Abstract
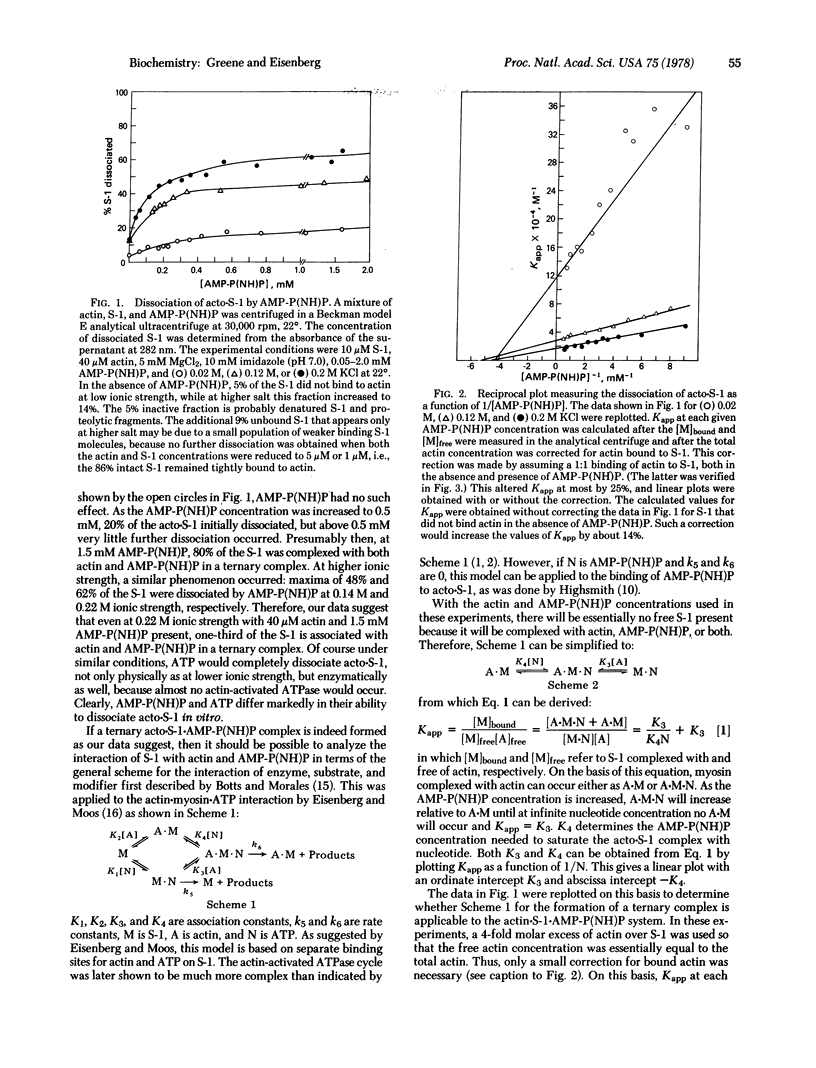
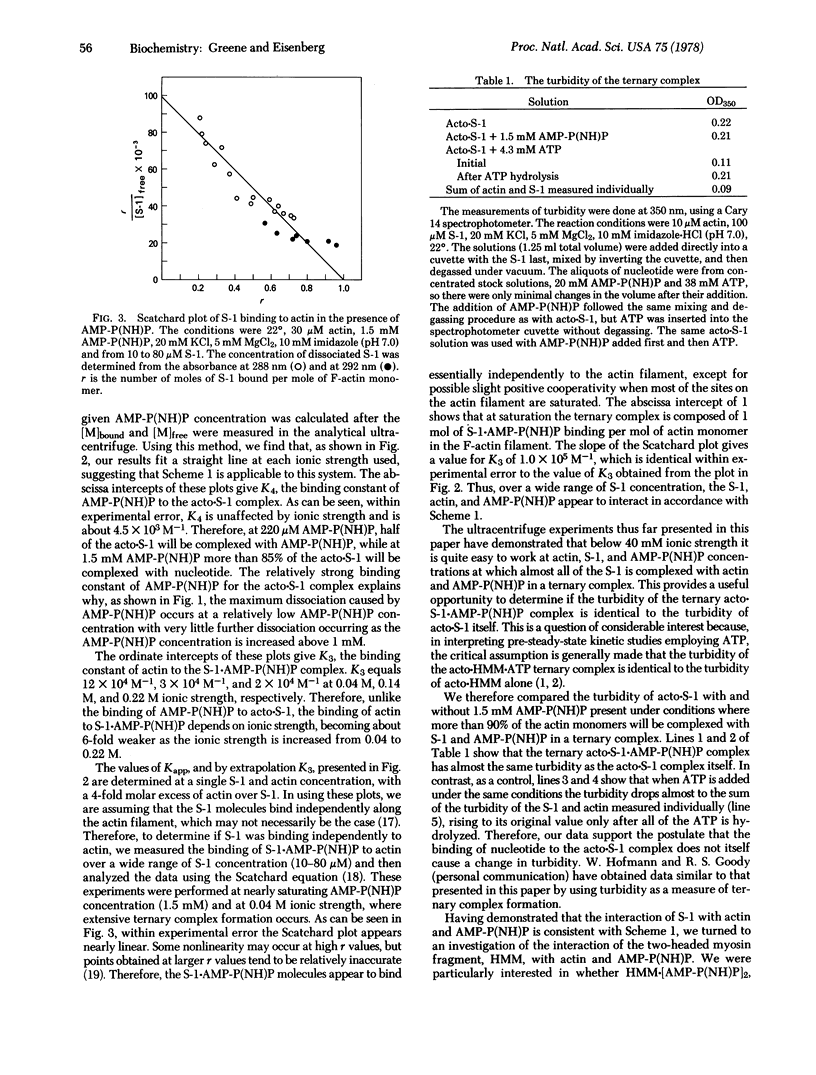
The formation of the ternary complex composed of actin, 5′-adenylyl imidodiphosphate [AMP-P(NH)P], and myosin subfragment 1 (S-1) was studied using the analytical ultracentrifuge with UV optics, which enabled the direct determination of the extent of dissociation of actin·S-1 (acto·S-1) by AMP-P(NH)P. In contrast to the reaction with ATP, at saturating levels of AMP-P(NH)P (1.5 mM), extensive formation of the ternary acto·S-1·AMP-P(NH)P complex occurs at 22°. With 40 μM actin present, AMP-P(NH)P causes almost no dissociation of the acto·S-1 complex at 0.04 M ionic strength, while even at 0.22 M ionic strength one-third of the S-1 remains associated with actin and AMP-P(NH)P in a ternary complex. A detailed study of the binding of S-1·AMP-P(NH)P to actin using the Scatchard plot analysis shows that, at saturation, 1 mol of S-1·AMP-P(NH)P binds per mol of actin monomer. There appears to be no cooperativity occurring as the S-1·AMP-P(NH)P binds along the actin filament, with the possible exception of a slight positive cooperativity when most of the sites on the actin filament are saturated. The turbidity of the ternary complex is identical to the turbidity of acto·S-1 alone. Preliminary experiments with the two-headed subfragment of myosin, heavy meromyosin (HMM), show that the binding of HMM·[AMP-P(NH)P]2 to actin is only about twice as strong as the binding of S-1·AMP-P(NH)P to actin, indicating that the second head contributes very little to the free energy of binding.
Keywords: myosin subfragment 1, heavy meromyosin, association constants, turbidity
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinfeld M. C., Martonosi A. N. Effect of F-actin upon the binding of ADP to myosin and its fragments. J Biol Chem. 1975 Oct 10;250(19):7871–7878. [PubMed] [Google Scholar]
- Chock S. P., Chock P. B., Eisenberg E. Pre-steady-state kinetic evidence for a cyclic interaction of myosin subfragment one with actin during the hydrolysis of adenosine 5'-triphosphate. Biochemistry. 1976 Jul 27;15(15):3244–3253. doi: 10.1021/bi00660a013. [DOI] [PubMed] [Google Scholar]
- Dos Remedios C. G., Yount R. G., Morales M. F. Individual states in the cycle of muscle contraction. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2542–2546. doi: 10.1073/pnas.69.9.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Dobkin L., Kielley W. W. Binding of actin to heavy meromyosin in the absence of adenosine triphosphate. Biochemistry. 1972 Dec 5;11(25):4657–4660. doi: 10.1021/bi00775a003. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Moos C. The adenosine triphosphatase activity of acto-heavy meromyosin. A kinetic analysis of actin activation. Biochemistry. 1968 Apr;7(4):1486–1489. doi: 10.1021/bi00844a035. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Zobel C. R., Moos C. Subfragment 1 of myosin: adenosine triphophatase activation by actin. Biochemistry. 1968 Sep;7(9):3186–3194. doi: 10.1021/bi00849a022. [DOI] [PubMed] [Google Scholar]
- Fraser A. B., Eisenberg E., Kielley W. W., Carlson F. D. The interaction of heavy meromyosin and subfragment 1 with actin. Physical measurements in the presence and absence of adenosine triphosphate. Biochemistry. 1975 May 20;14(10):2207–2214. doi: 10.1021/bi00681a025. [DOI] [PubMed] [Google Scholar]
- Goody R. S., Hofmann W., Mannherz G. H. The binding constant of ATP to myosin S1 fragment. Eur J Biochem. 1977 Sep;78(2):317–324. doi: 10.1111/j.1432-1033.1977.tb11742.x. [DOI] [PubMed] [Google Scholar]
- Goody R. S., Holmes K. C., Mannherz H. G., Leigh J. B., Rosenbaum G. Cross-bridge conformation as revealed by x-ray diffraction studies on insect flight muscles with ATP analogues. Biophys J. 1975 Jul;15(7):687–705. doi: 10.1016/S0006-3495(75)85848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody R. S., Leigh J. B., Mannherz H. G., Tregear R. T., Rosenbaum G. X-ray titration of binding of beta, gamma-imido-ATP to myosin in insect flight muscle. Nature. 1976 Aug 12;262(5569):613–615. doi: 10.1038/262613a0. [DOI] [PubMed] [Google Scholar]
- Highsmith S. Interactions of the actin and nucleotide binding sites on myosin subfragment 1. J Biol Chem. 1976 Oct 25;251(20):6170–6172. [PubMed] [Google Scholar]
- Highsmith S. The effects of temperature and salts on myosin subfragment-1 and F-actin association. Arch Biochem Biophys. 1977 Apr 30;180(2):404–408. doi: 10.1016/0003-9861(77)90054-6. [DOI] [PubMed] [Google Scholar]
- Lowey S., Luck S. M. Equilibrium binding of adenosine diphosphate to myosin. Biochemistry. 1969 Aug;8(8):3195–3199. doi: 10.1021/bi00836a010. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Low-angle x-ray diagrams from skeletal muscle: the effect of AMP-PNP, a non-hydrolyzed analogue of ATP. J Mol Biol. 1975 Dec 25;99(4):567–582. doi: 10.1016/s0022-2836(75)80172-0. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., de Bruin S. H., Gratzer W. B. An investigation of heavy meromyosin-ADP binding equilibria by proton release measurements. Biochemistry. 1977 Apr 19;16(8):1738–1742. doi: 10.1021/bi00627a034. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Rodger C. D., Tregear R. T. Changes in muscle crossbridges when beta, gamma-imido-ATP binds to myosin. J Mol Biol. 1976 Jun 14;104(1):263–276. doi: 10.1016/0022-2836(76)90012-7. [DOI] [PubMed] [Google Scholar]
- Marston S., Weber A. The dissociation constant of the actin-heavy meromyosin subfragment-1 complex. Biochemistry. 1975 Aug 26;14(17):3868–3873. doi: 10.1021/bi00688a021. [DOI] [PubMed] [Google Scholar]
- Schliselfeld L. H. Binding of adenylyl imidodiphosphate, an analog of adenosine triphosphate, to myosin and heavy meromyosin. J Biol Chem. 1974 Aug 10;249(15):4985–4989. [PubMed] [Google Scholar]
- Takeuchi K., Tonomura Y. Formation of acto-H-meromyosin and acto-subfragment-1 complexes and their dissociation by adenosine triphosphate. J Biochem. 1971 Dec;70(6):1011–1026. doi: 10.1093/oxfordjournals.jbchem.a129710. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]
- Wolcott R. G., Boyer P. D. The reversal of the myosin and actomyosin ATPase reactions and the free energy of ATP binding to myosin. Biochem Biophys Res Commun. 1974 Apr 8;57(3):709–716. doi: 10.1016/0006-291x(74)90604-4. [DOI] [PubMed] [Google Scholar]
- Woodrum D. T., Rich S. A., Pollard T. D. Evidence for biased bidirectional polymerization of actin filaments using heavy meromyosin prepared by an improved method. J Cell Biol. 1975 Oct;67(1):231–237. doi: 10.1083/jcb.67.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]



