Abstract
MicroRNAs (miRNA) regulate the synthesis of cytokines in response to Toll-like receptor (TLR) activation. Our recent microarray study comparing normal and inflamed human dental pulps showed that miRNA-181 (miR-181) family is differentially expressed in the presence of inflammation. Prior studies have reported that the dental pulp, which is composed primarily of TLR4/2+ fibroblasts, expresses elevated levels of cytokines including Interleukin-8 (IL-8) when inflamed. In this study, we employed an in-vitro model to determine the role of the miRNA 181 family in the TLR agonist-induced response in human fibroblasts. TLR4/2+ primary human dental pulp fibroblasts were stimulated with lipopolysaccharide from Porphyromonas gingivalis (Pg LPS), a known oral pathogen, and IL-8 and miR-181 expression measured. An inversely proportional relationship between IL-8 and miR-181a was observed. In-silico analysis identified a miR-181a binding site on the 3′UTR of IL-8 which was confirmed by dual-luciferase assays. MiR-181a directly binds to the 3′UTR of IL-8, an important inflammatory component of the immune response, and modulates its levels. This is the very first report demonstrating miR-181a regulation of IL-8.
Keywords: micro-RNA, Interleukin, immunoregulation, fibroblasts
INTRODUCTION
MicroRNAs (miRNA) have emerged as important post-transcriptional regulators of gene expression in diverse biological processes including inflammation, metabolism and healing 1. These key regulators of inflammation have been linked to homeostatic response to inflammatory stimuli by Toll-like receptor (TLR) -4 pathway activation 2 and in various TLR-mediated immune responses to bacterial infection where miRNAs either suppressed inflammatory response or reduced inflammatory triggers 3, 4.
Our recent microarray report showed differential expression of miRNAs in human dental pulp tissues that were clinically diagnosed to have pulpal inflammation (pulpitis) 5. The dental pulp is the tissue located in the in the middle of the tooth that contains mostly connective tissues, nerves and blood vessels for protection, nourishment and innervation of the tooth. Pulpitis is a relatively common and painful dental disease that represents an immune response to bacterial infection 6. Pulpitis and its sequela, periapical periodontitis have been directly linked to the pathogenicity of the oral microflora 6-10.
In our study that compared normal and inflamed human dental pulps, we reported the differential expression of 36 miRNAs 5. In another in-vivo study examining inflamed tissues surrounding the apices of teeth, 24 miRNAs were differently expressed 11. The miR-181 family, which regulates a wide range of gene targets, was differentially expressed in both of these tissues. They have also been recently implicated in TLR-induced in-vivo increase in cytokine levels 2. However, there have been no reports as yet on the role of these miRNAs in regulating the immune response in human fibroblasts.
In this study, we utilized an in-vitro infection model to determine the role of miR-181 family in regulating immune response to bacteria using primary cultures of human pulpal fibroblasts. These cells express TLRs 12 - a class of proteins that recognize conserved pathogen structures, triggers innate immune responses and primes antigen-specific adaptive immunity 13. TLR-4 for example recognizes bacterial lipopolysaccharide (LPS) 14 that stimulates pulp cells to produce cytokines and chemokines 7, 15-17.
The miR-181 family has been shown to control inflammation under physiological and pathological conditions, which are essentially the outcome its role in regulating various key aspects of growth, development, and activation 18. miR-181b, for example, targets importin-α3 that consequently was reported to inhibit downstream canonical NF-κB signaling pathway in endothelial cells, reduce vascular inflammation in vivo, decrease lung inflammation in endotoxemia mice and promote survival in LPS-induced septic mice 19. In addition, miR-181a regulates osteopontin-mediated vascular smooth muscle function that may be a novel therapeutic approach to modulate osteopontin that is found in abundance in atherosclerotic plaques 20. These validated roles of the miR-181 family show its relevance in other highly vascular and immunocompetent tissues in humans like the dental pulp.
Interleukin-8 (IL-8), like the miR-181 family, is also linked to TLR activation 21. IL-8 is a potent chemoattractant that is increased not only in major inflammatory conditions such as hepatitis 22, chronic obstructive pulmonary disease 23 and periodontitis 24 but also in pulpitis 8, 25 and in invitro models of pulpal infection 12, 15. Its essential involvement and causative role in acute inflammation by recruiting and activating neutrophils have been firmly established 21. Both IL-8 and miR-181 family have been associated with other inflammatory conditions and inflammatory responses of various cell types 19, 26, 27.
The purpose of this study is to determine if oral pathogens modulate the expression of miR-181 family and to correlate this expression with the production of IL-8.
RESULTS
MiR-181-a, -b and IL-8 expressions are modulated by Pg LPS
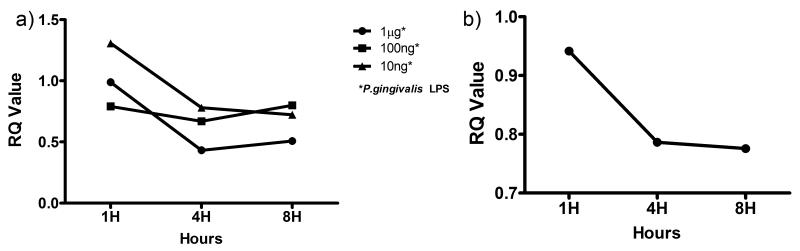
To examine if TLR activation affects the expression of miR-181 family in HPDF, cells were challenged with varying concentrations of Pg LPS over 8 hours. Figure 1a shows that miR-181a expression in fibroblasts is influenced by Pg LPS in a time- and dose-dependent manner. Compared with the one-hour time point, the expression of miR-181a decreased at 4-hours. At the 8-hour time point, the expression level either remained relatively similar (10 ng ml−1 LPS) or increased slightly (100 ng and 1 μg ml −1 LPS). This result is not surprising as a time- and dose-dependent stimulation by Pg LPS has been shown in other cell types 28, 29. MiR-181b expression was only noted in 1μg ml−1 Pg LPS (Figure 1b) while miR-181c was not detected in this study. 1μg ml−1 of Pg LPS was used in the subsequent experiments as this dose had been widely used in other studies 30 and had shown the most consistent result in the miR-181a and –b experiments, which were responsive only to 1μg ml−1 of Pg LPS.
Figure 1.
Dose and time responses of HDPF to Pg LPS. HDPF were challenged with 10ng, 100ng and 1μg ml−1 of Pg LPS for up to 8 hours and miR-181-a and –b expression analyzed by qRT-PCR. 10 nanograms of mRNA from each sample of HDPF cell lysates were reverse-transcribed and then subjected to RT-qPCR using inventoried primers and probe sets with the following mature miRNA sequences: AACAUUCAACGCUGUCGGUGAGU for 181-a (Figure 1a) and CUCACUGAACAAUGAAUGCAA for 181-b (Figure 1b). RNU6B control miRNA was used in all RT-qPCR experiments.
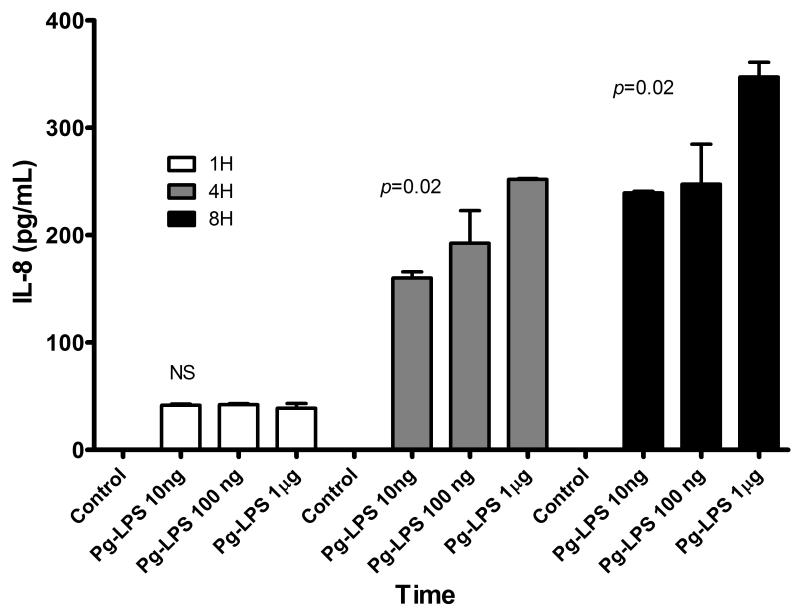
Induction of pro-inflammatory cytokines is a key feature of TLR signaling. We monitored the levels of IL-1β, IL-6, IL-8 and TNF-α in the supernatants of LPS stimulated HPDF. Among the cytokines assayed in this study, only IL-8 was detectable in the cell culture supernatant (Figure 2). IL-1β, IL-6 and TNF-α were all below minimum detectable dose. Interestingly, IL-8 was secreted in a time- and dose-dependent manner.
Figure 2.
Supernatant IL-8 levels in HDPF upon Pg LPS challenge at different time points and concentrations. Bars represent the mean of at least three experiments with SD. P values were calculated using one-way ANOVA.
IL-8 and miR-181a and b show an inversely proportional relationship
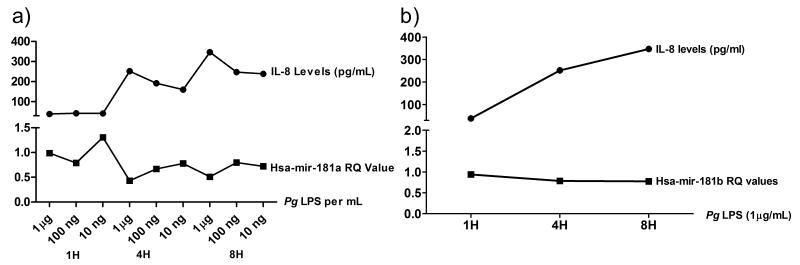
From the dose and time dependent data we observed an inversely proportional relationship between miR-181a gene expression and IL-8 protein levels (Figure 3a). The same relationship was observed between IL-8 and miR-181b (Figure 3b). A decrease in the miR-181a expression corresponded with an increase in IL-8 levels in culture supernatants.
Figure 3.
Juxtaposition of the relationship between secreted Interleukin-8 (IL-8) levels and miR-181a (A) and miR181b (B) expression. An inversely proportional relationship between the IL-8 and miR-181a and -b was observed.
IL-8 is modulated by miR-181a
To investigate the possible mechanistic role of miR-181 mediated regulation of IL8, we scanned its 3′UTR for potential miR-181 binding sites. Bioinformatics analysis identified a novel miRNA binding region spanning 346-368 nts of IL8 3′UTR (Figure 4a). Importantly, the seed sequence is conserved in all the four miRNAs of miR-181 family. To validate functional miRNA-target interactions, dual luciferase assays were performed. HEK293 cells transfected with miR-181a show reduced luciferase activity compared with scramble miRNA and miR-302a (Figure 4b). miR-302a also targets IL-8 31; however, in this study, it did not demonstrate a negative regulation of IL-8. This finding further confirms the potential of miR-181a in regulating IL-8. Increasing the miR-181a mimic concentration to five pmol had no further significant impact on the luciferase activity (Figure 4b). Thus, miR-181a appears to directly regulate IL8 levels by interacting with the 3′UTR.
Figure 4.

Modulation of Interleukin-8 (IL-8) activity by hsa-mir-181 family. IL8 3′UTR binding site for miR-181 family was scanned using miRWalk (Figure 4a). miSVR score: -0.0308 and PhastCons score: 0.5156. To validate this binding, dual Luciferase assay was performed to (Figure 4b) using mimic (scramble oligonucleotides) and miR-302a as controls. Bars represent the means of three independent assays with SD deviation. * P<0.05 compared with negative mimic analyzed using unpaired T-Test.
DISCUSSION
MiR-181 is a critical miRNA that was largely thought to regulate lymphocyte development and homeostasis, among other important functions 32. However, recent studies have also revealed its role in immunoregulation 2, 33. In this study, we have shown that miR-181 family is expressed in non-leukocytic cells and that interleukin-8 and miR-181a and -181-b expression is influenced by a TLR agonist in a time- and dose-dependent manner. Moreover, the inversely proportional relationship between miR-181a and IL-8 is likely due in some part, to miR-181a directly binding to the 3′UTR of IL-8. This relationship suggests a possible modulatory role of miR-181a and miR-181b on IL-8 expression.
The findings above reveal the important role of miRNA in the regulatory networks governing the response of various cell types to microbial insults. Fibroblasts are primarily structural in function but their ability to respond to infection as first or second line of defense (after epithelial cells) is critical in limiting the spread of infection and in wound healing 34. The presence of TLRs in fibroblasts confers immunocompetency on these cells 12, giving merit to the importance of studying how their response is regulated. As part of the initial responders to microbial entry, the capability of fibroblasts to recruit professional immune cells to the site of infection can be a double-edged sword that may halt or propagate the infectious process 35.
The polymicrobial etiology of pulpitis and the clinical implications associated with it make this disease an ideal model of immunoregulation. As a more gram negative, anaerobic bacterial species penetrate the pulp-dentin interface, pulpal cells, predominantly fibroblasts recognize the conserved microbial patters through TLRs 12. Consequently, various inflammatory mediators are expressed that initiate and enhance the inflammatory process. We mimicked this response by utilizing an in-vitro model using HDPF stimulated with Pg-LPS. Our results agree with previous studies on the immunocompetence of pulpal fibroblast 7, 12, 15, 17. In this study, the detectable levels of IL-8 in Pg-LPS stimulated cells may recruit circulating immune cells (e.g. neutrophils) to the site of infection as shown in-vivo by Izumi et al 36 and Olgart et al 37. In their study, bacteria induced inflammatory changes in the dental pulp characterized by infiltration of immune cells, activation of the complement system due to the development of a local immune reaction, and the accumulation of arachidonic acid metabolism with the destruction of cellular components.
Inflammation of the pulp and periapical tissues is commonly associated with pain, and approximately 90% of dental emergency visits with pain as the chief complaint are attributable to activation of pulpal or periapical nociceptors 38. The prevalence of periapical disease in the United States is estimated to be about 4.1% 39. Despite the prevalence of endodontic disease and the great discomfort associated with it, the fundamental molecular aspects of its pathogenesis are still not fully understood. The current literature on pulpal immune response to microbial infection continues to expand; however, very little is known on the regulatory mechanism behind pulpal disease.
A critical barrier to progress in treatment of pulpitis is the incomplete understanding of the regulatory network governing this disease. This study provides evidence that the dental pulp possesses immunocompetent cells with an active regulatory network capable of responding to microbial insults and modulating inflammatory response. Furthermore, the pathogenesis of pulpitis and its immunoregulation can serve as a model for other microbial diseases that provide less opportunity for ex-vivo or in-vitro studies due to their inaccessibility or critical location like the brain, lungs, or eyes.
We confirmed the regulation of IL-8 by miR-181a, a miRNA that has been shown to be differentially expressed in inflamed tissues 5, 19, 26, 27. MiRNAs post-transcriptionally regulate gene expression by targeting specific messenger RNAs (mRNAs) for degradation or translational repression 40. Their cytoplasmic levels directly influence the protein bioavailability of their targeted genes. We have shown in this study that the level of miR-181a is inversely proportional with secreted IL-8 level (Figure 3). We confirmed the repression of IL-8 by miR-181a using Luciferase assay (Figure 4b) and in-silico alignment analysis (Figure 4a). This pattern of negative regulation by miRNAs has been shown in other studies 41.
With the understanding that IL-8 can be directly regulated with a miRNA, a targeted therapy to control inflammation can be further investigated not only in dental pulp where treatment to pulpitis is currently limited to pulp extirpation but in other organs or tissue systems as well. This treatment method is already in progress in other fields 42.
MATERIALS AND METHODS
Dental Pulp Tissue Collection and Culture
The study was performed with the approval of and compliance to the guidelines set by the Institutional Regulatory Board of the UNC Office of Human Research Ethics. Dental pulp tissue from a clinically normal tooth of three healthy donors was collected immediately after extraction for orthodontic reason. The pulp tissue was obtained under informed consent.
Fibroblast cultures were established using a previously published protocol by Adachi et al 7. In brief, minced pieces of pulp tissues were explanted into 35-mm culture dishes containing Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Sigma, St. Louis, MO, USA), 1 mM sodium pyruvate (Gibco), and 50 IU/mL penicillin/50 3g/mL streptomycin (Gibco) at 37°C in a humidified atmosphere of 5% CO2. Confluent primary cultures were harvested and subcultured. Morphologically fibroblastic cells obtained by this method were used as fibroblast for experiments at passages four to six.
Fibroblast Stimulation
Fibroblasts were seeded in 6-well culture plates and incubated until confluent monolayers of approximately 106 cells were established. The cells were then challenged with 1μg, 100ng or 10 ng ml−1 of Porphyromonas gingivalis W83 LPS (Pg LPS) (Dr. David A. Scott, University of Louisville, KY, USA) for 1, 4 and 8 hours. Culture supernatants were collected and stored at -80 oC until use.
Reverse transcription-quantitative PCR
For reverse transcription-quantitative PCR (RT-qPCR), RNA was isolated from cell lysates using the miRNeasy Mini Kit (Qiagen) and was reverse transcribed with High-Capacity cDNA Archive Kit (Applied Biosystems or ABI) according to manufacturer’s instructions. cDNA was subjected to RT-qPCR using TaqMan® inventoried primers and probe sets and the 7500 Fast Real-Time PCR System (both from ABI). Normalization was performed using RNU-6B primer (ABI) 43. Relative quantification (RQ) values were analyzed using Excel spreadsheet. The experiments were carried out in three independent experimental set-ups.
ELISA
IL-1β, IL-6, IL-8 and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (R&D Systems) according to the manufacturer’s instructions. The absorbance was read at 450 nm.
Luciferase assay
IL8 3′UTR was amplified using forward (GCACTCGAGTGTGTGGGTCTGTTGTAGGG ) and reverse primers (ATGCGGCCGCTGACTGTGGAGTTTTGGCTGT) and the pGL3-IL8 construct (kind gift of Dr. Richard Pestell Jefferson Medical College, Philadelphia, PA) as template44. The amplified fragment (~950 bp) was digested by XhoI and NotI and cloned into psiCHEKTM2 vector (Promega). The transfection was performed as described previously 45. Briefly, actively growing Human Embryonic Kidney 293 cells (HEK cells) were seeded in 96-well plate at a density of 2 × 104 cells per well in complete DMEM. The next day, cells were cotransfected using Lipofectamine 2000 (Invitrogen) with 80 ng of IL8 construct and different concentration (2 pmol and 5 pmol) of miR-181a mimics. To confirm the negative regulation of IL-8 by miR-181a, two controls were used: 1) scramble oligonucleotides (Qiagen), which are claimed to have no potential binding sites on human genes; and 2) miR-302-a that has potential binding with IL-8 31. 36 h after transfection, luciferase activities were measured by Lumat (Turner BioSystems). The fold change in luciferase activity was calculated as previously described 45.
Statistical Analysis
One-way ANOVA with Tukey’s post-hoc analysis or t-test were employed where statistical difference is noted in the study using Graph Pad. P-values < 0.05 were considered significant
ACKNOWLEDGEMENT
This research is supported by the National Institute for Dental and Craniofacial Research (NIDCR) R01DE021052, NIDCR T90DE021986 and the UNC School of Dentistry’s Next Generation of Oral Health Researchers (NextGen). Special thanks to Dr. Richard Pestell (Jefferson Medical College, Philadelphia, PA).
Footnotes
COMPETING INTEREST The authors declare that they have no competing interests
REFERENCES
- 1.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circulation research. 2012;110(3):496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 2.Xie W, Li Z, Li M, Xu N, Zhang Y. miR-181a and inflammation: miRNA homeostasis response to inflammatory stimuli in vivo. Biochemical and biophysical research communications. 2013;430(2):647–52. doi: 10.1016/j.bbrc.2012.11.097. [DOI] [PubMed] [Google Scholar]
- 3.Case SR, Martin RJ, Jiang D, Minor MN, Chu HW. MicroRNA-21 inhibits toll-like receptor 2 agonist-induced lung inflammation in mice. Experimental lung research. 2011;37(8):500–8. doi: 10.3109/01902148.2011.596895. [DOI] [PubMed] [Google Scholar]
- 4.Liu G, Friggeri A, Yang Y, Park YJ, Tsuruta Y, Abraham E. miR-147, a microRNA that is induced upon Toll-like receptor stimulation, regulates murine macrophage inflammatory responses. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15819–24. doi: 10.1073/pnas.0901216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong S, Zhang S, Bair E, Nares S, Khan AA. Differential expression of microRNAs in normal and inflamed human pulps. Journal of endodontics. 2012;38(6):746–52. doi: 10.1016/j.joen.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Love RM, Jenkinson HF. Invasion of dentinal tubules by oral bacteria. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 2002;13(2):171–83. doi: 10.1177/154411130201300207. [DOI] [PubMed] [Google Scholar]
- 7.Adachi T, Nakanishi T, Yumoto H, Hirao K, Takahashi K, Mukai K, et al. Caries-related bacteria and cytokines induce CXCL10 in dental pulp. Journal of dental research. 2007;86(12):1217–22. doi: 10.1177/154405910708601215. [DOI] [PubMed] [Google Scholar]
- 8.Huang GT, Potente AP, Kim JW, Chugal N, Zhang X. Increased interleukin-8 expression in inflamed human dental pulps. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1999;88(2):214–20. doi: 10.1016/s1079-2104(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 9.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germfree and conventional laboratory rats. Journal - Southern California Dental Association. 1966;34(9):449–51. [PubMed] [Google Scholar]
- 10.Siqueira JF, Jr., Rocas IN, Alves FR, Silva MG. Bacteria in the apical root canal of teeth with primary apical periodontitis. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2009;107(5):721–6. doi: 10.1016/j.tripleo.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 11.Chan LT, Zhong S, Naqvi AR, Self-Fordham J, Nares S, Bair E, et al. MicroRNAs: New Insights into the Pathogenesis of Endodontic Periapical Disease. Journal of endodontics. doi: 10.1016/j.joen.2013.08.032. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirao K, Yumoto H, Takahashi K, Mukai K, Nakanishi T, Matsuo T. Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. Journal of dental research. 2009;88(8):762–7. doi: 10.1177/0022034509341779. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology. 2010;11(5):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 14.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. The Journal of experimental medicine. 1999;189(11):1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaoka S, Tokuda M, Sakuta T, Taketoshi Y, Tamura M, Takada H, et al. Interleukin-8 gene expression by human dental pulp fibroblast in cultures stimulated with Prevotella intermedia lipopolysaccharide. Journal of endodontics. 1996;22(1):9–12. doi: 10.1016/S0099-2399(96)80228-7. [DOI] [PubMed] [Google Scholar]
- 16.Park SH, Hsiao GY, Huang GT. Role of substance P and calcitonin gene-related peptide in the regulation of interleukin-8 and monocyte chemotactic protein-1 expression in human dental pulp. International endodontic journal. 2004;37(3):185–92. doi: 10.1111/j.0143-2885.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- 17.Tokuda M, Sakuta T, Fushuku A, Torii M, Nagaoka S. Regulation of interleukin-6 expression in human dental pulp cell cultures stimulated with Prevotella intermedia lipopolysaccharide. Journal of endodontics. 2001;27(4):273–7. doi: 10.1097/00004770-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Sun X, Sit A, Feinberg MW. Role of miR-181 family in regulating vascular inflammation and immunity. Trends in cardiovascular medicine. 2013 doi: 10.1016/j.tcm.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. The Journal of clinical investigation. 2012;122(6):1973–90. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Remus EW, Lyle AN, Weiss D, Landazuri N, Weber M, Searles C, et al. miR181a protects against angiotensin II-induced osteopontin expression in vascular smooth muscle cells. Atherosclerosis. 2013;228(1):168–74. doi: 10.1016/j.atherosclerosis.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. Journal of leukocyte biology. 1994;56(5):559–64. [PubMed] [Google Scholar]
- 22.Pollicino T, Bellinghieri L, Restuccia A, Raffa G, Musolino C, Alibrandi A, et al. Hepatitis B virus (HBV) induces the expression of interleukin-8 that in turn reduces HBV sensitivity to interferon-alpha. Virology. 2013;444(1-2):317–28. doi: 10.1016/j.virol.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Kaur M, Singh D. Neutrophil chemotaxis caused by chronic obstructive pulmonary disease alveolar macrophages: the role of CXCL8 and the receptors CXCR1/CXCR2. The Journal of pharmacology and experimental therapeutics. 2013;347(1):173–80. doi: 10.1124/jpet.112.201855. [DOI] [PubMed] [Google Scholar]
- 24.Noh MK, Jung M, Kim SH, Lee SR, Park KH, Kim DH, et al. Assessment of IL-6, IL-8 and TNF-alpha levels in the gingival tissue of patients with periodontitis. Experimental and therapeutic medicine. 2013;6(3):847–851. doi: 10.3892/etm.2013.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karapanou V, Kempuraj D, Theoharides TC. Interleukin-8 is increased in gingival crevicular fluid from patients with acute pulpitis. Journal of endodontics. 2008;34(2):148–51. doi: 10.1016/j.joen.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Hutchison ER, Kawamoto EM, Taub DD, Lal A, Abdelmohsen K, Zhang Y, et al. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013;61(7):1018–28. doi: 10.1002/glia.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie W, Li M, Xu N, Lv Q, Huang N, He J, et al. MiR-181a regulates inflammation responses in monocytes and macrophages. PloS one. 2013;8(3):e58639. doi: 10.1371/journal.pone.0058639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herath TD, Darveau RP, Seneviratne CJ, Wang CY, Wang Y, Jin L. Tetra- and penta acylated lipid A structures of Porphyromonas gingivalis LPS differentially activate TLR4-mediated NF-kappaB signal transduction cascade and immuno-inflammatory response in human gingival fibroblasts. PloS one. 2013;8(3):e58496. doi: 10.1371/journal.pone.0058496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabeta K, Yamazaki K, Akashi S, Miyake K, Kumada H, Umemoto T, et al. Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infection and immunity. 2000;68(6):3731–5. doi: 10.1128/iai.68.6.3731-3735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajishengallis G, Tapping RI, Harokopakis E, Nishiyama S, Ratti P, Schifferle RE, et al. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cellular microbiology. 2006;8(10):1557–70. doi: 10.1111/j.1462-5822.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Rennard S. MicroRNA and Cytokines. Mol Cell Pharmacol. 2011;3(3):143–151. [Google Scholar]
- 32.Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limon P, Kaech SM, et al. The MicroRNA miR-181 Is a Critical Cellular Metabolic Rheostat Essential for NKT Cell Ontogenesis and Lymphocyte Development and Homeostasis. Immunity. 2013;38(5):984–97. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo XK, Zhang Q, Gao L, Li N, Chen XX, Feng WH. Increasing expression of microRNA 181 inhibits porcine reproductive and respiratory syndrome virus replication and has implications for controlling virus infection. Journal of virology. 2013;87(2):1159–71. doi: 10.1128/JVI.02386-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDougall S, Dallon J, Sherratt J, Maini P. Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 2006;364(1843):1385–405. doi: 10.1098/rsta.2006.1773. [DOI] [PubMed] [Google Scholar]
- 35.Gura T. Chemokines take center stage in inflammatory ills. Science. 1996;272(5264):954–6. doi: 10.1126/science.272.5264.954. [DOI] [PubMed] [Google Scholar]
- 36.Izumi T, Kobayashi I, Okamura K, Sakai H. Immunohistochemical study on the immunocompetent cells of the pulp in human non-carious and carious teeth. Archives of oral biology. 1995;40(7):609–14. doi: 10.1016/0003-9969(95)00024-j. [DOI] [PubMed] [Google Scholar]
- 37.Olgart L, Brannstrom M, Johnson G. Invasion of bacteria into dentinal tubules. Experiments in vivo and in vitro. Acta odontologica Scandinavica. 1974;32(1):61–70. doi: 10.3109/00016357409002533. [DOI] [PubMed] [Google Scholar]
- 38.Hasselgren G, Calev D. Endodontics emergency treatment sound and simplified. The New York state dental journal. 1994;60(6):31–3. [PubMed] [Google Scholar]
- 39.Buckley M, Spangberg LS. The prevalence and technical quality of endodontic treatment in an American subpopulation. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1995;79(1):92–100. doi: 10.1016/s1079-2104(05)80081-2. [DOI] [PubMed] [Google Scholar]
- 40.Pauley KM, Chan EK. MicroRNAs and their emerging roles in immunology. Annals of the New York Academy of Sciences. 2008;1143:226–39. doi: 10.1196/annals.1443.009. [DOI] [PubMed] [Google Scholar]
- 41.Perry MM, Moschos SA, Williams AE, Shepherd NJ, Larner-Svensson HM, Lindsay MA. Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;180(8):5689–98. doi: 10.4049/jimmunol.180.8.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. The New England journal of medicine. 2013;368(18):1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 43.Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nature communications. 2012;3:1078. doi: 10.1038/ncomms2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu Z, Willmarth NE, Zhou J, Katiyar S, Wang M, Liu Y, et al. microRNA 17/20 inhibits cellular invasion and tumor metasis in breast cancer by herterotypic signalling. Proc Natl Acad Sci USA. 2010;107:8231–8236. doi: 10.1073/pnas.1002080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naqvi AR, Fordham JB, Khan A, Nares S. MicroRNAs responsive to Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis LPS modulate expression of genes regulating innate immunity in human macrophages. Innate immunity. 2013 doi: 10.1177/1753425913501914. [DOI] [PMC free article] [PubMed] [Google Scholar]