Abstract
Background
The incidence of transfusion-related acute lung injury (TRALI) in cardiac surgery patients is high and this condition contributes to an adverse outcome. Damage-associated molecular pattern (DAMP) molecules, HMGB1 and S100A12, are thought to mediate inflammatory changes in acute respiratory distress syndrome. We aimed to determine whether DAMP are involved in the pathogenesis of TRALI in cardiac surgery patients.
Materials and methods
This was a secondary analysis of a prospective observational trial in cardiac surgery patients admitted to the Intensive Care Unit of a university hospital in the Netherlands. Fourteen TRALI cases were randomly matched with 32 transfused and non-transfused controls. Pulmonary levels of HMGB1, S100A12 and inflammatory cytokines (interleukins-1β, -6, and -8 and tumour necrosis factor-α) were determined when TRALI evolved. In addition, systemic and pulmonary levels of soluble receptor for advanced glycation end products (sRAGE) were determined.
Results
HMGB1 expression and levels of sRAGE in TRALI patients did not differ from those in controls. There was a trend towards higher S100A12 levels in TRALI patients compared to the controls. Furthermore, S100A12 levels were associated with increased levels of markers of pulmonary inflammation, prolonged cardiopulmonary bypass, hypoxemia and duration of mechanical ventilation.
Conclusion
No evidence was found that HMGB1 and sRAGE contribute to the development of TRALI. S100A12 is associated with duration of cardiopulmonary bypass, pulmonary inflammation, hypoxia and prolonged mechanical ventilation and may contribute to acute lung injury in cardiac surgery patients.
Keywords: transfusion, TRALI, HMGB1, S100A12, cardiac surgery
Introduction
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-related morbidity and mortality. The incidence of TRALI is much higher in patients undergoing cardiac surgery than in the general hospital population and contributes to an adverse outcome1,2. TRALI is thought to result from the interaction of activated neutrophils with endothelial cells, thereby inducing endothelial damage, vascular leakage and pulmonary oedema. However, the precise pathways of TRALI are largely unknown.
The receptor for advanced glycation end products (RAGE) is pivotal in the pro-inflammatory response in acute lung injury. RAGE is a multi-ligand pattern recognition receptor expressed on type I alveolar cells, endothelium and neutrophils3, and ligation with damage-associated molecular pattern (DAMP) molecules, including high-mobility group box 1 (HMGB1) and S100A12 (also known as EN-RAGE) is thought to contribute to a pro-inflammatory response4.
In lung injury inflicted by mechanical ventilation or by trauma, increased levels of HMGB1 are associated with adverse outcome5–8. HMGB1 plays a role in neutrophil trafficking, as demonstrated by a study in which intratracheally instilled HMGB1 resulted in neutrophil accumulation, increased cytokine release and pulmonary oedema9. HMGB1 may, therefore, play an important role in neutrophil-mediated acute lung injury (ALI). Interestingly, HMGB1 was shown to accumulate during red blood cell storage10.
The DAMP molecule S100A12 is thought to mediate the early phase of ALI. In vitro experiments showed a direct pro-inflammatory effect of S100A12 on lung endothelial cells11–13. Soluble RAGE (sRAGE) is a marker of alveolar cell injury in ALI/acute respiratory distress syndrome (ARDS)14 and increased levels have been demonstrated in ALI following trauma15, injurious mechanical ventilation16 and lung transplantation17. Interestingly, sRAGE levels were associated with both blood transfusion as well as with the use of cardiopulmonary bypass17,18. Of note, advanced glycation end products formed in red blood cells were found to ligate to endothelial-bound RAGE, resulting in endothelial damage19. Thereby, RAGE ligands may play a role in the neutrophil-endothelial interactions in the pathogenesis of TRALI. We aimed to elucidate whether RAGE-activating DAMP contribute to a TRALI reaction in patients undergoing cardiac surgery. To do this, we assessed HMGB1 and S100A12 levels, as these are important RAGE-activating DAMP20 and were shown to be associated with transfusion, the use of cardiopulmonary bypass and ALI. In addition we determined sRAGE levels.
Materials and methods
Setting
This study is a secondary analysis of a trial in cardiac surgery patients performed in an Intensive Care Unit (ICU) of a university hospital in the Netherlands1 and was approved by the medical ethics committee of the Academic Medical Centre, Amsterdam, the Netherlands (06/201#08.17.1328as). The study was carried out in accordance with the Declaration of Helsinki. Patients 18 years or older were asked to give written informed consent prior to valvular and/or coronary artery surgery for participation in the study. Exclusion criteria were off-pump surgery and emergency surgery.
Design
Patients were prospectively screened for the onset of TRALI for up to 30 hours after surgery. Using the Canadian Consensus Conference definition21, TRALI was defined as new onset hypoxaemia or deterioration demonstrated by a PaO2/FiO2 <300, occurring within 6 hours after transfusion, with bilateral pulmonary changes, in the absence of cardiac pulmonary oedema21–23. Cardiogenic pulmonary oedema was identified when pulmonary arterial occlusion pressure was >18 mmHg. Chest radiographs taken before surgery and on arrival at the ICU were scored for the presence of new onset bilateral interstitial abnormalities by two independent physicians who were blinded to the predictor variables.
Sixteen TRALI cases were identified and non-directed bronchoalveolar lavage (NBL) fluid and plasma was available from 14 patients of these for analysis. Cases were randomly matched with controls. Transfused cardiac surgery patients who did not develop ALI and cardiac surgery patients who were not transfused and did not develop ALI served as controls.
Cardiothoracic surgery, anaesthesia procedures and Intensive Care Unit management
Patients were anaesthetised with lorazepam, etomidate, sufentanil and rocuronium for induction of anaesthesia and sevoflurane plus propofol for maintenance of anaesthesia. As part of standard care, a pulmonary artery catheter was inserted for peri-operative monitoring. In all patients, cardiopulmonary bypass was performed under mild to moderate hypothermia (28 °C–34 °C), using a membrane oxygenator and non-pulsatile blood flow. During the procedure, the lungs were deflated. After surgery, all patients were transferred to the ICU with mechanical ventilation. The postoperative ICU protocol involved fluid infusions with normal saline and starch solutions and transfusion of leucodepleted erythrocytes to maintain the haemoglobin level above 8.5 g/dL. If indicated, noradrenaline was used to maintain a mean arterial blood pressure of 65 mmHg and dobutamine and/or milrinone were used to achieve a cardiac index of ≥2.5 L/min/m2.
Data collection
The pre-operative European System for Cardiac Operative Risk Evaluation (EuroSCORE), physical status according to the American Society of Anaesthesiologists (ASA score), predicted vital capacity and forced expiratory volume in 1 second (FEV1) were determined. Left ventricular function was categorised, on the basis of ejection fraction (EF), into good (EF>45%), moderate (EF<45% but >30%) or poor (EF≤30%). Data on operation time, clamp time and time on cardiopulmonary bypass, the duration of mechanical ventilation, and the partial pressure of oxygen in arterial blood were all registered.
Plasma collection and analysis
Arterial blood samples, collected in test-tubes containing EDTA before cardiopulmonary bypass and after arrival at the ICU, were centrifuged at 1,500×g for 15 minutes at 4 °C. The supernatant was stored at −80 °C until sRAGE was measured.
Levels of sRAGE were determined by an enzyme-linked immunosorbent assay (ELISA) developed in our laboratory24. In short, 96-well plates were coated overnight with mouse anti-human RAGE antibody (R&D systems, Minneapolis, Minnesota, USA). Samples diluted as appropriate were added and incubated for 2 hours. Next, biotinylated goat anti-human RAGE antibody (R&D Systems) was added and incubated for another 2 hours. Streptavidin poly-horseradish peroxidase (HRP) was added for 30 minutes. Finally, sodium-acetate buffer (pH 5.5) containing 100 μ/mL tetramethylbenzidine and 0.003% H2O2 was added and the colour reaction was stopped by 1 NH2SO4. All measurements were made in duplicate.
Non-directed bronchoalveolar lavage technique
At the onset of TRALI, we performed NBL, with which we have ample experience25–28. Controls were lavaged within 30 hours of admission to the ICU. There was no significant difference in timing of the NBL between the groups1. As described previously25–28, a standard 50 cm, 14-gauge tracheal suction catheter was introduced via the endotracheal tube and advanced until significant resistance was encountered. Immediately after instillation of 20 mL of sterile 0.9% saline over 10–15 seconds, fluid was aspirated before withdrawal of the catheter. Generally, 4 to 5 mL of fluid were recovered. NBL samples were centrifuged at 1,500×g for 10 minutes at 4 °C. The supernatant was collected and stored at −80 °C until assays were performed. The cell pellet was re-suspended in phosphate-buffered saline and cell counts were determined using a haemocytometer (Beckman Coulter, Fullerton, CA, USA)26.
Analyses in non-directed bronchoalveolar lavage fluid
The correlation between HMGB1 concentration detected by ELISA and immunoblot is poor29. Since HMGB1 and HMGB2 show high homology there may be simultaneous determination by ELISA30. We, therefore, chose to use western blotting which is more specific for HMGB1. The NBL fluid samples were mixed with 3-fold concentrated sodium dodecyl sulphate (SDS) sample buffer containing 6% β-mercaptoethanol in a 2:1 ratio and denatured for 5 minutes at 95 °C. Twenty microliters of each sample were run on a 15% polyacrylamide gel and subsequently transferred to a polyvinylidene fluoride (PVDF) membrane (Pharmacia, Piscataway, NJ, USA). Cell extracts of 293T cells were included as a positive control. After blocking with 5% non-fat dry milk in phosphate-buffered saline +0.05% Tween-20 (PBS-T), the membrane was incubated overnight at room temperature with a rabbit polyclonal antibody directed against human HMGB1 (ab18256, Abcam, Cambridge, MA, USA) in PBS-T with 1% non-fat dry milk followed by secondary labelling with a HRP-labelled goat-anti-rabbit IgG polyclonal antibody (Bioké, Leiden, the Netherlands) in PBS-T with 1% non-fat dry milk. PVDF membranes were developed using Lumilight plus ECL substrate (Roche, Darmstadt, Germany) and a chemoluminescence detector with a cooled CCD camera (Syngene, Cambridge, UK). The density of HMGB1 bands at 35 kDa were measured using AIDA image analysis software (Raytest, Straubenhardt, Germany). HMGB1 levels were compared to a standard curve prepared by serial dilution of 293T cell lysates which were run on the same gel with a lower detection limit of 500 cells. NBL fluid HMGB1 was then expressed in cell units (band densitometry correlated to the number of lysed cells). Levels of sRAGE were determined by an ELISA, as described above24.
S100A12 was also detected by an ELISA (CircuLex™, Nagano, Japan). Diluted samples were added to a microplate pre-coated with a monoclonal antibody specific for S100A12/EN-RAGE and incubated for 1 hour. After washing, HRP-conjugated anti-S100A12/EN-RAGE polyclonal antibody was added and incubated for 1 hour. Next, the substrate reagent tetra-methylbenzidine was added for 20 minutes. The reaction was stopped with 1 NH2SO4. All measurements were made in duplicate. The lower limit of detection was 61 pg/mL.
As described previously, interleukin (IL)-1β, IL-6, IL-8 and tumour necrosis factor α (TNFα) were measured using specific commercially available ELISA (PeliKine-compact™ kit), according to the instructions of the manufacturer (Sanquin, Amsterdam, the Netherlands)26,31.
Statistical analysis
Continuous data are expressed as mean and standard deviation (SD) or as medians and interquartile ranges (IQR) according to their distribution. Categorical variables are expressed as numbers (%). TRALI patients were compared with the control groups using one-way ANOVA and Dunnett’s post-test or the Kruskall-Wallis test and Mann-Whitney U test depending on data distribution. The chi-square test was used to compare categorical variables. Correlations were determined by Spearman’s rho. Because of the small size of the individual groups (TRALI and controls), associations were determined for the total group of patients.
Statistical significance was defined as p<0.05. Statistical analysis was performed with SPSS 18.0 (SPSS, Inc, Chicago, IL, USA).
Results
The patients’ characteristics are shown in Table I. Patients in the TRALI group were older, had higher ASA scores and lower FEV1 values prior to surgery when compared to controls. There were no differences in cardiac function, nor in other risk factors for ALI (data not shown). TRALI patients more often underwent combined bypass and valve replacement surgery and operation time and duration of cardiopulmonary bypass were significantly longer than those in controls. Patients who developed TRALI received more red blood cells and fresh-frozen plasma compared to transfused controls. Cases had a lower PaO2/FiO2 ratio as well as a longer duration of mechanical ventilation.
Table I.
Patients’ characteristics.
| Non-transfused | Transfused | ||
|---|---|---|---|
|
|
|||
| No ALI n=16 |
No ALI n=16 |
TRALI n=14 |
|
| Patients’ data | |||
| Age, years* | 63±13 | 65±10 | 74±8a |
| Sex, male (%) | 13 (81) | 9 (56) | 12 (79) |
| EuroSCORE* | 3.8±2.8 | 5.4±3.2 | 6.4±2.9 |
| ASA score# | 3 (1) | 3 (0)d | 3 (1)b |
| FEV1, % predicted* | 100±17 | 83±17d | 81±19a |
|
| |||
| Left ventricular function | |||
| Poor (%) | 0 (0) | 3 (19) | 1 (7) |
| Moderate (%) | 3 (21) | 1 (6) | 6 (43) |
| Good (%) | 11 (79) | 12 (75) | 7 (50) |
|
| |||
| Surgery data | |||
| CABG (%) | 11 (69) | 9 (56) | 6 (43) |
| Valve replacement (%) | 3 (19) | 3 (19) | 0 |
| CABG + valve replacement (%) | 0 | 3 (19) | 8 (57)a |
| Other (%) | 2 (12) | 1 (6) | 0 |
|
| |||
| Peri-operative data | |||
| Red blood cells, units# | NA | 2.0 (1) | 2.5 (2)a |
| Fresh frozen plasma, units# | NA | 0.0 (2) | 2.0 (2.8)a |
| Platelets, units# | NA | 0.0 (0) | 0.5 (1) |
| Clamp time, min* | 74±38 | 92±37 | 114±45a |
| Pump time, min* | 104±43 | 137±51 | 165±58b |
| Operation time, min* | 313±66 | 381±104d | 414±81c |
| MV, hours* | 13.4±4.6 | 16.6±8,4 | 127.4±211a |
| PaO2/FiO2 ratio* | 223±108 | 170±78 | 118±44b |
ASA: American Society of Anaesthesiologists; FEV1: forced expiratory volume in 1 second; CABG: coronary artery bypass grafting; MV: mechanical ventilation.
Normally distributed data, expressed as means ± standard deviation (SD);
not normally distributed data, expressed as median and interquartile range (IQR);
p<0.05;
p<0.01;
p≤0.001 TRALI compared to transfused or non-transfused controls;
P<0.05 transfused controls compared to non-transfused controls
NA: not applicable.
Levels of cytokines in non-directed bronchoalveolar lavage fluid
Pulmonary levels of the pro-inflammatory cytokines IL-8 and IL-6 in NBL fluid were higher in TRALI patients than in controls26. Pulmonary levels (median [IQR]) of IL-1β did not differ between TRALI patients (29.5 [4.1–204.2] pg/mL) and either transfused (14.7 [10.2–44.5] pg/mL) or non-transfused controls (9.7 [1.4–14.7] pg/mL), (p=0.11). Likewise, the levels of TNF-α did not differ among the groups, being 17 (4–121) pg/mL in TRALI patients compared to 36 (10–59) pg/mL in transfused and 34 (13–44) pg/mL in non-transfused controls (p=0.19).
Levels of soluble receptor for advanced glycation end products in plasma and bronchoalveolar lavage fluid of cardiac surgery patients with transfusion-related acute lung injury and controls
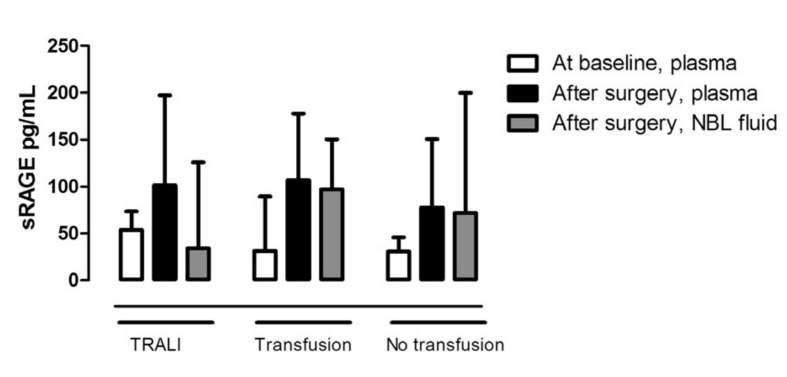
In all patients, cardiac surgery resulted in a modest increase in plasma sRAGE levels compared to baseline values (Figure 1). Following surgery, levels (median and IQR) of sRAGE in NBL fluid were low, being 34.1 (14.7–135.9) pg/mL in TRALI patients, 97.1 (19.3–150.4) pg/mL in transfused controls and 71.8 (27.3–200) pg/mL in non-transfused controls (p=0.57). Pulmonary levels of sRAGE were not elevated compared to plasma levels and none of the levels differed between TRALI patients and controls (Figure 1).
Figure 1.
sRAGE levels in plasma before and after cardiac surgery and sRAGE levels in non-directed bronchoalveolar lavage (NBL) fluid after surgery.
TRALI: TRALI cases. Transfusion: transfused patients who did not develop lung injury. No transfusion: patients not transfused and without lung injury. Data are expressed as median and interquartile range.
Levels of HMGB1 in bronchoalveolar lavage fluid of cardiac surgery patients with transfusion-related acute lung injury and controls
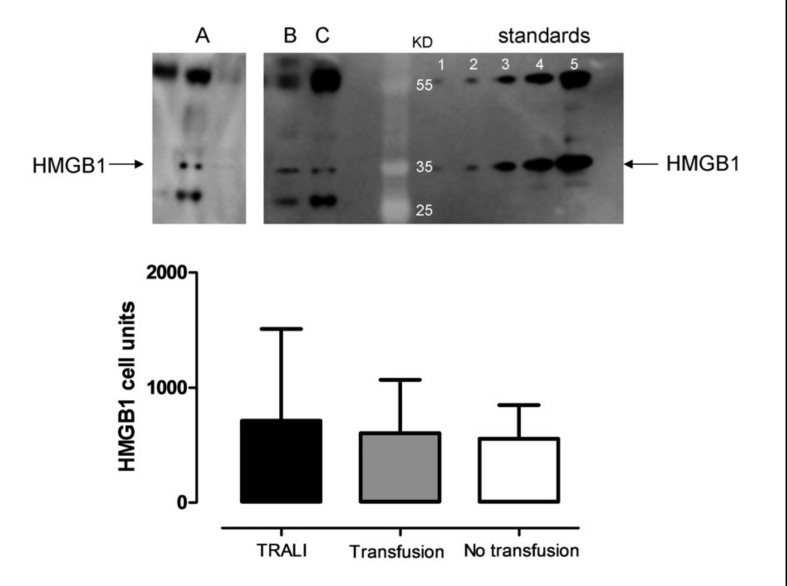
No differences were found in pulmonary HMGB1 expression (mean ± SD) between patients with TRALI (712±379 [cell units]) and either transfused (605±205 [cell units]) or non-transfused controls (556±123 [cell units]) (p=0.86; Figure 2). There was a wide variation in expression of HMGB1. If dichotomised (detected versus not detected), no differences were found among the three groups (data not shown).
Figure 2.
Levels of HMGB1 (high-mobility group box 1) in non-directed bronchoalveolar lavage (NBL) fluid after cardiac surgery.
TRALI: TRALI cases. Transfusion: transfused patients who did not develop lung injury. No transfusion: patients not transfused and without lung injury. Data expressed are as mean and standard deviation. The western blot is representative for HMGB1 from a patient with TRALI (A) and two control patients (B and C).
Levels of S100A12 in bronchoalveolar lavage fluid of cardiac surgery patients with transfusion-related acute lung injury and controls
S100A12 could be detected at substantial levels in NBL fluid of all patients, with the highest median levels measured in TRALI patients (702 [77–1,661] ng/mL) compared to 388 (217–1,199) ng/mL in transfused and 119 (71–349) ng/mL in non-transfused controls (Figure 3); this trend was, however, not statistically significant trend (p=0.15).
Figure 3.
Levels of S100A12 in non-directed bronchoalveolar lavage fluid after cardiac surgery.
TRALI: TRALI cases. Transfusion: transfused patients who did not develop lung injury. No transfusion: patients not transfused and without lung injury. Data expressed as median and interquartile range.
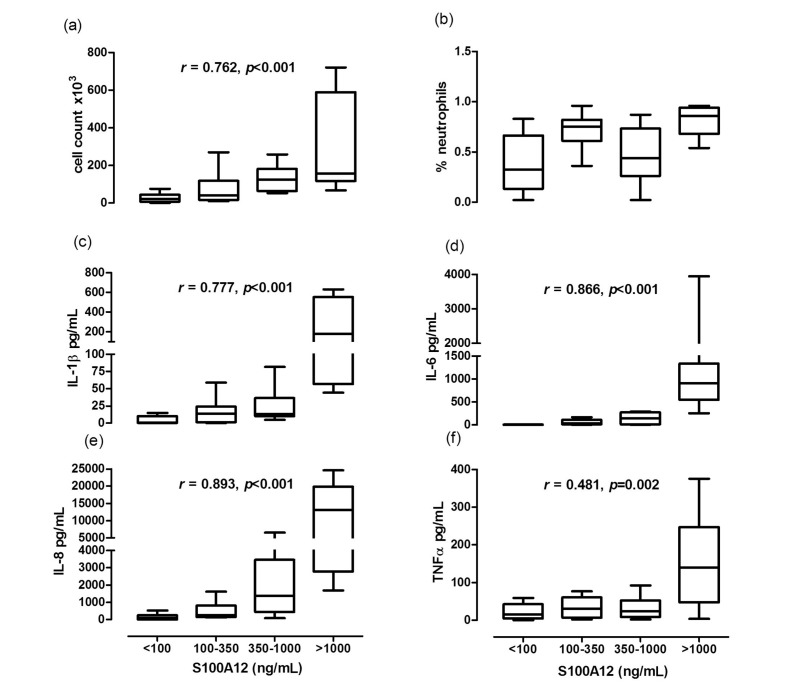
There was a negative correlation between S100A12 levels and pO2 6 hours post-operatively (r=−0.584, p=0.014) and a positive correlation between levels of S100A12 in NBL fluid and duration of mechanical ventilation (r=0.445, p=0.005). Furthermore, duration of ventilation was positively associated with the total number of units transfused (r=0.537, p=0.002). We then determined whether there was a correlation between the degree of pulmonary inflammation and pulmonary levels of S100A12. Patients with higher NBL levels of S100A12, had higher NBL cell counts and higher NBL levels of TNF-α, IL-1B, IL-6 and IL-8 (Figure 4). Levels of S100A12 were not significantly associated with EUROscore, ASA score or amount of transfusion. Levels of S100A12 did show a positive correlation with time on cardiopulmonary bypass (r=0.337, p<0.05).
Figure 4.
Pulmonary levels of inflammatory markers grouped by S100A12 in all patients.
Cell count in non-directed bronchoalveolar lavage (NBL) fluid was significantly higher in patients with higher S100A12 levels (a). Percentage of neutrophils in NBL fluid (b). Levels of IL-1β (c), IL-6 (d), IL-8 (e) and TNFα (f) were significantly higher in patients with high levels of S100A12. Data presented in quartiles.
Discussion
In the present study, cardiac surgery patients had markedly elevated pulmonary levels of S100A12, which were associated with longer time on cardiopulmonary bypass, hypoxaemia, prolonged mechanical ventilation and pulmonary inflammation. The levels of S100A12 were highest in those patients developing TRALI and this molecule may, therefore, play a role in the pathogenesis of TRALI.
Levels of S100A12 following cardiac surgery were very high compared to those in previous studies in other postoperative patients12. Furthermore, the level of pulmonary S100A12 was about 10-fold higher than that reported in ARDS11. Increased S100A12 correlated with decreased oxygenation, prolonged time on mechanical ventilation and elevated pulmonary levels of pro-inflammatory cytokines. This is in line with the recent finding that S100A12 actively contributes to the early inflammatory response by amplification of inflammatory processes32.
As time on cardiopulmonary bypass was significantly associated with elevated pulmonary levels of S100A12 in this study, use of the bypass machine may have contributed to this increase. Increased levels of S100A12 after cardiopulmonary bypass in children were found to correlate with severity of ALI33. Of note, levels in TRALI patients were clearly higher than those in patients with ALI after cardiopulmonary bypass33. Given that S100A12 is a pro-inflammatory mediator whose levels have been shown to rise early in the course of lung injury11,12 and the TRALI syndrome is classically a rapid pulmonary reaction to a blood transfusion, S100A12 might contribute to pulmonary inflammation in cardiac surgery patients with a TRALI reaction. TRALI is thought to be a “two-hit syndrome”, in which an inflammatory condition primes lung neutrophils, rendering them susceptible to lung injury inflicted by mediators in the blood product34. As S100A12 mediates inflammation via activated granulocytes32, we speculate that S100A12 may amplify the TRALI reaction or may contribute to the “first hit” in TRALI, thereby lowering the threshold for developing TRALI35. This may explain the high incidence of TRALI found previously1,36.
As shown before, plasma levels of sRAGE increased modestly in all patients following cardiac surgery. However, levels were low compared to those reported previously18,33. In addition, sRAGE in NBL fluid after surgery was not elevated and did not differ among TRALI patients and their controls. In line with this finding, sRAGE levels in NBL were not elevated in an experimental model of TRALI37. RAGE is expressed abundantly in pulmonary tissue and it has been proposed that S100A12 exerts its effects via RAGE. Interestingly, it was recently demonstrated that Toll-like receptor 4 (TLR4), not RAGE, is pivotal in S100A12-mediated inflammatory responses32. In the lung, TLR4 expression has been demonstrated on vascular endothelial cells and activation has shown to contribute to pulmonary neutrophil sequestration38. The relatively low levels of sRAGE in our patients do not, therefore, preclude S100A12-mediated pulmonary inflammation.
In this study, expression of HMGB1 was not increased in TRALI patients compared to that in controls. HMGB1 has been demonstrated to be an important mediator of inflammation in ARDS due to various causes5,6,9. However, its relation to outcome has not unequivocally been demonstrated. In trauma-induced ARDS, HMGB1 was associated with a poor outcome8. In another group of ARDS patients, HMGB1 levels did not differ between survivors and non-survivors, nor did they correlate with lung injury score or hypoxaemia6. These conflicting results may be related to the time course of the lung injury. A late increase in HMGB1 was seen in patients with ARDS, with peak levels occurring between day 1 and 7, but not at onset of the disease6. In addition, in mechanically ventilated patients, pulmonary HMGB1 release could only be demonstrated after days and not hours of ventilation7. Altogether, HMGB1 has been suggested to be a late mediator of inflammation during ALI and it does not seem to play a role in TRALI.
Taken together, no clear association between DAMP molecules and TRALI was found. However, S100A12 was associated with pulmonary inflammation, suggesting that this early DAMP molecule may be a strong driver of the inflammatory response in ALI following cardiac surgery. Although the increase in pulmonary S100A12 levels in TRALI patients was not statistically different from that in controls, the observed trend suggests that the study was underpowered to establish a clear association. Whether S100A12 is a mediator in TRALI does, therefore, remain to be established. Of note, other DAMP molecules have been shown to accumulate during the storage of blood products. These molecules include haem and lipid peroxidation products, both ligands for TLR4, and extracellular ATP and microparticles, which are capable of activating the NLRP3 inflammasome35. The latter has been shown to contribute to pulmonary inflammation39. Whether these DAMP molecules contribute to the inflammatory changes in TRALI should be addressed in future research.
There are limitations to our study. First, the number of patients is small, which may have underpowered the study. Second, NBL fluid was collected only once, precluding conclusions about the time course of the role of DAMP molecules in the maintenance and amplification of the inflammatory response in TRALI. However, this cohort of TRALI patients in whom lavage samples were obtained is the largest to date.
In conclusion, no evidence was found that HMGB1 and sRAGE contribute to the development of TRALI. The early DAMP molecule, S100A12, is associated with prolonged cardiopulmonary bypass, pulmonary inflammation, ventilation duration and hypoxaemia after cardiac surgery and may mediate the priming phase of ALI in cardiac surgery patients who develop this condition. Further research is warranted to establish the role of DAMP molecules in the inflammatory response in TRALI.
Footnotes
The Authors declare no conflicts of interest.
Reference
- 1.Vlaar AP, Hofstra JJ, Determann RM, et al. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study. Blood. 2011;117:4218–25. doi: 10.1182/blood-2010-10-313973. [DOI] [PubMed] [Google Scholar]
- 2.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–62. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 3.Morbini P, Villa C, Campo I, et al. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006;19:1437–45. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 4.van Zoelen MA, Achouiti A, van der Poll T. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit Care. 2011;15:208. doi: 10.1186/cc9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogawa EN, Ishizaka A, Tasaka S, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 2006;174:400–7. doi: 10.1164/rccm.200605-699OC. [DOI] [PubMed] [Google Scholar]
- 6.Ueno H, Matsuda T, Hashimoto S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–6. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 7.van Zoelen MA, Ishizaka A, Wolthuls EK, et al. Pulmonary levels of high-mobility group box 1 during mechanical ventilation and ventilator-associated pneumonia. Shock. 2008;29:441–5. doi: 10.1097/SHK.0b013e318157eddd. [DOI] [PubMed] [Google Scholar]
- 8.Bitto A, Barone M, David A, et al. High mobility group box-1 expression correlates with poor outcome in lung injury patients. Pharmacol Res. 2010;61:116–20. doi: 10.1016/j.phrs.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Abraham E, Arcaroli J, Carmody A, et al. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 10.Peltz ED, Moore EE, Eckels PC, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wittkowski H, Sturrock A, van Zoelen MA, et al. Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Crit Care Med. 2007;35:1369–75. doi: 10.1097/01.CCM.0000262386.32287.29. [DOI] [PubMed] [Google Scholar]
- 12.Kikkawa T, Sato N, Kojika M, et al. Significance of measuring S100A12 and sRAGE in the serum of sepsis patients with postoperative acute lung injury. Dig Surg. 2010;27:307–12. doi: 10.1159/000313687. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz E, Muhlebach MS, Tessier PA, et al. Different expression ratio of S100A8/A9 and S100A12 in acute and chronic lung diseases. Respir Med. 2008;102:567–73. doi: 10.1016/j.rmed.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–15. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen MJ, Brohi K, Calfee CS, et al. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63:1083–9. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie JD, Shah CV, Kawut SM, et al. Plasma levels of receptor for advanced glycation end products, blood transfusion, and risk of primary graft dysfunction. Am J Respir Crit Care Med. 2009;180:1010–5. doi: 10.1164/rccm.200901-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agostoni P, Banfi C, Brioschi M, et al. Surfactant protein B and RAGE increases in the plasma during cardiopulmonary bypass: a pilot study. Eur Respir J. 2011;37:841–7. doi: 10.1183/09031936.00045910. [DOI] [PubMed] [Google Scholar]
- 19.Mangalmurti NS, Chatterjee S, Cheng G, et al. Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products. Transfusion. 2010;50:2353–61. doi: 10.1111/j.1537-2995.2010.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuipers MT, van der Poll T, Schultz MJ, et al. Bench-to-bedside review: Damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit Care. 2011;15:235. doi: 10.1186/cc10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–89. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 22.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–6. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 23.Goldman M, Webert KE, Arnold DM, et al. Proceedings of a consensus conference: towards an understanding of TRALI. Transfus Med Rev. 2005;19:2–31. doi: 10.1016/j.tmrv.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Determann RM, Wolthuis EK, Choi G, et al. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L344–50. doi: 10.1152/ajplung.00268.2007. [DOI] [PubMed] [Google Scholar]
- 25.Cornet AD, Hofstra JJ, Vlaar AP, et al. Activated protein C attenuates pulmonary coagulopathy in patients with acute respiratory distress syndrome. J Thromb Haemost. 2013;11:894–901. doi: 10.1111/jth.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vlaar AP, Hofstra JJ, Determann RM, et al. Transfusion-related acute lung injury in cardiac surgery patients is characterized by pulmonary inflammation and coagulopathy: a prospective nested case-control study. Crit Care Med. 2012;40:2813–20. doi: 10.1097/CCM.0b013e31825b8e20. [DOI] [PubMed] [Google Scholar]
- 27.Schultz MJ, Determann RM, Royakkers AA, et al. Bronchoalveolar activation of coagulation and inhibition of fibrinolysis during ventilator-associated lung injury. Crit Care Res Pract. 2012;2012:961784. doi: 10.1155/2012/961784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofstra JJ, Vlaar AP, Knape P, et al. Pulmonary activation of coagulation and inhibition of fibrinolysis after burn injuries and inhalation trauma. J Trauma. 2011;70:1389–97. doi: 10.1097/TA.0b013e31820f85a7. [DOI] [PubMed] [Google Scholar]
- 29.Urbonaviciute V, Furnrohr BG, Weber C, et al. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol. 2007;81:67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]
- 30.Yamada S, Inoue K, Yakabe K, et al. High mobility group protein 1 (HMGB1) quantified by ELISA with a monoclonal antibody that does not cross-react with HMGB2. Clin Chem. 2003;49:1535–7. doi: 10.1373/49.9.1535. [DOI] [PubMed] [Google Scholar]
- 31.Tuinman PR, Vlaar AP, Cornet AD, et al. Blood transfusion during cardiac surgery is associated with inflammation and coagulation in the lung: a case control study. Crit Care. 2011;15:R59. doi: 10.1186/cc10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foell DMD, Wittkowski H, Kessel C, et al. Pro-inflammatory S100A12 can activate human monocytes via Toll-like receptor 4. Am J Respir Crit Care Med. 2013;187:1324–34. doi: 10.1164/rccm.201209-1602OC. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Chen Q, Shi S, et al. Plasma sRAGE enables prediction of acute lung injury after cardiac surgery in children. Crit Care. 2012;16:R91. doi: 10.1186/cc11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007;136:788–99. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 35.Land WG. Transfusion-related acute lung injury: the work of DAMPs. Transfus Med Hemother. 2013;40:3–13. doi: 10.1159/000345688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–62. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 37.Su X, Looney MR, Gupta N, et al. Receptor for advanced glycation end-products (RAGE) is an indicator of direct lung injury in models of experimental lung injury. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1–5. doi: 10.1152/ajplung.90546.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andonegui G, Bonder CS, Green F, et al. Endothelium-derived Toll-like receptor-4 is the key molecule in LPS-induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–20. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuipers MT, Aslami H, Janczy JR, et al. Ventilator-induced lung injury is mediated by the NLRP3 inflammasome. Anesthesiology. 2012;116:1104–15. doi: 10.1097/ALN.0b013e3182518bc0. [DOI] [PubMed] [Google Scholar]