Abstract
Background:
Kisspeptin is a critical hypothalamic regulator of reproductive function. Chronic kisspeptin administration causes profound tachyphylaxis in male monkeys and in women with functional hypothalamic amenorrhea. The pharmacological effects of chronic kisspeptin exposure in healthy women with normal menstrual cycles have not been studied previously.
Aim:
Our aim was to determine the effects of follicular-phase kisspeptin-54 treatment on menstrual cyclicity in healthy women.
Methods:
We performed a prospective, single-blinded, 1-way crossover study. Healthy women received twice-daily sc injections of kisspeptin (6.4 nmol/kg) or 0.9% saline during menstrual days 7–14 (n = 5 per treatment arm). Serial assessments of basal reproductive hormones, ultrasound parameters, LH pulsatility, and acute sensitivity to GnRH and kisspeptin-54 injection were performed.
Results:
Menstrual cyclicity persisted in all women after follicular-phase kisspeptin-54 treatment. Chronic exposure to kisspeptin-54 did not abolish acute stimulation of LH after injection of kisspeptin-54 or GnRH. In addition, kisspeptin-54 treatment was associated with a shorter mean length of the menstrual cycle (mean length of menstrual cycle was 28.6 ± 1.4 days with saline vs 26.8 ± 3.1 days with kisspeptin, P < .01), earlier onset of highest recorded serum LH (mean menstrual day of highest LH was 15.2 ± 1.3 with saline vs 13.0 ± 1.9 with kisspeptin, P < .05), and earlier onset of the luteal phase (mean menstrual day of progesterone increase was 18.0 ± 2.1 with saline vs 15.8 ± 0.9 with kisspeptin, P < .05).
Conclusion:
Our data suggest that 1 week of exogenous kisspeptin-54 does not abolish menstrual cyclicity in healthy women. Further work is needed to determine whether kisspeptin could be used to treat certain anovulatory disorders.
The kisspeptins are a group of arginine-phenylalanine (RF) amide peptides encoded by the KISS1 gene and are endogenous ligands for the kisspeptin receptor (KISS1R) (1–4). KISS1 and KISS1R are expressed predominantly in the hypothalamus, pituitary, and placenta (3, 5–7). Kisspeptin signaling exerts powerful effects on the mammalian reproductive system. Mice and humans lacking kisspeptin or the kisspeptin receptor fail to undergo puberty and are infertile (8–10). Central or peripheral administration of kisspeptin induces gonadotropin and sex steroid release in all mammalian species investigated, including rats (11–13), mice (14, 15), monkeys (16), and sheep (17, 18).
Administration of kisspeptin-54 or kisspeptin-10 acutely stimulates gonadotropin secretion in healthy male (19–22) and female (23, 24) volunteers and women with functional hypothalamic amenorrhea (HA) (25, 26). However, twice-daily sc administration of kisspeptin-54 to women with HA causes profound tachyphylaxis within 24 hours of commencing treatment (25). Furthermore, continuous iv infusion of kisspeptin-10 to monkeys is associated with tachyphylaxis within 3 hours of commencing administration (16). It has therefore been assumed that chronic treatment with kisspeptin-54 in healthy women may have limited therapeutic potential as a stimulator of human reproductive activity because tachyphylaxis has been observed after chronic administration in primates and women with HA. However, this hypothesis had not been tested previously.
In this study, we aimed to determine whether chronic exogenous kisspeptin was sufficient to alter menstrual cyclicity, using the exact dose previously demonstrated to cause tachyphylaxis within 24 hours in women with HA (6.4 nmol/kg) (25, 26). We performed a single-blinded placebo-controlled 1-way crossover study to determine the effects of twice-daily administration of kisspeptin for 7 days on the menstrual cycle in healthy human female subjects with regular menstrual cycles.
Subjects and Methods
Subjects
Ethical approval was granted by the Hammersmith and Queen Charlotte's and Chelsea Hospitals Research Ethics Committee (registration number 05/Q0406/142). Written informed consent was obtained from all subjects. This study was performed in accordance with the Declaration of Helsinki. Five healthy female subjects with regular menstrual cycles were recruited through advertisements placed in local newspapers (age 31.6 ± 2.6, range 24–37 years; weight 60.4 ± 2.5, range 50.4–63.8 kg) as described previously (22, 23).
Protocol
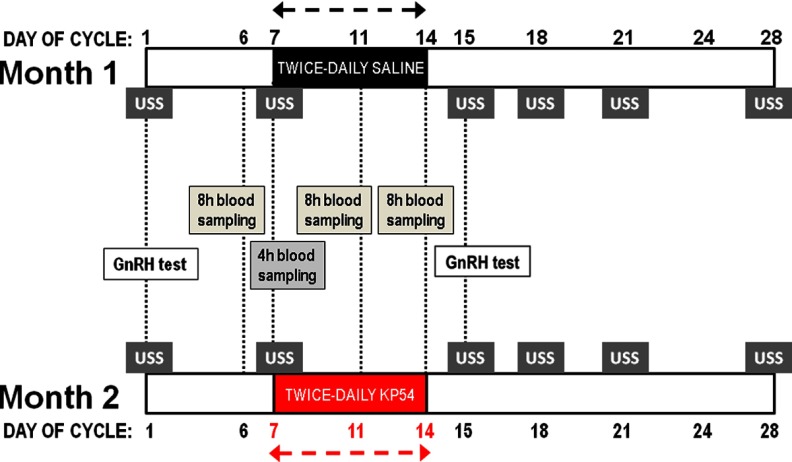
A single-blinded, placebo-controlled, 1-way crossover design study of 10 menstrual cycles was performed (Figure 1). During month 1 of the study protocol, five healthy female subjects self-administered twice-daily sc saline injections between days 7 and 14 of their menstrual cycle (see Subject Characteristics in Supplemental Table 1, published on The Endocrine Society's Journals Online website at http://jcem.endojournals.org). During month 2 of the study protocol, the same five healthy female subjects self-administered twice-daily sc kisspeptin-54 injections (6.4 nmol/kg; equivalent to 37 μg/kg) (19) between days 7 and 14 of their menstrual cycle; (see Supplemental Methods for kisspeptin-54 peptide synthesis and testing). Day 1 of each month was defined as the first day of menstrual bleeding.
Figure 1.
Study protocol diagram. Five healthy women underwent a 1-way crossover protocol. Twice-daily injections of saline and kisspeptin-54 (KP54) were administered between days 7 and 14 during months 1 and 2 of the protocol, respectively. During each month of the protocol, subjects underwent a baseline GnRH test (day 1) and 8-hour blood sampling (day 6) before commencing injections. During the injection period (days 7–14), subjects underwent a 4-hour study (day 7) and two additional 8-hour studies (days 11 and 14) immediately after injection of saline or kisspeptin-54. A GnRH test was performed 24 hours after cessation of saline or kisspeptin treatment (day 15). USS denotes ultrasound examination and measurement of reproductive hormones. Day 1 was defined as the first day of menstrual bleeding.
Kisspeptin injections
All subjects were trained in self-administration of sc injections by an investigator at the start of the study protocol. At the beginning of each week when injections were to be performed, a box containing unlabeled vials of freeze-dried saline (month 1) or vials of freeze-dried kisspeptin-54 (month 2), alcohol wipes, saline ampoules for reconstitution of freeze-dried vial contents, 0.5-mL insulin syringes with needles, and needle disposal bins was given to each subject. For injection, vial contents were reconstituted in 0.5 mL of 0.9% saline. A 0.5-mL insulin syringe was then used to inject saline alone (month 1) or 6.4 nmol/kg kisspeptin-54 (month 2) into the lower anterior abdominal region sc. This kisspeptin-54 dose was the same as used in our previous work in healthy women and women with HA (25, 26). Volunteers were instructed to prepare and perform injections in the morning after breakfast (unless attending for a study in which case it was done by the volunteer at time 0 of the study) and in the evening before bed. The volume of the saline or kisspeptin injections was identical. Subjects were instructed to refrigerate vials stored at home. Before commencing study visits, injection sites were inspected, and numbers of returned vials, insulin syringes, and saline or kisspeptin vials were counted to monitor compliance. Plasma kisspeptin immunoreactivity (IR) was also assessed throughout the study protocol to confirm compliance.
Baseline period
During menstrual days 1 to 6 of each month of the study protocol, pituitary responsiveness was assessed by a GnRH test (see Supplemental Methods for protocol), and baseline reproductive hormones and ultrasound markers were measured. The collection, processing, and analysis of blood samples are detailed in Supplemental Methods. The baseline period also allowed the acclimatization of subjects to study conditions. No injections were administered during this period.
Treatment period
During menstrual days 7 to 14 of each month of the study protocol, subjects self-administered twice-daily, single-blinded sc injections of saline (month 1 of study protocol) or kisspeptin-54 (month 2 of study protocol) as above.
Posttreatment period
During menstrual days 15 to 28 of each month of the study protocol, subjects underwent a posttreatment observation period to assess pituitary responsiveness (GnRH test) and measure circulating reproductive hormones and ultrasound markers. No injections were administered during this period.
Four-hour blood sampling after injection of saline or kisspeptin
All subjects underwent blood sampling during the 4-hour period immediately after the first injection of the saline (day 7, month 1) or kisspeptin-54 (day 7, month 2) treatment period to confirm that kisspeptin-54 acutely stimulated LH release as previously shown in healthy women (23). Saline or kisspeptin-54 (6.4 nmol/kg) was sc administered at 0 minutes by the subject, and blood was sampled for serum LH, FSH, estradiol, and plasma kisspeptin IR at −30, 0, 10, 20, 40, 60, 90, 120, 150, 180, 210, and 240 minutes.
Eight-hour blood sampling for assessment of LH pulsatility
Subjects underwent 3 assessments of LH pulsatility during month 1 and month 2 of the study protocol. Baseline assessment of LH pulsatility was performed on menstrual day 6 (1 day before commencing injections). LH pulsatility was also assessed after injection of saline or kisspeptin-54 on menstrual days 11 and 14. The saline or kisspeptin-54 was reconstituted using one of the vials given to each subject at the beginning of the treatment period for home storage. Blood was sampled every 10 minutes. Studies commenced between 8:00 and 9:00 am.
Assessment of pituitary sensitivity before and after injections of saline or kisspeptin
Subjects each underwent 2 GnRH tests during month 1 and month 2 of the study protocol (see Supplemental Methods for protocol). A baseline GnRH test was performed on menstrual day 1 and was repeated in each subject 24 hours after final saline or kisspeptin injection (menstrual day 15).
Basal measurement of reproductive hormones
Basal measurements of serum LH, FSH, estradiol, progesterone, and plasma kisspeptin IR were taken from subjects during days 1, 6, 7, 11, 14, 15, 18, 21, and 28 during month 1 and month 2 of the study protocol. On days 7, 11, and 14, the basal blood sample was taken before the morning injection.
Ultrasound scans
Transabdominal ultrasound scans were performed on days 1, 7, 15, 18, 21, and 28 during month 1 and month 2 of the study protocol. Transvaginal scans were not used to minimize discomfort to volunteers during repeated examinations. The ultrasonographer was blinded to treatment for all subjects. During each scan, the following parameters were measured: endometrial thickness (in millimeters), mean ovarian volume (in cubic centimeters), mean follicle number, and maximum diameter of largest follicle in each ovary (in millimeters). Ovulation was defined as a rise in serum progesterone >10 nmol/L together with suggestive radiological features (visualization of a dominant follicle with subsequent appearance of a preovulatory follicle and/or corpus luteum).
Data analysis
Investigators performing the clinical studies were blinded to results until all subjects had completed the study protocol. J.D.V. used an established, blinded deconvolution method with 93% sensitivity and specificity (27) to identify LH pulses and calculate the secretory mass of LH pulses (integral of LH secretion over time during a secretory burst normalized per liter of distribution volume). Cumulative levels of basal, pulsatile, and total (basal plus pulsatile) LH secretion were also estimated during each study. Data are presented as mean ± SEM. Kisspeptin IR data were log-transformed to normalize data before data analysis. All other analyses included data series, most which had normal distributions assessed using the Kolmogorov-Smirnov test with Dallal-Wilkinson-Lillie analysis. Hormone profiles during 4-hour blood sampling studies were analyzed using repeated-measures 2-way ANOVA with Bonferroni post hoc correction. Pairs of means were analyzed using the unpaired two-tailed t test. Multiple means were compared using 1-way ANOVA with Bonferonni's multiple-comparison test. In all cases, P < .05 was considered statistically significant.
Results
Acute effects of saline or kisspeptin-54 injection on plasma kisspeptin at the commencement, midpoint, and end of twice-daily administration in healthy women
Acute changes in plasma kisspeptin IR after injection of saline or kisspeptin-54
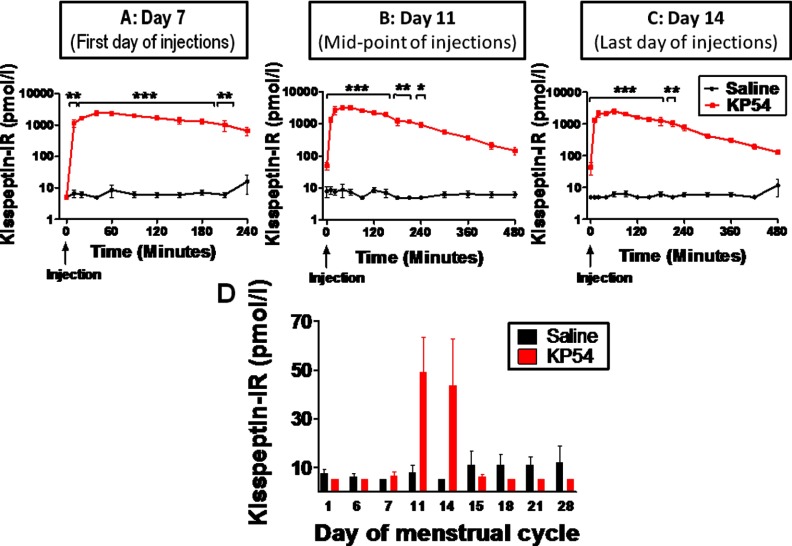
Plasma kisspeptin was unchanged at approximately 10 pmol/L after injection of saline on the first (Figure 2A), fourth (Figure 2B), and final (seventh; Figure 2C) days of twice-daily administration. Kisspeptin-54 injection acutely elevated plasma kisspeptin IR on the first day of administration, with peak mean kisspeptin IR levels of 2421 ± 392 pmol/L at 45 minutes after injection (P < .001 vs saline) (Figure 2A). Similar elevations of kisspeptin IR were observed after injection of kisspeptin on the fourth and last injection days (Figure 2, B and C).
Figure 2.
Acute changes in plasma kisspeptin levels in healthy women receiving twice-daily injections of saline or kisspeptin-54. A–C, Time profiles of plasma kisspeptin IR after sc bolus injection of saline or kisspeptin-54 (KP54) at time 0 on menstrual days 7 (A), 11 (B), and 14 (C) of the study protocol. D, Bar graph comparing mean preinjection kisspeptin IR after saline or kisspeptin-54 throughout the study protocol. All subjects commenced twice-daily treatment with saline or kisspeptin-54 on the morning of menstrual day 7 after their basal blood sample and finished treatment on the morning of day 14. Data are presented as mean ± SEM. *, P < .05; **, P < .01.
Plasma kisspeptin IR preinjection of saline or kisspeptin-54
In subjects receiving saline, plasma kisspeptin IR measured before the morning saline injection remained approximately 10 pmol/L throughout the study protocol (Figure 2D). In subjects receiving kisspeptin injections, plasma kisspeptin IR was not elevated on menstrual days 1, 6, or 7 because these blood samples were taken before the first kisspeptin-54 injection. Plasma kisspeptin IR was elevated on menstrual days 11 and 14 (during the twice-daily kisspeptin treatment period but just before the morning kisspeptin injection), which suggested compliance with kisspeptin injection the previous evening. As expected, plasma kisspeptin IR was not elevated on the morning of day 15, because it was approximately 24 hours after the final kisspeptin-54 injection. Furthermore, plasma kisspeptin IR remained <10 pmol/L on days 18 to 28 of the study protocol (Figure 1D).
Acute effects of saline or kisspeptin-54 injection on serum reproductive hormones at the commencement, midpoint, and end of twice-daily administration in healthy women
Commencement of treatment period (menstrual day 7)
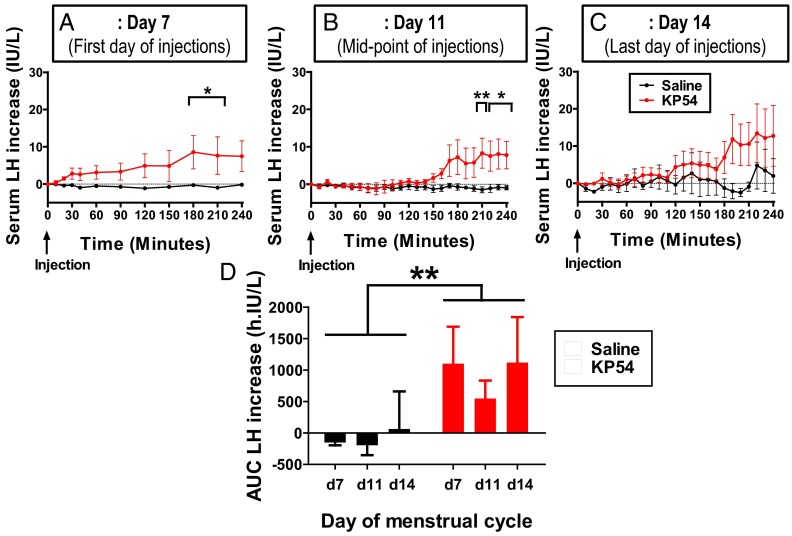
Saline injection did not change serum LH levels when compared with baseline (Figure 3A, baseline LH 5.20 ± 0.64 IU/L). Kisspeptin-54 injection acutely increased serum LH levels in subjects when compared with saline (Figure 3A, P < .05 at 180–210 minutes, baseline LH 5.68 ± 0.98 IU/L). The mean maximal increase in LH from baseline after kisspeptin injection was observed at 180 minutes and was 8.6 ± 3.4 IU/L above baseline.
Figure 3.
Acute changes in serum reproductive hormones in healthy women receiving twice-daily injections of saline or kisspeptin-54. A–C, Acute increases in serum LH after sc bolus injection of saline or kisspeptin-54 (KP54) at time 0 on menstrual days 7 (A), 11 (B), and 14 (C) of the study protocol. All subjects underwent treatment during menstrual days 7 and 14 with twice-daily sc bolus of saline or kisspeptin-54 injections. D, Summary bar graph comparing mean area under curve (AUC) LH increase after saline or kisspeptin-54 on days 7, 11, and 14 of the study protocol. Data are presented as mean ± SEM. *, P < .05; **, P < .01.
Midpoint of treatment period (menstrual day 11)
Saline injection did not change serum LH or FSH compared with baseline (Figure 3B, baseline LH 5.74 ± 1.45 IU/L). Kisspeptin-54 injection acutely increased serum LH levels in subjects when compared with saline (Figure 3B, P < .01 at 210 minutes, and P < .05 at 220 to 240 minutes, baseline LH 9.41 ± 4.89 IU/L). The mean maximal increase in LH from baseline after kisspeptin injection was observed at 210 minutes and was 8.3 ± 2.4 IU/L above baseline.
End of treatment period (menstrual day 14)
Saline injection did not change serum LH or FSH compared with baseline (Figure 3C, baseline LH 17.79 ± 9.69 IU/L). The mean maximal increase in LH from baseline after kisspeptin injection was observed at 220 minutes and was 12.7 ± 8.1 IU/L above baseline (baseline LH 6.01 ± 1.60 IU/L). Kisspeptin-54 injection showed a trend toward stimulating serum LH, but this did not reach statistical significance compared with saline. During days 7, 11, and 14 of the treatment period, total LH secretion was increased significantly after kisspeptin-54 when compared with saline (P < .01 using 2-way ANOVA) (Figure 3D).
We also compared the magnitude of serum LH increase 1 hour after kisspeptin-54 injection with the peak increase in serum LH after kisspeptin-54 injection (Supplemental Figure 1). On menstrual day 7, the peak LH response was 3-fold higher when compared with the LH response 1 hour after kisspeptin-54 injection (increase in serum LH was 9.2 ± 4.5 IU/L at 1 hour; peak 3.1 ± 1.8 IU/L, P = .063 vs 1 hour). On menstrual days 11 and 14, there was virtually no change in mean serum LH 1 hour after kisspeptin-54 injection (<1 IU/L), whereas peak increases in serum LH of 19.7 ± 9.5 IU/L (P = .059 vs 1 hour) and 23.0 ± 12.9 IU/L (P = .067 vs 1 hour) were later observed, respectively.
Effects of twice-daily saline or kisspeptin injections on length of the menstrual cycle and biochemical markers of reproductive activity in healthy women
Length of menstrual cycle
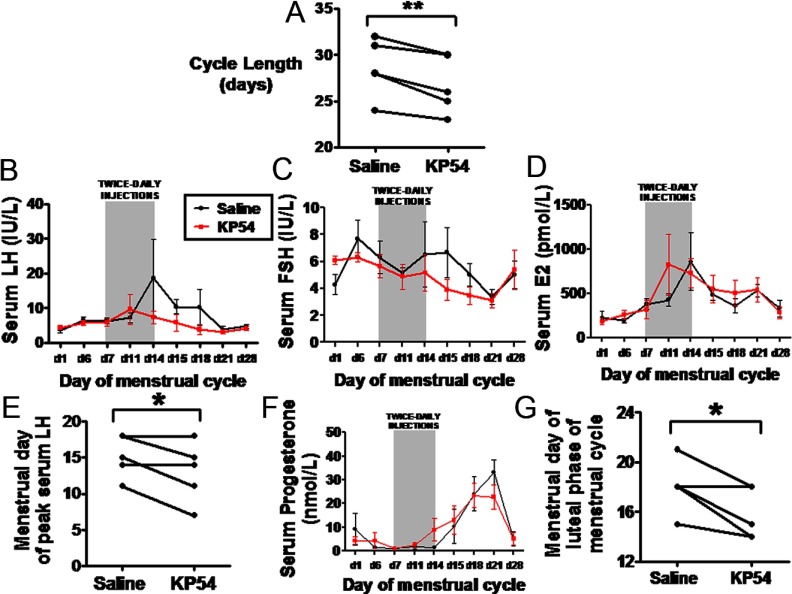
Subjects had the same menstrual cycle length before commencing the study when compared with menstrual cycle length during saline administration (mean menstrual cycle length was 28.6 ± 1.1 days before study commencement and 28.6 ± 1.4 days with saline; P = 1.00). However all subjects had a shorter menstrual cycle (by approximately 2 days) during kisspeptin-54 treatment when compared with saline (mean length of menstrual cycle was 28.6 ± 1.4 days with saline vs 26.8 ± 3.1 days with kisspeptin, P < .01) (Figure 4A).
Figure 4.
Levels of serum reproductive hormones throughout the menstrual cycle in healthy women receiving twice-daily injections of saline or kisspeptin-54. A, Comparison of cycle length in individual healthy women undergoing twice-daily sc bolus injections of saline or kisspeptin-54 (KP54) between menstrual days 7 and 14 of the study protocol. B–D, Levels of serum LH (B), FSH (C), and estradiol (D) during the menstrual cycle in healthy women undergoing twice-daily sc bolus injections of saline or kisspeptin-54 between menstrual days 7 and 14. E, Comparison of peak serum LH in individual healthy women undergoing twice-daily sc bolus injections of saline or kisspeptin-54 between menstrual days 7 and 14 of the study protocol. F and G, Levels of serum progesterone (F) and comparison of menstrual day of onset of the luteal phase (G) in individual healthy women undergoing twice-daily sc bolus injections of saline or kisspeptin-54 between menstrual days 7 and 14 of the study protocol. The luteal phase of the menstrual cycle was defined as beginning when serum progesterone was elevated >10 nmol/L. Data are presented as mean ± SEM. *, P < .05; **, P < .01.
Timing of peak serum LH
During kisspeptin-54 treatment, observed peak levels of serum LH and estradiol, but not FSH, were earlier during the menstrual cycle when compared with the saline group (Figure 4, B–D). Furthermore the menstrual day of highest recorded serum LH was approximately 2 days earlier during kisspeptin-54 treatment when compared with saline treatment (mean menstrual day of highest recorded serum LH was 15.2 ± 1.3 with saline vs 13.0 ± 1.9 with kisspeptin, P < .05) (Figure 4E).
Timing of luteal phase of menstrual cycle
We examined the onset of the luteal phase, which is characterized by release of a mature oocyte from the ovary and secretion of progesterone by the residual corpus luteum. During kisspeptin-54 treatment, levels of serum progesterone became elevated (>10 nmol/L) earlier during the menstrual cycle when compared with the saline group (Figure 4F). The menstrual day of onset of the luteal phase (defined as beginning when serum progesterone was elevated >10 nmol/L) was approximately 2 days earlier during kisspeptin-54 treatment when compared with saline treatment (mean menstrual day of serum progesterone increase >10 nmol/L was 18.0 ± 2.1 with saline vs 15.8 ± 0.9 with kisspeptin, P < .05) (Figure 4G).
Effects of twice-daily saline or kisspeptin injections on radiological markers of reproductive activity in healthy women
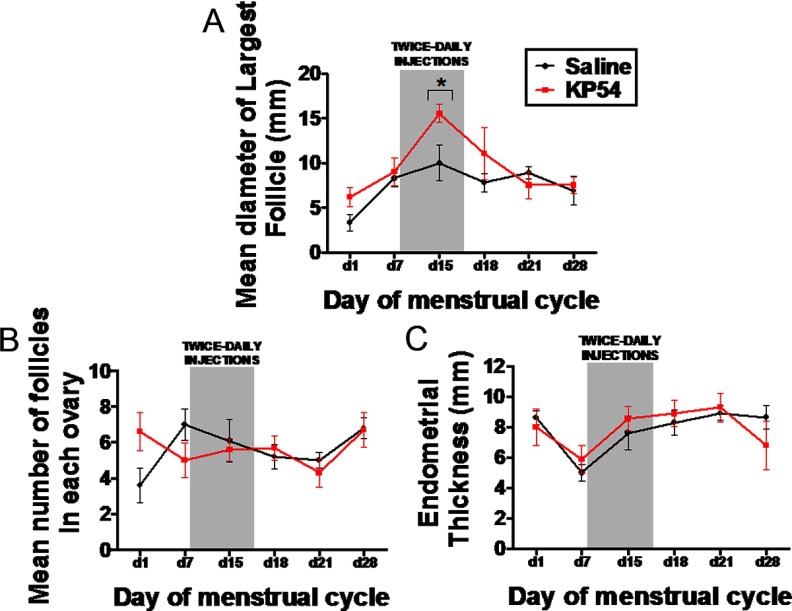
A corpus luteum, indicating recent ovulation, was observed in all women after kisspeptin-54 treatment and in 3 of 5 women after saline treatment. Elevated serum progesterone (>10 nmol/L) was observed in all subjects receiving saline or kisspeptin-54 treatment. Immediately after 7 days of kisspeptin-54 treatment (on menstrual day 15), the mean diameter of the largest follicle seen during ultrasonography was significantly higher when compared with women during saline treatment (mean diameter of largest follicle was 10.0 ± 2.2 mm with saline vs 15.5 ± 1.2 mm with kisspeptin, P < .05) (Figure 5A). No significant differences in number of follicles or endometrial thickness were observed during kisspeptin-54 treatment when compared with saline (Figure 5, B and C).
Figure 5.
Changes in radiological markers during the menstrual cycle of healthy women receiving twice-daily injections of saline or kisspeptin-54. A–C Mean values for diameter of largest ovarian follicle (A), number of ovarian follicles (B), and endometrial thickness (C) in healthy women undergoing twice-daily sc bolus injections of saline or kisspeptin-54 (KP54) (6.4 nmol/kg) between menstrual days 7 and 14. Data are presented as mean ± SEM.
Effects of twice-daily saline or kisspeptin on LH pulsatility in healthy women
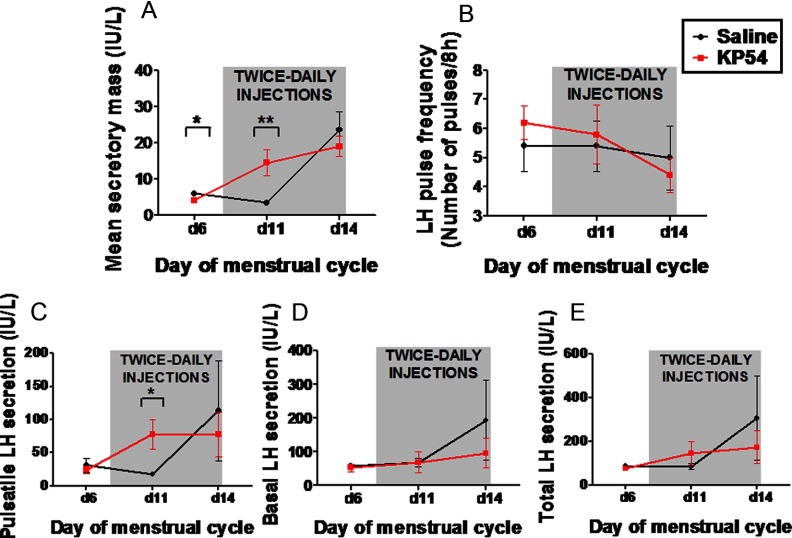
LH pulsatility was determined immediately before commencing saline or kisspeptin-54 treatment (menstrual day 6 of study protocol) and during saline or kisspeptin-54 treatment (menstrual days 11 and 14 of study protocol). Mean secretory mass was slightly lower before kisspeptin-54 treatment when compared with saline treatment (mean secretory mass was 5.9 ± 0.5 IU/L with saline vs 4.1 ± 0.4 IU/L with kisspeptin, P < .01) (Figure 6A). Despite this, mean secretory mass was increased significantly during menstrual day 11 (fourth day of treatment) during kisspeptin-54 treatment when compared with the saline treatment (mean secretory mass was 3.4 ± 0.4 IU/L with saline vs 14.5 ± 3.6 IU/L with kisspeptin, P < .01). On the last day of treatment (menstrual day 14), no significant differences in secretory mass were observed between saline or kisspeptin-54 treatment. No significant differences in pulse frequency were observed between saline and kisspeptin-54 treatment (Figure 6B). Pulsatile LH secretion was increased significantly during menstrual day 11 (fourth day of treatment) in the kisspeptin-54 group when compared with the saline group (mean secretory mass was 17.9 ± 3.6 IU/L with saline vs 78.2 ± 22.8 IU/L with kisspeptin, P < .05) (Figure 6C). However, basal and total LH secretion were not significantly different between groups (Figure 6, D and E).
Figure 6.
Changes in LH pulsatility during the menstrual cycle of healthy women receiving twice-daily injections of saline or kisspeptin-54. All women underwent frequent blood sampling for 8 hours on menstrual days 6, 11, and 14 of the study protocol. Twice-daily sc bolus injections of saline or kisspeptin-54 (KP54) (6.4 nmol/kg) were self-administered between menstrual days 7 and 14. A and B, Mean values for secretory mass (A) and estimated pulses per 24 hours (B). C–E, Levels of pulsatile (C), basal (D), and total (E) LH secretion during each 8-h sampling study. Data are presented as mean ± SEM. *, P < .05; **, P < .01.
Assessment of pituitary sensitivity before and after injections of saline or kisspeptin
We examined the sensitivity of all healthy female subjects to iv GnRH, both 6 days before (menstrual day 1 of study protocol) and 24 hours after saline or kisspeptin treatment (menstrual day 15 of study protocol). On menstrual day 1, no significant difference in pituitary sensitivity was observed after GnRH administration between subjects before commencing saline of kisspeptin treatment (mean maximal LH increase during first 2 hours after GnRH injection was 13.1 ± 1.1 IU/L before saline and 13.4 ± 1.1 IU/L before kisspeptin-54; P value was not significant) (Supplemental Figure 2A). Furthermore, 24 hours after cessation of twice-daily saline or kisspeptin-54 injections, no significant difference in pituitary sensitivity was observed after GnRH administration between saline and kisspeptin-54 treatment (mean maximal LH increase during first 2 hours after GnRH injection was 39.9 ± 8.9 IU/L after saline and 41.0 ± 16.4 IU/L after kisspeptin-54; P value was not significant) (Supplemental Figure 2B).
The expected physiological increase in pituitary sensitivity to GnRH during menstrual day 15 vs menstrual day 1 (28) was observed in healthy female subjects, whether receiving saline or kisspeptin treatment (Supplemental Figure 2C).
Discussion
Genetic studies demonstrate that kisspeptin peptides are necessary for pubertal maturation in humans (8–10). We and other investigators have recently demonstrated that exogenous kisspeptin acutely stimulates gonadotropin secretion in women (23–26). However, the pharmacological effects of chronic kisspeptin exposure in healthy women with regular menstrual cycles have not been studied previously. Chronic kisspeptin administration causes profound tachyphylaxis in male monkeys and in women in functional HA (16, 25, 26). We present novel data suggesting that menstrual cyclicity persists in healthy women after twice-daily kisspeptin-54 treatment during the follicular phase of the menstrual cycle.
In the current study, kisspeptin treatment was associated with an advanced timing of the serum progesterone increase and onset of menstrual bleeding by approximately 2 days when compared with saline. Animal studies demonstrate that kisspeptin stimulates gonadotropin secretion in a GnRH-dependent manner because its action is abolished by GnRH antagonist (14). Exogenous GnRH is sufficient to trigger ovulation (29). More studies with daily follicle morphology assessment and daily serum LH measurement would be required to confirm our findings. However, our study data raise the possibility that kisspeptin-54 administration may advance the onset of ovulation in healthy women by stimulating endogenous hypothalamic GnRH secretion. In addition to stimulating GnRH, kisspeptin itself is implicated in generation of the LH surge needed for ovulation. In rodents, a subpopulation of hypothalamic anteroventral periventricular nucleus kisspeptin neurons are implicated in generating the LH surge needed for ovulation (30) and are positively rather than negatively regulated by estradiol (31). Central administration of a monoclonal antibody to kisspeptin is sufficient to block ovulation in rats (32). Furthermore, administration of kisspeptin-10 has been shown to stimulate ovulation in the musk shrew (33), rat (34), and sheep (17). Humans have no anatomical equivalent of the anteroventral periventricular nucleus. Nevertheless, it is possible that exogenous kisspeptin-54 may have advanced the onset of the luteal phase in our female subjects by increasing kisspeptin signaling in a subpopulation of hypothalamic kisspeptin neurons that stimulate the LH surge. Several lines of evidence suggest that in humans, unlike in lower species, a change in pituitary responsiveness to GnRH rather than an actual GnRH surge is responsible for the LH surge (35, 36). It is therefore possible that kisspeptin-54 advances ovulation in women by increasing the prevailing levels of estradiol, which are needed to trigger the LH surge at a pituitary level.
Chronic kisspeptin administration has also been implicated as a potential novel therapy for inhibiting reproductive hormone secretion in contraception or the treatment of hormone-sensitive cancer; our data suggest that kisspeptin-54 may have limited therapeutic potential in this regard, at least in women at the dose of kisspeptin tested. However, our observation that kisspeptin may advance the menstrual cycle raises the possibility that women with certain forms of infertility could be treated with kisspeptin. A recent study suggests that kisspeptin is sufficient to restore ovulation in a mouse model of anovulatory hyperprolactinemia (37). More studies are required to determine whether kisspeptin-54 treatment could be used to stimulate ovulation in women with anovulatory reproductive disorders other than HA.
Traditional ovulatory drugs such as clomiphene citrate have low pregnancy rates (38, 39). Although efficacious, in vitro fertilization confers a risk of ovarian hyperstimulation (40). Kisspeptin acts by stimulating endogenous, rather than pharmacological, levels of reproductive hormone secretion. Kisspeptin might therefore offer a fertility treatment with lower risk of ovarian hyperstimulation syndrome when compared with in vitro fertilization.
Comparison of our data with other clinical studies of kisspeptin administration is complicated by the investigation of its 2 peptide forms (kisspeptin-54 and -10) and sexual dimorphism of the effects of kisspeptin-10; iv bolus administration consistently stimulates LH men (20–22), but women are much less sensitive to the effects of kisspeptin-10 during the follicular phase of the menstrual cycle when compared with the preovulatory phase (22, 24). Although kisspeptin-54 stimulates LH in both sexes, its effects have never been compared directly between men and women (19, 23). Consistent with our findings in healthy women, George et al (21) recently observed that a 22.5-hour iv infusion of the shorter form of kisspeptin, kisspeptin-10, stimulates LH secretion without apparent tachyphylaxis in healthy men. However, in contrast to the current findings, twice-daily kisspeptin-54 treatment has been shown to rapidly cause tachyphylaxis in women with HA. Women with HA are acutely 4-fold more sensitive to the effects of kisspeptin-54 when compared with healthy women in the follicular phase of their menstrual cycle. A higher dose of kisspeptin-54 may therefore be necessary to achieve tachyphylaxis in healthy female volunteers (23, 25). Nevertheless, our data suggest that menstrual cyclicity persists in healthy women using the currently tested regimen of follicular-phase kisspeptin-54 treatment. Preliminary data have emerged suggesting that chronic administration of a kisspeptin analog causes tachyphylaxis in healthy men (41). It would be interesting to determine whether tachyphlyaxis to kisspeptin-54 is also sexually dimorphic or dependent on the dose and precise form of kisspeptin receptor agonist administered.
It is interesting to note that although iv bolus injection of the shorter kisspeptin fragment, kisspeptin-10, acutely stimulates LH secretion within an hour of administration, we observed that sc injection of kisspeptin-54 acutely stimulated peak LH secretion 3 to 4 hours after administration despite a rapid elevation of kisspeptin IR within minutes of administration. On the first injection day, the increase in serum LH 1 hour after kisspeptin-54 injection was 3-fold lower when compared with the peak increase in serum LH after kisspeptin-54 injection. Furthermore, on the fourth and final injection days, there was negligible stimulation of LH secretion 1 hour after kisspeptin-54 injection; however, serum LH later increased to peak levels 3 to 4 hours after kisspeptin injection. By comparison, Chan et al (20) observed that kisspeptin-10 stimulated peak LH secretion within an hour of commencing administration in healthy men. It is possible that these data suggest differences between the biological actions of kisspeptin-10 and -54. For instance, one might speculate that kisspeptin-10 stimulates a readily releasable pool of GnRH stored within nerve terminals of the median eminence (42, 43), whereas kisspeptin-54 might act more proximally in the GnRH neuron to stimulate de novo GnRH synthesis. Alternatively, it is possible kisspeptin-54 might require breakdown into a smaller kisspeptin fragment before becoming biologically active. However, liquid chromatography has only identified kisspeptin-54 in the human circulation acutely after kisspeptin-54 administration (19).
It is interesting to appraise the evidence that exogenous kisspeptin stimulates basal and pulsatile GnRH secretion. Although kisspeptin stimulates rat pituitary gonadotropin secretion in vitro (44), animal studies using GnRH antagonists support the view that exogenous kisspeptin stimulates basal LH secretion through a GnRH-dependent mechanism (45, 46), which is possibly mediated through GnRH nerve terminals at the median eminence (42, 43). However, it is less clear whether exogenous kisspeptin stimulates endogenous GnRH pulsatility. Sustained exposure to exogenous kisspeptin-10 or -54 stimulates pulsatile LH secretion for several hours in healthy men (21) and patients with inactivating mutations of the neurokinin B signaling pathway (47). However, because these studies merely measure LH pulsatility, it is not possible to exclude that effects reflect increasing circulating estrogen and pituitary responsiveness to unchanged, endogenous GnRH pulsatility. Chan and colleagues (20) recently demonstrated that iv bolus kisspeptin-10 increased the time to the next endogenous LH pulse in healthy men; this may represent the only current data suggesting that exogenous kisspeptin can directly modulate endogenous GnRH pulsatility in humans (by increasing the latency period to the next LH/GnRH pulse).
It is important to recognize that the current study is based on observations within a small number of subjects so may have insufficient statistical power to reveal subtle effects of kisspeptin-54 administration on menstrual cyclicity or LH pulsatility. Furthermore, the gonadotropin response to kisspeptin treatment is known to alter with the phase of the menstrual cycle in healthy women (23, 24). A larger study is needed to confirm our findings and determine what factors influence the variability in the response of subjects to kisspeptin treatment. It is possible that chronic kisspeptin-54 treatment might have effects that last beyond the treatment period. For this reason, we designed a 1-way crossover protocol in which subjects self-administered saline during the first month followed by kisspeptin-54 during the second month. It therefore remains possible that an order effect might have contributed to our results. However, it is noteworthy that LH pulse secretory mass was marginally lower at the start of the second month when compared with LH pulse secretory mass at the start of the first month (when greater stress levels would be expected). Furthermore, subjects had the same menstrual cycle length before commencing the study when compared with menstrual cycle length during saline administration. We also recognize that although estradiol measurements were compared with placebo-controlled values, the automated platform assay used to analyze serum estradiol can cross-react with other steroids.
Mean peak levels of LH during the menstrual cycle were lower during kisspeptin treatment when compared with saline treatment. Furthermore, it is important to recognize that kisspeptin-54 did not acutely stimulate significant LH secretion during the final day of twice-daily kisspeptin-54 treatment. We therefore cannot exclude that kisspeptin-54 treatment caused partial tachyphylaxis in healthy female volunteers. Alternatively, the short baseline sampling period involving just 2 blood samples may have reduced our power to detect stimulation of LH after kisspeptin-54 injection on day 14. LH levels were highly variable during menstrual day 14, which may have been caused by some but not all subjects experiencing an LH surge and by the potential effect of kisspeptin-54 to advance the onset of the LH surge in subjects.
It is noteworthy that all subjects had peak progesterone levels ≥21 mmol/L after kisspeptin-54 treatment, which is highly suggestive of ovulation. Furthermore, a corpus luteum was observed in all 5 subjects during the month of kisspeptin-54 treatment (Supplemental Table 2). Our data therefore suggest that all 5 subjects had ovulatory cycles during kisspeptin treatment. More studies are required to determine whether menstrual cycles during kisspeptin therapy have subtle physiological differences when compared with natural menstrual cycles and to determine whether anovulation is observed at a different frequency during kisspeptin treatment when compared with placebo treatment.
In summary, our data have important pharmacological implications; menstrual cyclicity persists in healthy women during a treatment regime of kisspeptin-54 previously demonstrated to cause tachyphylaxis in women with HA. Furthermore, kisspeptin-54 treatment is associated with an advanced timing of the luteal phase of the menstrual cycle when compared with placebo. More studies are required to determine whether kisspeptin-54 therapy could have potential to treat patients with anovulatory reproductive disorders.
Acknowledgments
We are grateful to the National Institute for Health Research/Wellcome Trust Clinical Research Facility at Imperial College Healthcare NHS Trust for providing infrastructure for this study.
This work was funded by a Medical Research Council project grant. The department is funded by an Integrative Mammalian Biology Capacity Building Award and the National Institute for Health Research (NIHR) Biomedical Research Centre Funding Scheme. C.N.J. is supported by an NIHR Clinical Lectureship, an Academy of Medical Sciences/Wellcome Starter Grant for Clinical Lecturers, and a Society for Endocrinology Early Career Grant. A.N.C. and A.D.S. are supported by Wellcome/GlaxoSmithKline Clinical Research Fellowships. G.M.K.N. and A.A. are supported by Wellcome Clinical Research Training Fellowships. R.R. and C.I. are supported by NIHR Academic Clinical Fellowships. W.S.D. is supported by an NIHR Career Development Fellowship.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- HA
- hypothalamic amenorrhea
- IR
- immunoreactivity.
References
- 1. Clements MK, McDonald TP, Wang R, et al. FMRFamide-related neuropeptides are agonists of the orphan G-protein-coupled receptor GPR54. Biochem Biophys Res Commun. 2001;285:1189–1193 [DOI] [PubMed] [Google Scholar]
- 2. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636 [DOI] [PubMed] [Google Scholar]
- 3. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617 [DOI] [PubMed] [Google Scholar]
- 4. Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975 [DOI] [PubMed] [Google Scholar]
- 5. Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 7. Richard N, Galmiche G, Corvaisier S, Caraty A, Kottler ML. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–393 [DOI] [PubMed] [Google Scholar]
- 8. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 10. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635 [DOI] [PubMed] [Google Scholar]
- 11. Thompson EL, Patterson M, Murphy KG, et al. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858 [DOI] [PubMed] [Google Scholar]
- 12. Patterson M, Murphy KG, Thompson EL, Patel S, Ghatei MA, Bloom SR. Administration of kisspeptin-54 into discrete regions of the hypothalamus potently increases plasma luteinising hormone and testosterone in male adult rats. J Neuroendocrinol. 2006;18:349–354 [DOI] [PubMed] [Google Scholar]
- 13. Tovar S, Vázquez MJ, Navarro VM, et al. Effects of single or repeated intravenous administration of kisspeptin upon dynamic LH secretion in conscious male rats. Endocrinology. 2006;147:2696–2704 [DOI] [PubMed] [Google Scholar]
- 14. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077 [DOI] [PubMed] [Google Scholar]
- 15. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr, Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–2126 [DOI] [PubMed] [Google Scholar]
- 17. Caraty A, Smith JT, Lomet D, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–5267 [DOI] [PubMed] [Google Scholar]
- 18. Redmond JS, Macedo GG, Velez IC, Caraty A, Williams GL, Amstalden M. Kisspeptin activates the hypothalamic-adenohypophyseal-gonadal axis in prepubertal ewe lambs. Reproduction. 2011;141:541–548 [DOI] [PubMed] [Google Scholar]
- 19. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 20. Chan YM, Butler JP, Pinnell NE, et al. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96:E908–E915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. George JT, Veldhuis JD, Roseweir AK, et al. Kisspeptin-10 is a potent stimulator of LH and increases pulse frequency in men. J Clin Endocrinol Metab. 2011;96:E1228–E1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jayasena CN, Nijher GM, Comninos AN, et al. The effects of kisspeptin-10 on reproductive hormone release show sexual dimorphism in humans. J Clin Endocrinol Metab. 2011;96:E1963–E1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhillo WS, Chaudhri OB, Thompson EL, et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 24. Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97:E1458–E1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jayasena CN, Nijher GM, Chaudhri OB, et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab. 2009;94:4315–4323 [DOI] [PubMed] [Google Scholar]
- 26. Jayasena CN, Nijher GM, Abbara A, et al. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther. 2010;88:840–847 [DOI] [PubMed] [Google Scholar]
- 27. Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab. 2009;297:E538–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotrophin-releasing hormone. Science. 1980;207:1371–1373 [DOI] [PubMed] [Google Scholar]
- 29. Grimes EM, Taymor ML, Thompson IE. Induction of timed ovulation with synthetic luteinizing hormone-releasing hormone in women undergoing insemination therapy. I. Effect of a single parenteral administration at midcycle. Fertil Steril. 1975;26:277–282 [DOI] [PubMed] [Google Scholar]
- 30. Clarkson J, d'Anglemont de Tassigny X, et al. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–8697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adachi S, Yamada S, Takatsu Y, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53:367–378 [DOI] [PubMed] [Google Scholar]
- 33. Inoue N, Sasagawa K, Ikai K, et al. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus murinus). Proc Natl Acad Sci U S A. 2011;108:17527–17532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388 [DOI] [PubMed] [Google Scholar]
- 35. Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley WF., Jr Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proc Natl Acad Sci U S A. 1994;91:6894–6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adams JM, Taylor AE, Schoenfeld DA, Crowley WF, Jr, Hall JE. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab. 1994;79:858–864 [DOI] [PubMed] [Google Scholar]
- 37. Sonigo C, Bouilly J, Carré N, et al. Hyperprolactinemia-induced ovarian acyclity is reversed by kisspeptin administration. J Clin Invest. 2012;122:3791–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update. 1997;3:359–365 [DOI] [PubMed] [Google Scholar]
- 39. Hart R, Norman R. Polycystic ovarian syndrome–prognosis and outcomes. Best Pract Res Clin Obstet Gynaecol. 2006;20:751–778 [DOI] [PubMed] [Google Scholar]
- 40. Min JK, Breheny SA, MacLachlan V, Healy DL. What is the most relevant standard of success in assisted reproduction? The singleton, term gestation, live birth rate per cycle initiated: the BESST endpoint for assisted reproduction. Hum Reprod. 2004;19:3–7 [DOI] [PubMed] [Google Scholar]
- 41. Matsui H. 2012. Testosterone depletion by continuous administration of kisspeptin analogues. Paper presented at: Second World Conference of Kisspeptin Signaling in the Brain; November 9, 2012; Tokyo, Japan [Google Scholar]
- 42. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. d'Anglemont de Tassigny X, Ackroyd KJ, Chatzidaki EE, Colledge WH. Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J Neurosci. 2010;30:8581–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matsui H, Tanaka A, Yokoyama K, et al. Chronic administration of the metastin/kisspeptin analog KISS1–305 or the investigational agent TAK-448 suppresses hypothalamic pituitary gonadal function and depletes plasma testosterone in adult male rats. Endocrinology. 2012;153:5297–5308 [DOI] [PubMed] [Google Scholar]
- 45. Gutiérrez-Pascual E, Martínez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagón MM, Castaño JP. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;19:521–530 [DOI] [PubMed] [Google Scholar]
- 46. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102:2129–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young J, George JT, Tello JA, et al. Kisspeptin restores pulsatile LH secretion in patients with neurokinin B signaling deficiencies: physiological, pathophysiological and therapeutic implications. Neuroendocrinology. 2013;97:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]