Introduction
Drug-induced immune haemolytic anaemia (DIIHA) is a rare but serious complication of drug treatment. To date, more than 130 drugs have been reported to cause haemolytic anaemia with the three drugs currently most commonly responsible for causing DIIHA being piperacillin, cefotetan, and ceftriaxone1. Although ceftazidime is a commonly used antibiotic, it is a rare cause of DIIHA, and only three cases of DIIHA due to ceftazidime have previously been reported2–4. Here we report on a patient who developed severe DIIHA due to ceftazidime. Since this drug is widely used in clinical practice, this report may help to increase the index of suspicion for the diagnosis of DIIHA. Early recognition and proper treatment of the condition are essential and likely to improve the outcome of patients with DIIHA who present with what would be otherwise rapidly fatal haemolysis.
Case report
A 77-year old man was transferred to our hospital for the treatment of a sudden episode of macroscopic haemoglobinuria. Four days before admission, the patient complained of coughing with sputum and was diagnosed with bronchitis after a chest X-ray at the community hospital. The patient was treated with ceftazidime (4 g/day i.v.) for 3 days, and on the fourth day after ceftazidime treatment, he had a sudden episode of haematuria with jaundice. The patient did not receive any other medications besides ceftazidime. On admission, vital signs were steady and scleral icterus and generalised jaundice were noted on physical examination. Laboratory studies showed that the haemoglobin (Hb) level was 125.0 g/L (normal range, 120–160 g/L), the lactate dehydrogenase (LDH) concentration was 1,040 U/L (normal range, 110–300 U/L), the reticulocyte count was 0.9% (normal range, 0.5–1.5%), and the serum total bilirubin concentration was 127.9 μmol/L (normal range, 0–25.0 μmol/L), with the indirect bilirubin concentration being 123.1 μmol/L (normal range, 1.5–18 μmol/L).
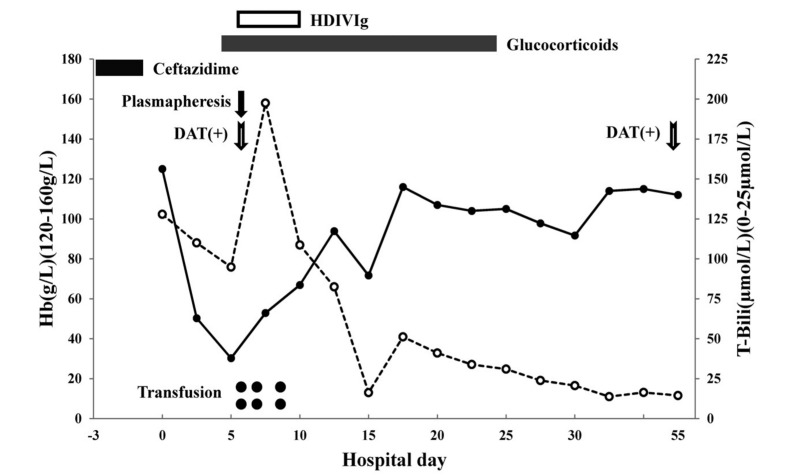
Only 3 days later, the Hb had dropped to 50.3 g/L, and 10 days after admission the reticulocyte count increased to 10.3%. A peripheral blood smear showed no schistocytes and tests for paroxysmal nocturnal hemoglobinuria were negative. The glucose-6-phosphate dehydrogenase (G6PD) fluorescent spot test, osmotic fragility of red blood cells, and haemoglobin electrophoresis analysis were normal. The hospital course is summarised in Figure 1.
Figure 1.
Hospital course: trends of Hb (solid line) and total bilirubin (dashed line).
The day the patient was admitted to our hospital was considered as hospitalisation day 0.
HDIVIg: High-dose intravenous immunoglobulin; DAT: direct antiglobulin test.
The patient’s blood type was B Rh+ and samples were positive for irregular antibodies. Direct antiglobulin testing was performed using the standard microtube column agglutination technique (DG Gel DC Scan, Diagnostic Grifols, Barcelona, Spain) according to the manufacturer’s instructions. A positive test (4+) was observed for IgG and C3d. Direct antiglobulin testing was repeated on day 55 after cess ation of ceftazidime, and the results were positive for IgG (2+) but negative for C3d. Drug -dependent antibody testing was performed, using previously described methods2, on serum from day 9 of treatment with ceftazidime. In brief, equal volumes of ceftazidime solution (1 mg/ mL in buffered saline) and patient’s serum were added to donors’ pooled group O red blood cells. Reactions without the drug or without patient’s serum were used as negative controls. All were read for agglutination after incubation for 1 hour at 37 °C and with the addition of antihuman globulin. The patient’s serum was non-reactive when tested in negative controls, but displayed direct agglutination of red blood cells when tested in the presence of ceftazidime.
The patient was treated with dexamethasone (10 mg/day), together with supportive care, including oxygen and nutritional support. Five days after admission, his Hb dropped to 30.3 g/L, indicating that the patient had a life-threatening complication with persistent haemolysis. Transfusion was delayed because of positive results of cross-matching tests with the patient’s blood samples. Plasm apheresis was used to eliminate possible antibodies against red blood cells. When 1,000 mL of plasma had been exchanged, the patient briefly lost consciousness, and plasmapheresis was stopped. Two units of red blood cells were ordered, and before transfusion, high-dose immunoglobin (0.4 g/kg/day) and methylprednisolone (0.5 g/day) were administered. No transfusion reactions were observed, and after several transfusions (6 units of red blood cells in total), the patient’s Hb increased gradually and he regained consciousness. Transfusion therapy was not continued and glucocorticoid was given with a tapered dose. Before discharge, the patient’s Hb level was 112.0 g/L and the total bilirubin concentration had returned to a normal level.
Discussion
DIIHA is an uncommon condition characterised by a sudden drop in haemoglobin following exposure to causative drugs. The incidence of DIIHA has been estimated to be about 1 case per million of the population, but it is likely underdiagnosed5. There are two main types of antibodies associated with DIIHA: drug-independent antibodies and drug-dependent antibodies6. Drug-independent antibodies are true red blood cell autoantibodies, not antibodies to a drug, and the laboratory and clinical findings can be indistinguishable from those associated with idiopathic autoimmune haemolytic anaemia. Drug-dependent antibodies only react when the drug is present. However, some drugs may show the characteristics of more than one mechanism of action.
The number of drugs causing DIIHA increased from 30 in 1980 to 130 in 20111. A 20-year retrospective review showed that the drugs most commonly causing DIIHA are cephalosporins, followed by penicillin and/or penicillin derivatives, non-steroidal anti-inflammatory drugs and quinine and/or quinidine7. Ceftazidime is a commonly used drug but has not yet been widely reported to be responsible for DIIHA. However, the Food and Drug Administration received 27 reports of haemolytic anaemia possibly related to ceftazidime between 1998 and 20118 and DIIHA due to ceftazidime is probably under-recognised.
The clinical manifestations of DIIHA are quite variable. Affected patients may have symptoms and signs such as mild, moderate or severe anaemia with pallor, weakness, palpitations, dyspnoea, and haemolysis (jaundice and, rarely, haemoglobinuria with chills). Other life-threatening complications, such as acute renal failure, disseminated intravascular coagulation and shock may be observed in some patients. Early diagnosis and proper treatment are essential for DIIHA patients with severe complications. The most effective treatment of patients who develop life-threatening DIIHA is immediate discontinuation of all suspected drugs.
Severe forms of haemolysis with rapidly falling Hb levels and haemoglobinuria are extremely rare. The patient presented here had a sudden episode of haemoglobinuria and his Hb dropped from 125.0 g/L to 30.3 g/L over 5 days. Transfusion was delayed because of a compatibility problem and a concern over the possibility of finding compatible red blood cells. However, red blood cell transfusions should not be precluded for patients with life-threatening haemolysis, regardless of a positive blood cross-matching reaction. Preventive measures, such as high-dose immunoglobin and methylprednisolone, may be helpful for a successful transfusion. The indication for blood transfusion in patients with DIIHA does not differ from that for other types of haemolytic anaemia, but the presence of antibodies and/or residues of the drug in the patient’s serum may lead to serological incompatibility in laboratory antibody screening tests, including cross-matching.
In conclusion, ceftazidime should be used with caution because of its rare but potentially serious adverse effect of haemolysis. Early recognition of DIIHA and institution of supportive care, including transfusion, is likely to improve the outcome.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Garratty G. Immune hemolytic anemia caused by drugs. Expert Opin Drug Saf. 2012;11:635–42. doi: 10.1517/14740338.2012.678832. [DOI] [PubMed] [Google Scholar]
- 2.Chambers LA, Donovan LM, Kruskall MS. Ceftazidime-induced hemolysis in a patient with drug-dependent antibodies reactive by immune complex and drug adsorption mechanisms. Am J Clin Pathol. 1991;95:393–6. doi: 10.1093/ajcp/95.3.393. [DOI] [PubMed] [Google Scholar]
- 3.Garratty G. Immune cytopenia associated with antibiotics. Transfus Med Rev. 1993;7:255–67. doi: 10.1016/s0887-7963(93)70145-5. [DOI] [PubMed] [Google Scholar]
- 4.Fueger JT, Bell JA, Gottschall JL, et al. Ceftazidime-dependent antibody reacting with untreated red cells in the presence of drug [abstract] Transfusion. 2001;41:104S. [Google Scholar]
- 5.Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24:143–50. doi: 10.1016/j.blre.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Pierce A, Nester T. Pathology consultation on drug-induced hemolytic anemia. Am J Clin Pathol. 2011;136:7–12. doi: 10.1309/AJCPBVLJZH6W6RQM. [DOI] [PubMed] [Google Scholar]
- 7.Johnson ST, Fueger JT, Gottschall JL. One center’s experience: the serology and drugs associated with drug-induced immune hemolytic anemia--a new paradigm. Transfusion. 2007;47:697–702. doi: 10.1111/j.1537-2995.2007.01173.x. [DOI] [PubMed] [Google Scholar]
- 8.eHealthMe study from FDA reports. [Accessed on 02/10/2013]. Available at: http://www.ehealthme.com/print/ds17070694.