Graphical Abstract
Two new highly oxygenated limonoids, flexuosoids A (1) and B (2), and three new arylnaphthalene lignan glycosides, phyllanthusmins D–F (3–5), were isolated from the roots of Phyllanthus flexuosus, in addition to three known lignans, phyllanthusmin C, arabelline, and (+)-diasyringaresinol. Their structures were elucidated on the basis of detailed spectroscopic analysis and chemical methods. Compounds 1 and 2, two new decaoxygenated limonoids with a C-19/29 lactol bridge and heptaoxygenated substituents at C-1, C-2, C-3, C-7, C-11, C-17, and C-30, represent the second example of limonoids in the Euphorbiaceae family. Most of the isolates were tested for their antifeedant, anti-herpes simplex virus 1, and cytotoxic activities. The new limonoids 1 and 2 showed promising antifeedant activity against the beet army worm (Spodoptera exigua) with EC50 values of 25.1 and 17.3 μg/cm2, respectively. In addition, both of them displayed moderate cytotoxicity against the ECA109 human esophagus cancer cell line, along with the known lignan glycoside, phyllanthusmin C, with the IC50 values of 11.5 (1), 8.5 (2), and 7.8 (phyllanthusmin C) μM, respectively.
Two new highly oxygenated limonoids, flexuosoids A (1) and B (2), and three new arylnaphthalene lignan glycosides, phyllanthusmins D–F (3–5), were isolated from the roots of Phyllanthus flexuosus, along with three known lignans. Flexuosoids A (1) and B (2) showed promising antifeedant activity against the beet army worm (Spodoptera exigua) with EC50 values of 25.1 and 17.3 μg/cm2, respectively. In addition, both of them and the known lignan glycoside, phyllanthusmin C, displayed moderate cytotoxicity against the ECA109 human esophagus cancer cell line, with the IC50 values of 11.5, 8.5, and 7.8 μM, respectively.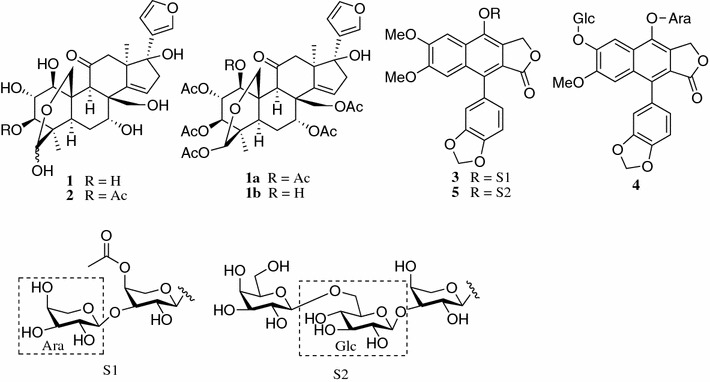
Electronic supplementary material
The online version of this article (doi:10.1007/s13659-014-0026-2) contains supplementary material, which is available to authorized users.
Keywords: Phyllanthus flexuosus, Euphorbiaceae, Limonoids, Lignan glycosides, Antifeedant, Antiviral, Cytotoxicity
Introduction
The genus Phyllanthus (Euphorbiaceae), comprising about 600 species, is widespread throughout the tropical and subtropical countries of the world, with about 30 species growing in the South of China. Several species have been used as traditional medicines [1], and flavonoids, alkaloids, sesquiterpenoids, triterpenoids, lignans, and tannins have been reported from the genus [2–6]. Among these, some sesquiterpenoids showed a wide range of biological properties including cytotoxic and antiviral effects [5–7].
Phyllanthus flexuosus (Sieb. et Zucc.) Muell. Arg (Euphorbiaceac), a shrub up to 3 m height, mainly grows in the southern parts of the Yangtze River, People’s Republic of China. The whole plants have been utilized medicinally for treating infectious diseases by the Dai people in Yunnan Province, China [8]. Chemical studies on the leaves and stem barks of P.flexuosus have revealed the occurrence of triterpenoids, tannins and coumarins [9–12]. Previously we obtained three phenylacetylene-bearing tricyclic diterpenes from this species, among which phyllanflexoids A was the first example of phenylacetylene-bearing 18-nor-diterpenoid glycoside [13]. As a part of our continuing research for new bioactive secondary metabolites from the genus Phyllanthus [6, 14–17], five new compounds, including two highly oxygenated limonoids, flexuosoids A (1) and B (2), and three arylnaphthalene lignan glycosides, phyllanthusmins D–F (3–5), were isolated from the roots of P. flexuosus, together with three known lignans. Their structures were elucidated by extensive spectroscopic analysis and chemical method. Most of the isolated compounds were evaluated for their antifeedant, anti HSV-1, and cytotoxic activities, and the results obtained are discussed herein.
Results and Discussion
The MeOH extract of the air-dried roots of P. flexuosus was applied to repeated column chromatography over macroporous resin D101, Sephadex LH-20 and silica gel, followed by semi-preparative HPLC, to afford five new compounds (1–5), in addition to three known lignans. The known compounds were identified as phyllanthusmin C [18], arabelline [19], and (+)-diasyringaresinol [20] by comparison of their spectroscopic data with literature values (Fig. 1).
Fig. 1.
Structures of compounds from the roots of P. flexuosus
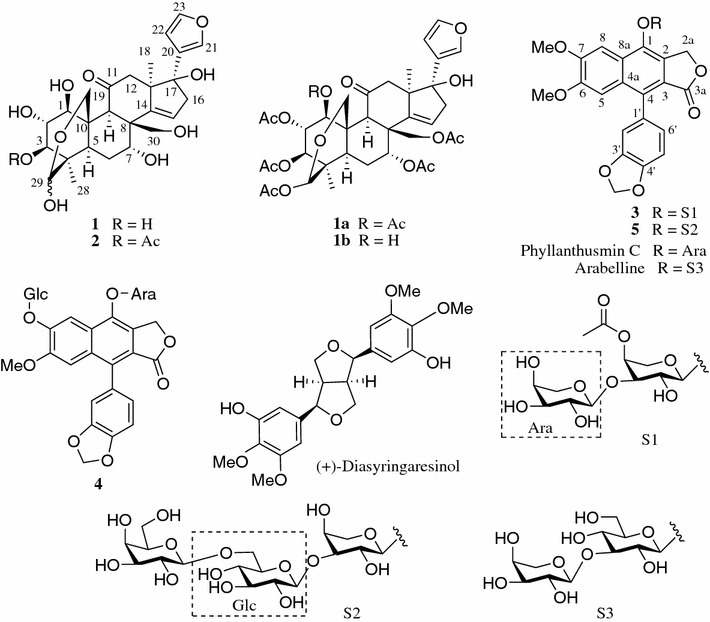
Compound 1 was obtained as a white amorphous powder. The negative HRESIMS (m/z 505.2072 [M − H]−, calcd for C26H33O10, 505.2073) and the 13C NMR (DEPT) spectra of 1 indicated a molecular formula of C26H34O10, requiring for 10 degrees of unsaturation. The IR spectrum of 1 indicated the presence of hydroxy (3424 cm−1) and carbonyl (1691 cm−1) groups. The 13C NMR (DEPT) data for 1 (Table 1) revealed 26 carbon signals, including characteristic signals due to a hemiacetal ring unit (δC 97.36 and 64.81) [21], a β-substituted furan ring (δC 128.55, 141.25, 110.63 and 144.33) [22], a trisubstituted double bond (δC 153.48 and 121.76) and a ketone (δC 214.73). The hemiacetal ring [H2-19: δH 4.20 and 4.52 (each 1H, J = 12.9 Hz); H-29: δH 4.83] [21] and the β-substituted furan ring protons (δH 6.42, 7.52, 7.47) [22] were also distinguished from the 1H NMR spectrum (Table 2), in addition to two methyl singlets at δH 0.97 and 1.03. The aforementioned NMR data for 1 were closely related to those of 3α-deacetylamoorastatin [23]. However, a major difference between these two compounds was observed as the oxidation of a methyl and a methylene in 3α-deacetyl-amoorastatin core to an oxymethylene (δC 71.92, δH 3.60, 3.89 (each 1H, d, J = 10.7 Hz) and an oxymethine [δC 68.60, δH 4.54 (dd, J = 4.4, 3.4 Hz] in 1, respectively. Moreover, the 14,15-epoxide and the C-17 methane in 3α-deacetyl-amoorastatin were replaced with a Δ14(15) double bond (δC 153.48 and 121.76) and an oxygen-bearing quaternary carbon (δC 84.21, C-17) in 1, respectively. The above data were further assigned unambiguously by HSQC, 1H-1H COSY and HMBC analysis (Fig. 2 and Electronic supplementary material). The 1H-1H COSY spectrum verified the presence of -CH(O)(1)–CH(O)(2)–CH(O)(3)–, –CH(5)–CH2(6)–CH(O)(7)–, and =CH(15)–CH2(16) fragments in 1 (bold lines in Fig. 2). The HMBC correlations (Fig. 2) from the oxymethylene at δH 3.60 and 3.89 (H2-30) to δC 69.54 (C-7)/δC 52.89 (C-8)/δC 46.06 (C-9)/δC 153.48 (C-14), and from the oxymethine at δH 4.54 (H-2) to δC 75.84 (C-1)/δC 43.96 (C-4)/δC 43.41 (C-10) indicated that C-30 and C-2 of 1 were oxygenated, respectively. The Δ14(15) double bond was confirmed by the HMBC correlations of H2-12, H2-16 and Me-18 to the olefinic carbon at δC 153.48 (C-14). Moreover, HMBC correlations from H-15, H2-16, Me-18, H-21 and H-22 to the oxygen-bearing quaternary carbon (δC 84.21, C-17) revealed the hydroxylated C-17 of D ring, on which the furan ring was located in 1. Other HMBC correlations confirmed the planar structure of 1 as shown.
Table 1.
13C NMR spectroscopic data of 1 and 2 (methanol-d4, δ in ppm)
| Position | 1a | 2a | ||
|---|---|---|---|---|
| 29-exo | 29-endo | 29-exo | 29-endo | |
| 1 | 75.84, CH | 75.76, CH | 74.36, CH | 74.36, CH |
| 2 | 68.60, CH | 68.81, CH | 67.88, CH | 67.99, CH |
| 3 | 75.78, CH | 79.27, CH | 76.69, CH | 79.83, CH |
| 4 | 42.96, C | 43.17, C | 42.65, C | 42.58, C |
| 5 | 27.69, CH | 25.06, CH | 29.15, CH | 27.76, CH |
| 6 | 25.62, CH2 | 27.77, CH2 | 25.70, CH2 | 26.67, CH2 |
| 7 | 69.54, CH | 69.79, CH | 69.61, CH | 69.88, CH |
| 8 | 52.89, C | 52.95, C | 52.96, C | 53.01, C |
| 9 | 46.06, CH | 46.36, CH | 46.06, CH | 46.39, CH |
| 10 | 43.41, C | 43.51, C | 43.41, C | 43.41, C |
| 11 | 214.73, C | 214.89, C | 214.71, C | 214.89, C |
| 12 | 44.31, CH2 | 44.31, CH2 | 44.37, CH2, | 44.52, CH2 |
| 13 | 53.03, C | 52.96, C | 53.15, C | 52.90, C |
| 14 | 153.48, C | 153.55, C | 153.55, C | 153.60, C |
| 15 | 121.76, CH | 121.69, CH | 121.89, CH | 121.80, CH |
| 16 | 44.45, CH2 | 44.39, CH2 | 44.59, CH2 | 44.45, CH2 |
| 17 | 84.21, C | 84.21, C | 84.33, C | 84.33, C |
| 18 | 26.12, CH3 | 26.05, CH3 | 26.21, CH3 | 26.15, CH3 |
| 19 | 64.81, CH2 | 59.49, CH2 | 65.17, CH2 | 59.32, CH2 |
| 20 | 128.55, C | 128.55, C | 128.79, C | 128.79, C |
| 21 | 141.25, CH | 141.25, CH | 141.34, CH | 141.34, CH |
| 22 | 110.63, CH | 110.63, CH | 110.73, CH | 110.73, CH |
| 23 | 144.33, CH | 144.33, CH | 144.42, CH | 144.42, CH |
| 28 | 20.45, CH3 | 19.73, CH3 | 19.75, CH3 | 19.32, CH3 |
| 29 | 97.36, CH | 97.09, CH | 96.88, CH | 96.80, CH |
| 30 | 71.92, CH2 | 72.01, CH2 | 72.04, CH2 | 72.08, CH2 |
| COMe | 173.31, C | 173.25, C | ||
| COMe | 21.23, CH3 | 21.18, CH3 | ||
aData were measured at 125 MHz
Table 2.
1H NMR spectroscopic data of 1 and 2 (methanol-d4, δ in ppm)
| Position | 1a | 2a | ||
|---|---|---|---|---|
| 29-exo | 29-endo | 29-exo | 29-endo | |
| 1 | 4.35, d (4.4) | 4.35, d (4.4) | 4.37, d (4.6) | 4.37, d (4.6) |
| 2 | 4.54, dd (4.4, 3.4) | 4.50b | 4.59b | 4.54b |
| 3 | 3.93, d (3.4) | 3.42, d (3.2) | 5.50, d (4.8) | 4.97, d (4.8) |
| 5 | 2.63, dd (13.9, 3.0) | 2.48b | 2.64, dd (10.9, 2.9) | 2.71b |
| 6 |
1.78, dd (13.7, 3.5) 1.90, br. d (14.5) |
1.81, br. d 2.48b |
1.76, dd (10.9, 3.5) 1.88, br. d (14.1) |
1.78b 2.45b |
| 7 | 4.05, br. d | 4.02, br. d | 4.04, br. d | 4.00, br. d |
| 9 | 4.01, s | 4.03, s | 4.01, s | 4.03, s |
| 12 |
1.99, d (19.2) 3.25, d (17.9) |
1.99, d (19.2) 3.25, d (17.9) |
3.25, d (19.3) 1.89, d (19.1) |
3.25, d (19.3) 1.89, d (19.1) |
| 15 | 5.73, br. d | 5.73, br. d | 5.73, br. d | 5.73, br. d |
| 16 |
2.52, dd (16.3, 3.3) 3.25, d (17.9) |
2.52, dd (16.3, 3.3) 3.25, d (17.9) |
2.51, dd, (16.3, 3.5) 3.24, d (16.2) |
2.51, dd, (16.3, 3.5) 3.24, d (16.2) |
| 18 | 1.03, s | 1.03, s | 1.04, s | 1.02, s |
| 19 |
4.20, d (12.9) 4.52, d (12.9) |
4.52b 4.56b |
4.18, d (10.2) 4.56b |
4.38b 4.38b |
| 21 | 7.52, s | 7.52, s | 7.53, s | 7.53, s |
| 22 | 6.42, s | 6.42, s | 6.43, s | 6.43, s |
| 23 | 7.47, s | 7.47, s | 7.47, s | 7.47, s |
| 28 | 0.97, s | 1.00, s | 0.82, s | 0.83, s |
| 29 | 4.83, s | 4.62, s | 4.80, s | 4.72, s |
| 30 |
3.60, d (10.7) 3.89, d (10.7) |
3.61, d (9.8) 3.93, d (9.8) |
3.60, d (11.1) 3.87, d (11.1) |
3.63b 3.92, d (11.1) |
| COMe | 2.09, s | 2.08, s | ||
aData were measured at 500 MHz
bOverlapping 1H NMR signals are reported without designated multiplicity
Fig. 2.
Key 1H-1H COSY (–) and HMBC ( ) correlations of 1
) correlations of 1
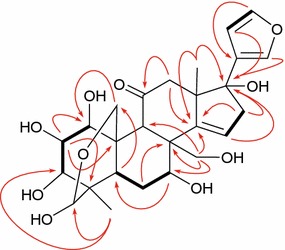
The relative stereochemistry of 1 was fixed by the proton coupling constants observed in the NMR spectrum and ROESY experiment (Fig. 3). The ROESY correlations of H-5/H-9, H-1/H-9, H-5/Me-28, H-3/Me-28, H-3/H-5, and H-2/H-19 supported the relative configuration of the fused A/B rings as shown in Fig. 1, and the hemiacetal ring between C-19 and C-29 was on the same side to those of 1-OH and 3-OH. The small values of J1,2 (4.4 Hz) and J2,3 (3.4 Hz) indicated the equatorial H-1, H-2 and H-3, corresponding to β-configurations for 1-OH, and 3-OH, and α-configurations for 2-OH. In the 1H NMR spectrum of 1, the broad singlet H-7 suggested an α-configuration of 7-OH, which was further confirmed by ROESY correlations of H-29/H-19, H-19/H-30 and H-30/H-7. The 17-OH was assigned as β by a ROESY correlation of H-22 with Me-18. In addition, it is noted that compound 1 was isolated as a mixture of two tautomers in a ratio of 4:1 as estimated by 1H NMR spectrum. The 1H and 13C NMR spectral features of the mixture (Tables 1, 2) showed two sets of H-atom and C-atom resonances (partially overlapped) [24]. This could be explained by the equilibrium due to the hemiacetal unit. Acetylation of 1 yielded the 1,2,3,7,29,30-hexa-acetylated (1a) and 2,3,7,29,30-penta-acetylated (1b) adducts of 1, whose structures (Fig. 1) were characterized by detailed spectroscopic analysis (see Electronic supplementary material). The 29-exo configurations for both 1a and 1b were supported by their chemical shifts of H-3 (δH 5.41), since H-3 resonated at δH 4.9–5.1 for 29-endo and 5.3–5.6 for 29-exo [25]. Based on the above evidence, compound 1 was determined as shown in Fig. 1 and named as flexuosoid A.
Fig. 3.
Key ROESY ( ) correlations of 1
) correlations of 1
Compound 2 was obtained as a white amorphous powder and possessed a molecular formula C28H36O11, as deduced from the HRESIMS (m/z 547.2182 [M − H]−). The 1H and 13C NMR data of 2 were similar to those of 1, except for the appearance of an additional acetyl group [δH 2.08/2.09 (3H, s) and δC 173.31/173.25 (CO), 21.23/21.18 (CH3)] and the down-field shift of C-3 and H-3 to δC 76.69 and δH 5.50, respectively, suggesting that the hydroxy group at C-3 in 1 was acetylated in 2. This was confirmed by HMBC correlation of δH 5.50 (H-3) with the acetyl carbonyl carbon at δC 173.31 (Fig. 1S, Electronic supplementary material). In the ROESY spectrum, correlations of H-5/Me-28, H-5/H-9, H-19/H-29 and H-22/Me-18 supported the relative configurations of rings system of 2 as shown in Fig. 1. Moreover, the broad singlet H-7 in the 1H NMR spectrum and the ROESY correlations of H-30/H-7 of 2 revealed that 7-OH had the α-configuration. The ROESY correlation of H-22 with Me-18 supported the assignment of the β-configuration to 17-OH, and the correlations of H-1/H-9 and H-3/H-28 relieved the β-configuration to 1-OH and 3-OH. Due to the severe overlapping of H2-19 and H-2 signals in 2, the assignment of α-orientation for hydroxy group at C-2 was achieved by acetylation of 2 to give 1,2,3,7,29,30-hexa-acetyl flexuosoid A (1a) and 2,3,7,29,30-penta-acetyl flexuosoid A (1b). In the 1H NMR spectrum of 1a, the small values of J1,2 (4.4 Hz) and J2,3 (4.4 Hz) revealed the equatorial H-1, H-2, and H-3 in 2. Furthermore, the 29-exo configuration for 2 was supported by chemical shifts of H-3 of 1a and 1b, the acetylated products of 2. Thus, 2 was assigned as shown in Fig. 1 and named as flexuosoid B.
Compound 3, a white amorphous powder, possessed a molecular formula C33H34O16, as deduced by the HRESIMS at m/z 685.1763 [M − H]− (calcd for C33H33O16, 685.1768). The IR spectrum showed the presence of hydroxy (3423 cm−1) and benzene rings (1622, 1507, and 1435 cm−1). The 13C NMR and DEPT spectra (Table 3) displayed the presence of 33 carbon resonances, due to two carboxyls (δC 172.09 and 172.5), 16 aromatic carbons (δC 102–153), one methylenedioxy (δC 102.6), one oxymethylene (δC 69.0), two methoxys (δC 56.7 and 56.0), two pentosyl moieties and an acetyl methyl (δC 21.2). In the 1H NMR spectrum, two singlet aromatic protons at δH 6.89 and 8.03 (each 1H, s) and a set of ABX coupled signals [δH 6.87 (1H, d, J = 8.0 Hz), 6.64 (1H, d, J = 1.5 Hz), 6.59 (1H, dd, J = 8.0, 1.5 Hz)] arising from an 1,3,4-trisubstituted benzene ring were observed, in addition to characteristic proton signals due to a methylenedioxy (δH 6.02 and 6.00, each 1H, s), two methoxys [δH 3.94, 3.63 (each 3H, s)], and two anomeric protons [δH 4.74 (d, J = 7.7 Hz) and 4.49 (d, J = 7.0 Hz)]. Acid hydrolysis of 3 afforded L-arabinose as the soly monosaccharide, which was confirmed by GC analysis of its corresponding trimethylsilylated l-cysteine adducts. The aforementioned data of 3 were closely related to those of phyllanthusmin C, a known arylnaphthalene lignan glycoside from Phyllanthus oligospermus [18]. The major difference was the presence of one more arabinosyl and an additional acetyl group in 3, compared with phyllanthusmin C. All signals of 3 were assigned completely on the basis of HSQC, HMBC and 1H-1H COSY experiments. In the HMBC spectrum of 3 (Fig. 4), correlation of an anomeric proton at δH 4.74 with C-1 (δC 146.1) confirmed the linkage of an arabinosyl moiety at C-1 of lignan aglycone. Subsequently, HMBC correlations of the additional arabinosyl anomeric proton at δH 4.49 with the inner arabinosyl C-3′ (δC 81.8) and the inner arbinosyl H-4′′ (δH 5.16) with the acetyl carbonyl carbon (δC 172.5), indicated that the additional arabinosyl and acetyl units were linked to the inner arabinosyl C-3′ and C-4′ in 3, respectively. This resulted in deshielded resonances of the inner arabinosyl C-3′ and C-4′ of 3 by 7.4 and 2.8 ppm, respectively, and shielded resonances of C-5′ by 2.2 ppm, compared with those of phyllanthusmin C. On the basis of these observations and other HMBC correlations (Fig. 4 and Electronic supplementary material), the structure of phyllanthusmin D (3) was established.
Table 3.
1H and 13C NMR spectroscopic data of 3 and 4 (methanol-d4, δ in ppm)
| Position | 3a | 4b | ||
|---|---|---|---|---|
| δH (J in Hz) δC | δH (J in Hz) δC | |||
| 1 | 146.14/146.13c, C | 146.5, C | ||
| 2 | 119.85/119.76c, C | 120.71/120.72c, C | ||
| 3 | 132.19/132.10c, C | 132.70/132.72c, C | ||
| 4 | 137.47/137.40c, C | 137.5, C | ||
| 4a | 131.69/131.66c, C | 132.3, C | ||
| 5 | 6.89c, s | 106.75/106.70c, CH | 7.13c,s | 107.7, CH |
| 6 | 151.51/151.47c, C | 151.7, C | ||
| 7 | 153.14/153.11c, C | 150.6, C | ||
| 8 | 8.03, s | 102.63/102.59c, CH | 8.25, s | 106.5, C |
| 8a | 128.64/128.61c, C | 128.5, C | ||
| 1′ | 129.74/129.80c, C | 130.2, C | ||
| 2′ | 6.64, d (1.5) | 111.65/111.71c, CH | 6.81c, d (1.7) | 111.86/111.89c, CH |
| 3′ | 148.86/148.90c, C | 149.2, C | ||
| 4′ | 148.81/148.90c, C | 149.2, C | ||
| 5′ | 6.87c, d (8.0) | 108.84/108.88c, CH | 6.96c, d (7.8) | 109.18/109.20c, CH |
| 6′ | 6.59c, dd (8.0, 1.5) | 124.71/124.74c, CH | 6.77c, dd (7.8, 1.7) | 124.9, CH |
| 2a |
5.48c, d (15.2) 5.39c, d (15.2) |
69.0, CH2 |
5.47c, d (15.1) 5.62c, d (15.1) |
69.4, CH2 |
| 3a | 172.09/172.05c, C | 172.3, C | ||
| 6-OMe | 3.63c, s | 56.0, CH3 | ||
| 7-OMe | 3.94, s | 56.7, CH3 | 3.76, s | 56.3, CH3 |
| O–CH2–O |
6.02c, s 6.00c, s |
102.6, CH2 |
6.06c, s 6.05c, s |
102.8, CH2 |
| 1″ | 4.74, d (7.7) | 106.9, CH | 4.76c, d (7.4) | 107.3, CH |
| 2″ | 4.12, dd (8.6, 7.7) | 72.2, CH | 3.99, dd (9.3, 7.4) | 72.9, CH |
| 3″ | 3.83d | 81.8, CH | 3.65, dd (9.3, 3.5) | 74.4, CH |
| 4″ | 5.16, br. s | 72.5, CH | 3.86, br. s | 70.0, CH |
| 5″ |
3.98d 3.52d |
65.4, CH2 |
3.50, d (12.5) 3.93, dt (12.5, 1.9) |
67.8, CH2 |
| 1‴ | 4.49, d (7.0) | 106.5, CH | 5.31, d (7.5) | 101.3, CH |
| 2‴ | 3.66, dd (8.8, 7.0) | 72.6, CH | 3.60, dd (9.2, 7.6) | 74.9, CH |
| 3‴ | 3.54d | 74.1, CH | 3.55, t (9.2) | 78.2, CH |
| 4‴ | 3.81, br. s | 69.5, CH | 3.41, t (9.2) | 71.5, CH |
| 5‴ |
3.86d 3.52d |
67.0, CH2 | 3.75d | 78.0, CH |
| 6‴ |
3.70, dd (11.9, 6.2) 3.97, dd (11.9, 2.0) |
62.8, CH2 | ||
| COMe | 172.5, C | |||
| COMe | 2.15, s | 21.2, CH3 | ||
aData were measured at 500 and 125 MHz for 1H and 13C, respectively
bData were measured at 600 and 150 MHz for 1H and 13C, respectively
cExisting as very close pairs at room temperature (21 °C)
dOverlapping 1H NMR signals are reported without designated multiplicity
Fig. 4.
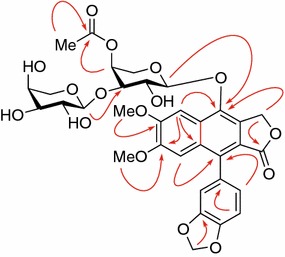
Key HMBC ( ) correlations of 3
) correlations of 3
Compound 4 possessed a molecular formula of C31H32O16, on the basis of HRESIMS (m/z 661.1766 [M + H]+). Acid hydrolysis of 4 afforded L-arabinose and d-glucose as sugar residue, which was confirmed by GC analysis of its corresponding trimethylsilated l-cysteine adducts. The 1H and 13C NMR spectroscopic data (Table 3) of 4 showed close resemblance with those of phyllanthusmin C, except for the presence of only one methoxy group and an additional set of signals assignable to a β-glucosyl moiety [δH 5.31 (d, J = 7.5 Hz, δC 101.3, 74.9, 78.2, 71.5, 78.0 and 62.8] in 4. In the HMBC experiment (Fig. 2S, Electronic supplementary material), correlations of H2-2a [δH 5.47, (d, J = 15.1); 5.62, (d, J = 15.1)] and δH 8.25 (H-8) with the quaternary carbon at δC 146.5 (C-1) allowed assignment of C-1 and H-8. The HMBC correlation from the arabinosyl anomeric proton (δH 4.76, Ara-1″) to C-1 (δC 146.5) indicated that the arabinosyl moiety was attached to C-1 of the lignan core. Moreover, HMBC correlation of the anomeric proton (δH 5.31, Glc-1‴) with C-7 (δC 150.6) supported linkage of the additional glucosyl moiety to C-7, which was further confirmed by the ROESY correlation between the anomeric proton (δH 5.31, Glc-1‴) with H-8 (δH 8.25). On the basis of these observations, the structure of 4 was established as phyllanthusmin E.
Compound 5 was isolated as a white amorphous powder. Its molecular formula was deduced to be C38H44O21 by HRESIMS (m/z 871.2077 [M − H]−). Acid hydrolysis of 5 afforded L-arabinose, d-glucose and d-galactose as sugar residue, which was confirmed by GC analysis of their corresponding trimethylsilylated l-cysteine adducts. The 1H and 13C NMR data (Table 4) of 5 were very similar to those of phyllanthusmin C, except for the appearance of two additional hexosyl units attributable to one glucosyl [δH 5.55 (d, J = 7.9 Hz), δC 105.5, 75.3, 79.0, 72.3, 78.9 and 68.6] and one galactosyl [δH 5.02 (d, J = 7.4 Hz), δC 106.2, 73.0, 75.5, 70.2, 76.9 and 63.1] units [26]. Linkage of the additional glucosyl unit to the arabinosyl C-3″ position was confirmed by the long range correlations between the glucosyl anomeric proton at δH 5.55 (H-1‴) and the arabinosyl C-3″ (δC 81.0). Moreover, HMBC correlation of the galactosyl H-1‴ (δH 5.02) with the glucosyl C-6‴ (δC 68.6) was also observed (Fig. 2S, Electronic supplementary material). Therefore, the structure of phyllanthusmin F (5) was elucidated as shown in Fig. 1.
Table 4.
1H and 13C NMR spectroscopic data of 5 (methanol-d4, δ in ppm)a
| Position | δH (J in Hz) | δ C | Position | δH (J in Hz) | δ C |
|---|---|---|---|---|---|
| 1 | 146.60/146.63b, C | O–CH2–O | 5.93, s | 102.3, CH2 | |
| 2 | 120.46, C | 6.03, s | |||
| 3 | 132.30/132.36b, C | Ara-1″ | 5.35, d (7.7) | 107.4, CH | |
| 4 | 136.7, C | 2″ | 4.26c | 75.6, CH | |
| 4a | 131.5, C | 3″ | 4.59c | 81.0, CH | |
| 5 | 7.36, s | 107.4, CH | 4″ | 4.01, br. d (9.5) | 75.7, CH |
| 6 | 151.6, C | 5″ |
4.63c 4.75, t (9.5) |
68.9, CH2 | |
| 7 | 153.2, C | Glc-1‴ | 5.55, d (7.9) | 105.5, CH | |
| 8 | 8.85, s | 103.3, CH | 2‴ | 4.08c | 75.5, CH |
| 8a | 128.9, C | 3‴ | 4.39, t (8.3) | 79.0, CH | |
| 1′ | 129.99/130.02b, C | 4‴ | 4.22c | 72.3, CH | |
| 2′ | 7.17/7.25, d (1.4) | 112.09/112.20b, CH | 5‴ | 4.22c | 78.9, CH |
| 3′ | 148.6, C | 6‴ | 4.24c | 68.6, CH2 | |
| 4′ | 148.5, C | 3.68, d (11.3) | |||
| 5′ | 7.16/7.14, d (7.8) | 125.0, CH | Gal-1″″ | 5.02, d (7.4) | 106.2, CH |
| 6′ | 7.13/7.08, dd (7.8, 1.4) | 109.0, CH | 2″″ | 4.49, t (8.2) | 73.0, CH |
| 2a | 5.70, dd (15.2, 3.7) | 68.6, CH2 | 3″″ | 4.06c | 75.1, CH |
| 6.11, dd (15.7, 10.9) | 4″″ | 4.22c | 70.2, CH | ||
| 3a | 170.7, C | 5″″ | 4.28c | 76.9, CH | |
| 6-OMe | 3.66, s | 55.9, CH3 | 6″″ | 4.56, d (16.3) | 63.1, CH2 |
| 7-OMe | 4.17, s | 56.9, CH3 | 4.30c |
aData were measured at 600 and 150 MHz for 1H and 13C, respectively
bExisting in pairs at room temperature (21 °C)
cOverlapping 1H NMR signals are reported without designated multiplicity
It was noted that the 1H and 13C NMR spectra of the new arylnaphthalene lignans (3–5) recorded at room temperature exhibited doubling of the signals, of which most aromatic signals existed as very close pairs, whose chemical shifts are different only at the second decimal places, due to the equilibrium between the two conformational isomers resulting from the slow rotation of sugar unit at room temperature around the glycosidic linkage [19]. The energy barrier about the glycosidic bond was sufficiently high to prevent fast exchange between the two rotamers at room temperature [27].
Most of the isolated compounds were tested for their antifeedant, anti HSV-1 and cytotoxic activities. The two new limonoids 1 and 2 displayed promising antifeedant activity against Spodoptera exigua with EC50 values of 25.1 and 17.3 μg/cm2, respectively, which were less active than that of the positive control (EC50 = 3.7 μg/cm2), a commericial neem oil containing 1 % azadirachtin, while slightly more active than aphanamixoid A (EC50 = 25.8 μg/cm2), a potential natural product with antifeedant activity [28]. Furthermore, both compounds 1 and 2, together with the known lignan, phyllanthusmin C, showed moderate cytotoxicity against the ECA109 cell line, with the IC50 values of 11.5, 8.5, and 7.8 μM, respectively, compared to the positive control, 17-AAG (IC50 = 1.1 μM). However, all the tested compounds showed no inhibitory effects against HSV-1.
Limonoids, mainly found in the Meliaceae and Rutaceae families, are of rare occurrence in the Euphorbiaceae family [29]. The new deca-oxygenated flexuosoids A (1) and B (2) represent the second example of limonoids in the Euphorbiaceae family [30]. It is noteworthy that the new limonoids 1 and 2 bear a C-19/29 lactol bridge and oxygen substituents at C-1, C-2, C-3, C-7, C-11, C-17, and C-30 positions, of which the oxygen substituents at C-17 and C-30 are of rare occurrence among the reported limonoids [29]. Furthermore, compounds 1 and 2 showed not only promising antifeedant activity but also moderate cytotoxicity against the ECA109 human esophagus cancer cell line.
Experimental Section
General Experimental Procedures
Optical rotations were performed on a P-1020 polarimeter (JASCO, Tokyo, Japan). IR spectra were measured on a Bruker Tensor 27 spectrometer with KBr pellets. 1D and 2D NMR spectra were run on Bruker DRX-500 and AV-600 instruments operating at 500 and 600 MHz for 1H, and 125 and 150 MHz for 13C, respectively. Coupling constants are expressed in Hertz and chemical shifts are given on a ppm scale with tetramethylsilane as internal standard. The MS data were recorded on a VG Auto Spec-3000 spectrometer (VG, Manchester, U.K.) with glycerol as the matrix. HRESIMS were recorded on an API Qstar Pulsa LC/TOF spectrometer. GC analysis was run on a Shimadzu GC-14C gas chromatograph. Column chromatography (CC) was performed with macroporous resin D101 (Mitsubishi Chemical Industry, Ltd., Tokyo, Japan), silica gel (200–300 mesh, Qingdao Haiyang Chemical Co., Ltd., Qingdao, People’s Republic of China), Sephadex LH-20 (25–100 μm, Pharmacia Fine Chemical Co. Ltd. Japan). Thin-layer chromatography (TLC) was carried out on silica gel H-precoated plates (Qingdao Haiyang Chemical Co., Ltd., Qingdao, People’s Republic of China) with CHCl3/MeOH/H2O (8.5:1.5:0.1, 8:2:0.2 or 7:3:0.5, v/v). Spots were detected by spraying with 10 % H2SO4 in EtOH followed by heating. Semi-preparative HPLC separation was performed on an Agilent 1260 liquid chromatography with a 5 μm Waters Sunfire-C18 column (10 × 250 mm, Waters, Sunfire TM, USA). GC analysis was run on Agilent Technologies HP5890 gas chromatography equipped with an H2 flame ionization detector. The column was 30QC2/AC-5 quartz capillary column (30 m × 0.32 mm) with the following conditions: column temperature: 180–280 °C; programmed increase, 3 °C/min; carrier gas: N2 (1 mL/min); injection and detector temperature: 250 °C; injection volume: 4 μL, split ratio: 1/50.
Cell Lines and Biochemicals
African green monkey kidney cells (Vero, ATCC CCL81), provided by Wuhan Institute of Virology, Chinese Academy of Sciences, were propagated in DMEM supplemented with 10 % heat-inactivated FBS. The constituents of the maintenance medium were the same as those of the growth medium except that only 2 % FBS was added. The cells were cultured at 37 °C in a humid atmosphere with 5 % CO2. HSV-1 strain F (ATCC VR-733), obtained from Hong Kong University, was propagated in Vero cells and stored at −80 °C until use. The human esophagus cancer cell ECA109 was cultured in RPMI 1640 containing 10 % heat inactivated FBS and 100 U/mL penicillin/streptomycin in a humidified incubator in a 5 % CO2 atmosphere at 37 °C.
Plant Material
The roots of P. flexuosus were collected from Yunnan Province, People’s Republic of China, in June 2010. Voucher specimens (HITBC_015077) were deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, and were identified by Mr. Shi-Shun Zhou from Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences.
Extraction and Isolation
The air-dried and powdered roots of P. flexuosus (2.0 kg) were extracted with MeOH (3 times, 3 h each time) under reflux at 60 °C. The methanol extracts (320 g) were subjected to macroporous resin D101 column chromatography (CC) and eluted with the following gradient: MeOH/H2O (3:7, 6:4, 9:1) and finally MeOH, to give 4 fractions (Fr. 1–4). Fr. 2 (45.0 g) and Fr. 3 (16.6 g) were subjected separately to repeated CC on silica gel (CHCl3/MeOH/H2O, 9:1:0.1–7:3:0.5) and Sephadex LH-20 (MeOH/H2O, 1:9–8:2) to yield 3 (13 mg), phyllanthusmins C (20 mg), arabelline (12 mg), (+)-diasyringaresinol (8 mg) and subfraction F2A (32 mg). Subfraction 2A was purified by semi-preparative HPLC (MeCN/H2O, 25:75) to afford 4 (4 mg). Fr. 3 was repeatedly chromatographed over MCI-gel CHP-20P (MeOH/H2O, 5–45 % with a 5 % increment) and silica gel (CHCl3/MeOH/H2O, 9:1:0.1–6:4:1) to get subfractions F3A (170 mg) and F3B (180 mg). Subfraction F3A was purified by semi-preparative HPLC (MeCN/H2O, 25:75) to give 5 (4 mg). Subfraction F3B was purified by semi-preparative HPLC (MeCN/H2O, 14:86) to furnish 1 (14 mg) and 2 (25 mg).
Flexuosoid A (1)
White amorphous powder; −41.2 (c 0.30, MeOH); UV (MeOH) λmax (log ε): 203.8 (3.06) nm; IR (KBr) νmax 3424, 2965, 2931, 1691, 1629, 1455, 1382, 1263, 1159, 1137, 1058 cm−1; 1H and 13C NMR data, see Tables 1 and 2; ESIMS (pos. ion mode) m/z 529 [M + Na]+; negative HR ESIMS m/z 505.2072 [M − H]− (calcd for C26H33O10, 505.2073).
Flexuosoid B (2)
White amorphous powder; −41.7 (c 0.68, MeOH); UV (MeOH) λmax (log ε) 204.0 (3.95) nm; IR (KBr) νmax 3424, 2967, 2933, 1695, 1455, 1380, 1257, 1037 cm−1; 1H and 13C NMR data, see Tables 1 and 2; ESIMS (neg. ion mode) m/z 583 [M + Cl]−; negative HRESIMS m/z 547.2182 [M − H]− (calcd for C28H35O11, 547.2179).
Phyllanthusmin D (3)
White amorphous powder; +10.5 (c 0.07, MeOH); UV (MeOH) λmax (log ε): 201.0 (4.86), 260.4 (4.94) nm; IR (KBr) νmax 3423, 2908, 1742, 1622, 1507, 1480, 1455, 1435, 1386, 1377, 1342, 1264, 1216, 1169, 1080, 1025, 1012 cm−1; 1H and 13C NMR data, see Table 3; ESIMS (neg. ion mode) m/z 721 [M + Cl]−; negative HRESIMS m/z 685.1763 [M − H]− (calcd for C33H33O16, 685.1768).
Phyllanthusmin E (4)
White amorphous powder; −48.7 (c 0.28, MeOH); UV (MeOH) λmax (log ε): 203.2 (4.24), 259.0 (4.55) nm; IR (KBr) νmax 3442, 2923, 1630, 1535, 1468, 1416, 1385, 1203, 1169, 1128, 1073, 1039 cm−1; 1H and 13C NMR data see Table 3; ESIMS (neg. ion mode) m/z 695 [M + Cl]−; positive HRESIMS m/z 661.1766 [M + H]+ (calcd for C31H33O16, 661.1763).
Phyllanthusmin F (5)
White amorphous powder; −40.6 (c 0.30, MeOH); UV (MeOH) λmax (log ε): 202.8 (4.41), 262.2 (4.56) nm; IR (KBr) νmax 3426, 2925, 1730, 1627, 1507, 1480, 1458, 1435, 1386, 1341, 1265, 1230, 1168, 1075, 1049, 1009 cm−1; 1H and 13C NMR data see Table 4; ESIMS (neg. ion mode) m/z 871 [M + Cl]−; negative HRESIMS m/z 871.2077 [M + Cl]− (calcd for C38H44O21Cl, 871.2063).
Acetylation of Compounds 1 and 2
See Electronic supplementary material.
Acid Hydrolysis of Compounds 3–5
See Electronic supplementary material.
Antifeedant Activity Assay
The insects, beet armyworm (Spodoptera exigua) purchased from the Pilot-Scale Base of Bio-Pesticides, Institute of Zoology, Chinese Academy of Sciences. A dual-choice bioassay modified from previous methods was performed for antifeedant tests [31]. The larvae were reared on an artificial diet in the laboratory under controlled photoperiod (light:dark, 12:8 h) and temperature (25 ± 2 °C). Larvae were star ved 4–5 h prior to each bioassay. Fresh leaf discs were cut from Brassica chinensis, using a cork borer (1.1 cm in diameter). Treated leaf discs were painted with 20 μL of acetone solution containing the test compounds, and control leaf discs with the same amount of acetone. After air drying, two tested leaf discs and two control ones were set in alternating position in the same Petri dish (90 mm in diameter), with moistened filter paper at the bottom. Two-thirds of instars were placed at the center of the Petri dish. Five replicates were run for each treatment. After feeding for 24 h, areas of leaf discs consumed were measured. The antifeedant index (AFI) was calculated according to the formula AFI = [(C − T)/(C + T)] × 100, where C and T represent the control and treated leaf areas consumed by the insect. The insect antifeedant potency of the test compound was evaluated in terms of the ED50 value (the effective dosage for 50 % feeding reduction) which was determined by Probit analysis for the insect species. The positive control was served as commercial neem oil containing 1 % azadirachtin produced by Kunming Rixin Dachuan Technology Co., Ltd.
Cytotoxicity Assay
The cytotoxicity of the test compounds on Vero cells was determined by an MTT assay. Vero cells were seeded in 96-well plates at a density of 1 × 104 cells/well, and incubated at 37 °C in a 5 % CO2 atmosphere for 24 h, until 90 % or greater confluence of the monolayers was reached. Increasing concentrations of the test compounds were added to cells, with a replicate number of three wells per concentration and 17-AAG served as positive control. After a 2-day incubation period in such conditions, a MTT solution was added (final concentration 0.5 mg/mL) and the plates were incubated for another 4 h to allow formazan production. The solid precipitate was dissolved with DMSO and the absorbance at 570 nm was measured using a 96-well Spectrophotometer (Bio-Rad 550) with a reference wavelength of 630 nm. The cytotoxicity of the test compounds on the ECA109 human esophagus cancer cell and Vero cell lines was determined by an MTT assay. The ECA109 human esophagus cancer cell line was cultured in RPMI 1640 containing 10 % heat inactivated FBS and 100 U/mL penicillin/streptomycin in a humidified incubator in a 5 % CO2 atmosphere at 37 °C. Cells (5 × 103/well) were plated in 96-well plates in 100 μL medium, cultured overnight and exposed to a range of concentrations of compounds for 48 h. After the addition of 20 μL MTT solution (5 mg/mL) per well, the plates were incubated for 4 h, the medium were removed, the formazan crystals were solubilized in 100 μL DMSO per well and the absorbance values were read at 570 nm.
Anti-HSV-1 Assay
Vero cells were seeded in 96-well plates at a density of 1 × 104 cell/well. Confluent cell monolayers were treated with increasing non-cytotoxic concentrations of the plant extract. Four wells were used for each concentration. Afterwards, the cells were infected with HSV-1 (100 TCID50), incubated at 37 °C in a 5 % CO2 humidified atmosphere and observed daily for cytopathic effect (CPE) using a light microscope. Acyclovir at concentration 20 μg/mL served as positive control. The EC50 value was calculated by MTT method.
Electronic supplementary material
Below is the link to the electronic supplementary material.
The Acetylation of compounds 1 and 2, and Acid hydrolysis of compounds 3-5 sections, the Key 1H-1H COSY, HMBC and ROESY correlations of 2, Key HMBC correlations of 4 and 5, the physicochemical and spectroscopic data of 1a and 1b, and the 1H and 13C NMR, HSQC, ROESY, HMBC and 1H-1H COSY spectra for compounds 1 - 5, 1a, and 1b are available as supporting information. (PDF 971 kb)
Acknowledgments
We are grateful to the members of the analytical group of our institute for the measurement of spectroscopic data. This work was supported by the NSFC 21002105, the 973 Program of Science and Technology of P. R. China (2011CB915503), the Fourteenth Batch Candidates of the Young Academic Leaders of Yunnan Province (Min XU, 2011CI044) and by West Light Foundation of the Chinese Academy of Sciences.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Jian-Qiang Zhao and Yan-Ming Wang contributed equally to this paper.
Contributor Information
Min Xu, Email: xumin@mail.kib.ac.cn.
Ying-Jun Zhang, Email: zhangyj@mail.kib.ac.cn.
References
- 1.Unander DW, Webster GL, Blumberg BS. J. Ethnopharmacol. 1995;45:1–18. doi: 10.1016/0378-8741(94)01189-7. [DOI] [PubMed] [Google Scholar]
- 2.Zhang YJ, Abe T, Tanaka T, Yang CR, Kouno I. J. Nat. Prod. 2001;64:1527–1532. doi: 10.1021/np010370g. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YJ, Tanaka T, Iwamoto Y, Yang CR, Kouno I. J. Nat. Prod. 2000;63:1507–1510. doi: 10.1021/np000135i. [DOI] [PubMed] [Google Scholar]
- 4.Ratnayake R, Covell D, Ransom TT, Gustafson KR, Beutler JA. Org. Lett. 2009;11:57–60. doi: 10.1021/ol802339w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettit GR, Schaufel-berger DE, Nieman RA, Dufresne C, Saenz-Renauld JA. J. Nat. Prod. 1990;53:1406–1413. doi: 10.1021/np50072a002. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Wang YF, Chen RJ, Zhang MY, Wang YF, Yang CR, Zhang YJ. J. Nat. Prod. 2009;72:969–972. doi: 10.1021/np800792d. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YJ, Nagao T, Tanaka T, Yang CR, Okabe H, Kouno I. Biol. Pharm. Bull. 2004;27:251–255. doi: 10.1248/bpb.27.251. [DOI] [PubMed] [Google Scholar]
- 8.B.T. Li, Flora of China, vol. 44. (Science Press, Beijing, 1994), pp. 86
- 9.Tanaka R, Kinouchi Y, Wada S, Tokuda H. Planta Med. 2004;70:1234–1236. doi: 10.1055/s-2004-835858. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka R, Tabuse M, Matsunaga S. Phytochemistry. 1988;27:3563–3567. doi: 10.1016/0031-9422(88)80769-6. [DOI] [Google Scholar]
- 11.Tanaka R, Masuda K, Matsunaga S. Phytochemistry. 1993;32:472–474. doi: 10.1016/S0031-9422(00)95021-0. [DOI] [Google Scholar]
- 12.Tanaka R, In Y, Ishida T, Matsunaga S. J. Nat. Prod. 1994;57:1123–1128. [Google Scholar]
- 13.Zhao JQ, Lv JJ, Wang YM, Xu M, Zhu HT, Wang D, Yang CR, Wang YF, Zhang YJ. Tetrahedron Lett. 2013;54:4670–4674. doi: 10.1016/j.tetlet.2013.06.082. [DOI] [Google Scholar]
- 14.Pei Y, Xiang YF, Chen JN, Lu CH, Hao J, Du Q, Lai CC, Qu C, Li S, Ju HQ, Ren Z, Liu QY, Xiong S, Qian CW, Zeng FL, Zhang PZ, Yang CR, Zhang YJ, Xu J, Kitazato K, Wang YF. Antiviral Res. 2011;89:98–108. doi: 10.1016/j.antiviral.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Tian LW, Pei Y, Zhang YJ, Wang YF, Yang CR. J. Nat. Prod. 2009;72:1057–1060. doi: 10.1021/np800760p. [DOI] [PubMed] [Google Scholar]
- 16.Ju HQ, Wang SY, Pei Y, Xiang YF, Li S, Zhang YJ, Yang CR, Wang YFJ. Chin. Med. Mater. 2011;34:242–245. [PubMed] [Google Scholar]
- 17.Liu G, Xiong S, Xiang YF, Guo CW, Ge F, Yang CR, Zhang YJ, Wang YF, Kitazato K. Arch. Virol. 2011;156:1359–1369. doi: 10.1007/s00705-011-0989-9. [DOI] [PubMed] [Google Scholar]
- 18.Wu SJ, Wu TS. Chem. Pharm. Bull. 2006;54:1223–1225. doi: 10.1248/cpb.54.1223. [DOI] [PubMed] [Google Scholar]
- 19.Al-Abed Y, Sabri S, Zarga MA, Shah Z, Atta-Ur-Rahman J. Nat. Prod. 1990;53:1152–1161. doi: 10.1021/np50071a003. [DOI] [Google Scholar]
- 20.Chang FR, Chao YC, Teng CM, Wu YC. J. Nat. Prod. 1998;61:863–866. doi: 10.1021/np970348g. [DOI] [PubMed] [Google Scholar]
- 21.Polonsky J, Varon Z, Arnoux B, Pascard C, Pettit GR, Schmidt JM, Lange LM. J. Am. Chem. Soc. 1978;22:7731–7733. doi: 10.1021/ja00492a051. [DOI] [Google Scholar]
- 22.Govindachari TR, Viswanathan N, Pai BR, Savitri TS. Tetrahedron Lett. 1964;16:901–906. doi: 10.1016/S0040-4039(00)90405-2. [DOI] [Google Scholar]
- 23.Vardamidesa JC, Dongo E, Nkengfack AE, Fomum ZT, Ngando TM, Vogler B, Kraus W. Fitoterapia. 2001;72:386–393. doi: 10.1016/S0367-326X(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 24.Huang RC, Okamura H, Iwagawa T, Nakatani M. Bull. Chem. Soc. Jpn. 1994;67:2468–2472. doi: 10.1246/bcsj.67.2468. [DOI] [Google Scholar]
- 25.Takeya K, Qiao ZS, Hirobe C, Itokawa H. Bioorg. Med. Chem. 1996;4:1355–1359. doi: 10.1016/0968-0896(96)00128-9. [DOI] [PubMed] [Google Scholar]
- 26.Kitagawa I, Saito M, Taniyama T, Yoshikawa M. Chem. Pharm. Bull. 1985;33:598–608. doi: 10.1248/cpb.33.598. [DOI] [Google Scholar]
- 27.Lewis KC, Maxwell AR, McLean S, Reynolds WF, Enriquez RG. Magn. Reson. Chem. 2000;38:771–774. doi: 10.1002/1097-458X(200009)38:9<771::AID-MRC729>3.0.CO;2-4. [DOI] [Google Scholar]
- 28.Cai JY, Zhang Y, Luo SH, Chen DZ, Tang GH, Yuan CM, Di YT, Li SH, Hao XJ, He HP. Org. Lett. 2012;14:2524–2527. doi: 10.1021/ol3008149. [DOI] [PubMed] [Google Scholar]
- 29.Tan QG, Luo XD. Chem. Rev. 2011;111:7437–7522. doi: 10.1021/cr9004023. [DOI] [PubMed] [Google Scholar]
- 30.Zhao JQ, Wang YM, He HP, Li SH, Li XN, Yang CR, Wang D, Zhu HT, Xu M, Zhang YJ. Org. Lett. 2013;15:2414–2417. doi: 10.1021/ol400875n. [DOI] [PubMed] [Google Scholar]
- 31.Isman MB, Koul O, Luczynski A, Kaminski J. J. Agric. Food Chem. 1990;38:1406–1411. doi: 10.1021/jf00096a024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Acetylation of compounds 1 and 2, and Acid hydrolysis of compounds 3-5 sections, the Key 1H-1H COSY, HMBC and ROESY correlations of 2, Key HMBC correlations of 4 and 5, the physicochemical and spectroscopic data of 1a and 1b, and the 1H and 13C NMR, HSQC, ROESY, HMBC and 1H-1H COSY spectra for compounds 1 - 5, 1a, and 1b are available as supporting information. (PDF 971 kb)


