Abstract
Background
Recent advances in red blood cell metabolomics have paved the way for further improvements of storage solutions.
Materials and methods
In the present study, we exploited a validated high performance liquid chromatography-mass spectrometry analytical workflow to determine the effects of vitamin C and N-acetylcysteine supplementation (anti-oxidants) on the metabolome of erythrocytes stored in citrate-phosphate-dextrose saline-adenine-glucose-mannitol medium under blood bank conditions.
Results
We observed decreased energy metabolism fluxes (glycolysis and pentose phosphate pathway). A tentative explanation of this phenomenon could be related to the observed depression of the uptake of glucose, since glucose and ascorbate are known to compete for the same transporter. Anti-oxidant supplementation was effective in modulating the redox poise, through the promotion of glutathione homeostasis, which resulted in decreased haemolysis and less accumulation of malondialdehyde and oxidation by-products (including oxidized glutathione and prostaglandins).
Discussion
Anti-oxidants improved storage quality by coping with oxidative stress at the expense of glycolytic metabolism, although reservoirs of high energy phosphate compounds were preserved by reduced cyclic AMP-mediated release of ATP.
Keywords: red blood cells, vitamin C, N-acetyl cysteine, mass spectrometry, metabolomics
Introduction
Despite decades of significant technological improvements, red blood cell (RBC) storage under blood bank conditions is still accompanied by the exacerbation of in vivo ageing phenomena, a process that is often referred to as “storage lesion”1–4. Blood bank storage conditions impose a significant challenge for the maintenance of RBC metabolism5, and especially of high energy phosphate compounds, such as adenosine triphosphate (ATP) and 2,3-diphosphoglycerate (DPG)1–6. Storage lesion also includes a well-documented list of reversible and irreversible modifications to RBC morphology and biochemistry1–4, such as alterations to RBC metabolism and ion homeostasis5, accumulation of oxidative stress, especially to the lipid (malondialdehyde accumulation) and protein fractions (carbonylation, protein fragmentation)6,7, increased vesiculation rate8 ultimately resulting in abnormal morphology, which in turn promotes osmotic fragility9. These anomalies, although reversible to some extent, are related to the promotion of apoptosis-like phenomena that compromise RBC survival upon transfusion10. Two main interventions have been suggested to be able to improve the quality, safety and efficacy of RBC concentrates that are stored for a long time: (i) oxygen removal in order to pursue anaerobic storage and/or (ii) the formulation of alternative additive/rejuvenation solutions.
Anaerobic storage of RBC through deoxygenation of packed red cell units has been demonstrated to have the potential to extend the shelf-life of erythrocyte concentrates up to 63 days11, by preserving ATP and DPG reservoirs better11. However, almost counter-intuitively, oxygen removal does not result in lower levels of oxidative stress upon reoxygenation12,13. Conversely, hypoxia limits the anti-oxidant capacity of RBC as it blocks the metabolic shift towards the pentose phosphate pathway (PPP)12,13, which is responsible for the production of the reducing coenzyme NAPDH that, in turn, is essential to maintain the homeostasis of several anti-oxidant enzymes and pathways (e.g., glutathione homeostasis).
In parallel, alternative storage strategies envisage the formulation of novel additive/rejuvenation solutions, which are mostly based upon pH modulation (affecting the activity of glycolytic enzymess, which in turn promotes the replenishment of ATP and DPG reservoirs14–16) and/or supplementation of carbon substrates to refuel energy production17.
Blood preservation studies have been also conducted to understand the potential benefits arising from the addition of anti-oxidants, such as vitamin C (ascorbate), to storage solutions18–20. Ascorbate levels in RBC in vivo are the same as those in plasma21, although RBC also display a high capacity to regenerate the vitamin from its two electron-oxidized form, dehydroascorbic acid22. Besides, ascorbate helps to preserve alpha-tocopherol (vitamin E) from oxidation, a compound that is found in lipoproteins and in the RBC membrane23.
While thiol compounds are known to protect directly against oxidative stress and it has already been demonstrated that they can permeate RBC membranes24, only glutathione loading has so far been proposed as a potential additive to storage solution formulations25. From this point of view, N-acetylcysteine (NAC), a precursor to the tripeptide glutathione (GSH), might be an ideal candidate for inclusion in additive solutions. Indeed, NAC has important anti-oxidant activity, as it has been demonstrated to reduce oxidative stress in sickle cell patients26.
Decades of investigations in the field of RBC biochemistry27 have paved the way for a “Systems biology”-oriented28 understanding of RBC physiology and metabolism. These in silico models have allowed dissection of RBC metabolism under in vitro ageing (storage under blood bank conditions), enabling nuclear magnetic resonance29 or, more recently, mass spectrometry (MS)-based metabolomics investigations5,6,12,30–32.
Taking advantage of a novel high performance liquid chromatography (HPLC)-time of flight-quadrupole (micro-TOF-Q) mass spectrometry (MS) approach, a workflow that recently contributed precious insights into the understanding of RBC metabolism under control and anaerobic blood banking conditions5,6,12, we present the results of a pilot laboratory study to investigate the effects on RBC metabolome when packed red cell units stored in the presence of citrate-phosphate-dextrose ([CPD] saline-adenine-glucose-mannitol SAGM) were supplemented with vitamin C and NAC.
Materials and methods
Collection of samples
Red blood cell units were drawn from healthy donor volunteers in accordance with guidelines from the Italian National Blood Centre (Blood Transfusion Service for donated blood) and after having received informed consent in conformity with the declaration of Helsinki. We studied RBC units collected from 10 male, healthy, volunteer donors (age 39.4±7.5 [mean ± SD]) at the “Celio” Military Hospital in Rome (Italy). Units were stored under standard conditions at 4 °C for up to 42 days in the presence of: (i) CDP-SAGM, or (ii) CPD-SAGM with the addition of ascorbic acid (0.23 mM - Sigma Aldrich, Milan, Italy) and NAC (0.5 mM - Sigma-Aldrich, Milan, Italy). In detail, whole blood was collected into CPD-containing units and handled in accordance with standard European Council guidelines, as it was centrifuged, leucofiltered (log4) and packed erythrocytes were recovered. However, packed erythrocytes recovered from each donated unit were split in two satellite bags (36±4 mL of packed erythrocytes +20 mL of either supplemented or non-supplemented SAGM additive solution; ≈66.7 haematocrit-satellite PVC bag, plasticiser TEHTM, PL 1240 - Fenwal). This workflow has already been exploited by our group12 and other groups, and is the only viable strategy to assay the effects of a specific treatment to donated blood from the same donor while pairing treated and untreated groups. It is also worth noting that recent evidence suggests that the lack of diethylhexyl phthalate (DEHP) in plastic satellite bags, such as in the case of paediatric bags, promotes more stress-induced oxidative haemolysis (although still significantly below the 0.8% threshold) than in the parent units33. Since the aim of the present study was to assess the effectiveness of ascorbic acid and NAC in preventing haemolysis and oxidative injury through the modulation of metabolic fluxes, such an exacerbation would have helped us to highlight any treatment-specific response. In addition, since statistically significant (p<0.05 ANOVA) better results were obtained in terms of haemolysis, reactive oxygen species (ROS) accumulation and pH when both anti-oxidants were added, rather than either of them alone (Table I), the experiments in this study were performed on units supplemented with both vitamin C and NAC. Dosing experiments for vitamin C and NAC were performed to minimise haemolysis at the end of the storage period. Vitamin C concentrations below 0.5 mM were considered as they best preserved erythrocytes from oxidative hemolysis34. Sterility was assessed throughout the whole storage period.
Table I.
Haemolysis, ROS and pH levels in units supplemented with either vitamin C, NAC or both. *p-value<0.05 ANOVA.
| Haemolysis (%) | ROS (nmol/mL) | pH (units) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Day 0 | Day 42 | Day 0 | Day 42 | Day 0 | Day 42 | |
| Control | 0.16 | 0.92 | 2.98 | 9.26 | 6.67 | 5.97 |
| Vitamin C | 0.15 | 0.95 | 3.76 | 6.70* | 6.75 | 6.20* |
| NAC | 0.12 | 0.70* | 2.98 | 7.52 | 6.74 | 6.22* |
| Both | 0.16 | 0.51* | 2.03 | 6.50* | 6.66 | 6.33* |
ROS: reactive oxygen species
Samples were removed aseptically for analysis on a weekly basis. Samples for metabolomics analyses were collected after 0, 7, 21, 28 and 42 days of storage.
Acetonitrile, formic acid, and HPLC-grade water and standards (≥98% chemical purity) were purchased from Sigma Aldrich.
Determination of haemolysis, malondialdehyde and intracellular pH
Levels of haemolysis, malondialdehyde and pH were determined as previously reported6.
Haemolysis was calculated following the method of Harboe, with minor modifications as previously reported6. Samples were diluted in distilled water and incubated at room temperature for 30 minutes to lyse RBC. Samples of the lysed RBC were diluted 1/300 while supernatants were diluted 1/10 in distilled water. After stabilisation for 30 minutes and vortex mixing (Titramax 100, Heidolph Elektro, Kelheim, Germany), the absorbance of the haemoglobin was measured at 380 nm, 415 nm and 450 nm (PowerWave 200 Spectrophotometer, Bio-Tek Instruments, Winooski, Vermont, USA). The mean blank value was subtracted and the corrected optical density (OD) was calculated as follows: 2×OD415 –OD380 –OD450.
In order to determine malondialdehyde levels, 0.2 mL of packed RBC were suspended in 3.0 mL of Krebs’ Ringer’s phosphate buffer solution (pH 7.4) and 1 mL of the cell suspension was treated with 1 mL of 10% trichloroacetic acid and centrifuged at 1,000×g for 5 min. Next, 1 mL of supernatant was mixed with 1 mL of 0.67% thiobarbituric acid and heated over a water bath for 20 minutes at 85–90 °C. The solution was cooled and read against a complementary blank at 532 nm (OD1) and 600 nm (OD2). A blank was prepared separately without packed RBC. The net OD was calculated after subtracting absorbance at OD2 from that at OD1. The malondialdehyde level was determined from the standard plot and expressed as nmol/mL of packed RBC.
For the determination of intracellular pH, red cell pellets obtained by centrifuging 600 μL of suspension in a nylon tube at 30,000×g for 10 min, were frozen, thawed over 5 minutes and then refrozen. To prevent the acid shift observed when samples are kept unfrozen, triplicate measurements of pH were made immediately after a second thawing of each lysate with a Radiometer pH glass capillary electrode maintained at 20 °C and linked to a Radiometer pH acid-base analyser (Radiometer, Copenhagen, Denmark).
Untargeted metabolomics analyses
Metabolite extractions and metabolomics analyses were performed as previously reported35. For each sample, 0.5 mL of the pooled erythrocyte stock were transferred into a microcentrifuge tube (Eppendorf®, Hamburg, Germany) and centrifuged at 1,000 g for 2 minutes at 4 °C. The tube was then placed on ice while the supernatant was carefully aspirated, paying attention not to remove any erythrocytes at the interface. The erythrocytes were resuspended in 0.15 mL of ice cold ultra-pure water (18 MΩ) to lyse the cells and then the tube was plunged into a water bath at 37 °C for 0.5 minutes. Samples were mixed with 0.6 mL of −20 °C methanol and then with 0.45 mL chloroform. Subsequently, 0.15 mL of ice cold ultra-pure water were added to each tube and the tubes were transferred to a freezer at −20 °C for 2–8 hours. An equivalent volume of acetonitrile was added to the tubes which were then transferred to a refrigerator (4 °C) for 20 minutes. Samples with precipitated proteins were thus centrifuged for 10,000×g for 10 minutes at 4 °C. The stability of oxidation-prone compounds, such as GSH, under the experimental conditions described above, was confirmed at 1 hour, 1 day and 1 week after preparation of the samples (samples stored at −80 °C).
Since only 0.5 mL per biological replicate per time point was necessary to perform metabolic extraction and subsequent technical replicate runs, the final volume in each assayed unit did not decrease substantially throughout the monitored time span.
Analyses were performed with an Ultimate 3000 Rapid Resolution HPLC system (LC Packings, DIONEX, Sunnyvale, CA, USA) and an electrospray hybrid MicroTOF-Q mass spectrometer (Bruker-Daltonik, Bremen, Germany) equipped with an ESI-ion source. The procedures and technical settings used were consistent with those in our previous investigations5,6,12. Mass spectra analyses were performed with the software MAVEN (Princeton, USA)36, which enables interrogation of the KEGG37 database for metabolite identification. Results were plotted through GraphPad Prism 5.03 (GraphPad Software, La Jolla, CA, USA) as fold-changes in values against those in the day 0 controls.
Results and discussions
Addition of ascorbic acid and N-acetylcysteine influenced pH by modulating glycolysis
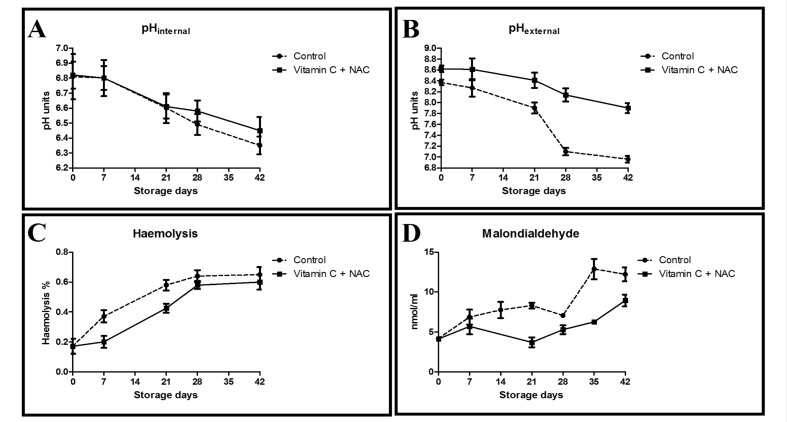
The pH of the SAGM additive solution prior to supplementation with vitamin C and NAC was consistent with values reported in the literature38, while supplementation with the anti-oxidants resulted in acidification of the solution (−0.2 pH units, on average). However, when packed erythrocytes were exposed to either of the solutions (supplemented or non-supplemented) no major deviations from the control values were observed within the first 3 hours (Figure 1A, B). Of note, despite the measured acidification of SAGM induced by the supplements, in the long-term, RBC exposure to supplemented SAGM resulted in higher pH levels than exposure to non-supplemented controls, in terms of both internal (Figure 1A) and external (Figure 1B) pH, the former showing higher than control levels starting from storage day 21 onwards.
Figure 1.
A time course overview of internal pH, external pH, haemolysis and malondialdehyde accumulation for control (dashed line) and vitamin C+NAC-supplemented (continuous line) CPD-SAGM erythrocyte concentrates stored at 4 °C for up to 42 days (n=10).
Supplementation with vitamin C and NAC had beneficial effects on haemolysis (Figure 1C), especially up to storage day 28.
Malondialdehyde levels increased progressively in both control and supplemented erythrocyte concentrates, although control units showed constantly higher levels than their supplemented counterparts (Figure 1D).
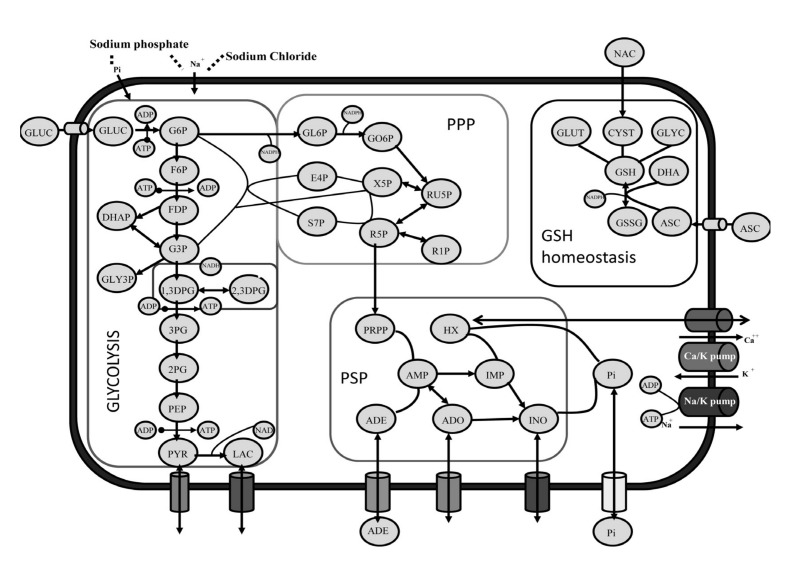
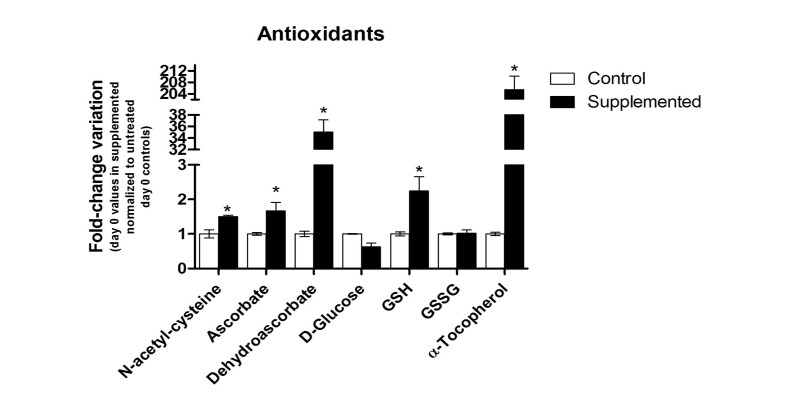
The underlying hypothesis of the present investigation was that supplementation with vitamin C and NAC could influence RBC metabolism (an overview of the main metabolic pathways in mature erythrocytes is provided in Figure 2). Three hours after supplementation with vitamin C and NAC (day 0), we observed intracellular accumulation of NAC (1.5±0.04 fold-change against controls), ascorbate (1.67±0.24), dehydroascorbate (35.00±2.14), GSH (2.24±0.41) and α-tocopherol (205.48±4.73), unaltered levels of oxidized glutathione (GSSG: 1.02±0.09), and apparently decreased levels of intracellular glucose (0.62±0.11), as illustrated in Figure 3. The decreased levels of glucose are consistent with documented competition between ascorbate and D-glucose for GLUT transporters for membrane transport (internalisation) in human RBC21. However, it is worth stressing that the data presented here only provide support for a negative correlation between glucose and ascorbate levels in supplemented units. Immediate benefits on the levels of GSH and α-tocopherol were expected as well, on the basis of the NAC-promoted biosynthesis of the former26, and the antioxidant and protective action of ascorbic acid on the latter23. Slower internalisation of glucose in supplemented erythrocyte concentrates (Figure 3) might also explain the shallower decrease of the extracellular pH curves (Figure 1A, B), and is consistent with the lesser lactate accumulation (Figure 4). In order to investigate this hypothesis, mass spectrometry-based metabolomics analyses were performed to assay metabolic fluxes for glucose consumption.
Figure 2.
An overview of red blood cell metabolic pathways, including the Emden Meyerhoff glycolytic pathway, the pentose phosphate pathway (PPP), the purine salvage pathway (PSP), and GSH homeostasis. We also highlight how supplementation with N-acetylcysteine (NAC) and ascorbate (ASC) contributes to glutathione (GSH) homeostasis.
Figure 3.
An overview of relative quantities of a subset of metabolites involved in redox metabolism poise at day 0 and 3 hours after supplementation with vitamin C and NAC. Results are plotted as fold-change variations (mean±SD) against untreated controls (n=10). *p-value <0.05 ANOVA.
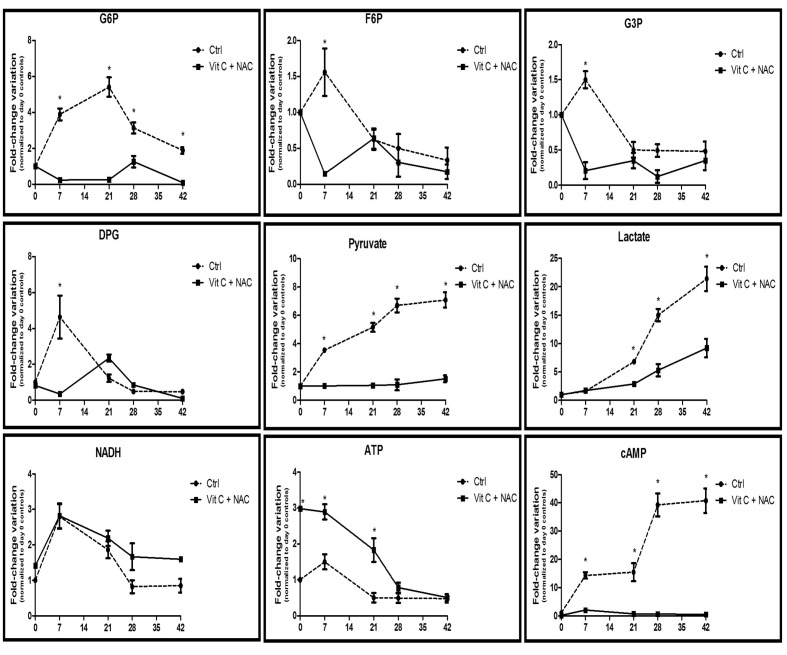
Figure 4.
Time course metabolomics analysis of glycolysis in RBC stored under control conditions (dashed line) or in CPD-SAGM supplemented with vitamin C and NAC (continuous line). G6P: glucose 6-phosphate; F6P: fructose 6-phosphate; G3P: glyceraldehyde 3-phosphate; DPG: diphosphoglycerate; cAMP: cyclic AMP. *p-value <0.05 ANOVA.
Metabolites were plotted as fold-change variations of time course measurements, normalised against day 0 controls. In this view, it is worth stressing that the day 0 levels of specific metabolites already differed by 3 hours after supplementation, as also illustrated in Figure 3 for a subset of redox poise-related metabolites. Results are presented graphically, dividing metabolites into different pathways, including: (i) glycolysis (Figure 4), (ii) the pentose phosphate pathway (PPP) (Figure 5), (iii) glutathione homeostasis (Figure 6); (iv) lipid peroxidation (Figure 7), and (v) purine metabolism (Figure 8).
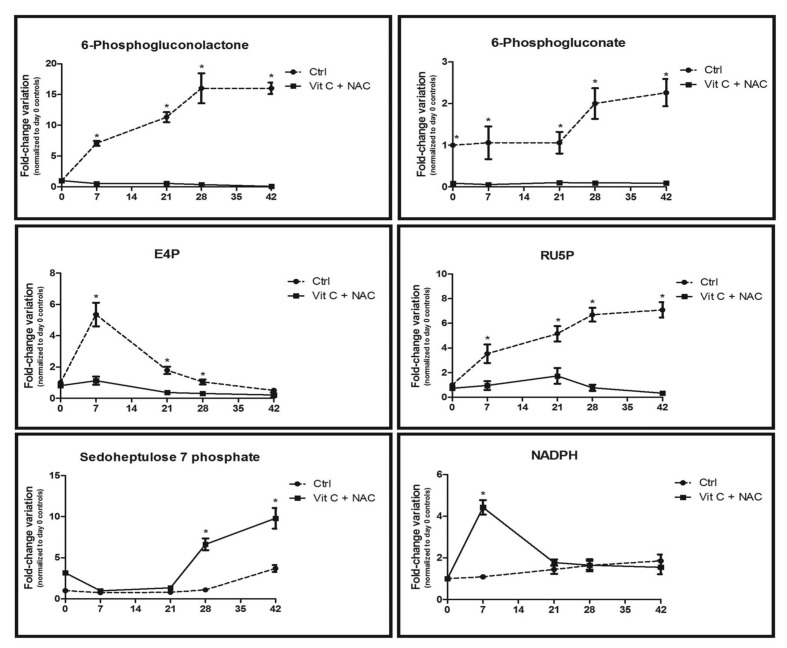
Figure 5.
Time course metabolomics analysis of the pentose phosphate pathway in RBC stored under control conditions (dashed line) or in CPD-SAGM supplemented with vitamin C and NAC (continuous line). Results are plotted on a weekly basis (storage days 0, 7, 21, 28 and 42), as fold-change variations (means±SD) normalised against day 0 controls (n = 10). E4P: erythrose 4-phosphate; RU5P: ribulose 5-phosphate. *p-value <0.05 ANOVA.
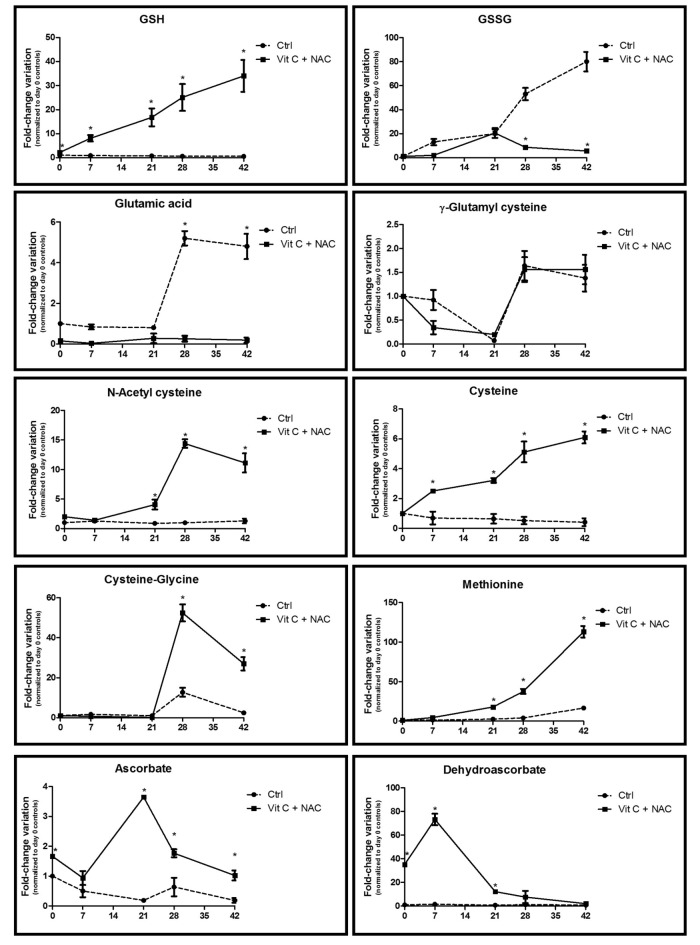
Figure 6.
Time course metabolomics analysis of glutathione homeostasis-related metabolites in RBC stored under control conditions (dashed line) or in CPD-SAGM supplemented with vitamin C and NAC (continuous line). GSH: reduced glutathione; GSSG: oxidized glutathione. *p-value <0.05 ANOVA.
Figure 7.
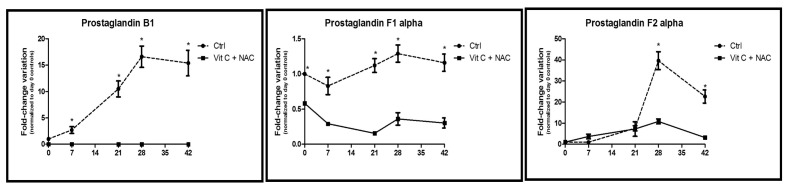
Time course metabolomics analysis of prostaglandins in RBC stored under control conditions (dashed line) or in CPD-SAGM supplemented with vitamin C and NAC (continuous line). *p-value <0.05 ANOVA.
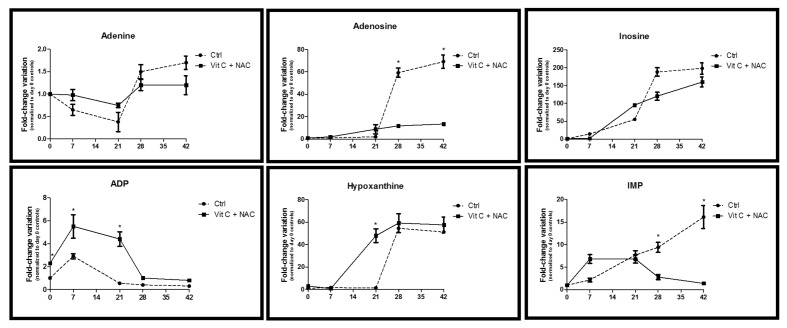
Figure 8.
Time course metabolomics analysis of purine metabolism in RBC stored under control conditions (dashed line) or in CPD-SAGM supplemented with vitamin C and NAC (continuous line). ADP: adenosine diphosphate; IMP: inosine monophosphate. *p-value <0.05 ANOVA.
Figure 4 shows how glycolytic intermediates, including glucose 6-phosphate (G6P), fructose 6-phosphate (F6P), glyceraldehyde 3-phosphate (G3P), pyruvate, and byproducts of lactic fermentation (lactate) consistently decreased upon supplementation with vitamin C and NAC.
On the other hand, DPG levels followed a peculiar trend, with a rapid decrease in supplemented units within the first week of storage, while day 21 levels in supplemented units were higher than in controls (Figure 4), suggesting a long-term positive effect of NAC-vitamin C supplementation on RBC, in agreement with previous studies on ascorbate18,19.
Higher levels of NADH in vitamin C+NAC-supplemented erythrocyte concentrates might be explained in the light of two considerations: (i) a reduced rate of glycolysis and slower lactate production are accompanied by slower oxidation of NADH back to NAD+; (ii) NADH is also an essential cofactor for cytochrome b5 reductase - methaemoglobin reductase, which is responsible for the reduction of oxidized iron in methaemoglobin back to the ferrous state. Higher NADH levels might, therefore, also represent indirect proof of a lower necessity of RBC to cope with haemoglobin oxidation in vitamin C+NAC-supplemented units.
In the control arm of the study, the increases in ATP and DPG at 7 days, followed by the rapid consumption of both the high energy phosphate compounds, are consistent with the findings of our previous mass spectrometry-based investigations5,12, and only partly with those of analogous investigations relying on spectrophotometric approaches39. Higher levels of ATP in supplemented units are consistent with the positive effect on ATP preservation observed throughout the whole blood storage period in the presence of ascorbic acid18,19.
A tentative explanation of this phenomenon involves the relative concentrations of cyclic AMP (cAMP - Figure 4), which constantly increase in control RBC over the duration of storage, while they remain constant and slowly decrease in units supplemented with vitamin C+NAC. A progressive decrease of high energy phosphate compounds (including ATP) might reflect cAMP-mediated ATP release by RBC in response to deoxygenation, a phenomenon that occurs in vivo to promote vasodilation in hypoxic districts40. In the present study, higher ATP and DPG levels despite slower glycolytic rates might, therefore, be explained by the lower cAMP levels in supplemented units (Figure 4), although further studies are mandatory.
Glycolytic fluxes were not redirected towards the pentose phosphate pathway
We wondered whether the observed lower levels of lactate were to be attributed to a slower rate of glucose consumption via the Embden-Meyerhof pathway or whether they hid a metabolic branching toward the PPP. This pathway is devoted to protecting RBC from oxidative stress12,13.
Figure 5 shows the results for PPP intermediate metabolites, including 6-phosphogluconolactone, 6-phosphogluconate, erythrose 4-phosphate (E4P), ribulose 5-phosphate (RU5P), sedoheptulose 7-phosphate and the reduced coenzyme NADPH, as a by-product of oxidative phase reactions. For all the tested metabolites we observed lower relative levels of each compound in the supplemented units than in the non-supplemented controls, except for sedoheptulose 7-phosphate and NADPH. For NADPH, in particular, we observed a 4-fold increase after 7 days of storage in supplemented units, while later on NADPH levels in the supplemented units were similar to those in their untreated counterparts. Since NADPH is an essential coenzyme in anti-oxidant reactions, including the reduction of GSSG to GSH, this finding could have been due to a decreased consumption of NADPH, promoted by milder oxidative stress conditions in supplemented units (also confirmed by the lesser accumulation of malondialdehyde - Figure 1D), rather than to an actual increased production of this metabolite.
Increased relative levels of sedoheptulose 7-phosphate in the units supplemented with vitamin C+NAC might be a consequence of a regular carbon flux from the oxidative to the non-oxidative phase of the PPP, which results in metabolic fluxes re-entering glycolysis. Conversely, in control RBC, prolonged storage results in a progressive flux towards the purine salvage pathway, as previously reported5.
Additives promoted anti-oxidant responses related to the glutathione system and homeostasis
Vitamin C and NAC were carefully selected for their expected potential benefits on the anti-oxidant defence systems, above all glutathione homeostasis18–23.
Figure 6 shows the results for the main metabolites involved in the maintenance of glutathione homeostasis and biosynthesis41, including GSH, GSSG, glutamic acid, γ-glutamyl cysteine, acetyl-cysteine, cysteine, cysteine-glycine, methionine, ascorbate and dehydroascorbate.
Day 0 levels of GSH, acetyl-cysteine, ascorbate and dehydroascorbate were different between controls and supplemented units (Figures 3 and 6). It should be considered that the results in Figure 6 are plotted as fold-change variations against day 0 control values, which further highlights the significance of the observed trends towards increases (GSH, acetyl-cysteine, ascorbate) and decreases (dehydroascorbate - the oxidized form of ascorbic acid) of the metabolites in supplemented units. It is interesting to note that despite NAC being added at concentrations of 0.5 mM already on day 0, its uptake is not inhibited in erythrocytes until it reaches concentrations as high as 10 mM,42 which justifies its time-dependent progressive accumulation in supplemented units. Furthermore, cysteine (a rate-limiting precursor of GSH biosynthesis) and thiol metabolism was up-regulated (cysteine, cysteine-glycine, methionine) in supplemented units (Figure 6), in agreement with the higher supplementation of NAC (a cysteine precursor) in these units. This is relevant in the light of the emerging concept of a significant correlation between cysteine efflux and erythrocyte ageing in vivo43. This effect might represent a clear advantage over supplementation with vitamin C alone18–20, which does directly help to cope with oxidative stress via ascorbate oxidation, but does not promote replenishment of the GSH reservoirs via up-regulation of cysteine levels.
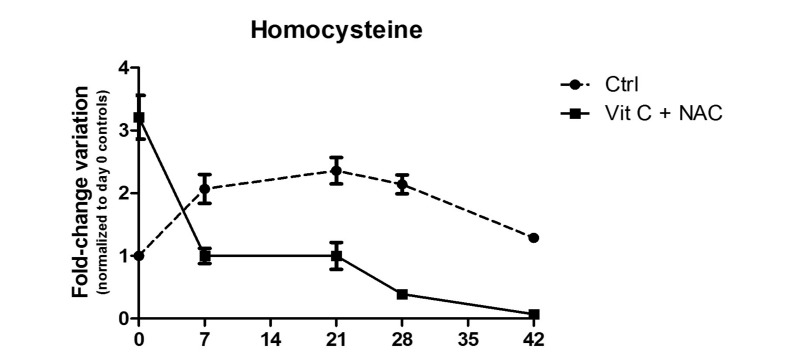
On the other hand, NAC supplementation promoted the accumulation of the potentially toxic compound homocysteine immediately after supplementation (Figure 9). Indeed, homocysteine is a precursor of cysteine as it represents the thiol group donor in cysteine biosynthesis from serine. However, while storage resulted in the accumulation of homocysteine in control units, the levels of this amino acid decreased in a storage-dependent fashion in supplemented units. In the light of this observation, we can conclude that further increasing NAC and ascorbic acid concentrations would have promoted an overdose of GSH, which could result in feedback inhibition of upstream biosynthetic pathways and accumulation of their potentially toxic intermediates (such as homocysteine).
Figure 9.
Homocysteine levels in control (dashed line) or vitamin C and NAC-supplemented (continuous line) RBC units.
Recently, it has been shown that anaerobic storage of RBC results in deoxyhaemoglobin-dependent blockade of the metabolic shift towards the PPP12,13, which impairs the capacity of RBC to cope with oxidative stress by negatively affecting glutathione homeostasis12. In the present study, we observed that glutathione homeostasis is boosted by vitamin C and NAC supplementation (Figure 6).
Furthermore, the preservation of thiol groups by improved glutathione homeostasis should also affect the activity of several key metabolic enzymes that rely upon thiol groups in functional active sites, such as glyceraldehyde 3-phosphate dehydrogenase (glycolysis)44 and peroxiredoxin 2 (anti-oxidant defences)45, the latter becoming oxidized and progressively migrating to the membrane over the duration of storage under blood bank conditions6,46.
The beneficial effects of vitamin C+NAC supplementation are also evident when focusing on lipid oxidation. In the light of the decrease in malondialdehyde in supplemented units (Figure 1D), we further focused on prostaglandin metabolism (prostaglandin B1, F1α and F2α [8-isoprostane]) (Figure 7). 8-isoprostane, in particular, is a widely accepted marker of lipid peroxidation46 and has been shown to accumulate (especially in the supernatant) during storage of RBC1–5. Consistently, supplemented units displayed lower levels of prostaglandins throughout the whole storage period.
Supplementation with vitamin C and N-acetylcysteine promoted the purine salvage pathway
RBC cannot synthesise 5-phosphoribosylamine de novo and thus rely upon salvage reactions to replenish purine reservoirs which serve as substrates for high energy phosphate purine compounds (such as ATP and adenine nucleotides, accounting for 70–80% of cellular nucleotides)46. Erythrocyte membranes allow adenine and adenosine transport via facilitated diffusion, which enables the entry of adenine in additive solutions (such as in SAGM)47.
Storage of RBC supplemented with vitamin C and NAC resulted in progressive accumulation of both adenine and adenosine (although at a lower rate than in untreated controls, Figure 8).
Inosine, a major substrate for salvage reactions, increased (albeit not significantly) during storage (Figure 7), in analogy to standard storage conditions6. Inosine accumulation might stem from deamination of adenosine, a documented process within the framework of RBC storage1. Inosine may further undergo phosphorolysis to form hypoxanthine (increasing both in controls and supplemented units, Figure 8) and ribose 1-phosphate (R1P), a reaction that enables the introduction of a phosphorylated sugar (through non-oxidative phase PPP intermediates) into the RBC without ATP consumption. On the other hand, in a recently investigated mouse model, Zimring and colleagues have linked the storage-dependent inosine conversion to hypoxanthine (and xanthine) to an impaired capacity to synthesise ATP via salvage reactions30.
ADP levels were higher in supplemented units than in untreated controls (Figure 8), almost paralleling relative quantitative trends observed for ATP.
While it has been reported that inosine monophosphate accumulates in erythrocyte concentrates over the standard storage5, vitamin C and NAC supplementation resulted in higher than control levels up to day 21 of storage, which then decreased back to control levels by days 28 and 42 of storage (Figure 8).
Conclusion
Storage lesions to RBC are to some extent influenced by the additive solutions in which packed erythrocytes are preserved in the blood bank48. Recent strides in the field of mass-spectrometry-based metabolomics have prompted a thorough update and integration of decades of accurate biochemical observations with spectrophotometry and nuclear magnetic resonance studies. In particular, a role for mass spectrometry-based metabolomics is rapidly emerging in the field of RBC research.
The present, albeit preliminary, study, provides a few hints about the potential beneficial effects of supplementing RBC storage solutions with anti-oxidants. It should be noted that the results were obtained from a limited cohort of donors of the same gender (10 male donors). Nevertheless, we found that supplementation with vitamin C and NAC enabled replenishment of the RBC anti-oxidant battery and generation of new GSH. This resulted in an improvement of the quality of stored RBC, in terms of reduced oxidative stress and haemolysis. On the other hand, energy metabolism was depressed in terms of glycolytic fluxes, although ATP preservation was promoted by reduced cAMP-mediated ATP release.
Although metabolomics provides an exhaustive description of the biochemical scenario arising from vitamin C and NAC supplementation, further information will be soon collected through the application of other “-omics” disciplines, above all proteomics, to this topic.
Footnotes
Authorship contributions
Valeria Pallotta and Federica Gevi have contributed equally to this manuscript and share first authorship.
The Authors declare no conflicts of interest.
Funding
Valeria Pallotta, Federica Gevi, Angelo D’Alessandro and Lello Zolla are supported by funds from the Italian National Blood Centre (Centro Nazionale Sangue, CNS, Istituto Superiore Sanità, Rome, Italy). Angelo D’Alessandro was supported by a mobility studentship and post-doctoral funds from the Interuniversity Consortium for Biotechnologies (CIB). The authors are grateful to Dr. Francesca Caravello for her technical assistance during the experiments, within the framework of her M.Sc. internship training.
References
- 1.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–9. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 2.D’Alessandro A, Liumbruno G, Grazzini G, Zolla L. Red blood cell storage: the story so far. Blood Transfus. 2010;8:82–8. doi: 10.2450/2009.0122-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lion N, Crettaz D, Rubin O, Tissot JD. Stored red blood cells: a changing universe waiting for its map(s) J Proteomics. 2010;73:374–85. doi: 10.1016/j.jprot.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Karon BS, Van Buskirk CM, Jaben EA, et al. Temporal sequence of major biochemical events during blood bank storage of packed red blood cells. Blood Transfus. 2012;28:1–9. doi: 10.2450/2012.0099-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:10–27. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 6.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored erythrocyte concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonelou MH, Tzounakas VL, Velentzas AD, et al. Effects of pre-storage leukoreduction on stored red blood cells signaling: a time-course evaluation from shape to proteome. J Proteomics. 2012;76:220–38. doi: 10.1016/j.jprot.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 9.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 10.Lang F, Qadri SM. Mechanisms and significance of eryptosis, the suicidal death of erythrocytes. Blood Purif. 2012;33:125–30. doi: 10.1159/000334163. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T, AuBuchon JP, Tryzelaar L, et al. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007;92:22–31. doi: 10.1111/j.1423-0410.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 12.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosystems. 2013;9:1196–209. doi: 10.1039/c3mb25575a. [DOI] [PubMed] [Google Scholar]
- 13.Rogers SC, Said A, Corcuera D, et al. Hypoxia limits antioxidant capacity in red blood cells by altering glycolytic pathway dominance. FASEB J. 2009;23:3159–70. doi: 10.1096/fj.09-130666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger P, Korsten H, De Korte D, et al. An improved red blood cell additive solution maintains 2,3-diphosphoglycerate and adenosine triphosphate levels by an enhancing effect on phosphofructokinase activity during cold storage. Transfusion. 2010;50:2386–92. doi: 10.1111/j.1537-2995.2010.02700.x. [DOI] [PubMed] [Google Scholar]
- 15.Hess JR, Hill HR, Oliver CK, et al. Alkaline CPD and the preservation of RBC 2,3-DPG. Transfusion. 2002;42:747–52. doi: 10.1046/j.1537-2995.2002.00115.x. [DOI] [PubMed] [Google Scholar]
- 16.Veale MF, Healey G, Sparrow RL. Effect of additive solutions on red blood cell (RBC) membrane properties of stored RBCs prepared from whole blood held for 24 hours at room temperature. Transfusion. 2011;51:25S–33S. doi: 10.1111/j.1537-2995.2010.02960.x. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, AuBuchon JP, Dumont LJ, et al. The effects of additive solution pH and metabolic rejuvenation on anaerobic storage of red cells. Transfusion. 2008;48:2096–105. doi: 10.1111/j.1537-2995.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- 18.Dawson RB, Hershey RT, Myers CS, Miller RM. Blood preservation 35. Red cell 2,3-DPG and ATP maintained by DHA-ascorbate-phosphate. Transfusion. 1981;21:219–23. doi: 10.1046/j.1537-2995.1981.21281178161.x. [DOI] [PubMed] [Google Scholar]
- 19.Dawson RB, Hershey RT, Myers CS, Eaton JW. Blood preservation XLIV. 2,3-DPG maintenance by dehydroascorbate better than D-ascorbic acid. Transfusion. 1980;20:321–3. doi: 10.1046/j.1537-2995.1980.20380214899.x. [DOI] [PubMed] [Google Scholar]
- 20.Stowell SR, Smith NH, Zimring JC, et al. Addition of ascorbic acid solution to stored murine red blood cells increases posttransfusion recovery and decreases microparticles and alloimmunization. Transfusion. 2013;53:2248–57. doi: 10.1111/trf.12106. [DOI] [PubMed] [Google Scholar]
- 21.May JM. Ascorbate function and metabolism in the human erythrocyte. Front Biosci. 1998;3:d1–10. doi: 10.2741/a262. [DOI] [PubMed] [Google Scholar]
- 22.Rizvi SI, Pandey KB, Jha R, Maurya PK. Ascorbate recycling by erythrocytes during aging in humans. Rejuvenation Res. 2009;12:3–6. doi: 10.1089/rej.2008.0787. [DOI] [PubMed] [Google Scholar]
- 23.May JM, Qu ZC, Mendiratta S. Protection and recycling of alpha-tocopherol in human erythrocytes by intracellular ascorbic acid. Arch Biochem Biophys. 1998;349:281–9. doi: 10.1006/abbi.1997.0473. [DOI] [PubMed] [Google Scholar]
- 24.Mazor D, Golan E, Philip V, et al. Red blood cell permeability to thiol compounds following oxidative stress. Eur J Haematol. 1996;57:241–6. doi: 10.1111/j.1600-0609.1996.tb01370.x. [DOI] [PubMed] [Google Scholar]
- 25.Dumaswala UJ, Wilson MJ, Wu YL, et al. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic Res. 2000;33:517–29. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- 26.Nur E, Brandjes DP, Teerlink T, et al. CURAMA study group. N-acetylcysteine reduces oxidative stress in sickle cell patients. Ann Hematol. 2012;91:1097–105. doi: 10.1007/s00277-011-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy JR. Erythrocyte metabolism. II. Glucose metabolism and pathways. J Lab Clin Med. 1960;55:286–302. [PubMed] [Google Scholar]
- 28.Jamshidi N, Palsson BØ. Systems biology of the human red blood cell. Blood Cells Mol Dis. 2006;36:239–47. doi: 10.1016/j.bcmd.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Messana I, Misiti F, el-Sherbini S, et al. Quantitative determination of the main glucose metabolic fluxes in human erythrocytes by 13C- and 1H-MR spectroscopy. J Biochem Biophys Methods. 1999;39:63–84. doi: 10.1016/s0165-022x(99)00005-6. [DOI] [PubMed] [Google Scholar]
- 30.Zimring JC, Smith N, Stowell SR, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2014;54:137–48. doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino T, Yachie-Kinoshita A, Hirayama A, et al. Dynamic simulation and metabolome analysis of long-term erythrocyte storage in adenine-guanosine solution. Plos One. 2013;8:e71060. doi: 10.1371/journal.pone.0071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darghouth D, Koehl B, Heilier JF, et al. Alterations of red blood cell metabolome in overhydrated hereditary stomatocytosis. Haematologica. 2011;96:1861–5. doi: 10.3324/haematol.2011.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanias T, Lanteri M, Derek S, et al. Red blood cell storage in pediatric transfer bags is correlated with increased levels of haemolysis and altered osmotic fragility. 55th ASH Annual Meeting and Exposition; New Orleans, LA. December 7–10, 2013; Abstract 2403. [Google Scholar]
- 34.Ibrahim IH, Sallam SM, Omar H, Rzik M. Oxidative haemolysis of erythrocytes induced by various vitamins. Int J Biomed Sci. 2006;2:295–8. [PMC free article] [PubMed] [Google Scholar]
- 35.D’Alessandro A, Gevi F, Zolla L. A robust high resolution reversed-phase HPLC strategy to investigate various metabolic species in different biological models. Mol Biosyst. 2011;7:1024–32. doi: 10.1039/c0mb00274g. [DOI] [PubMed] [Google Scholar]
- 36.Melamud E, Vastag L, Rabinowitz JD. Metabolomic analysis and visualization engine for LC-MS data. Anal Chem. 2010;82:9818–26. doi: 10.1021/ac1021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burger P, Korsten H, De Korte D, et al. An improved red blood cell additive solution maintains 2,3-diphosphoglycerate and adenosine triphosphate levels by an enhancing effect on phosphofructokinase activity during cold storage. Transfusion. 2010;50:2386–92. doi: 10.1111/j.1537-2995.2010.02700.x. [DOI] [PubMed] [Google Scholar]
- 39.Sparrow RL. Time to revisit red blood cell additive solutions and storage conditions: a role for “omics” analyses. Blood Transfus. 2012;10(Suppl 2):s7–11. doi: 10.2450/2012.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–64. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 41.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raftos JE, Whillier S, Chapman BE, Kuchel PW. Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Int J Biochem Cell Biol. 2007;39:1698–706. doi: 10.1016/j.biocel.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Kumar P, Maurya PK. L-cysteine efflux in erythrocytes as a function of human age: correlation with reduced glutathione and total antioxidant potential. Rejuvenation Res. 2013;16:179–84. doi: 10.1089/rej.2012.1394. [DOI] [PubMed] [Google Scholar]
- 44.Li YK, Boggaram J, Byers LD. Alkylation of glyceraldehyde-3-phosphate dehydrogenase with haloacetylphosphonates. An unusual pH-dependence. Biochem J. 1991;275:767–73. doi: 10.1042/bj2750767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsikas D, Suchy MT, Niemann J, et al. Glutathione promotes prostaglandin H synthase (cyclooxygenase)-dependent formation of malondialdehyde and 15(S)-8-iso-prostaglandin F(2α) FEBS Lett. 2012;586:3723–30. doi: 10.1016/j.febslet.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Schuster S, Kenanov D. Adenine and adenosine salvage pathways in erythrocytes and the role of S-adenosylhomocysteine hydrolase. A theoretical study using elementary flux modes. FEBS J. 2005;272:5278–90. doi: 10.1111/j.1742-4658.2005.04924.x. [DOI] [PubMed] [Google Scholar]
- 48.Sparrow RL, Sran A, Healey G, et al. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: a paired study. Transfusion. 2013 doi: 10.1111/trf.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]