Introduction
Immune-mediated anaemia is characterized by the production of autoantibodies against different erythrocyte antigens resulting in red blood cells being destroyed, mainly in the spleen. First-line therapy consists of immune suppression by steroids, while the second choice of treatment, in resistant or relapsed patients, is splenectomy.
The World Health Organisation (WHO) World Malaria Report 2012 summarises data received from 104 malaria endemic countries and territories and updates the 2011 report. According to these latest estimates, there were about 219 million cases of malaria in 2010 and an estimated 660,000 deaths. The disease is still highly endemic in the tropics but has been completely eradicated in some temperate areas, including southern Europe, and partially eradicated in northern Africa1–3.
In some malaria cases a concomitant, positive direct antiglobulin test (DAT) can increase disease severity and interfere with a correct diagnosis4. Plasmodium vivax is unique among human malarias in that erythrocyte invasion is almost entirely dependent on the red cell surface receptor, known as the Duffy blood group antigen (Fy)5.
When steroids fail, the treatment of choice for immune-mediated chronic anaemia is splenectomy, although this operation can carry the risk of reactivating the infection in the case of underlying silent forms of malaria6,7.
Case report
The 29-year old female patient had arrived from Morocco in 2008 and was seen at our outpatient clinic with a long history immunological anaemia (DAT positive), dating from 2004 when she was still living in her native country. Her medical record was consistent with chronic anaemia as she had been treated previously with transfusions and steroids; she had no history of past fever crises were absent, but no further documentation was available. She returned to Morocco only once, in 2010, to visit relatives. In 2008, when she was seen for the first time at our outpatient clinic, blood tests confirmed severe anaemia and DAT positivity, specifically anti-Cd3. The peripheral blood smear was consistent with haemolytic anaemia with the presence of several spherocytes and occasional schistocytes, but no parasites were seen. An abdominal ultrasound scan showed no abnormalities and in particular the spleen volume was normal. Standard schedule prednisone steroid treatment8 was started again, administered using multiple cycles and continued for nearly 3 years with unsatisfactory results until, in February 2012, the decision was made to carry out a laparoscopic splenectomy. Post-operative recovery was good and the patient was discharged after 10 days, although her anaemia had still not improved.
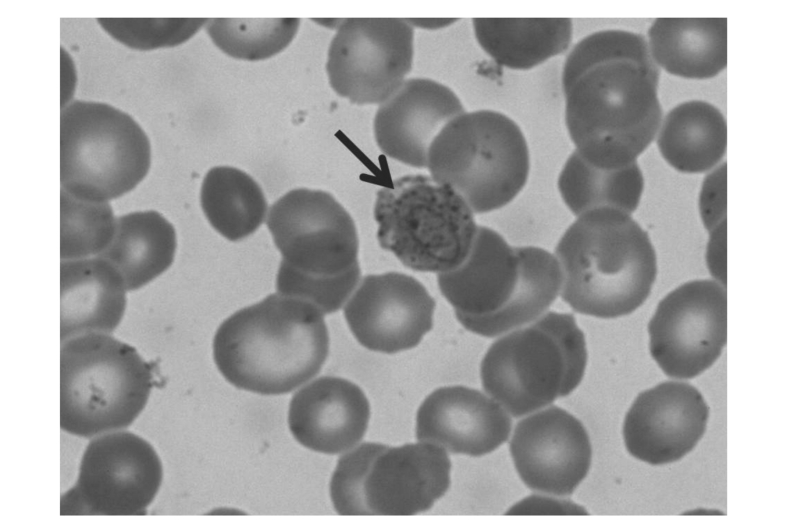
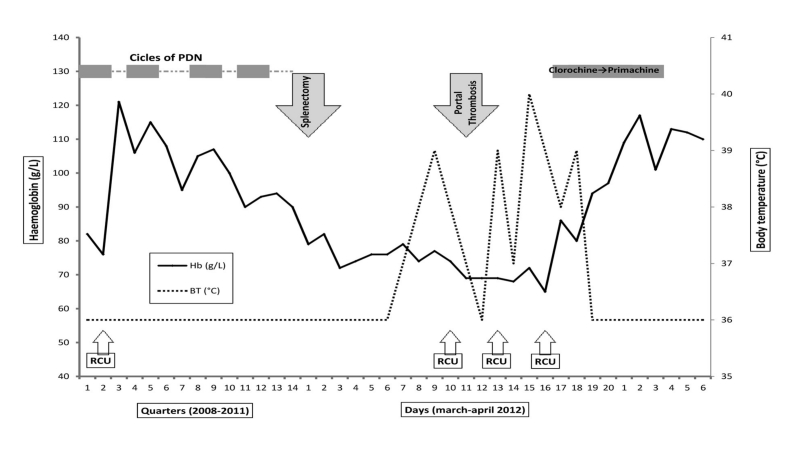
Two weeks later she was readmitted with abdominal pain and fever; an ultrasound scan showed portal thrombosis. The overall clinical picture had further deteriorated due to fever >39 °C and severe anaemia requiring transfusion. Antibiotic treatment was started and multiple blood cultures were performed, all of which were negative. The fever continued and her general clinical condition deteriorated requiring additional transfusions. As no aetiological agents were isolated from the cultures, blood smears were examined to look for the possibility of a protozoan infection; these revealed the presence of erythrocytes infected by Plasmodium vivax (Figure 1). Chloroquine treatment followed by primaquine was started immediately. The anaemia quickly improved and the fever disappeared resulting in a resolution of the general clinical picture. The entire 4-year clinical and laboratory findings are reported in Figure 2.
Figure 1.
Patient's post-splenectomy peripheral blood smear showing typical P. vivax trophozoites with large chromatin dots.
Figure 2.
The 4-year clinical and biochemical course of the patient’s haemolytic anaemia.
Four cycles of prednisone, at a dose of 1 mg/kg/day, were administered. Portal thrombosis occurred 2 weeks after splenectomy. Chloroquine was administered at a dose of 30 mg/kg bw/day for 4 days followed by primaquine 15 mg/day for 14 days. Raised levels of lactate dehydrogenase, reticulocytes, haptoglobin and bilirubin were consistent with haemolytic crises, but were within normal limits during remission (data not reported in the figure). BT= body temperature. RBCU= red blood cell unit.
Discussion
Moroccans are the most common immigrants in Italy, there now being nearly 400,000. In 1965 a National Moroccan Malaria Control Programme was launched and had a rapid effect on Plasmodium falciparum transmission, the last autochthonous case being notified in 1973. The country is no longer high risk for malaria although there are some areas in which it is still possible to contract the disease. The WHO has considered Morocco free of Plasmodium falciparum since the 1970s and prophylaxis is not recommended anymore for travellers. Thirty years later it became possible to prevent Plasmodium vivax transmission and in 2008 Morocco underwent certification to obtain malaria-free status according to WHO criteria3.
Geographically, Plasmodium vivax is the most widespread species of Plasmodium causing human disease. Duffy is an important minor blood group antigen that has two immunologically distinct alleles, Fy(a) or Fy(b), resulting from a single-point mutation occurring within the binding domain of the Plasmodium vivax red cell invasion ligand, as in the case of this patient. The parasite’s main biological characteristic is the presence of liver and spleen hypnozoites responsible for frequent relapses which add considerably to the already substantial number of cases of the disease. Strict surveillance must be maintained to prevent Plasmodium. vivax being spread, mainly from central Africa, to other favourable environmental areas such as Morocco.
Complement activation, confirmed by anti-Cd3 positivity, may contribute to anaemia in malaria, possibly by inducing erythrophagocytosis and haemolysis9. Splenectomy can induce fever, increase parasitaemia (with circulating mature parasites) and reactivate latent infections. In conclusion, even when DAT positivity suggests the diagnosis of autoimmune anaemia, the possibility of underlying malaria should be considered, even in patients coming from areas thought to be free of risk.
Acknowledgements
This work was in part supported by Associazione Italiana Leucemie Treviso (AIL Treviso), Laura Candiotto is a fellow of AIL Treviso.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Tatem AJ, Smith DL, Gething PW, et al. Ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010;376:1579–91. doi: 10.1016/S0140-6736(10)61301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabatinelli G, Majori G. Malaria surveillance in Italy: 1986–1996 analysis and 1997 provisional data. Euro Surveill. 1998;3:38–40. doi: 10.2807/esm.03.04.00106-en. [DOI] [PubMed] [Google Scholar]
- 3.Adlaoui E, Faraj C, El Bouhmi M, et al. Mapping malaria transmission risk in northern morocco using entomological and environmental data. Malar Res Treat. 2011;2011:391–463. doi: 10.4061/2011/391463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwood BM, Stratton D, Williamson WA, Mohammed I. A study of the role of immunological factors in the pathogenesis of the anaemia of acute malaria. Trans R Soc Trop Med Hyg. 1978;72:378–85. doi: 10.1016/0035-9203(78)90131-1. [DOI] [PubMed] [Google Scholar]
- 5.King CL, Adams JH, Xianli J, et al. Fy(a)/Fy(b) antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria. Proc Natl Acad Sci USA. 2011;108:20113–8. doi: 10.1073/pnas.1109621108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidegain F, Berry A, Alvarez M, et al. Acute Plasmodium falciparum malaria following splenectomy for suspected lymphoma in 2 patients. Clin Infect Dis. 2005;40:e97–100. doi: 10.1086/430061. [DOI] [PubMed] [Google Scholar]
- 7.Vasin VA, Martynov VA, Vasin IV, Orlov VA. Outbreak of latent malaria following splenectomy for trauma. Klin Med. 1986;64:136–7. [PubMed] [Google Scholar]
- 8.Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831–8. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- 9.Helegbe GK, Goka BQ, Kurtzhals JA, et al. Complement activation in Ghanaian children with severe Plasmodium falciparum malaria. Malar J. 2007;6:165. doi: 10.1186/1475-2875-6-165. [DOI] [PMC free article] [PubMed] [Google Scholar]