Abstract
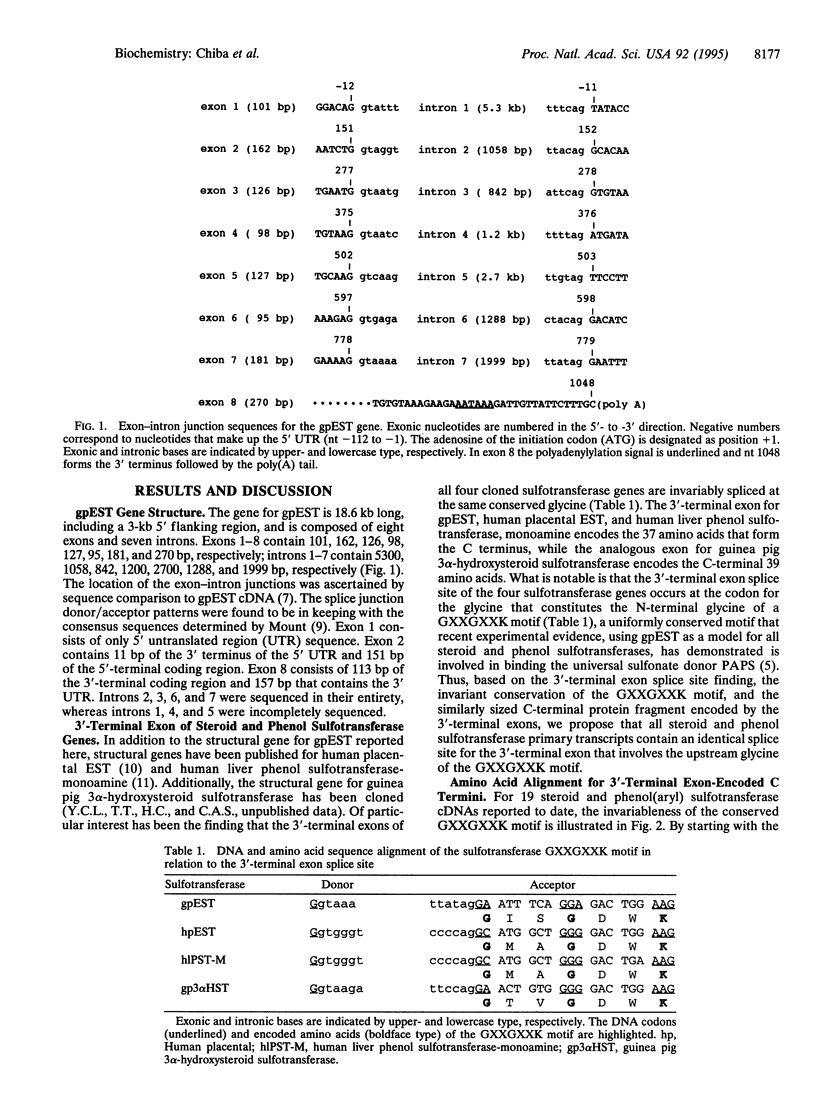
The guinea pig estrogen sulfotransferase gene has been cloned and compared to three other cloned steroid and phenol sulfotransferase genes (human estrogen sulfotransferase, human phenol sulfotransferase, and guinea pig 3 alpha-hydroxysteroid sulfotransferase). The four sulfotransferase genes demonstrate a common outstanding feature: the splice sites for their 3'-terminal exons are identically located. That is, the 3'-terminal exon splice sites involve a glycine that constitutes the N-terminal glycine of an invariably conserved GXXGXXK motif present in all steroid and phenol sulfotransferases for which primary structures are known. This consistency strongly suggests that all steroid and phenol sulfotransferase genes will be similarly spliced. The GXXGXXK motif forms the active binding site for the universal sulfonate donor 3'-phosphoadenosine 5'-phosphosulfate. Amino acid sequence alignment of 19 cloned steroid and phenol sulfotransferases starting with the GXXGXXK motif indicates that the 3'-terminal exon for each steroid and phenol sulfotransferase gene encodes a similarly sized C-terminal fragment of the protein. Interestingly, on further analysis of the alignment, three distinct amino acid sequence patterns emerge. The presence of the conserved functional GXXGXXK motif suggests that the protein domains encoded by steroid and phenol sulfotransferase 3'-terminal exons have evolved from a common ancestor. Furthermore, it is hypothesized that during the course of evolution, the 3'-terminal exon further diverged into at least three sulfotransferase subdivisions: a phenol or aryl group, an estrogen or phenolic steroid group, and a neutral steroid group.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy I. A., Wood T. C., Weinshilboum R. Human liver estrogen sulfotransferase: identification by cDNA cloning and expression. Biochem Biophys Res Commun. 1994 May 16;200(3):1621–1629. doi: 10.1006/bbrc.1994.1637. [DOI] [PubMed] [Google Scholar]
- Bernier F., Leblanc G., Labrie F., Luu-The V. Structure of human estrogen and aryl sulfotransferase gene. Two mRNA species issued from a single gene. J Biol Chem. 1994 Nov 11;269(45):28200–28205. [PubMed] [Google Scholar]
- Comer K. A., Falany J. L., Falany C. N. Cloning and expression of human liver dehydroepiandrosterone sulphotransferase. Biochem J. 1993 Jan 1;289(Pt 1):233–240. doi: 10.1042/bj2890233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demyan W. F., Song C. S., Kim D. S., Her S., Gallwitz W., Rao T. R., Slomczynska M., Chatterjee B., Roy A. K. Estrogen sulfotransferase of the rat liver: complementary DNA cloning and age- and sex-specific regulation of messenger RNA. Mol Endocrinol. 1992 Apr;6(4):589–597. doi: 10.1210/mend.6.4.1374839. [DOI] [PubMed] [Google Scholar]
- Dooley T. P., Probst P., Munroe P. B., Mole S. E., Liu Z., Doggett N. A. Genomic organization and DNA sequence of the human catecholamine-sulfating phenol sulfotransferase gene (STM). Biochem Biophys Res Commun. 1994 Dec 15;205(2):1325–1332. doi: 10.1006/bbrc.1994.2810. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Orellana A., Gil G., Hirschberg C. B. Molecular cloning and expression of rat liver N-heparan sulfate sulfotransferase. J Biol Chem. 1992 Aug 5;267(22):15744–15750. [PubMed] [Google Scholar]
- Khan A. S., Taylor B. R., Chung K., Etheredge J., Gonzales R., Ringer D. P. Genomic structure of rat liver aryl sulfotransferase IV-encoding gene. Gene. 1993 Dec 31;137(2):321–326. doi: 10.1016/0378-1119(93)90028-2. [DOI] [PubMed] [Google Scholar]
- Komatsu K., Driscoll W. J., Koh Y. C., Strott C. A. A P-loop related motif (GxxGxxK) highly conserved in sulfotransferases is required for binding the activated sulfate donor. Biochem Biophys Res Commun. 1994 Nov 15;204(3):1178–1185. doi: 10.1006/bbrc.1994.2587. [DOI] [PubMed] [Google Scholar]
- Komatsu K., Oeda T., Strott C. A. Cloning and sequence analysis of the 5'-flanking region of the estrogen sulfotransferase gene: steroid response elements and cell-specific nuclear DNA-binding proteins. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1297–1304. doi: 10.1006/bbrc.1993.1965. [DOI] [PubMed] [Google Scholar]
- Kong A. N., Ma M., Tao D., Yang L. Molecular cloning of cDNA encoding the phenol/aryl form of sulfotransferase (mSTp1) from mouse liver. Biochim Biophys Acta. 1993 Jan 23;1171(3):315–318. doi: 10.1016/0167-4781(93)90073-m. [DOI] [PubMed] [Google Scholar]
- Kong A. N., Tao D., Ma M., Yang L. Molecular cloning of the alcohol/hydroxysteroid form (mSTa1) of sulfotransferase from mouse liver. Pharm Res. 1993 Apr;10(4):627–630. doi: 10.1023/a:1018926825475. [DOI] [PubMed] [Google Scholar]
- Kong A. N., Yang L., Ma M., Tao D., Bjornsson T. D. Molecular cloning of the alcohol/hydroxysteroid form (hSTa) of sulfotransferase from human liver. Biochem Biophys Res Commun. 1992 Aug 31;187(1):448–454. doi: 10.1016/s0006-291x(05)81514-1. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Park C. S., Strott C. A. Molecular cloning of a chiral-specific 3 alpha-hydroxysteroid sulfotransferase. J Biol Chem. 1994 Jun 3;269(22):15838–15845. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. R., Glenn W. K., Moore S. S., Kerr J., Thompson A. R., Thompson E. O. Oestrogen sulfotransferase: molecular cloning and sequencing of cDNA for the bovine placental enzyme. Aust J Biol Sci. 1988;41(4):507–516. doi: 10.1071/bi9880507. [DOI] [PubMed] [Google Scholar]
- Oeda T., Lee Y. C., Driscoll W. J., Chen H. C., Strott C. A. Molecular cloning and expression of a full-length complementary DNA encoding the guinea pig adrenocortical estrogen sulfotransferase. Mol Endocrinol. 1992 Aug;6(8):1216–1226. doi: 10.1210/mend.6.8.1406700. [DOI] [PubMed] [Google Scholar]
- Ogura K., Kajita J., Narihata H., Watabe T., Ozawa S., Nagata K., Yamazoe Y., Kato R. Cloning and sequence analysis of a rat liver cDNA encoding hydroxysteroid sulfotransferase. Biochem Biophys Res Commun. 1989 Nov 30;165(1):168–174. doi: 10.1016/0006-291x(89)91050-4. [DOI] [PubMed] [Google Scholar]
- Ogura K., Kajita J., Narihata H., Watabe T., Ozawa S., Nagata K., Yamazoe Y., Kato R. cDNA cloning of the hydroxysteroid sulfotransferase STa sharing a strong homology in amino acid sequence with the senescence marker protein SMP-2 in rat livers. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1494–1500. doi: 10.1016/0006-291x(90)91036-r. [DOI] [PubMed] [Google Scholar]
- Otterness D. M., Her C., Aksoy S., Kimura S., Wieben E. D., Weinshilboum R. M. Human dehydroepiandrosterone sulfotransferase gene: molecular cloning and structural characterization. DNA Cell Biol. 1995 Apr;14(4):331–341. doi: 10.1089/dna.1995.14.331. [DOI] [PubMed] [Google Scholar]
- Otterness D. M., Wieben E. D., Wood T. C., Watson W. G., Madden B. J., McCormick D. J., Weinshilboum R. M. Human liver dehydroepiandrosterone sulfotransferase: molecular cloning and expression of cDNA. Mol Pharmacol. 1992 May;41(5):865–872. [PubMed] [Google Scholar]
- Ozawa S., Nagata K., Gong D. W., Yamazoe Y., Kato R. Nucleotide sequence of a full-length cDNA (PST-1) for aryl sulfotransferase from rat liver. Nucleic Acids Res. 1990 Jul 11;18(13):4001–4001. doi: 10.1093/nar/18.13.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Sibbald P. R., Wittinghofer A. The P-loop--a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci. 1990 Nov;15(11):430–434. doi: 10.1016/0968-0004(90)90281-f. [DOI] [PubMed] [Google Scholar]
- Tomizuka T., Oeda T., Tamura Y., Yoshida S., Strott C. A. Characterization of guinea pig estrogen sulfotransferase expressed by Chinese hamster ovary cell-K1 stable transfectants. Endocrinology. 1994 Sep;135(3):938–943. doi: 10.1210/endo.135.3.8070389. [DOI] [PubMed] [Google Scholar]
- Wilborn T. W., Comer K. A., Dooley T. P., Reardon I. M., Heinrikson R. L., Falany C. N. Sequence analysis and expression of the cDNA for the phenol-sulfating form of human liver phenol sulfotransferase. Mol Pharmacol. 1993 Jan;43(1):70–77. [PubMed] [Google Scholar]
- Wood T. C., Aksoy I. A., Aksoy S., Weinshilboum R. M. Human liver thermolabile phenol sulfotransferase: cDNA cloning, expression and characterization. Biochem Biophys Res Commun. 1994 Feb 15;198(3):1119–1127. doi: 10.1006/bbrc.1994.1159. [DOI] [PubMed] [Google Scholar]
- Zhu X., Veronese M. E., Bernard C. C., Sansom L. N., McManus M. E. Identification of two human brain aryl sulfotransferase cDNAs. Biochem Biophys Res Commun. 1993 Aug 31;195(1):120–127. doi: 10.1006/bbrc.1993.2018. [DOI] [PubMed] [Google Scholar]
- Zhu X., Veronese M. E., Sansom L. N., McManus M. E. Molecular characterisation of a human aryl sulfotransferase cDNA. Biochem Biophys Res Commun. 1993 Apr 30;192(2):671–676. doi: 10.1006/bbrc.1993.1467. [DOI] [PubMed] [Google Scholar]


