Abstract
Purpose
Effective sensitizing strategies potentially can extend the benefit of TMZ therapy in GBM patients. We previously demonstrated that robust TMZ-sensitizing effects of the PARP inhibitor veliparib (ABT-888) are restricted to TMZ-sensitive GBM xenografts. The focus of this study is to provide an understanding for the differential sensitization in paired TMZ-sensitive and -resistant GBM models.
Experimental Design
The impact of veliparib on TMZ-induced cytotoxicity and DNA damage was evaluated in vitro and in vivo in models of acquired TMZ-resistance (GBM12TMZ-mgmtHigh, GBM12TMZ-mgmtLow, U251TMZ), inherent TMZ-resistance (T98G), and TMZ-sensitive (U251, GBM12). In vivo drug efficacy, pharmacokinetics, and pharmacodynamics were analyzed using clinically relevant dosing regimens.
Results
Veliparib enhanced TMZ cytotoxicity and DNA damage signaling in all GBM models in vitro with more pronounced effects in TMZ-resistant lines at 3-10 μM veliparib. In vivo, combined TMZ/veliparib, compared to TMZ alone, significantly delayed tumor growth and enhanced DNA damage signaling and γH2AX levels in the sensitive GBM12 xenograft line but not in the resistant GBM12TMZ lines. The pharmacokinetic profile of veliparib was similar for GBM12 and GBM12TMZ tumors with Cmax (~1.5 μM) in tissue significantly lower than concentrations associated with optimal in vitro sensitizing effects for resistant tumors. In contrast, robust suppression of PARP-1 expression by shRNA significantly increased TMZ sensitivity of U251TMZ in vitro and in vivo.
Conclusions
In vitro cytotoxicity assays do not adequately model the therapeutic index of PARP inhibitors, as concentrations of veliparib and TMZ required to sensitize TMZ-resistant cancer cells in vivo cannot be achieved using a tolerable dosing regimen.
Introduction
Temozolomide (TMZ) is an important component of conventional chemotherapy for glioblastoma multiforme (GBM), but inherent and acquired resistance significantly limits its therapeutic efficacy (1-3). The main cytotoxic lesions induced by TMZ are N7-methylguanine (N7-MeG), N3-methyladenine (N3MeA), and O6-methylguanine (O6-MeG). N7-MeG and N3MeA are repaired by base-excision repair (BER), while the O6-MeG lesion is repaired by O6-methylguanine-DNA-methyl transferase (MGMT) (3-7). Disruption of either repair process can sensitize tumors to TMZ cytotoxicity, but BER generally is robust in cells and is a minor determinant of overall TMZ responsiveness. In contrast, MGMT expression is silenced by promoter methylation in approximately one third of GBM, and high expression of MGMT is a common mechanism of inherent TMZ resistance and likely contributes to mechanisms of acquired resistance (7-9). In tumors lacking MGMT, unrepaired O6-MeG mispairs with thymidine and triggers futile cycles of mismatch repair (MMR) during replication, resulting in replication fork-associated DNA double strand breaks and cytotoxicity (7, 10). Although uncommon in untreated tumors, defects in MMR are an important mechanism of acquired TMZ-resistance in recurrent GBM (11-13). Collectively, MGMT over-expression and MMR deficiency contribute to TMZ resistance in GBM, and defining strategies to sensitize resistant tumors to TMZ could significantly extend the survival gains associated with TMZ therapy.
Inhibitors of poly ADP-ribose polymerase 1 and 2 (PARP1/2) are potent TMZ-sensitizing agents being studied in clinical trials for GBM and other solid tumors (14, 15). PARP1/2 enzymes are responsible for poly ADP-ribosylation (PARylation) of numerous proteins and play crucial role in modulating DNA repair. However, in context of BER, PARP1 also functions as a scaffold that recruits XRCC1 and DNA polymerase-β to apurinic sites, and the TMZ-sensitizing effects of PARP inhibition mainly have been ascribed to this function (4, 16-19). Consistent with this concept, genetic or pharmacologic inhibition of BER or PARP1/2 can significantly sensitize TMZ resistant tumors in vitro (16, 17, 20-26). Contrary to these observations, we previously reported robust in vivo TMZ sensitizing effects with the PARP inhibitor, veliparib that were limited to TMZ-sensitive primary GBM xenograft lines and these effects were lost in derivative TMZ-resistant xenograft models (27). The focus of the current study is to evaluate both in vitro and in vivo chemo-sensitizing effects of veliparib in GBM models with differential TMZ sensitivities and to provide an understanding for the lack of sensitizing effects of veliparib in TMZ resistant GBMs in vivo.
Materials and Methods
Cell culture, drugs & antibodies
Short-term explant cultures (at passage 2-5) of GBM12 and derivative GBM12TMZ sub-lines were grown in neurobasal media (Life Technologies) (9, 28). The generation of the TMZ resistant GBM12 sublines with differential MGMT expression, GBM12TMZ-mgmtLow (#5476 or #5920) and GBM12TMZ-mgmtHigh (#3080), were reported previously (9). U251 and T98G cell lines were obtained from the American Type Culture Collection (ATCC) and authenticated by short tandem repeat analysis (29) performed by the ATCC in November 2013. The U251TMZ model was reported previously (28). U251, U251TMZ and T98G malignant glioma cell lines were maintained in DMEM supplemented with 10% fetal bovine serum (FBS). Cyquant and neurosphere formation assays were performed as described previously (9).TMZ was purchased from the Mayo Clinic Pharmacy and veliparib obtained from the Cancer Therapy Evaluation Program at NCI. For in vivo studies, TMZ was suspended in Ora-plus (Perrigo, Minneapolis, MN) and veliparib diluted in saline, and both were administered orally. Antibodies for phospho-S345-Chk1, phospho-T68-Chk2, γH2AX, Histone H3, β-Actin and PARP1 were obtained from Cell Signaling Technologies; Chk1, Chk2, replication protein-A (RPA) from Millipore; phospho-S824-KAP1 from Abcam; PAR from Trivigen; KAP1 and GAPDH from Santa Cruz Biotech; and MGMT from R&D Systems.
Lentivirus production and cell transduction
Lentivirus (pGIPZ) encoding shRNA for PARP1 and a non-targeting (NT) control obtained from the Mayo RNA Interference Shared Resource and packaged in HEK293T cells. Transduction was performed in the presence of 5 μg/ml polybrene (Millipore) for 24 hours with subsequent selection with puromycin.
Western blotting
Cells were processed for protein extraction and subsequent SDS-poly acrylamide gel electrophoresis as previously described (9, 28). Acid extraction of nuclear proteins was performed to analyze γH2AX and Histone H3; cells were suspended in ice cold PBS/0.5% TritonX100 for 10 min, centrifuged and supernatant discarded. Remaining nuclei were extracted with 0.2 N hydrochloric acid and soluble nuclear proteins were recovered.
Immunocytochemistry
Cells cultured on coverslips were fixed, permeabilized and immuno-stained with replication protein A and/or γH2AX (Cell Signaling) antibodies in 1% bovine serum albumin at 4°C overnight. Cells were washed and then incubated with anti-mouse Alexa-Fluor-488 or anti-rabbit Alexa-Fluor-594 conjugated IgG (BD Biosciences) prior to mounting with Slowfade/DAPI (Invitrogen). Immuno-stained cells were analyzed by confocal microscopy (Zeiss LSM510; 63X objective lenses) and the nuclei positive for foci were quantified.
Efficacy studies in vivo
Studies were reviewed and approved by the Mayo Institutional Animal Care and Use Committee. Subcutaneous xenografts were established by injecting the flank of athymic nude mice with 1×106 cells suspended in matrigel/PBS. Mice with established tumors of ~100 ±15 mm3 size were randomized and treated with placebo, TMZ (50 mg/kg/d) with or without veliparib (25 mg/kg/d, delivered in 2 divided doses) for 5 days every 28 days for 3 cycles. Tumor volume was measured thrice weekly until euthanasia. Intracranial xenografts were established from virally transduced U251TMZ cells as previously described (9); one week after implantation, mice were treated as above, observed daily and euthanized upon reaching a moribund state. For pharmacokinetic analysis, mice with tumors were treated with TMZ and veliparib for 5 days, and tumor harvested 30 min, 2, 4 or 6 hours after the last dose. For pharmacodynamic assessment, tumors were harvested at 2 or 72 hours after the last drug dose.
Tissue analysis of veliparib concentrations
Tumor samples were homogenized, extracted with chilled acetonitrile, and chromatographic separations were achieved on an Aquasil C18 5μm (250 × 4.6mm) column (Thermo Fisher) using an acetonitrile/water mixture containing 0.1% formic acid delivered at a flow rate of 1.2 ml/min. The fractionated column effluent was monitored using fluorescence detection with an excitation and emission of 320 nm and 390 nm, respectively. Veliparib concentrations were determined using standard curves. Pharmacokinetics was analyzed using Phoenix WinNonLin software (Certara).
Statistical analyses
In vitro data presented are the mean ± standard error from 3 or more experiments. Two-tailed Student t tests were used to measure statistical differences. IC50s were calculated by fitting the experimental data to a sigmoidal curve using GraphPad software or by linear regression specifically for GBM12TMZ-mgmtHigh. Statistical analysis of animal survival and tumor progression was performed using log-rank test.
Results
Veliparib sensitizes GBM cells to TMZ
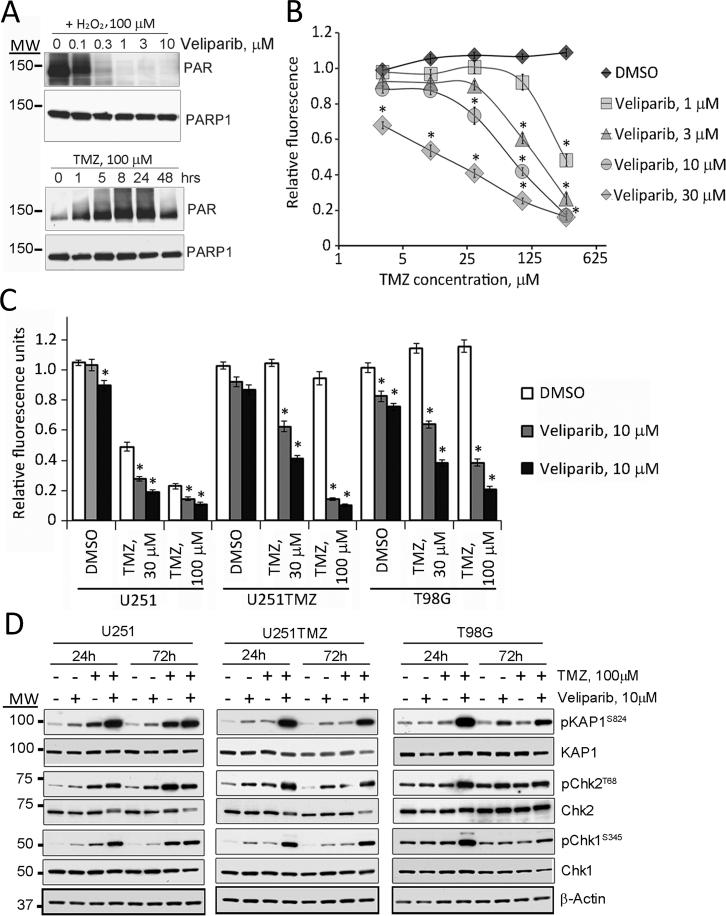
The dose-response for inhibition of PARP activity was initially assessed in T98G cells. Using hydrogen peroxide (H2O2) to maximally stimulate PARP, veliparib resulted in marked suppression of PARP-induced poly ADP-ribosylation (PARylation) at concentrations greater than 1 μM (Figure 1A, upper panel). At this concentration, veliparib effectively suppressed PARP activity for up to 6 days, which suggests that the drug is highly stable in culture media (Supplementary Figure S1A). Therapeutically achievable concentrations of TMZ (100 μM) (30) induced PARP activity in T98G cells with a steady increase in PARylation up to 24 hours (Figure 1A, lower panel). Consistent with these data, veliparib alone had minimal growth inhibitory effects, while combined veliparib/TMZ treatment significantly decreased the growth of T98G cells (Figure 1B). At the lowest concentration of veliparib tested (1 μM), no enhanced TMZ cytotoxicity was observed except at 300 μM TMZ, and only a marginal increase in growth inhibition was observed with repeated co-administration of 100 μM TMZ (Supplementary Figure S1B). In contrast, significantly enhanced cytotoxicity was seen with 30 μM veliparib at all concentrations of TMZ (Figure 1B). The effects of combination therapy also were evaluated in the U251 cell line and the derivative TMZ-resistant U251TMZ line developed by our laboratory (28). Combining veliparib with TMZ showed enhanced effects in U251 cells and U251TMZ line (Figure 1C). In combination with 10 μM veliparib, the difference in cytotoxicity for 100 μM TMZ was more profound in the TMZ-resistant U251TMZ cell line (relative fluorescence 0.95±0.04 for TMZ alone versus 0.14±0.01 for the combination, p<0.01) and T98G (1.15±0.02 versus 0.38±0.02, respectively, p<0.01) as compared to U251 cells (0.23±0.02 versus 0.15±0.01, respectively, p<0.01). Similarly, veliparib enhanced TMZ induced apoptosis in all 3 lines with more pronounced effects seen in TMZ resistant T98G and U251TMZ (Supplementary Figure S2). Taken together, these data suggest that inhibition of PARP by veliparib effectively sensitizes both sensitive and resistant GBM cells to TMZ, and at high concentrations of veliparib, the sensitizing effects are more evident in TMZ-resistant models.
Figure 1. Veliparib sensitizes GBM cell lines to TMZ in vitro.
A) Veliparib dose-response: T98G cells were pre-treated with the indicated concentrations of veliparib and stimulated with 100 μM H2O2 or incubated with 100 μM TMZ. Immunoblotting for PAR and total PARP1 on the same membrane are shown. B) Cytotoxicity assay: T98G cells were incubated with graded concentrations of veliparib and TMZ for 6 days and then analyzed in a CyQuant assay. Results shown are relative fluorescence for treated vs. untreated cells. C) Similar TMZ-sensitizing studies were performed for U251 and U251TMZ cells and results for all 3 lines at selected concentrations of drugs are shown. D) DNA damage signaling: U251, U251TMZ and T98G cells were treated as indicated for 24 or 72 hours and processed for immunoblotting with the indicated antibodies. Results for A and D are representative of 3 independent experiments, and in B and C, data plotted represent the mean ± SEM from a minimum of 3 independent experiments. * indicates a p-value<0.05 and MW, molecular weight.
Veliparib promotes TMZ mediated DNA damage in GBM cells
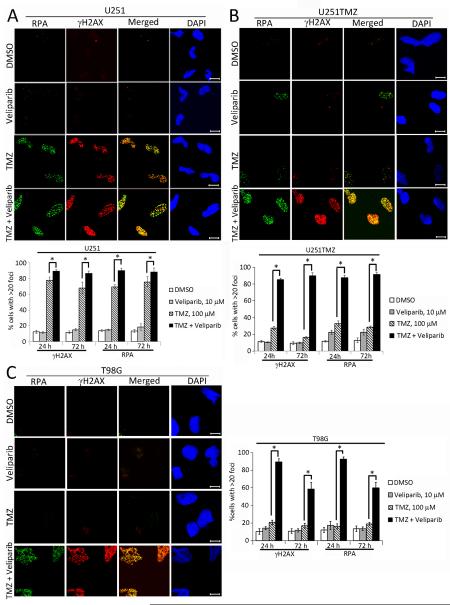
The impact of veliparib on TMZ-induced DNA damage signaling was evaluated in vitro. Treatment with 100 μM TMZ alone resulted in reproducible increased phosphorylation of Chk1, Chk2 and KAP1 only in U251 cells, while monotherapy with veliparib in U251 cells and veliparib or TMZ in U251TMZ resulted in nominal changes in DNA damage signaling. In contrast, co-treatment with TMZ and 10 μM veliparib resulted in markedly elevated phosphorylation of these proteins in all 3 tumor lines after 24 hours of treatment. The phosphorylation signals were maintained 72 hours after treatment in U251 and U251TMZ cells and reduced in intensity in T98G cells (Figure 1D). As a second measure of DNA damage, the effects of drug treatment on induction of stalled replication forks (RPA foci) and DNA double-strand breaks (γH2AX foci) were evaluated by immunofluorescence. As seen in Figure 2, treatment with TMZ in U251 and TMZ/veliparib in all 3 cells lines induced γH2AX and RPA foci. Although, TMZ treatment induced RPA and γH2AX foci in all lines tested, the fraction of cells with >20 γH2AX foci 24 hours after treatment was markedly lower in the resistant U251TMZ and T98G as compared to U251 cells. Co-treatment with veliparib and TMZ, compared to TMZ alone, resulted in a modest increase in the fraction of U251 cells with γH2AX foci 24 hours after treatment (90.6±1.9% versus 78.1±7.6%, respectively; p=0.042), while the combination had more profound effects in both resistant models: U251TMZ (85.0±1.8% versus 24.7±4.2%, respectively; p<0.002), and T98G (89.3±3.6% versus 20.7±2.3%, respectively; p<0.001). Similar results were observed for γH2AX 72 hours after treatment, and evaluation of RPA foci resulted in a highly similar result at both time-points. Collectively, the differences in cell survival and the extent of DNA damage induced by TMZ alone versus the TMZ/veliparib combination are greater in the TMZ-resistant glioma lines as compared to a TMZ-sensitive line.
Figure 2. Veliparib promotes TMZ induced γH2AX and RPA foci GBM cell lines.
A. U251, B. U251TMZ, and C. T98G cells cultured on glass cover slips were treated with DMSO, 10 μM veliparib for 30 min and subsequently with or without 100 μM TMZ for 24 h or 72 h and immunostained for RPA32 and γH2AX and counterstained with DAPI. Shown on the top are representative images (bar =10 μm) and graphs depicted below present the mean ± SEM from 3 independent experiments for percentage of cells with >20 foci/nuclei for each treatment. * indicates a p-value ≤ 0.05.
Veliparib potentiates cytotoxic effects of TMZ in primary xenograft GBM lines in vitro
Combination therapy was evaluated further in a patient derived xenograft line that faithfully maintains the molecular features of the derivative patient tumor (31) and in two derivative TMZ-resistant sub-lines we developed by treating GBM12 tumors with a clinically relevant TMZ dosing regimen (9). The effects of veliparib on TMZ cytotoxicity were evaluated in neurosphere cultures, which maintain a pluripotent phenotype (Supplementary Figure S3). Parental GBM12 was markedly sensitive to even 10 μM TMZ with only 20.5±2.5% neurosphere formation compared to control and no surviving neurospheres after 100 μM TMZ. In contrast, GBM12TMZ-mgmtLow and GBM12TMZ-mgmtHigh sub-lines were significantly resistant to 100 μM TMZ (39.4±1.4% and 95.7±3.1% relative neurosphere formation, respectively; Figure 3A). The addition of veliparib significantly enhanced the efficacy of TMZ in all 3 xenograft lines with more pronounced effects observed at higher concentrations of TMZ and/or veliparib (Figure 3A and Supplementary Figure S4A). At each concentration tested, veliparib treatment was associated with a reduced IC50 for TMZ in all 3 xenograft lines. The differences in IC50 with and without veliparib were more pronounced in the TMZ resistant sub-lines (Figure 3B), although at a concentration of 1 μM veliparib, the TMZ IC50 for the GBM12TMZ-mgmtLow (38 μM) and GBM12TMZ-mgmtHigh (207 μM) are significantly higher than the IC50 for TMZ only in GBM12 (6.9 μM). Similar chemo- potentiating effects of veliparib also were observed in other xenograft lines with differential TMZ sensitivities (Supplementary Figure S4B). Thus, despite significant cytotoxic enhancing effects in each cell line, the level of TMZ sensitivity in the resistant lines co-treated with veliparib did not approach the level of inherent TMZ sensitivity of the parental GBM12 line.
Figure 3. Veliparib sensitizes GBM12 derivative TMZ-resistant xenograft lines in vitro.
A, B) Cytotoxicity assay: the sensitive GBM12 and derivative TMZ resistant sub-lines, GBM12TMZ-mgmtLow and GBM12TMZ-mgmtHigh were analyzed for neurosphere growth following treatment with graded concentrations of veliparib and/or TMZ, A) the relative neurosphere counts for the indicated treatments are graphed as the mean ± SEM from 3 independent experiments. B) The calculated IC50 for TMZ at different concentrations of veliparib are plotted as the mean ± SD from 3 independent experiments. C) DNA damage signaling: GBM12 and derivative sub-lines were treated for 24 hours with the indicated doses of veliparib and/or TMZ and then processed for immunoblotting with the indicated antibodies. D) Cells were analyzed for γH2AX foci as in Figure 2 after drug treatment for 24 hours. Results are plotted as the mean ± SEM from a minimum of 3 independent experiments. * indicates a p-value ≤ 0.05 and NS a p-value > 0.05 and MW, molecular weight.
Veliparib enhances TMZ induced DNA damage signaling
The impact of graded veliparib concentrations on TMZ-induced damage signaling was evaluated in vitro for the GBM12 sub-lines. TMZ induced robust activation of PARP activity, and veliparib was equally effective at suppressing PAR levels in all 3 sub-lines with complete suppression of detectable PAR at concentrations of 0.3 μM veliparib (Figure 3C). A similar concentration range (1 to 3 μM) combined with TMZ was associated with reproducibly increased phosphorylation of Chk1, Chk2 and KAP1 as compared to TMZ alone in all 3 xenograft lines. Furthermore, veliparib equally enhanced TMZ induced DNA damage signaling in undifferentiated (neurospheres) or differentiated (FBS supported monolayer) cell cultures of each xenograft line (Supplementary Figure S5). Treatment with TMZ alone resulted in significantly increased γH2AX foci only in GBM12 cells. Co-treatment resulted in significantly increased γH2AX foci compared to TMZ alone in all 3 lines: GBM12 (74.2±2.1% cell with > 20 foci vs. 59.4±2.0% with TMZ alone; p=0.002), GBM12TMZ-mgmtLow (77.3±2.8% vs. 17.6±3.7 %, respectively; p=0.0005), and GBM12T-mgmtHigh (64.2±3.7% vs. 12.9±1.9%, respectively; p=0.0009) (Figure 3D). Taken together, these results indicate that veliparib enhances TMZ-induced DNA damage signaling response in each of the xenograft lines regardless of their differential sensitivity to TMZ.
Differential TMZ sensitizing effects of veliparib in vivo
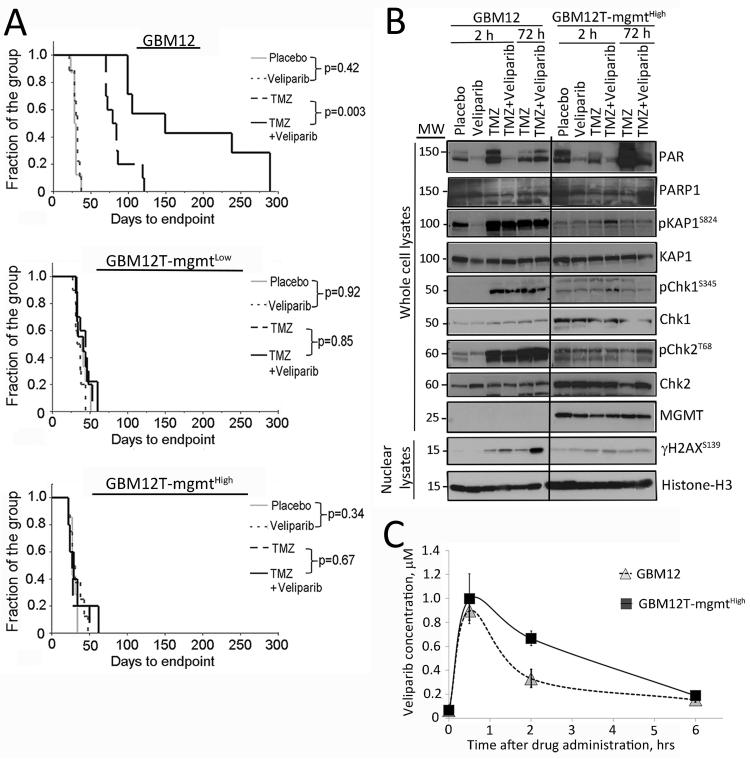
The efficacy of combined veliparib and TMZ was assessed in the GBM12 sub-lines as subcutaneous xenografts to eliminate issues of drug delivery across the blood-brain barrier. Consistent with observed chemo-sensitization in vitro, the combination of veliparib and TMZ resulted in significant delay in tumor progression in parental GBM12 as compared to treatment with TMZ alone (difference in median time to endpoint 68 days for TMZ vs. TMZ/veliparib, p=0.003) (Figure 4A and Supplementary Figure S6). However, tumor progression in both GBM12TMZ sub-lines was unaffected by the combination as compared to TMZ alone (difference in median time to end point -4 days for GBM12TMZmgmtLow (p=0.85) and +1 day for GBM12TMZmgmtHigh (p = 0.67) (Figure 4A and Supplementary Figure S3). These data demonstrate a marked discordance between the profound sensitizing effects observed in vitro compared to a lack of sensitizing effect in vivo for the resistant lines.
Figure 4. Veliparib enhances the efficacy of TMZ in vivo in GBM12 but not in TMZ-resistant sub-lines.
A) Tumor regrowth analysis: Mice with established flank tumor xenografts were randomized into groups of 10 mice each and treated for 3 cycles with the indicated drugs. Time for reaching critical tumor volume of 1500 mm3 is shown as endpoint for each group as Kaplan-Meier plots. B) Pharmacodynamic analysis: mice with GBM12 or GBM12TMZ-mgmtHigh xenografts were treated for 5 days and euthanized either 2 hours or 72 hours after the last dose of TMZ/veliparib. Three tumors were processed for each treatment/time point, and equal amounts of protein from these tumors were pooled for analysis in any given lane. All samples were run on the same gel, but 2 unloaded intervening lanes have been cropped from the images, MW indicates molecular weight. C) Pharmacokinetic profile of veliparib in tumor tissue: Groups of mice with flank xenografts were treated for 5 days with TMZ/veliparib and euthanized at time-points ranging from 0 to 6 hours after the last dose of veliparib. The values plotted are the mean ± SD veliparib concentrations in 5 tumors at each time point.
Pharmacodynamic effects were evaluated in the primary GBM12 line and the GBM12TMZ-mgmtHigh sub-line to compare the effects of veliparib on TMZ-induced damage signaling after 5 days of therapy. As expected, veliparib alone or in combination with TMZ efficiently suppressed PARP activity 2 hours, but not as effectively at 72 hours, after the last drug treatment. In the GBM12 xenografts, there was robust induction of phospho-KAP1, phospho-Chk1 and phospho-Chk2 associated with TMZ treatment with or without veliparib at both time points (Figure 4B). In contrast, discrete but marginal increases in DNA damage signaling were observed in the GBM12TMZ-mgmtHigh sub-line only 2 hours after the last dose of TMZ/veliparib combination for phospho-KAP1 and phospho-Chk1 with no evidence of persistent activation at 72 hours (Figure 4B). Analysis of nuclear extracts demonstrated enhanced γH2AX levels with combination TMZ/veliparib, as compared to TMZ alone in GBM12 samples harvested 72 hours after drug treatment. In contrast, TMZ alone or TMZ/veliparib had a marginal increase in γH2AX signal 2 hours but not 72 hours after the last drug dose (Figure 4B). Collectively, these data demonstrate that lack of sensitization in vivo by veliparib in the TMZ resistant GBM12TMZ-mgmtHigh sub-line is associated with nominal induction of DNA damage.
Pharmacokinetic evaluation
The tumor tissue concentration-time profile of veliparib was determined to evaluate whether lack of tumor response in the resistant tumor model was associated with differences in achievable drug levels. For GBM12, tumors harvested at 30 min (Tmax determined previously) revealed a tumor tissue Cmax of 1.23±0.11 μM and an AUC0-6 hr value of 3.04 μM*hr (Figure 4C). The tumor tissue concentration-time profile in GBM12T-mgmtHigh was similar with a slightly higher Cmax of 1.36±0.28 μM (p=0.55 versus Cmax for GBM12) and AUC0-6 hr of 4.4 μM* hr (p=0.10 versus AUC for GBM12). The half-life values of the drug in GBM12 and GBM12TMZ-mgmtHigh tumors were 2.6 and 2.3 hrs, respectively (p=0.60) (Figure 4C). In comparison to the in vitro concentration-response curves for the GBM12 sub-lines, the maximum concentration of veliparib (1.2-1.4 μM) in vivo is much lower than optimal concentrations associated with robust TMZ-sensitizing effects in vitro.
PARP knockdown potentiates TMZ response in TMZ-resistant model
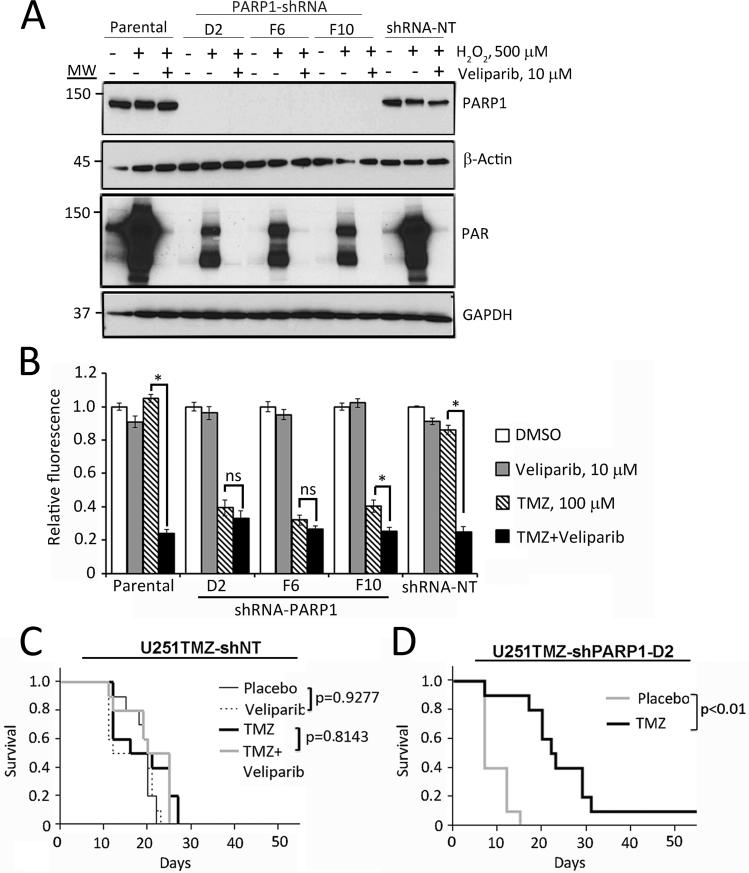
Results from our pharmacologic analyses implied that lack of efficient chemo-sensitization in resistant tumors may be related to the biologically achievable/tolerable concentrations of veliparib. To test this possibility, the effects of PARP1 knockdown were assessed in the U251TMZ model. As seen in Figure 5A, effective PARP1 knockdown was achieved using 3 different lentiviral shRNA constructs (D2, F6 and F10), and PARP1 knockdown significantly suppressed PAR accumulation in response to H2O2 as compared to non-transduced or cells transduced with a non-targeted (NT) shRNA (Figure 5A). In a cell growth assay, PARP1 knockdown had no significant effect on cell growth or survival, but was associated with significantly enhanced sensitivity to TMZ to an extent similar to that seen with veliparib in the parental U251TMZ or U251TMZ-NT cells (Figure 5B). Moreover, in the PARP1 knockdown cells (U251TMZ-D2 or F6), treatment with TMZ alone was as effective as TMZ/veliparib. These data demonstrate that PARP1 knockdown in U251TMZ cells can provide equivalent TMZ-sensitizing effects as an optimal concentration of veliparib in vitro.
Figure 5. PARP1 knockdown facilitates TMZ efficacy in U251TMZ in vitro and in vivo.
A) PARP1 knockdown: U251TMZ cells and derivative stable transductants expressing PARP1-specific shRNA (D2, F6, F10) or non-targeted shRNA (shRNA-NT) were treated with DMSO or veliparib and subsequently stimulated with H2O2 for 10 min. Samples were processed for immunoblotting with the indicated antibodies, MW (molecular weight). B) TMZ-sensitizing effects: parental and shRNA-expressing U251TMZ cells were treated with TMZ and/or veliparib, and cell growth was compared using a CyQuant assay. The data plotted represent the mean ± SEM from a minimum of 3 independent experiments. * indicates a p-value ≤0.05, NS p-value >0.05. C & D) The indicated cells were used to establish orthotopic tumors and groups of 10 mice with established tumors were randomized to therapy as indicated. Survival for mice in each treatment group is plotted in the Kaplan-Meier graphs and differences evaluated using log-rank test.
The U251TMZ-NT and U251TMZ-D2 sub-lines then were used in an intracranial tumor model to evaluate the effects of PARP1 knockdown on TMZ responsiveness in vivo. As expected the U251TMZNT sub-line was highly resistant to TMZ therapy with no significant gain in survival compared to placebo treatment (p=0.30), and similar to previous experience with GBM12TMZ lines, the combination of veliparib with TMZ was equally ineffective (p=0.81 compared to TMZ alone, and p=0.09 compared to placebo; Figure 5C). In contrast, the U251TMZ-D2 sub-line was remarkably sensitive to TMZ compared to placebo with a significant extension in survival (median survival 26 days vs. 9 days, p<0.001; Figure 5D). These results suggest that effective PARP suppression in TMZ-resistant tumors may provide significant gain in TMZ sensitivity similar to that seen in vitro with the combination of TMZ and veliparib.
Discussion
The combination of PARP inhibitors with specific chemotherapies can provide significant sensitizing effects in pre-clinical models, and there is intense interest in moving the most promising regimens into clinical trials. The data presented here demonstrate profound in vitro TMZ-sensitizing effects of veliparib in 4 GBM models of acquired (U251TMZ, GBM12TMZ-mgmtHigh or GBM12TMZ-mgmtLow) or inherent (T98G) TMZ resistance as compared to more modest sensitizing effects in TMZ sensitive models (U251, GBM12). Multiple other studies have observed a similar phenomenon with more pronounced in vitro TMZ-sensitizing effects of PARP inhibitors observed in TMZ-resistant models (14, 25, 32). Although direct comparison of in vitro and in vivo results for sensitive versus resistant lines have not been published previously, the in vivo sensitizing effects of PARP inhibition generally are limited in TMZ resistant models and more profound in TMZ sensitive models (14, 15, 18, 22, 33-39). Clear understanding of the basis for this striking difference between in vitro and in vivo sensitizing effects has important implications for understanding the spectrum of tumors that may respond to this novel therapeutic strategy.
The discordance between cell culture and animal studies at least partially can be explained by examining in vitro sensitizing effects relative to tolerable drug concentrations in animals or humans. TMZ is clinically dosed at 150-200 mg/m2 to achieve peak concentrations approximating 30-100 μM in humans, and the dosing schedule used in the animal studies reported here provide a similar pharmacokinetic profile in mice as in humans (30). Conversely, hematologic toxicities limit the maximally tolerated dose of veliparib in humans to 40 mg/kg twice daily when given concurrently with TMZ. This dose of veliparib is associated with peak plasma concentrations of less than 1.5 μM in humans, which is similar to the drug concentrations achieved in mice in the current studies (Figure 4C) (33, 40). The initial dose-response evaluation presented in Figure 1B demonstrates that effective sensitization of T98G cells with 1 μM veliparib was only achieved at supra-therapeutic concentrations of 300 μM TMZ, and the more profound sensitizing effects within the clinically achievable TMZ concentration range were limited to supra-therapeutic levels of veliparib. A similar effect was seen in the primary GBM12 xenograft line and derivative resistant models in which the sensitizing effects were nominal at 1 μM veliparib (Figure 3B). In this same context, the profound TMZ-sensitizing effects of PARP inhibitors in TMZ-resistant models shown in multiple studies mostly are limited to clinically unachievable concentrations of either TMZ and/or PARP inhibitor (14, 18, 25, 32, 38, 41). These data highlight the importance of interpreting in vitro results in the context of clinically relevant drug concentrations.
The in vitro veliparib/TMZ combination data analysis suggests that the limited in vivo efficacy observed in TMZ-resistant models may be related to inadequate suppression of PARP activity in animals. Consistent with this idea, PARP1 knockdown effectively sensitized the U251TMZ sub-line to TMZ in vitro to an extent similar as supra-physiologic levels (10 μM) of veliparib. These data suggest that inhibition of PARP1 specifically may be important for sensitizing effects at these drug concentrations, and the residual PAR-modifications seen in Figure 6A for the shPARP1-treated cells may reflect basal PARP2 activity. Similar studies in HeLa and B16 tumor cell lines have demonstrated robust TMZ-sensitizing effects in vitro and in vivo following PARP1 knockdown without additional sensitizing effects when cells were co-treated with the PARP inhibitor GPI15427 (26). These data exclude veliparib-mediated PARP trapping onto DNA as a probable mechanism of the in vitro sensitizing effects observed here (42). Similar to the in vitro results, the PARP knockdown in U251TMZ-shPARP-D2 xenografts significantly sensitized this highly resistant model to TMZ while veliparib had no impact on TMZ efficacy in the corresponding non-targeted shRNA sub-line (Figure 5D). While the biochemical effects of shRNA knock-down versus pharmacologic inhibition are distinct, the shRNA approach allowed robust suppression of PARP1 activity without associated systemic toxicity when combined with TMZ. Considering that tolerable dosing of veliparib with TMZ limits the exposure to ~1 μM veliparib (Figure 4C), the shRNA data presented suggest that the achievable drug levels provide insufficient suppression of PARP1 activity to effectively sensitize resistant tumor models to TMZ.
The impact of veliparib on various PARP-mediated DNA repair processes, and the mechanism of TMZ-sensitization at clinically achievable concentrations remain unclear. PARP1/2 facilitates the recruitment of BER proteins to abasic sites, and PARP1 knockdown or robust inhibition can efficiently suppress BER (16). Since over 80% of TMZ-induced lesions are repaired by BER and disruption of BER significantly enhances the lethality of TMZ, the disruption of BER by PARP inhibition is widely viewed as the likely mechanism of sensitization (43). Consistent with this notion, robust sensitization was observed in the TMZ-resistant tumor lines at high concentrations of veliparib. However, at clinically relevant concentrations of ≤1 μM veliparib, the efficacy of TMZ therapy was enhanced only in TMZ-sensitive models (Figure 4A), and the extent of in vitro enhancement was modest compared to more profound sensitizing effects observed in vivo (Figures 3A and 4A). In comparing the extent of DNA damage induction in vivo (Figure 4B), TMZ with or without veliparib did not induce significant DNA damage signaling in the MGMT over-expressing GBM12TMZ sub-line, while the same treatment induced robust damage signaling in the parental GBM12 line. MGMT directly removes O6MeG lesions, which otherwise mispair with thymidine, trigger futile cycles of mismatch repair, and ultimately lead to replication-associated DNA double-strand breaks. Recent studies have demonstrated a role for PARP in preventing MRE-11-mediated degradation of stalled replication forks (9, 44), and we hypothesize that the effects of veliparib on the processing of O6MeG-induced stalled replication forks may be an important contributor to the sensitizing effects of this drug in vivo.
The results of the current study provide critical insight into design of clinical trials with veliparib and possibly other PARP inhibitors. The lack of efficacy of veliparib combined with TMZ in resistant models suggests this treatment regimen is unlikely to be useful in patients that have progressed on a TMZ-based therapy or in tumors that are inherently resistant to TMZ. Based on these and other data, the Alliance cooperative group is launching a randomized Phase II/III clinical trial to test the efficacy of veliparib combined with TMZ in newly diagnosed GBM patients. Using MGMT promoter hypermethylation as a biomarker for TMZ-sensitive tumors, this trial only will enroll patients with tumor MGMT promoter hypermethylation. Our data would suggest that robust TMZ sensitizing effects could be achieved in either TMZ sensitive or resistant tumors if PARP activity can be sufficiently suppressed. This is especially interesting in the context of the next generation of highly potent and specific PARP inhibitors (45-47). Combinations of TMZ with these more potent PARP inhibitors are less well tolerated in animals than veliparib and require significant dose-reductions of either TMZ or the PARP inhibitor (unpublished data). Thus, similar to previous clinical studies with the MGMT inhibitor O6-benzylguanine, successful clinical integration of these more potent PARP inhibitors with TMZ may be limited by a narrow therapeutic window (48). These observations reinforce the importance of evaluating the potential clinical benefit for specific PARP inhibitor/TMZ combinations in the context of clinically achievable concentrations of the combination partners.
Supplementary Material
Translational relevance.
Numerous pre-clinical studies have suggested that PARP inhibitors including veliparib can enhance the efficacy of temozolomide (TMZ) in both sensitive and resistant tumor models. However, we previously demonstrated that the in vivo TMZ-sensitizing effects of veliparib were restricted exclusively to TMZ-sensitive GBM xenograft models. Using paired sensitive and derivative resistant models, robust sensitization was observed in vitro for TMZ-resistant models at supra-therapeutic drug concentrations. In contrast, using the maximally tolerable in vivo regimen that approximates clinically achievable drug exposures, robust sensitizing effects with veliparib only were observed in TMZ sensitive models. While these results have implications for understanding the spectrum of tumors that may respond to this novel therapeutic strategy, they also highlight the importance of evaluating PARP inhibitors or other novel chemosensitizing strategies in the context of clinically achievable drug concentrations.
Acknowledgments
We thank Jenny L Pokorny, Karen E. Parrish and Dr. Gasper Kitange for technical assistance and discussion, Drs. Larry Karnitz and Vincent Giranda for critical review of the manuscript and Mayo Optical Morphology Core for imaging. Veliparib was generously provided by AbbVie and the National Cancer Institute, NIH.
Grant support: This work was supported by the Mayo Clinic, funding from the NIH RO1 CA127716, RO1 CA141121 and the Mayo Brain Tumor SPORE P50 CA108961
Footnotes
Disclosure of Potential Conflicts of Interest: JNS has commercial research grant from Merck, Basilea, Sanofi and Genentech. No potential conflicts of interest were disclosed by other authors.
References
- 1.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–97. [PubMed] [Google Scholar]
- 4.Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- 5.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA repair. 2007;6:1079–99. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Kitange GJ, Carlson BL, Mladek AC, Decker PA, Schroeder MA, Wu W, et al. Evaluation of MGMT promoter methylation status and correlation with temozolomide response in orthotopic glioblastoma xenograft model. J Neurooncol. 2009;92:23–31. doi: 10.1007/s11060-008-9737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14:2900–8. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 9.Kitange GJ, Mladek AC, Carlson BL, Schroeder MA, Pokorny JL, Cen L, et al. Inhibition of histone deacetylation potentiates the evolution of acquired temozolomide resistance linked to MGMT upregulation in glioblastoma xenografts. Clin Cancer Res. 2012;18:4070–9. doi: 10.1158/1078-0432.CCR-12-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Atri S, Tentori L, Lacal PM, Graziani G, Pagani E, Benincasa E, et al. Involvement of the mismatch repair system in temozolomide-induced apoptosis. Mol Pharmacol. 1998;54:334–41. doi: 10.1124/mol.54.2.334. [DOI] [PubMed] [Google Scholar]
- 11.Cahill DP, Levine KK, Betensky RA, Codd PJ, Romany CA, Reavie LB, et al. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin Cancer Res. 2007;13:2038–45. doi: 10.1158/1078-0432.CCR-06-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsberg J, Thon N, Eigenbrod S, Hentschel B, Sabel MC, Westphal M, et al. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011;129:659–70. doi: 10.1002/ijc.26083. [DOI] [PubMed] [Google Scholar]
- 13.Yip S, Miao J, Cahill DP, Iafrate AJ, Aldape K, Nutt CL, et al. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res. 2009;15:4622–9. doi: 10.1158/1078-0432.CCR-08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 15.Thomas HD, Calabrese CR, Batey MA, Canan S, Hostomsky Z, Kyle S, et al. Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol Cancer Ther. 2007;6:945–56. doi: 10.1158/1535-7163.MCT-06-0552. [DOI] [PubMed] [Google Scholar]
- 16.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387–93. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortini P, Pascucci B, Parlanti E, D'Errico M, Simonelli V, Dogliotti E. The base excision repair: mechanisms and its relevance for cancer susceptibility. Biochimie.;2003;85:1053–71. doi: 10.1016/j.biochi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Shi Y, Guan R, Donawho C, Luo Y, Palma J, et al. Potentiation of temozolomide cytotoxicity by poly(ADP)ribose polymerase inhibitor ABT-888 requires a conversion of single-stranded DNA damages to double-stranded DNA breaks. Mol Cancer Res. 2008;6:1621–9. doi: 10.1158/1541-7786.MCR-08-0240. [DOI] [PubMed] [Google Scholar]
- 19.Masson M, Niedergang C, Schreiber V, Muller S, Menissier-de Murcia J, de Murcia G. XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol Cell Biol. 1998;18:3563–71. doi: 10.1128/mcb.18.6.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svilar D, Goellner EM, Almeida KH, Sobol RW. Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. Antioxid Redox Signal. 2011;14:2491–507. doi: 10.1089/ars.2010.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agnihotri S, Gajadhar AS, Ternamian C, Gorlia T, Diefes KL, Mischel PS, et al. Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients. J Clin Invest. 2012;122:253–66. doi: 10.1172/JCI59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miknyoczki S, Chang H, Grobelny J, Pritchard S, Worrell C, McGann N, et al. The selective poly(ADP-ribose) polymerase-1(2) inhibitor, CEP-8983, increases the sensitivity of chemoresistant tumor cells to temozolomide and irinotecan but does not potentiate myelotoxicity. Mol Cancer Ther. 2007;6:2290–302. doi: 10.1158/1535-7163.MCT-07-0062. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers AJ. Overcoming resistance of glioblastoma to conventional cytotoxic therapies by the addition of PARP inhibitors. Anticancer Agents Med Chem. 2010;10:520–33. doi: 10.2174/187152010793498627. [DOI] [PubMed] [Google Scholar]
- 24.Goellner EM, Grimme B, Brown AR, Lin YC, Wang XH, Sugrue KF, et al. Overcoming temozolomide resistance in glioblastoma via dual inhibition of NAD+ biosynthesis and base excision repair. Cancer Res. 2011;71:2308–17. doi: 10.1158/0008-5472.CAN-10-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horton TM, Jenkins G, Pati D, Zhang L, Dolan ME, Ribes-Zamora A, et al. Poly(ADP-ribose) polymerase inhibitor ABT-888 potentiates the cytotoxic activity of temozolomide in leukemia cells: influence of mismatch repair status and O6-methylguanine-DNA methyltransferase activity. Mol Cancer Ther. 2009;8:2232–42. doi: 10.1158/1535-7163.MCT-09-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tentori L, Muzi A, Dorio AS, Scarsella M, Leonetti C, Shah GM, et al. Pharmacological inhibition of poly(ADP-ribose) polymerase (PARP) activity in PARP-1 silenced tumour cells increases chemosensitivity to temozolomide and to a N3-adenine selective methylating agent. Curr Cancer Drug Targets. 2010;10:368–83. doi: 10.2174/156800910791208571. [DOI] [PubMed] [Google Scholar]
- 27.Clarke MJ, Mulligan EA, Grogan PT, Mladek AC, Carlson BL, Schroeder MA, et al. Effective sensitization of temozolomide by ABT-888 is lost with development of temozolomide resistance in glioblastoma xenograft lines. Mol Cancer Ther. 2009;8:407–14. doi: 10.1158/1535-7163.MCT-08-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nadkarni A, Shrivastav M, Mladek AC, Schwingler PM, Grogan PT, Chen J, et al. ATM inhibitor KU-55933 increases the TMZ responsiveness of only inherently TMZ sensitive GBM cells. J Neurooncol. 2012;110:349–57. doi: 10.1007/s11060-012-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capes-Davis A, Reid YA, Kline MC, Storts DR, Strauss E, Dirks WG, et al. Match criteria for human cell line authentication: where do we draw the line? Int J Cancer. 2013;132:2510–9. doi: 10.1002/ijc.27931. [DOI] [PubMed] [Google Scholar]
- 30.Middlemas DS, Stewart CF, Kirstein MN, Poquette C, Friedman HS, Houghton PJ, et al. Biochemical correlates of temozolomide sensitivity in pediatric solid tumor xenograft models. Clinical Cancer Research. 2000;6:998–1007. [PubMed] [Google Scholar]
- 31.Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–76. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curtin NJ, Wang LZ, Yiakouvaki A, Kyle S, Arris CA, Canan-Koch S, et al. Novel poly(ADP-ribose) polymerase-1 inhibitor, AG14361, restores sensitivity to temozolomide in mismatch repair-deficient cells. Clin Cancer Res. 2004;10:881–9. doi: 10.1158/1078-0432.ccr-1144-3. [DOI] [PubMed] [Google Scholar]
- 33.Donawho CK, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, et al. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res. 2007;13:2728–37. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 34.Tentori L, Leonetti C, Scarsella M, Muzi A, Mazzon E, Vergati M, et al. Inhibition of poly(ADP-ribose) polymerase prevents irinotecan-induced intestinal damage and enhances irinotecan/temozolomide efficacy against colon carcinoma. Faseb J. 2006;20:1709–11. doi: 10.1096/fj.06-5916fje. [DOI] [PubMed] [Google Scholar]
- 35.Tentori L, Leonetti C, Scarsella M, D'Amati G, Vergati M, Portarena I, et al. Systemic administration of GPI 15427, a novel poly(ADP-ribose) polymerase-1 inhibitor, increases the antitumor activity of temozolomide against intracranial melanoma, glioma, lymphoma. Clin Cancer Res. 2003;9:5370–9. [PubMed] [Google Scholar]
- 36.Daniel RA, Rozanska AL, Thomas HD, Mulligan EA, Drew Y, Castelbuono DJ, et al. Inhibition of poly(ADP-ribose) polymerase-1 enhances temozolomide and topotecan activity against childhood neuroblastoma. Clin Cancer Res. 2009;15:1241–9. doi: 10.1158/1078-0432.CCR-08-1095. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Han EK, Anderson M, Shi Y, Semizarov D, Wang G, et al. Acquired resistance to combination treatment with temozolomide and ABT-888 is mediated by both base excision repair and homologous recombination DNA repair pathways. Mol Cancer Res. 2009;7:1686–92. doi: 10.1158/1541-7786.MCR-09-0299. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Palma J, Kinders R, Shi Y, Donawho C, Ellis PA, et al. An enzyme-linked immunosorbent poly(ADP-ribose) polymerase biomarker assay for clinical trials of PARP inhibitors. Anal Biochem. 2008;381:240–7. doi: 10.1016/j.ab.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Penning TD, Zhu GD, Gandhi VB, Gong J, Liu X, Shi Y, et al. Discovery of the Poly(ADP-ribose) polymerase (PARP) inhibitor 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide (ABT-888) for the treatment of cancer. J Med Chem. 2009;52:514–23. doi: 10.1021/jm801171j. [DOI] [PubMed] [Google Scholar]
- 40.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–11. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tentori L, Turriziani M, Franco D, Serafino A, Levati L, Roy R, et al. Treatment with temozolomide and poly(ADP-ribose) polymerase inhibitors induces early apoptosis and increases base excision repair gene transcripts in leukemic cells resistant to triazene compounds. Leukemia. 1999;13:901–9. doi: 10.1038/sj.leu.2401423. [DOI] [PubMed] [Google Scholar]
- 42.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72:5588–99. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 44.Bryant HE, Petermann E, Schultz N, Jemth AS, Loseva O, Issaeva N, et al. PARP is activated at stalled forks to mediate Mre11-dependent replication restart and recombination. Embo J. 2009;28:2601–15. doi: 10.1038/emboj.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calvert H, Azzariti A. The clinical development of inhibitors of poly(ADP-ribose) polymerase. Ann Oncol. 2011;22(Suppl 1):i53–9. doi: 10.1093/annonc/mdq667. [DOI] [PubMed] [Google Scholar]
- 46.Ekblad T, Camaioni E, Schuler H, Macchiarulo A. PARP inhibitors: polypharmacology versus selective inhibition. Febs J. 2013;280:3563–75. doi: 10.1111/febs.12298. [DOI] [PubMed] [Google Scholar]
- 47.Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN 673, a novel and highly potent PARP1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19:5003–15. doi: 10.1158/1078-0432.CCR-13-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quinn JA, Jiang SX, Reardon DA, Desjardins A, Vredenburgh JJ, Friedman AH, et al. Phase II trial of temozolomide (TMZ) plus irinotecan (CPT-11) in adults with newly diagnosed glioblastoma multiforme before radiotherapy. Journal of neuro-oncology. 2009;95:393–400. doi: 10.1007/s11060-009-9937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.