19 |
| 29 | Chronic | M | 11 | 38 | 6 | Yes | No | Yes | 11 |
| 30 | Chronic | F | 2 | 36 | 0 | Yes | Yes | Yes | 20.6 |
| 31 | Chronic | F | 11 | 43 | 9 | Yes | Yes | Yes | 116 |
| 32 | Chronic | M | 7 | 19 | 7 | Yes | Yes | Yes | 34 |
| 33 | Chronic | F | 5 | 14 | 4 | No | No | X | 36.5 |
| 34 | Chronic | M | 12 | 48 | 7 | Yes | Yes | Yes | 111 |
Overall response: platelet count >50×109/L and at least 30×109/L over the baseline count and remission of active bleeding.
Complete response: platelet count >150×109/L.
Data were recorded for a total of 74 courses. All the patients with newly diagnosed ITP and 9/22 patients with chronic/persistent ITP received a single course of combined therapy. The 13 remaining patients with chronic/persistent ITP were treated more than once (up to 13 courses). For these 13 children who were treated more than once, only the first course was considered and analysed.
Definitions
The overall response was defined as a platelet count increase above 50×109/L and at least 30×109/L over the baseline count and remission of active bleeding. A complete response was defined as a platelet count increase above 150×109/L.
The duration of response was the time from the day of the initial infusion of combined therapy to the first day of a new course of combined therapy or additional therapy administered for a recurrence of bleeding.
Patients who did not achieve a platelet count above 50×109/L and did not achieve a remission of bleeding were classified as non-responders.
Statistical analysis
Data were analysed with the statistical software R release 2.15.1 for Windows. The Shapiro-Wilk test was used to test normality. Results are expressed as medians and ranges for continuous variables and as absolute numbers and percentages for categorical ones. The associations between categorical variables were assessed with Fisher’s exact test and are presented with the 95% confidence intervals (CI) for the odds ratio. Differences between groups were checked with the Wilcoxon rank sum and Wilcoxon signed-rank test. Possible associations between continuous variables were assessed by the Kendall tau index.
All statistical tests were two-sided and p values <0.05 were considered statistically significant.
Results
Newly diagnosed primary immune thrombocytopenia
Response
The overall response rate was 67% (8/12 patients), while 2/8 (25%) responding patients had a complete response. Four out of 12 (33%) patients did not achieve platelet counts above 50×109/L and did not achieve a remission of bleeding and were, therefore, classified as non-responders.
Factors predictive of response
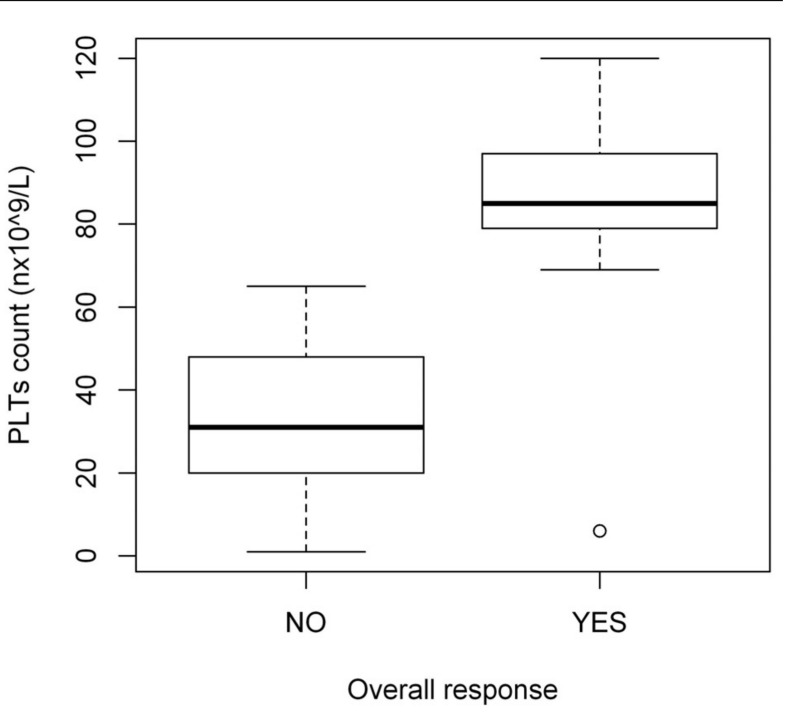
The overall and complete response rates were not influenced by gender, age at diagnosis, time between ITP diagnosis and treatment or pre-treatment platelet count (data not shown). The median platelet count on day 3 was significantly higher in responding patients than in non-responders (84.5×109/L versus 39.5×109/L, respectively; p=0.028) (Figure 1).
Figure 1.
Median platelet count on day +3 in patients with newly diagnosed ITP.
PLTs: platelets.
Duration of response
Four out of the 8 responders required a new course of therapy for a recurrence of bleeding at a median of 30 days (range, 22–46 days) after the first day of the infusion. The remaining four patients did not develop recurrent bleeding after the first combined therapy, which could be because of spontaneous, long-term remission of the disease.
There was no significant association between the duration of response and age at diagnosis or pre-treatment platelet count. The duration of response was not significantly associated with the platelet count at day +3 (p=0.083).
Side effects
No side effects were reported in any of the 12 patients. Likewise, no delayed toxicities attributable to combined therapy were experienced by any patients during the follow-up period.
Persistent and chronic primary immune thrombocytopenia
Response
The overall response rate was 82% (18/22 patients). A response was observed in 6/8 (75%) patients with persistent ITP and in 12/14 (86%) patients in the chronic phase of the disease. A complete response was documented in 13/18 (72%) responding patients (4/6 children with persistent ITP and 9/12 children with chronic ITP). Four out of 22 (18%) patients were classified as non-responders because they did not achieve platelet counts above 50×109/L and did not have relief from symptoms.
Factors predictive of response
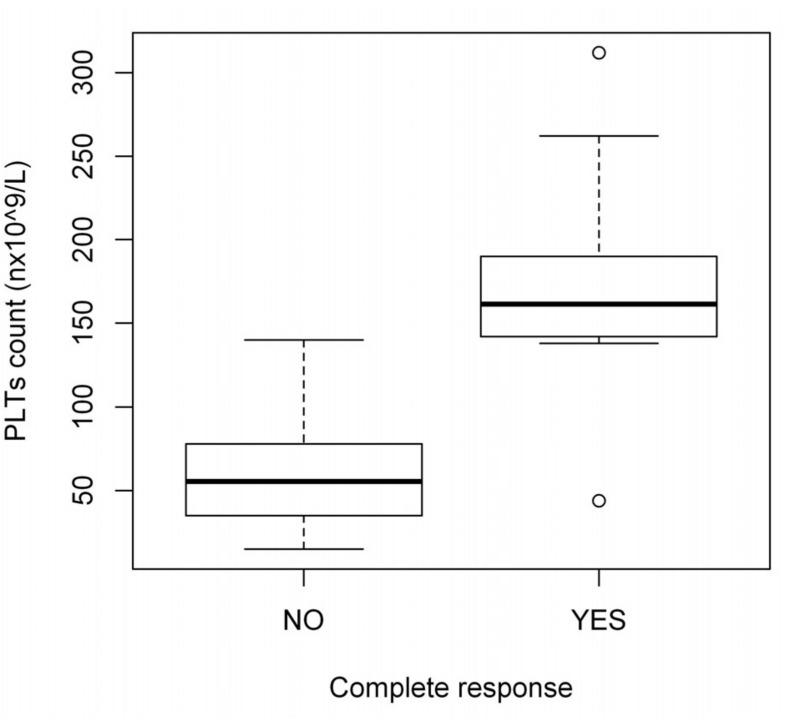
The complete response rate was not influenced by gender, age at diagnosis, time between ITP diagnosis and treatment or pre-treatment platelet count (data not shown). The platelet count on day +3 was significantly higher in patients who achieved a complete response than in those who did not (150.0×109/L versus 55.5×109/L, respectively; p=0.006) (Figure 2). The overall response rate was not analysed in patients with chronic ITP because only 4 of the 22 patients were non-responders.
Figure 2.
Median platelet count on day +3 in patients with persistent/chronic ITP.
PLTs: platelets.
Duration of response
All 18 responding patients required a new course of therapy for a recurrence of bleeding at a median of 31.5 (range 11–191) days after the first day of the first infusion. The duration of the response to the first treatment was not significantly associated with age at diagnosis, gender, pre-treatment or day +3 platelet counts (p=0.791). The median of duration of the response to the first treatment in the patients who obtained a complete response was significantly longer than 30 days (p=0.016) (a period previously considered as the longest remission).
Side effects
Two out of 22 children (9%) experienced mild hyperglycaemia, which did not develop into glycosuria in either case. One patient (4%) developed hypertension immediately after the infusion of methyl-prednisone, which promptly resolved with appropriate therapy. No delayed toxicities attributable to combined therapy were recorded during the follow-up period.
Discussion
We report here the clinical data of 34 paediatric ITP patients with active, persistent bleeding and platelet counts below 20×109/L who were treated with a combination of intravenous immunoglobulins and steroids.
The effect of the proposed schedule was investigated in 12 patients with newly diagnosed ITP who were unresponsive to the administration of single therapy and who had persistent, symptomatic bleeding, and in 22 patients with persistent or chronic ITP who complained of an early recurrence of active bleeding, and required frequent courses of either intravenous immunoglobulins or steroids administered at the same standard dosages. Despite the limitation of the small sample size, the response rate of 67% in children with newly diagnosed ITP who had not responded to the individually administered agents is extremely encouraging.
Moreover, the observation that responders achieve a platelet count sufficient to stop bleeding after the first day of infusion supports the feasibility of this therapeutic approach, even in an emergency setting, as was recently indicated by the AIEOP consensus guidelines regarding the management of chronic ITP5.
Our data did not show any apparent difference in the clinical presentation between responders and non-responders. Furthermore, since the overall response rate was not influenced by the duration of the disease before treatment, the combined approach might represent a worthwhile therapeutic option for patients with severe symptoms in the very early stages in order to avoid more aggressive treatments, including splenectomy, while waiting for a possible remission.
As far as concerns maintenance treatment in children without a satisfactory control of bleeding symptoms, administration of the combined therapeutic regimen resulted in a significant lengthening of the remission period.
Before starting the study, we could not rule out that the therapy might cause hyperglycaemia and/or hypertension. We therefore chose, as a precautionary measure, to exclude patients with increased susceptibility to these complications and children who already had hyperglycaemia or hypertension. However, in this study the schedule was well tolerated, as only three patients (all in the chronic phase of the disease and who had previously been treated more than once with steroids) developed mild, transient side effects.
Although the use of new generation drugs such as rituximab and thrombopoietin receptor agonists has been extremely encouraging in symptomatic refractory children with ITP7,8, the cost of these therapies should not be underestimated.
The proposed schedule was developed in order to allow administration of the treatment in an outpatient setting with a consequent reduction in direct costs (drugs, hospitalisation) as well as indirect ones (i.e. loss of working days for parents, loss of school days for children). The most cost-effective schedule is still to be found. To shed light on these aspects, a prospective multicentre study is ongoing to quantify the health-related quality of life and costs of this therapeutic approach as a maintenance therapy in children with persistent/chronic ITP.
Overall, despite the small numbers (particularly when considering sub-anaylses), the data from this pilot study suggest that a combined approach is a well-tolerated therapeutic option for children with primary immune thrombocytopenia and persistent bleeding symptoms which can be used in both emergency and maintenance settings.
Acknowledgements
We thank all the participants of the AIEOP (Associazione Italiana di Ematologia ed Oncologia Pediatrica)-ITP-Study Group.
This work was partially supported by “Regione Piemonte, Ricerca Sanitaria Finalizzata” and “Banca del Piemonte” grants to UR.
We are also grateful to Andrew Martin Garvey of the University of Torino for his patient revision of our paper.
Footnotes
Authorship contribution
The Authors contributed to this paper as follows: Emilia Parodi and Paola Giordano contributed equally to write the draft version. All Authors read, reviewed and approved the paper.
The Authors declare no conflicts of interest.
References
- 1.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 2.Eden OB, Lilleyman JS. Guidelines for management of idiopathic thrombocytopenic purpura. The British Paediatric Haematology Group. Arch Dis Child. 1992;67:1056–8. doi: 10.1136/adc.67.8.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88:3–40. [PubMed] [Google Scholar]
- 4.De Mattia D, Del Principe D, Del Vecchio GC, et al. Acute childhood idiopathic thrombocytopenic purpura: AIEOP consensus guidelines for diagnosis and treatment. Associazione Italiana di Ematologia e Oncologia Pediatrica. Haematologica. 2000;85:420–4. [PubMed] [Google Scholar]
- 5.De Mattia D, Del Vecchio GC, Russo G, et al. Management of chronic childhood immune thrombocytopenic purpura: AIEOP consensus guidelines. Acta Haematol. 2010;123:96–109. doi: 10.1159/000268855. [DOI] [PubMed] [Google Scholar]
- 6.Blanchette V, Imbach P, Andrew M, et al. Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet. 1994;344:703–7. doi: 10.1016/s0140-6736(94)92205-5. [DOI] [PubMed] [Google Scholar]
- 7.Kalpatthi R, Bussel JB. Diagnosis, pathophysiology and management of children with refractory immune thrombocytopenic purpura. Curr Opin Pediatr. 2008;20:8–16. doi: 10.1097/MOP.0b013e3282f45bb9. [DOI] [PubMed] [Google Scholar]
- 8.Kühne T, Imbach P. Management of children and adolescents with primary immune thrombocytopenia: controversies and solutions. Vox Sang. 2013;104:55–66. doi: 10.1111/j.1423-0410.2012.01636.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuhne T, Blanchette V, Buchanan GR, et al. Splenectomy in children with idiopathic thrombocytopenic purpura: a prospective study of 134 children from the Intercontinental Childhood ITP Study Group. Pediatr Blood Cancer. 2007;49:829–34. doi: 10.1002/pbc.21108. [DOI] [PubMed] [Google Scholar]
- 10.Jayabose S, Mahmoud M, Levendoglu-Tugal O, et al. Corticosteroid prophylaxis for neurologic complications of intravenous immunoglobulin G therapy in childhood immune thrombocytopenic purpura. J Pediatr Hematol Oncol. 1999;21:514–7. [PubMed] [Google Scholar]