Abstract
Background. We examined risk of newly detected human papillomavirus (HPV) infection and cervical abnormalities in relation to HPV type 16/18 antibody levels at enrollment in PATRICIA (Papilloma Trial Against Cancer in Young Adults; NCT00122681).
Methods. Using Poisson regression, we compared risk of newly detected infection and cervical abnormalities associated with HPV-16/18 between seronegative vs seropositive women (15–25 years) in the control arm (DNA negative at baseline for the corresponding HPV type [HPV-16: n = 8193; HPV-18: n = 8463]).
Results. High titers of naturally acquired HPV-16 antibodies and/or linear trend for increasing antibody levels were significantly associated with lower risk of incident and persistent infection, atypical squamous cells of undetermined significance or greater (ASCUS+), and cervical intraepithelial neoplasia grades 1/2 or greater (CIN1+, CIN2+). For HPV-18, although seropositivity was associated with lower risk of ASCUS+ and CIN1+, no association between naturally acquired antibodies and infection was demonstrated. Naturally acquired HPV-16 antibody levels of 371 (95% confidence interval [CI], 242–794), 204 (95% CI, 129–480), and 480 (95% CI, 250–5756) EU/mL were associated with 90% reduction of incident infection, 6-month persistent infection, and ASCUS+, respectively.
Conclusions. Naturally acquired antibodies to HPV-16, and to a lesser extent HPV-18, are associated with some reduced risk of subsequent infection and cervical abnormalities associated with the same HPV type.
Keywords: HPV, naturally acquired antibodies, infection, cervical abnormality, risk reduction
(See the editorial commentary by Franceschi and Baussano on pages 507–9.)
Human papillomavirus (HPV) types 16 and 18 cause approximately 70% of invasive cervical cancer worldwide [1]. Two prophylactic vaccines against HPV infection are available and have been shown to prevent persistent HPV infection and precancerous cervical abnormalities associated with HPV-16/18 [2–6].
Approximately 50%–70% of women develop serum antibodies after naturally acquired infection with HPV-16 or HPV-18 [7–13]. Naturally acquired antibodies can remain detectable for at least 4–5 years, albeit at much lower levels than those induced by vaccination [14]. Whereas some studies have not shown an immune protection role for naturally acquired antibodies [15–18], others have shown that they may provide protection against future infection [19–21]. Underpowering of studies and differences in methodology may explain these discrepancies. In addition, the levels of naturally acquired antibodies that may provide protection have not yet been established.
Vaccine efficacy data from the Papilloma Trial Against Cancer in Young Adults (PATRICIA) of the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®) have been reported previously [3, 22–24]. The intensive follow-up of the control arm of large vaccine trials provides an opportunity to evaluate the natural history of HPV infection, including the impact of naturally induced antibodies on infection and cervical abnormalities. The present paper describes an analysis of the risk of newly detected HPV infection and development of cervical abnormalities according to the baseline level of naturally acquired antibodies to HPV-16 or HPV-18 in women from the control group of PATRICIA over a 4-year follow-up.
METHODS
This analysis was based on data obtained from the control arm of the multinational (14 countries), double-blind, randomized , controlled PATRICIA trial. The objective was to investigate whether naturally acquired antibodies to HPV-16 or HPV-18 reduced the risk of newly detected infection and/or associated cervical abnormalities with the same HPV type.
Study Population and Procedures
The clinical trial methodology has been described in detail [3, 22]. Women aged 15–25 years with no more than 6 lifetime sexual partners were enrolled and randomized to the HPV-16/18 AS04-adjuvanted vaccine or control hepatitis A vaccine (both supplied by GlaxoSmithKline Vaccines, Rixensart, Belgium). Data from the control arm are reported here. Cervical liquid-based cytology samples were collected at baseline and every 6 months and used to perform HPV DNA typing every 6 months and for cytopathological examination (Bethesda system) every 12 months. A prespecified algorithm for abnormal cytology and colposcopy referral was used [23]. Cervical samples and biopsy material were tested for HPV DNA as previously described [25]. Serum antibodies to HPV-16 and HPV-18 were determined at baseline by enzyme-linked immunosorbent assay (ELISA) targeting L1-based virus-like particles (VLPs) [26]. Seropositivity was defined as an antibody level greater than or equal to the assay threshold: 8 ELISA units (EU)/mL for HPV-16 and 7 EU/mL for HPV-18 [26].
Endpoints were incident infection (which may include newly acquired infections and recurrent infections present below detection levels at baseline), 6- and 12-month persistent infection, atypical squamous cell of undetermined significance or greater (ASCUS+), cervical intraepithelial neoplasia (CIN) grade 1 or greater (CIN1+), and CIN grade 2 or greater (CIN2+) associated with HPV-16 or HPV-18. All CIN cases were reviewed by an independent endpoint committee [22]. Women completed a behavioral questionnaire, which asked about experience with sexual intercourse (age at first sexual intercourse and number of partners over the past 12 months) and lifetime tobacco exposure at the second study visit, 1 month after the first vaccination, and yearly thereafter. The term sexual intercourse included penetrative, genital-to-genital, or oral–genital sexual contact.
Written informed consent or assent was obtained and the protocol and other materials were approved by local independent ethics committees or institutional review boards. The trial was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and is registered at ClinicalTrials.gov under registration number NCT00122681.
Statistical Analysis
Analysis Cohort
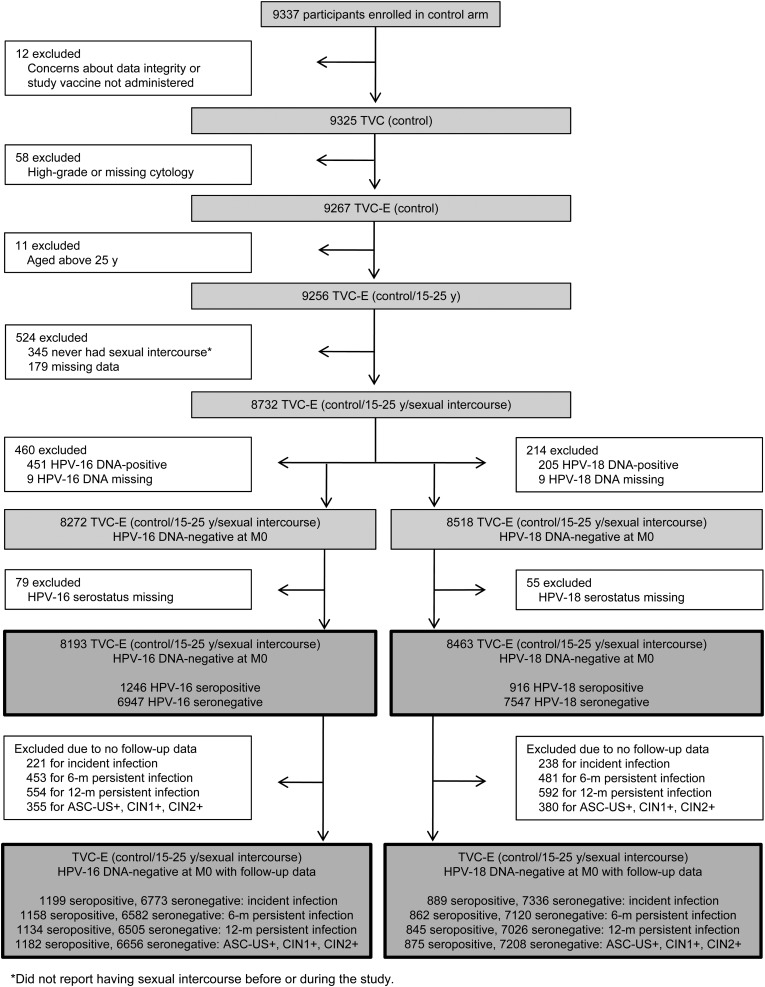
The analysis was performed in the control group of the total vaccinated cohort for efficacy, including women who received at least 1 dose of control vaccine and had normal or low-grade cytology at baseline. The analysis included only women who had potentially been exposed to HPV infection via sexual intercourse and those without current HPV infections at baseline (Figure 1). Thus, only newly detected infections (new infections or possibly recurrent infections present below detectable levels at baseline) were accounted for in the analysis.
Figure 1.
Participant disposition. Abbreviations: ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; MO, Month 0; TVC-E, total vaccinated cohort for efficacy.
Exposure Variables
The main exposure variables were HPV-16 and HPV-18 serostatus at baseline. Serostatus was analyzed as (1) a binary variable (seropositive or seronegative) according to ELISA assay threshold, and (2) according to quartiles in seropositive women (8–12, >12–21, >21–59, and >59–2805 EU/mL for HPV-16; 7–10, >10–17, >17–43, and >43–1086 EU/mL for HPV-18). We report both univariate and multivariable analyses. Based on established risk factors known to influence the risk of HPV infection, and a previous analysis of risk factors for HPV infection and cervical abnormalities in the PATRICIA study [27], the following covariates were taken into account in the multivariable analyses: marital status, tobacco exposure (number of pack-years), age at first sexual intercourse, number of sexual partners, history of sexually transmitted infection (STI), at least 1 previous pregnancy, and region. Other covariates known to influence the risk of HPV infection were not included in the multivariable analyses because of strong correlations with the above covariates: condom use was correlated with STI, age at first sexual intercourse was correlated with age at baseline, and at least 1 previous pregnancy was correlated with at least 1 delivery. The hazard ratio estimates were found to be very similar using both approaches, and therefore discussion of the results focuses on the multivariable analyses.
General Statistical Considerations and Multivariable Regression Analyses
All analyses were performed using SAS version 9.2. The incidence rate was calculated as the number of incident events divided by the total person-time. Person-years were calculated as the sum of the follow-up for each participant expressed in years. The follow-up period started on the day after first vaccination and ended on the first occurrence of the endpoint or the last visit (whichever occurred first).
The relationship between the exposure variables and the risk of newly detected infection or cervical abnormalities was assessed using Poisson regression analyses. First, the effect of the exposure variables on the endpoints was evaluated based on the relative incidence rates (rate ratios) and their 95% confidence intervals (CIs) obtained by Poisson regression. The final multivariable analysis allowed estimation of the relative role of initial HPV-16 or HPV-18 serostatus while adjusting for the simultaneous effects of the 7 covariates selected as potential confounders. Only subjects with no missing data were included in the multivariable analyses; however, the analyses were also performed taking into account missing data as a specific category for each covariate. The results were very similar (data not shown).
Second, the relationship between the risk of newly detected infection or cervical abnormalities and the baseline antibody level was analyzed using Poisson regression including antibody titer as a continuous variable. Seronegative women were assigned a value of half the ELISA assay cutoff level. Predicted antibody titers corresponding to a 50%, 70%, and 90% risk reduction were derived. Age at first sexual intercourse and smoking were included as covariates in a sensitivity analysis. Because of the large proportion (85%) of seronegative subjects at baseline, sensitivity analyses including a subset of 100 randomly selected seronegative subjects were also carried out.
RESULTS
Participant Characteristics at Study Entry
A total of 8193 (1246 HPV-16 seropositive [15.2%] and 6947 HPV-16 seronegative [84.8%]) and 8463 (916 HPV-18 seropositive [10.8%] and 7547 HPV-18 seronegative [89.2%]) women were included in the analysis of HPV-16– and HPV-18–related endpoints, respectively (Figure 1; Table 1).
Table 1.
Frequency Distribution of Exposure Variables, Age Group, and Country According to Initial Serostatus in Women Who Were DNA Negative for the Corresponding Human Papillomavirus Type at Baseline
| Exposure Variablea | Categories | HPV-16 Initial Serostatus, No. (%) |
HPV-18 Initial Serostatus, No. (%) |
||
|---|---|---|---|---|---|
| Seronegative (n = 6947) | Seropositive (n = 1246) | Seronegative (n = 7547) | Seropositive (n = 916) | ||
| Initial antibody titer (seropositive only) | Geometric mean titer (95% CI) | 28.9 (27.2–30·7) | 23.0 (21.5–24.6) | ||
| Median (range) | 21.0 (8–2805) | 17.0 (7–1086) | |||
| Quartile (Q1-Q3) | 12.0–59.0 | 10.0–43.0 | |||
| Age at baseline | 15–17 y | 2349 (33.8) | 259 (20.8) | 2516 (33.3) | 185 (20.2) |
| 18–25 y | 4598 (66.2) | 987 (79.2) | 5031 (66.7) | 731 (79.8) | |
| Country | Finland | 1925 (27.7) | 203 (16.3) | 2060 (27.3) | 135 (14.7) |
| Philippines | 956 (13.8) | 156 (12.5) | 1004 (13.3) | 119 (13.0) | |
| United States | 832 (12.0) | 233 (18.7) | 979 (13.0) | 141 (15.4) | |
| Thailand | 726 (10.5) | 117 (9.4) | 725 (9.6) | 121 (13.2) | |
| Brazil | 601 (8.7) | 206 (16.5) | 675 (8.9) | 167 (18.2) | |
| Taiwan | 557 (8.0) | 70 (5.6) | 586 (7.8) | 50 (5.5) | |
| Mexico | 354 (5.1) | 81 (6.5) | 393 (5.2) | 53 (5.8) | |
| Germany | 286 (4.1) | 47 (3.8) | 324 (4.3) | 37 (4.0) | |
| Australia | 201 (2.9) | 33 (2.7) | 229 (3.0) | 21 (2.3) | |
| Canada | 168 (2.4) | 44 (3.5) | 196 (2.6) | 38 (4.2) | |
| Spain | 165 (2.4) | 15 (1.2) | 180 (2.4) | 8 (0.9) | |
| United Kingdom | 90 (1.3) | 30 (2.4) | 105 (1.4) | 22 (2.4) | |
| Belgium | 71 (1.0) | 9 (0.7) | 74 (1.0) | 4 (0.4) | |
| Italy | 15 (0.2) | 2 (0.2) | 17 (0.2) | 0 | |
| Marital status | Living or lived with partner | 2166 (31.2) | 461 (37.0) | 2315 (30.7) | 358 (39.1) |
| Singleb | 4677 (67.3) | 761 (61.1) | 5118 (67.8) | 539 (58.8) | |
| Missing | 104 (1.5) | 24 (1.9) | 114 (1.5) | 19 (2.1) | |
| Tobacco exposure, No. of pack-years | None or <6 mo (<0.5) | 4942 (71.1) | 785 (63.0) | 5243 (69.5) | 597 (65.2) |
| At least 6 mo (≥0.5) | 1937 (27.9) | 447 (35.9) | 2231 (29.6) | 308 (33.6) | |
| Missing | 68 (1.0) | 14 (1.1) | 73 (1.0) | 11 (1.2) | |
| Age at first sexual intercourse | ≥18 y | 2844 (40.9) | 434 (34.8) | 3033 (40.2) | 314 (34.3) |
| 15–17 y | 3321 (47.8) | 615 (49.4) | 3589 (47.6) | 479 (52.3) | |
| <15 y | 769 (11.1) | 192 (15.4) | 909 (12.0) | 122 (13.3) | |
| Missing | 13 (0.2) | 5 (0.4) | 16 (0.2) | 1 (0.1) | |
| No. of sexual partners prior to the past 12 moc | 0 | 1664 (24.0) | 132 (10.6) | 1700 (22.5) | 117 (12.8) |
| 1 | 3396 (48.9) | 542 (43.5) | 3608 (47.8) | 407 (44.4) | |
| 2–3 | 1347 (19.4) | 358 (28.7) | 1572 (20.8) | 248 (27.1) | |
| ≥4 | 499 (7.2) | 204 (16.4) | 619 (8.2) | 139 (15.2) | |
| Missing | 41 (0.6) | 10 (0.8) | 48 (0.6) | 5 (0.6) | |
| No. of sexual partners during the past 12 moc | 0 | 1067 (15.4) | 88 (7.1) | 1095 (14.5) | 67 (7.3) |
| 1 | 4605 (66.3) | 888 (71.3) | 4971 (65.9) | 656 (71.6) | |
| 2–3 | 1081 (15.6) | 216 (17.3) | 1243 (16.5) | 161 (17.6) | |
| ≥4 | 171 (2.5) | 47 (3.8) | 212 (2.8) | 27 (3.0) | |
| Missing | 23 (0.3) | 7 (0.6) | 26 (0.3) | 5 (0.6) | |
| Condom use prior to the past 12 moc | No partner | 1694 (24.4) | 140 (11.2) | 1737 (23.0) | 119 (13.0) |
| Yes | 1682 (24.2) | 366 (29.4) | 1839 (24.4) | 264 (28.8) | |
| No | 3448 (49.6) | 719 (57.7) | 3846 (51.0) | 511 (55.8) | |
| Missing | 123 (1.8) | 21 (1.7) | 125 (1.7) | 22 (2.4) | |
| Condom use during the past 12 moc | No partner | 1049 (15.1) | 87 (7.0) | 1076 (14.3) | 67 (7.3) |
| Yes | 2151 (31.0) | 451 (36.2) | 2357 (31.2) | 336 (36.7) | |
| No | 3642 (52.4) | 690 (55.4) | 4003 (53.0) | 498 (54.4) | |
| Missing | 105 (1.5) | 18 (1.4) | 111 (1.5) | 15 (1.6) | |
| At least 1 previous pregnancy | Yes | 2139 (30.8) | 515 (41.3) | 2312 (30.6) | 391 (42.7) |
| No | 4778 (68.8) | 724 (58.1) | 5202 (68.9) | 520 (56.8) | |
| Missing | 30 (0.4) | 7 (0.6) | 33 (0.4) | 5 (0.6) | |
| At least 1 delivery | Yes | 1481 (21.3) | 332 (26.7) | 1576 (20.9) | 267 (29.2) |
| No | 5430 (78.2) | 907 (72.8) | 5933 (78.6) | 643 (70.2) | |
| Missing | 36 (0.5) | 7 (0.6) | 38 (0.5) | 6 (0.7) | |
| STI history | No | 6633 (95.5) | 1099 (88.2) | 7156 (94.8) | 814 (88.9) |
| Yes–Chlamydia trachomatis | 93 (1.3) | 61 (4.9) | 123 (1.6) | 41 (4.5) | |
| Yes–other | 210 (3.0) | 84 (6.7) | 257 (3.4) | 58 (6.3) | |
| Missing | 11 (0.2) | 2 (0.2) | 11 (0.2) | 3 (0.3) | |
| Contraceptive used | No contraception | 2526 (36.4) | 314 (25.2) | 2660 (35.3) | 229 (25.0) |
| Hormonal | 4188 (60.3) | 874 (70.1) | 4639 (61.5) | 638 (69.7) | |
| Intrauterine device | 327 (4.7) | 92 (7.4) | 367 (4.9) | 65 (7.1) | |
| Sterilized | 63 (0.9) | 20 (1.6) | 67 (0.9) | 15 (1.6) | |
Information on tobacco exposure, age at first sexual intercourse, number of sexual partners, pregnancy, and condom use was obtained from the behavioral questionnaire at baseline. Data from the behavioral questionnaires administered yearly during the follow-up period were used to estimate age at first sexual intercourse when sexual activity began during follow-up and to calculate the number of pregnancies during follow-up. Information on age at baseline (grouped as 15–17 and 18–25 years), marital status, country, contraceptive use (hormonal, intrauterine device, and sterilization), and STIs was obtained from case record forms. Countries were grouped by geographic region (Europe, Asia Pacific, Latin America, and North America).
Abbreviations: CI, confidence interval; HPV, human papillomavirus; STI, sexually transmitted infection.
a Only the following were considered as potential confounders in the multivariable analyses (as shown in Tables 2 and 3): marital status, tobacco exposure (number of pack-years), age at first sexual intercourse, number of sexual partners, STI history, at least 1 previous pregnancy, and region. Data on other variables are descriptive only; these variables were not included in the multivariable analysis because of strong correlations with other variables: condom use was correlated with STI; age at first sexual intercourse with age at baseline; at least 1 previous pregnancy with at least 1 delivery. Therefore, only STI, age at first sexual intercourse, and at least 1 previous pregnancy were kept in the multivariable analysis.
b Not married, widowed, divorced, separated, living with or had lived with partner.
c For analysis of HPV infection, the number of sexual partners during the past 12 months was considered; for analysis of atypical squamous cells of undetermined significance or greater, cervical intraepithelial neoplasia grade 1/2 or greater, the lifetime number of sexual partners prior to the past 12 months was considered.
d Women could be using >1 method of contraception.
Risk of Newly Detected HPV-16/18 Infection and Associated Cervical Abnormalities According to Levels of Naturally Acquired Antibodies
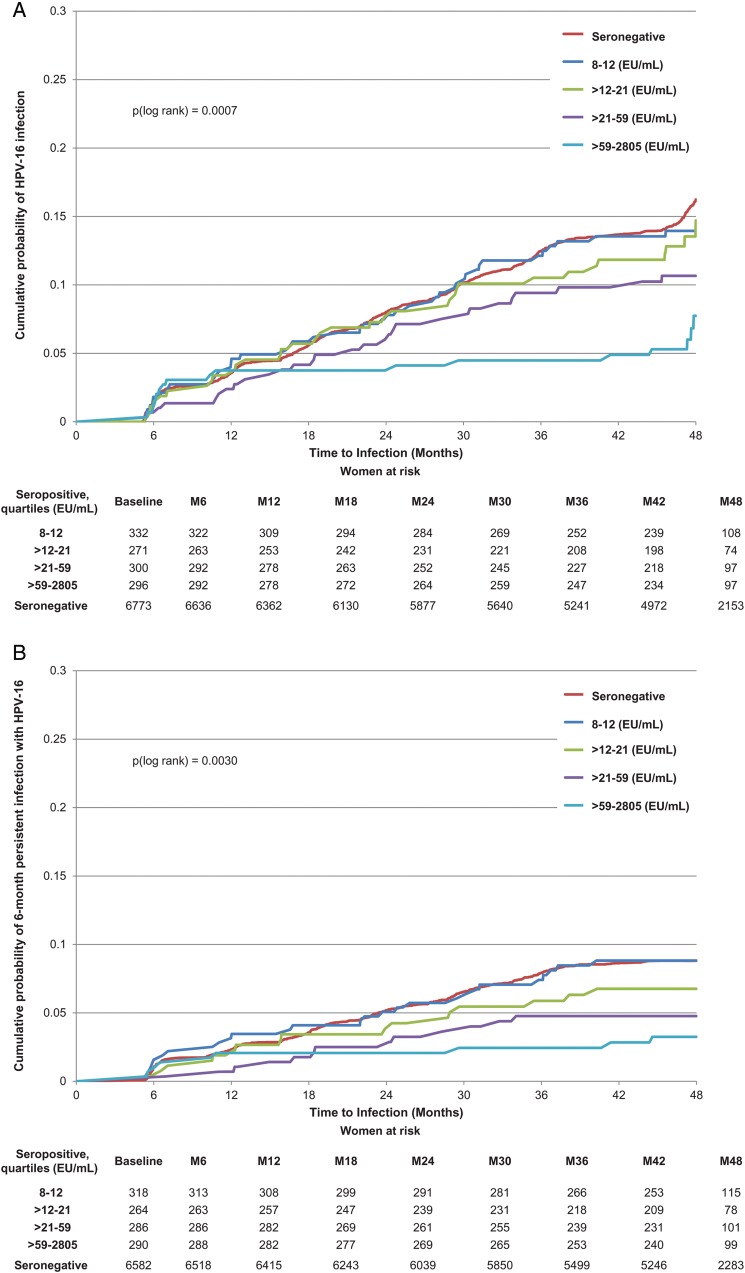
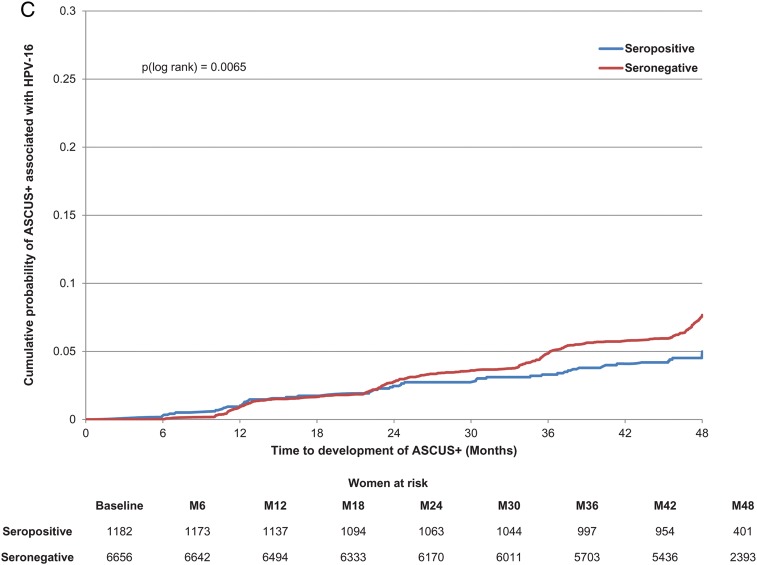
The multivariable analysis showed that the presence of naturally acquired antibodies to HPV-16 at baseline was associated with a lower risk of newly detected incident infection, 6- and 12-month persistent infection, and ASCUS+ associated with HPV-16 (Table 2; Figure 2A–C). The risk was gradually reduced as antibody levels rose. Although HPV-16 serostatus (positive vs negative) did not show a significant association with CIN1+, participants with an antibody level in the highest quartile at baseline did have a significantly reduced risk of developing CIN1+ compared with seronegative subjects, and the linear trend by quartile was statistically significant (P = .0006; Table 2). The linear trend by antibody quartile was also significant for CIN2+ (P = .018), although none of the individual quartile groups showed a significant reduction in risk (Table 2).
Table 2.
Risk of Newly Detected Human Papillomavirus Type 16 (HPV-16) Infections or Cervical Abnormalities Associated With HPV-16 According to Initial Serostatus (Univariate and Multivariable Regression Analyses) in Women Who Were HPV-16 DNA Negative at Baseline
| Endpoint | Serostatus at Baseline | No. Cases With New Endpoint | No. Cases Without New Endpoint | PY | Incidence per 100 PY | Univariate Analysis | Final Multivariable Analysis Adjusted on Confounders Retained |
|
|---|---|---|---|---|---|---|---|---|
| Rate Ratio (95% CI) | Rate Ratio (95% CI) | P Value | ||||||
| New HPV-16 incident infection (cases = 1185, noncases = 6787)a | Seronegative | 1059 | 5714 | 23 299 | 4.55 | 1.00 | 1.00 | |
| Seropositive | 126 | 1073 | 4114 | 3.06 | 0.67 (.56–.81) | 0.64 (.53–.78) | <.0001 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 8–12 | 43 | 289 | 1125 | 3.82 | 0.84 (.62–1.14) | 0.89 (.65–1.21) | .45 | |
| >12–21 | 36 | 235 | 923 | 3.90 | 0.86 (.62–1.20) | 0.80 (.57–1.11) | .18 | |
| >21–59 | 29 | 271 | 1016 | 2.85 | 0.63 (.43–.91) | 0.58 (.40–.85) | .0057 | |
| >59–2805 | 18 | 278 | 1050 | 1.71 | 0.38 (.24–.60) | 0.34 (.21–.54) | <.0001 | |
| P value for linear trend < .0001 | ||||||||
| New HPV-16 six-mo persistent infection (cases = 626, noncases = 7114)a | Seronegative | 560 | 6022 | 23 787 | 2.35 | 1.00 | 1.00 | |
| Seropositive | 66 | 1092 | 4195 | 1.57 | 0.67 (.52–.86) | 0.67 (.52–.87) | .0025 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 8–12 | 27 | 291 | 1146 | 2.36 | 1.00 (.68–1.47) | 1.09 (.74–1.61) | .67 | |
| >12–21 | 17 | 247 | 947 | 1.79 | 0.76 (.47–1.23) | 0.71 (.44–1.16) | .17 | |
| >21–59 | 13 | 273 | 1037 | 1.25 | 0.53 (.31–.92) | 0.55 (.31–.95) | .033 | |
| >59–2805 | 9 | 281 | 1065 | 0.85 | 0.36 (.19–.69) | 0.34 (.17–.65) | .0013 | |
| P value for linear trend < .0001 | ||||||||
| New HPV-16 twelve-mo persistent infection (cases = 405, noncases = 7234)a | Seronegative | 362 | 6143 | 24 113 | 1.50 | 1.00 | 1.00 | |
| Seropositive | 43 | 1091 | 4213 | 1.02 | 0.68 (.50–.93) | 0.68 (.49–.94) | .019 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 8–12 | 21 | 293 | 1152 | 1.82 | 1.21 (.78–1.89) | 1.31 (.84–2.04) | .23 | |
| >12–21 | 10 | 248 | 955 | 1.05 | 0.70 (.37–1.31) | 0.65 (.35–1.23) | .19 | |
| >21–59 | 9 | 269 | 1034 | 0.87 | 0.58 (.30–1.12) | 0.59 (.30–1.15) | .12 | |
| >59–2805 | 3 | 281 | 1073 | 0.28 | 0.19 (.06–.58) | 0.17 (.06–.55) | .0027 | |
| P value for linear trend < .0001 | ||||||||
| New ASCUS+ associated with HPV-16 (cases = 536, noncases = 7302)a | Seronegative | 484 | 6172 | 24 347 | 1.99 | 1.00 | 1.00 | |
| Seropositive | 52 | 1130 | 4251 | 1.22 | 0.62 (.46–.82) | 0.60 (.45–.80) | .0006 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 8–12 | 20 | 309 | 1176 | 1.70 | 0.86 (.55–1.34) | 0.91 (.58–1.42) | .67 | |
| >12–21 | 13 | 253 | 959 | 1.36 | 0.68 (.39–1.18) | 0.66 (.38–1.14) | .14 | |
| >21–59 | 11 | 281 | 1049 | 1.05 | 0.53 (.29–.96) | 0.51 (.28–.93) | .028 | |
| >59–2805 | 8 | 287 | 1067 | 0.75 | 0.38 (.19–.76) | 0.33 (.16–.67) | .0022 | |
| P value for linear trend < .0001 | ||||||||
| New CIN1+ associated with HPV-16 (cases = 177, noncases = 7661)a | Seronegative | 157 | 6499 | 24 939 | 0.63 | 1.00 | 1.00 | |
| Seropositive | 20 | 1162 | 4326 | 0.46 | 0.73 (.46–1.17) | 0.70 (.44–1.14) | .15 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 8–12 | 10 | 319 | 1204 | 0.83 | 1.32 (.70–2.50) | 1.38 (.72–2.64) | .33 | |
| >12–21 | 5 | 261 | 973 | 0.51 | 0.82 (.34–1.99) | 0.78 (.32–1.90) | .58 | |
| >21–59 | 4 | 288 | 1063 | 0.38 | 0.60 (.22–1.61) | 0.56 (.21–1.52) | .26 | |
| >59–2805 | 1 | 294 | 1086 | 0.09 | 0.15 (.02–1.05) | 0.13 (.02–.90) | .039 | |
| P value for linear trend = .0006 | ||||||||
| New CIN2+ associated with HPV-16 (cases = 121, noncases = 7717)a | Seronegative | 109 | 6547 | 24 984 | 0.44 | 1.00 | 1.00 | |
| Seropositive | 12 | 1170 | 4339 | 0.28 | 0.63 (.35–1.15) | 0.62 (.34–1.15) | .13 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 8–12 | 6 | 323 | 1208 | 0.50 | 1.14 (.50–2.59) | 1.21 (.53–2.76) | .66 | |
| >12–21 | 2 | 264 | 980 | 0.20 | 0.47 (.12–1.89) | 0.45 (.11–1.83) | .26 | |
| >21–59 | 3 | 289 | 1065 | 0.28 | 0.65 (.21–2.03) | 0.63 (.20–1.99) | .43 | |
| >59–2805 | 1 | 294 | 1086 | 0.09 | 0.21 (.03–1.51) | 0.19 (.03–1.38) | .10 | |
| P value for linear trend = .018 | ||||||||
Incident infection was defined as a new detection of the HPV type at any time during the follow-up period; 6- and 12-month persistent infection were defined as detection of the same HPV type in 2 consecutive samples over a minimum of 150 days and 300 days, respectively; ASCUS+ was defined as ASCUS, low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells/high-grade ASCUS, does not exclude high-grade squamous intraepithelial lesion (HSIL), atypical glandular cells of undetermined significance (AGUS), or HSIL. CIN1+ was defined as CIN1, CIN2, CIN3, adenocarcinoma in situ, or invasive cervical cancer; CIN2+ excluded CIN1.
Confounders (exposure variables) retained for the multivariable analysis were marital status, tobacco exposure (number of pack-years), age at first sexual intercourse, number of sexual partners prior to the past 12 months (ASCUS+, CIN1+, CIN2+), number of sexual partners during the past 12 months (incident and persistent infections), at least 1 previous pregnancy, sexually transmitted infection history, and region.
Linear trend was evaluated across 5 classes: (1) seronegative; (2) first quartile; (3) second quartile; (4) third quartile; (5) fourth quartile.
Abbreviations: ASCUS, atypical squamous cell of undetermined significance; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; PY, patient-years.
a No. of cases and noncases and patient-years reported for the univariate analysis.
Figure 2.
Cumulative probability of detecting an incident or 6-month persistent infection or developing atypical squamous cells of undetermined significance or greater (ASCUS+) associated with human papillomavirus type 16 (HPV-16). A, Incident HPV-16 infection. B, HPV-16 six-month persistent infection. C, ASCUS+ associated with HPV-16.
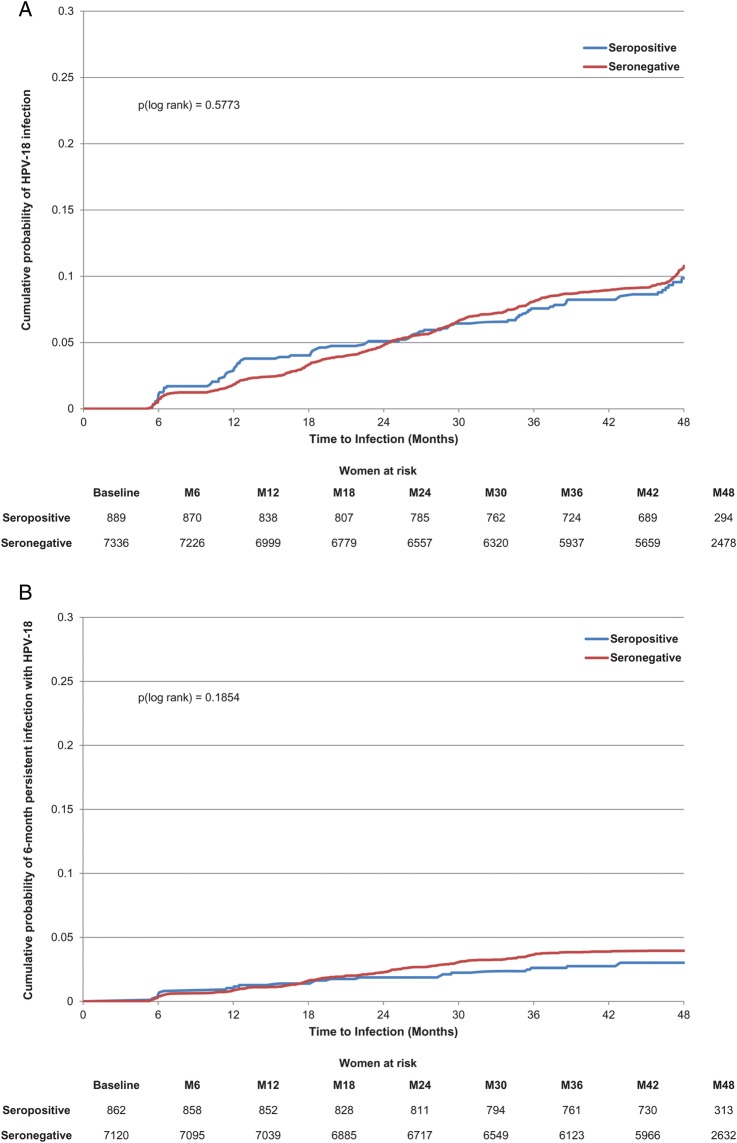
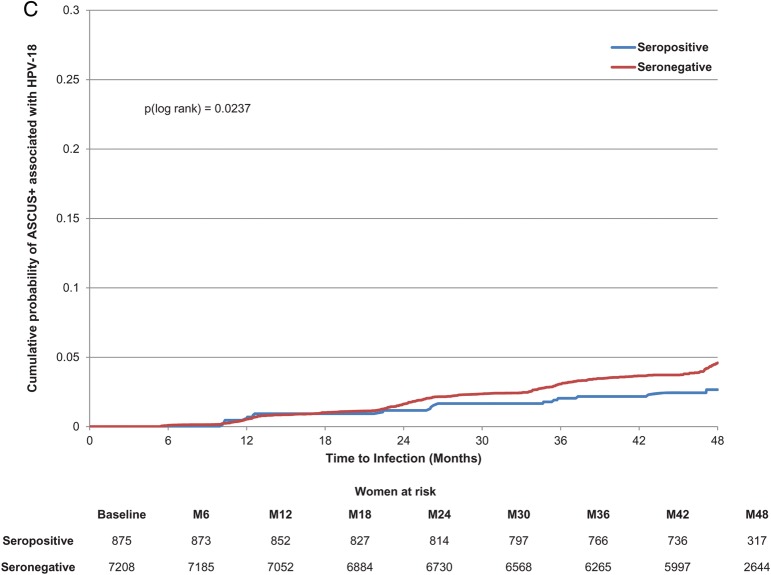
Seropositivity to HPV-18 at baseline was not significantly associated with a lower risk of newly detected incident infection or persistent infection (Table 3; Figure 3A and 3B). However, baseline HPV-18 serostatus (positive vs negative) showed a significant association with ASCUS+ and CIN1+ (Table 3; Figure 3C). There was no apparent effect of naturally acquired antibodies according to quartile (Table 3).
Table 3.
Risk of Newly Detected Human Papillomavirus Type 18 (HPV-18) Infections or Cervical Abnormalities Associated With HPV-18 According to Initial Serostatus (Univariate and Multivariable Regression Analyses) in Women Who Were HPV-18 DNA Negative at Baseline
| Endpoint | Serostatus at Baseline | No. Cases With New Endpoint | No. Cases Without New Endpoint | PY | Incidence Per 100 PY | Univariate Analysis | Final Multivariable Analysis Adjusted on Confounders Retained |
|
|---|---|---|---|---|---|---|---|---|
| Rate Ratio (95% CI) | Rate Ratio (95% CI) | P Value | ||||||
| New HPV-18 incident infection (cases = 837, noncases = 7388)a | Seronegative | 755 | 6581 | 25 917 | 2.91 | 1.00 | 1.00 | |
| Seropositive | 82 | 807 | 3121 | 2.63 | 0.90 (.72–1.13) | 0.94 (.75–1.19) | .61 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 7–10 | 27 | 223 | 859 | 3.14 | 1.08 (.74–1.58) | 1.24 (.84–1.82) | .28 | |
| >10–17 | 16 | 188 | 739 | 2.16 | 0.74 (.45–1.22) | 0.75 (.46–1.23) | .26 | |
| >17–43 | 21 | 194 | 761 | 2.76 | 0.95 (.61–1.46) | 0.96 (.62–1.49) | .86 | |
| >43–1086 | 18 | 202 | 762 | 2.36 | 0.81 (.51–1.29) | 0.81 (.50–1.30) | .38 | |
| P value for linear trend = .22 | ||||||||
| New HPV-18 six-mo persistent infection (cases = 297, noncases = 7685)a | Seronegative | 272 | 6848 | 26 426 | 1.03 | 1.00 | 1.00 | |
| Seropositive | 25 | 837 | 3202 | 0.78 | 0.76 (.50–1.14) | 0.86 (.56–1.30) | .46 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 7–10 | 8 | 235 | 887 | 0.90 | 0.88 (.43–1.77) | 1.09 (.54–2.21) | .80 | |
| >10–17 | 7 | 192 | 756 | 0.93 | 0.90 (.43–1.91) | 0.94 (.44–2.01) | .88 | |
| >17–43 | 6 | 204 | 780 | 0.77 | 0.75 (.33–1.68) | 0.81 (.36–1.83) | .61 | |
| >43–1086 | 4 | 206 | 779 | 0.51 | 0.50 (.19–1.34) | 0.56 (.21–1.51) | .25 | |
| P value for linear trend = .17 | ||||||||
| New HPV-18 twelve-mo persistent infection (cases = 147, noncases = 7724)a | Seronegative | 137 | 6889 | 26 592 | 0.52 | 1.00 | 1.00 | |
| Seropositive | 10 | 835 | 3210 | 0.31 | 0.60 (.32–1.15) | 0.66 (.34–1.26) | .21 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 7–10 | 2 | 235 | 894 | 0.22 | 0.43 (.11–1.75) | 0.56 (.14–2.26) | .41 | |
| >10–17 | 3 | 193 | 756 | 0.40 | 0.77 (.25–2.42) | 0.79 (.25–2.49) | .69 | |
| >17–43 | 2 | 206 | 786 | 0.25 | 0.49 (.12–2.00) | 0.52 (.13–2.13) | .37 | |
| >43–1086 | 3 | 201 | 774 | 0.39 | 0.75 (.24–2.36) | 0.75 (.23–2.39) | .63 | |
| P value for linear trend = .68 | ||||||||
| New ASCUS+ associated with HPV-18 (cases = 338, noncases = 7745)a | Seronegative | 316 | 6892 | 26 571 | 1.19 | 1.00 | 1.00 | |
| Seropositive | 22 | 853 | 3222 | 0.68 | 0.57 (.37–.88) | 0.64 (.41–.99) | .043 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 7–10 | 7 | 237 | 892 | 0.79 | 0.66 (.31–1.40) | 0.81 (.38–1.72) | .58 | |
| >10–17 | 5 | 195 | 756 | 0.66 | 0.56 (.23–1.34) | 0.60 (.25–1.46) | .26 | |
| >17–43 | 4 | 209 | 789 | 0.51 | 0.43 (.16–1.14) | 0.46 (.17–1.25) | .13 | |
| >43–1086 | 6 | 212 | 786 | 0.76 | 0.64 (.29–1.44) | 0.67 (.29–1.51) | .33 | |
| P value for linear trend = .17 | ||||||||
| New CIN1+ associated with HPV-18 (cases = 67, noncases = 8016)a | Seronegative | 66 | 7142 | 27 026 | 0.24 | 1.00 | 1.00 | |
| Seropositive | 1 | 874 | 3266 | 0.03 | 0.13 (.02–.90) | 0.13 (.02–.94) | .043 | |
| Seropositive, quartiles, EU/mL | ||||||||
| 7–10 | 0 | 244 | 904 | 0.00 | ND | ND | ND | |
| >10–17 | 0 | 200 | 769 | 0.00 | ND | ND | ND | |
| >17–43 | 0 | 213 | 796 | 0.00 | ND | ND | ND | |
| >43–1086 | 1 | 217 | 797 | 0.13 | 0.51 (.07–3.70) | ND | ND | |
| New CIN2+ associated with HPV-18 (cases = 34, noncases = 8049)a | Seronegative | 34 | 7174 | 27 062 | 0.13 | 1.00 | ND | ND |
| Seropositive | 0 | 875 | 3267 | 0.00 | ND | ND | ND | |
| Seropositive, quartiles, EU/mL | ||||||||
| 7–10 | 0 | 244 | 904 | 0.00 | ND | ND | ND | |
| >10–17 | 0 | 200 | 769 | 0.00 | ND | ND | ND | |
| >17–43 | 0 | 213 | 796 | 0.00 | ND | ND | ND | |
| >43–1086 | 0 | 218 | 798 | 0.00 | ND | ND | ND | |
Incident infection was defined as a new detection of the HPV type at any time during the follow-up period; 6- and 12-month persistent infection were defined as detection of the same HPV type in 2 consecutive samples over a minimum of 150 days and 300 days, respectively; ASCUS+ was defined as ASCUS, low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells/high-grade ASCUS, does not exclude high-grade squamous intraepithelial lesion (HSIL), atypical glandular cells of undetermined significance (AGUS), or HSIL. CIN1+ was defined as CIN1, CIN2, CIN3, adenocarcinoma in situ, or invasive cervical cancer; CIN2+ excluded CIN1.
Confounders (exposure variables) retained for the multivariable analysis were marital status, tobacco exposure (number of pack-years), age at first sexual intercourse, number of sexual partners prior to the past 12 months (ASCUS+, CIN1+, CIN2+), number of sexual partners during the past 12 months (incident and persistent infections), at least 1 previous pregnancy, sexually transmitted infection history, and region.
Linear trend was evaluated across 5 classes: (1) seronegative; (2) first quartile; (3) second quartile; (4) third quartile; (5) fourth quartile.
Abbreviations: ASCUS, atypical squamous cell of undetermined significance; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ND, analysis not performed because of too few cases for inferential analysis; PY, patient years.
a No. of cases and noncases and patient-years reported for the univariate analysis.
Figure 3.
Cumulative probability of detecting an incident or 6-month persistent infection or developing atypical squamous cells of undetermined significance or greater (ASCUS+) associated with human papillomavirus type 18 (HPV-18). A, Incident HPV-18 infection. B, HPV-18 six-month persistent infection. C, ASCUS+ associated with HPV-18.
Several covariates were shown to be significantly associated with new HPV-16 and HPV-18 incident infections in the multivariable analysis (Supplementary Tables 1 and 2).
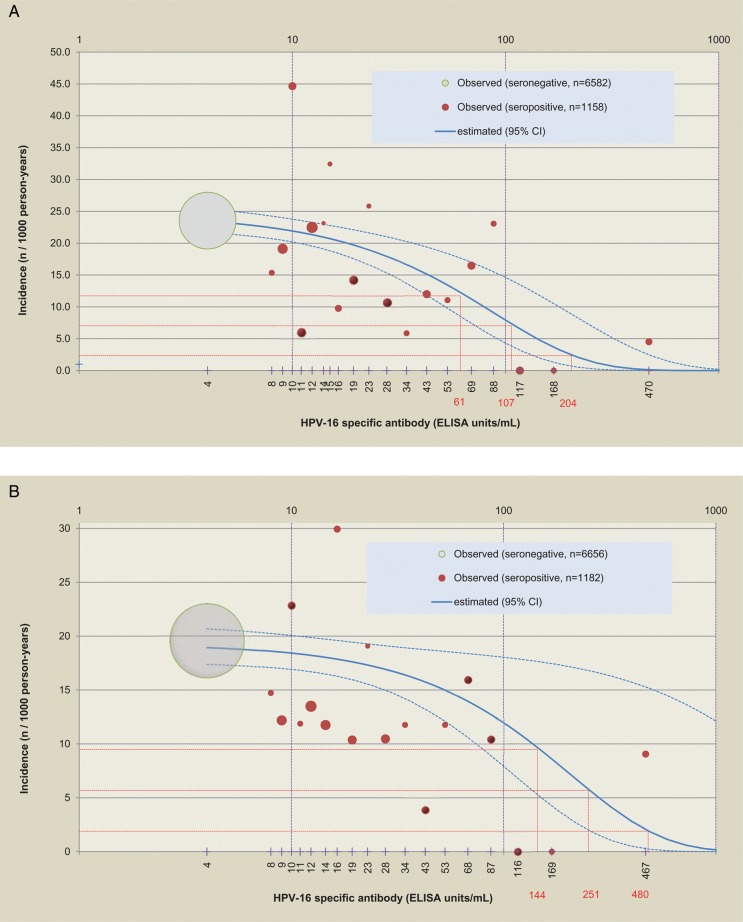
Figure 4A and 4B show the relationship between HPV-16 antibody level and 6-month persistent infection or ASCUS+ associated with HPV-16. The frequency of 6-month persistent HPV-16 infection declined as antibodies rose. An HPV-16 antibody level of 204 (95% CI, 129–480) EU/mL yielded a 90% reduction in 6-month persistent infection; values for a 70% and 50% reduction were 107 (95% CI, 68–251) and 61 (95% CI, 39–144) EU/mL, respectively (Figure 4A). A corresponding analysis for ASCUS+ showed that an HPV-16 antibody level of 480 (95% CI, 250–5756), 251 (95% CI, 131–3010) and 144 (95% CI, 75–1733) EU/mL yielded 90%, 70%, and 50% reductions, respectively (Figure 4B). With regard to incident HPV infection, an HPV-16 antibody level of 371 (95% CI, 242–794) EU/mL yielded a 90% reduction in incident infection; values for a 70% and 50% reduction were 194 (95% CI, 118–415) and 112 (95% CI, 73–415) EU/mL, respectively. Sensitivity analyses produced similar results.
Figure 4.
Relationship between initial antibody level and 6-month persistent infection or atypical squamous cells of undetermined significance or greater (ASCUS+) associated with human papillomavirus type 16. A, 6-month persistent infection. B, ASCUS+. The dot size is proportional to the number of subjects, the gray dot represents all seronegative subjects, and red dots represent approximately 5-percentile classes of seropositive subjects. The solid blue line corresponds to the Poisson regression model (the dotted lines are 95% confidence limits). The dotted red lines correspond to a 50%, 70%, and 90% reduction of the incidence of the endpoint (6-month persistent infection or ASCUS+), and the values in red are the corresponding threshold values of antibody titer. Sensitivity analyses including the covariates of age at first sexual intercourse and smoking history, or including only a subset of 100 seronegative subjects, produced similar results. For example, including the covariates of age at first sexual intercourse and smoking history for all seronegative subjects, the estimated antibody titers (with 95% confidence interval) yielding 90%, 70%, and 50% reductions in 6-month persistent infection were 180 (118–377), 94 (62–197), and 54 (36–114) EU/mL, respectively. Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; HPV-16, human papillomavirus type 16.
DISCUSSION
This study focused on the role of naturally acquired antibodies in prevention of newly detected HPV infection and associated cervical abnormalities in sexually active women who had no severe cervical abnormalities and no evidence of an active HPV infection (ie, were DNA negative for the type under consideration) at study entry. The results show that women with higher levels of naturally acquired antibodies to HPV-16 detected using an L1-based VLP assay had a lower risk of a subsequent newly detected infection or cervical abnormality associated with HPV-16. There was a similar effect for HPV-18, although the association was weaker and the relationship between the antibody level against HPV-18 L1-based VLPs and newly detected infection and associated abnormalities was not significant.
There was a clear pattern of reduced risk of a newly detected incident infection, persistent infection, ASCUS+, or CIN1+ associated with HPV-16 as baseline antibody levels rose, and the linear trend by antibody quartile was significant for all endpoints. Only the highest level of natural antibodies reduced new CIN1+ associated with HPV-16, whereas natural antibodies above the median (21 EU/mL) reduced the occurrence of ASCUS+. The evidence was less clear for CIN2+, due to fewer cases, but the linear trend by antibody quartile was also significant.
The present results did not show a reduced risk of infection or disease with higher levels of HPV-18 antibodies. However, the analysis of HPV-18 serostatus (positive vs negative) showed some evidence for a reduced incidence of ASCUS+ and, with a lower number of cases, of CIN1+ among seropositive women, suggesting that naturally acquired HPV-18 antibodies might play some protective role in the prevention of associated abnormalities. The same analyses for HPV-31, HPV-33, and HPV-45, which are closely related to HPV-16 or HPV-18, did not show a significant association (data not shown); these analyses were, however, severely limited by the low number of related cases.
It was interesting to note the very similar results obtained with the univariate and multivariable models. A number of covariates were taken into account in the multivariable analyses, based on established risk factors known to influence the risk of HPV infection, but results were independent of potential confounders accounted for. The similarity of the two approaches reinforces the robustness of the results. As expected, no cases of cervical cancer were seen, due to the young age of women in the study and the intensive follow-up for CIN2 detection.
Several studies have been unable to draw clear conclusions regarding the role of naturally acquired antibodies in preventing subsequent HPV infection [15–19, 28]. However, the findings of the Costa Rica Vaccine Trial indicated that women with antibody titers in the highest tertile for HPV-16 and HPV-18 (≥60 and ≥28 EU/mL, respectively) had a significantly lower risk of an incident cervical infection with the same HPV type [20]. Although the analyses of the Costa Rica Vaccine Trial and our study were conducted in a similar way, used the same ELISA technique, and controlled for similar demographic and behavioral characteristics, there are some differences, including subjects' age (18–25 years in the Costa Rica Vaccine Trial), and a wider geographic distribution and higher number of subjects in our study, which gave our study a greater power to identify significant effects. In addition, for the women included in the control arms, HPV-16 and HPV-18 DNA was detected in 8.6% and 3.1%, respectively, of women in the Costa Rica Vaccine Trial, compared with 5.2% and 2.3% in our study. Similarly, HPV-16 and HPV-18 seroprevalence was lower in our study (15.2% and 10.8%, respectively) than in the Costa Rica Vaccine Trial (25% for both HPV-16 and HPV-18). These differences may indicate that women in the Costa Rica Vaccine Trial might have been more likely to have had a past exposure to HPV and thus more time to mount a natural antibody response. Our results extend those of the Costa Rica Vaccine trial to persistent infection, ASCUS+, CIN1+, and CIN2+.
The present study is the first to demonstrate a statistically significant quantitative relationship between titers of naturally acquired antibodies to HPV-16 and the incidence of newly detected and 6-month persistent HPV-16 infection and HPV-16-associated ASCUS+. Antibody titers of approximately 370, 204, and 480 EU/mL were associated with a 90% risk reduction of incident infection, 6-month persistent infection, and ASCUS+, respectively. However, these values do not represent correlates of protection with regard to antibody levels induced by vaccination, because there are certainly some differences between naturally acquired vs vaccine-induced antibody production. For example, the mechanism of exposure of the immune system to HPV antigens via vaccination allows induction of a higher antibody level. In PATRICIA, antibody titer 1 month after the full vaccination course was 9341.5 (95% CI, 8760.4–9961.1) and 4769.6 (95% CI, 4491.2–5065.3) EU/mL for HPV-16 and HPV-18 antibodies, respectively [22]. Antibody properties such as affinity, avidity, and specificity may also be different. In addition, naturally acquired HPV infections potentially enable broad exposure of many HPV-specific proteins during the virus life cycle, unlike HPV vaccines based on L1 VLPs. Thus, natural infections are likely not restricted to the generation of L1 antibody responses but would be expected to include a spectrum of HPV-specific cell-mediated and humoral immune responses that could contribute to reduction in new infection.
In the Costa Rica vaccine study, the HPV-16 antibody titer at 48 months after 1 dose was 137 EU/mL; the 90th percentile was 703 EU/mL [29]. In the present study, the HPV-16 antibody levels producing a 90% reduction in incident infection, 6-month persistent infection, and ASCUS+ were, respectively, 371 EU/mL, 204 EU/mL, and 480 EU/mL. Corresponding values for a 70% reduction were 194 EU/mL, 107 EU/mL, and 251 EU/mL. These estimates might be useful as we move toward understanding minimal levels of HPV protective antibodies.
Analyses of PATRICIA and the Costa Rica Vaccine Trial strongly suggest that higher levels of naturally acquired antibodies play a role in preventing newly detected HPV infections and associated abnormalities. Although a correlate of protection for vaccine-induced antibodies cannot firmly be inferred, as described above, it is worth noting that the levels that appear to be associated with some reduction of risk are considerably below the levels of vaccine-induced antibodies found in clinical trials of the HPV-16/18 vaccine in which protection against CIN2+, CIN3+, and adenocarcinoma in situ has been demonstrated [2, 3, 22, 23], and also considerably below the levels sustained up to 8.4 years after vaccination [30].
The analysis had some unavoidable limitations. We do not know when women were exposed to HPV infection prior to the study, so we could not distinguish whether the antibody level at baseline was the peak from a recent exposure, or had declined over time from a higher level. Many women with HPV infection never develop detectable antibodies [7–13, 31, 32], so some seronegative women may have been previously exposed to infection. These women may have mounted a cell-mediated immune response against HPV, which may have reduced the risk of CIN2+ development. This could have underestimated the protective effect. However, this limitation would not apply to the observation of increasing protection with titer. A further limitation is that it is not possible to distinguish between reinfection (a new HPV infection) and recurrence of an existing infection that was quiescent or had persisted at an undetectable level. It has been shown that women can experience recurrence of a type-specific infection after a period of nondetection [33]. Our findings suggest that the reduction of risk associated with naturally acquired antibodies might apply to both new and recurrent HPV infections. We have focused on ASCUS+ and CIN1+ as cervical abnormalities because few CIN2+ cases were detected. However, the limited utility of ASCUS+ and CIN1+ as disease-related endpoints should be noted.
The quality of the serologic assay is important in classifying women as previously exposed or unexposed, and measurement of the antibody response is dependent upon the assay, its specificity, and the cutoff value. There is variability in the measurement of the lower antibody titers as shown in the quantitative models (Figure 4A and 4B). Therefore, some women with antibody levels close to the threshold value could have been misclassified as seropositive or seronegative. The ELISA assay used in PATRICIA and the Costa Rica study has two disadvantages [34]: it potentially measures nonneutralizing antibodies (which would not be protective as neutralizing antibodies are likely to form the main basis for vaccine-induced protection against HPV infection), and it detects reactivity only to antibodies of the immunoglobulin G class (thus protection conferred by other antibody classes is not evaluated). These problems can be overcome by the pseudovirion-based neutralization assay (PBNA), which measures only potentially protective neutralizing antibodies of all immunoglobulin classes [26, 34]; however, this assay is currently too laborious for routine use in large clinical trials. Notably, a good correlation has been found between the PBNA and the ELISA assay for titers of vaccine-induced antibodies to HPV-16 and HPV-18 [26] and for titers of naturally acquired antibodies to HPV-16 [35].
In conclusion, this study confirms the utility of control arm data from vaccine efficacy trials in understanding acquisition and progression of HPV infections and related cervical abnormalities. The results suggest that naturally acquired antibodies to HPV-16, and to a lesser extent HPV-18, reduce the risk of subsequent infection and associated cervical abnormalities with the same HPV type.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all study participants and their families. We gratefully acknowledge the work of the central and local study coordinators, and staff members of the sites that participated in this study.
Contribution to statistical support was provided by D. Rosillon (GlaxoSmithKline Vaccines, Wavre, Belgium), A. Raillard, S. Collas De Souza, M.-C. Bozonnat (4Clinics, Paris, France, Contract Research Organization on behalf of GlaxoSmithKline Vaccines). F. Dessy (GlaxoSmithKline Vaccines, Wavre, Belgium) is acknowledged for valuable discussions during manuscript development. The manuscript Core Writing Team included X. Castellsagué, P. Naud, S.-N. Chow, C.M. Wheeler, M. J. V. Germar, D. Rosillon, and L. Baril. Writing support services were provided by M. Greenacre, PhD, An Sgriobhadair Ltd, on behalf of GlaxoSmithKline Vaccines, Wavre, Belgium; editing and publication coordinating services were provided by J. Andersson PhD, CROMSOURCE Ltd, UK, and V. Delpire, Words and Science, Brussels, Belgium, on behalf of GlaxoSmithKline Vaccines, Wavre, Belgium.
The PATRICIA Study Group
Principal investigators/coinvestigators. Australia: I. Denham, S. M. Garland, A. Mindel, S. R. Skinner. Belgium: P. De Sutter, W. A. J. Poppe, W. Tjalma. Brazil: N. S. De Carvalho, P. Naud, J. C. Teixeira. Canada: F. Y. Aoki, F. Diaz-Mitoma, M. Dionne, L. Ferguson, M. Miller, K. Papp, B. Ramjattan, B. Romanowski, R. Somani, P. H. Orr. Finland: D. Apter, T. Karppa, N. Kudjoi, M. Lehtinen, L. Niemi, J. Paavonen, J. Palmroth, T. Petaja, U. Romppanen, M. Siitari-Mattila, L. Tuomivaara. Germany: K. H. Belling, T. Gent, F. Gieseking, T. Grubert, W. Harlfinger, W. D. Höpker, U. Kohoutek, S. Jensen-El Tobgui, G. Merder, K. Peters, S. Schoenian, K. Schulze, T. F. Schwarz. Italy: F. Boselli, C. A. Liverani. Mexico: J. Salmerón. Philippines: C. Crisostomo, R. Del Rosario-Raymundo, E. Raymundo, M. J. Germar, G. Limson, C. Remollino, G. Villanueva, S. Villanueva, J. D. Zamora, L. A. Zamora. Spain: J. Bajo, J. Bayas, M. Campins, X. Castellsagué, M. Castro, C. Centeno, L. Rodríguez de la Pinta, A. Torné, J. A. Vidart. Taiwan: S. N. Chow, M. H. Yu, C. C. Yuan. Thailand: S. Angsuwathana, U. Jaisamrarn, K. Wilawan. United Kingdom: M. Cruickshank, S. Govindraj, E. A. Hakim, H. Kitchener, D. Lewis, A. Szarewski (deceased). United States: N. Bennett, M. Caldwell, C. Chambers, A. Chatterjee, L. DeMars, L. Downs, D. Ferris, P. Fine, S. Gall, J. Hedrick, M. Hiraoka, W. Huh, T. Klein, W. Koltun, J. Lalezari, P. Lee, S. Luber, M. Martens, C. Peterson, J. Rosen, W. Rosenfeld, M. Scutella, L. Seidman, M. Sperling, R. Sperling, M. Stager, J. Stapleton, L. Twiggs, A. Waldbaum, C. M. Wheeler, E. Zbella.
GlaxoSmithKline clinical study support. A. Camier, B. Colau, V. Xhenseval, Z. Issaka (VALESTA), P. Marius, N. Martens, T. Ouammou, M. Rahier, N. Smoes, B. Spiessens, A. Meurée, N. Houard, S. Poncelet, A. Tonglet, C. Van Hoof (Xpe Pharma), A. S. Vilain, T. Zahaf, H. Bhat.
Laboratory contributions. E. Alt, B. Iskaros, A. Limaye, X. Liu-Jarin, R. D. Luff, M. McNeeley, C. Provenzano (Quest Diagnostics Clinical Trials, Teterboro, New Jersey). A. Molijn, W. Quint, L. Struijk, M. Van de Sandt, L. J. Van Doorn (DDL Diagnostic Laboratory, Voorburg, the Netherlands).
Endpoint committee. N. Kiviat, K. P. Klugman, P. Nieminen.
Independent data monitoring committee. C. Bergeron, E. Eisenstein, R. Marks, T. Nolan.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA (NCT00122681). Cervarix is a registered trademark of the GlaxoSmithKline group of companies.
Potential conflicts of interest. L. B., D. D., G. D., F. S., and D. R. are employees of the GlaxoSmithKline group of companies. L. B., D. D., G. D., and F. S. own stock in GlaxoSmithKline Biologicals SA, and G. D. has received royalty payments for patents from Wyeth, GlaxoSmithKline Biologicals SA. S. M. G. owned stocks in C. S. L. All investigators at study clinical sites were funded through their institutions to do the study protocol. F. Y. A. has received grants via his institution from Health Sciences Centre, Winnipeg, Manitoba. A. S., C. M. W., D. A., H. K., J. C. T., M. J. V. G., and X. C. have received funding through their institutions to do HPV vaccine studies for GlaxoSmithKline Biologicals SA. S. M. G. has received grants/grants pending from GlaxoSmithKline Biologicals SA and CSL. M. L. has received grants for HPV vaccination studies through his institution and from GlaxoSmithKline Biologicals SA and Merck Sharp & Dohme (MSD). S. R. S. has received grants/grants pending via her institutions from GlaxoSmithKline Biologicals SA. X. C. has received grants/grants pending via his institution from GlaxoSmithKline Biologicals SA, MSD, and Sanofi Pasteur. N. S. D. C. has received grants/grants pending from IPAMI. F. Y. A., J. S., U. J., and B. R. have received research grants via their institutions. F. X. B. has received research and educational grants from GlaxoSmithKline Biologicals SA and MSD, Sanofi Pasteur, and Qiagen. J. P. has received a research grant through the Helsinki University Hospital Research Institute to conduct clinical trials on HPV vaccination. Through the University of New Mexico, C. M. W. has received equipment and reagents for HPV genotyping from Roche Molecular Systems and funding for HPV vaccine studies from GlaxoSmithKline Biologicals SA (in addition to the present study) and Merck and Co, Inc. W. A. J. P., N. S. D. C., B. R., and T. F. S. have received consulting fees. A. R.'s employer, 4Clinics, has received consulting fee or honoraria and fees for statistical analysis from GlaxoSmithKline Biologicals SA. F. X. B., W. A. A. T., and X. C. have received consulting fees from GlaxoSmithKline Biologicals SA, MSD, and Sanofi Pasteur. A. S. has received consulting fee from GlaxoSmithKline Biologicals SA and MSD. S. R. S. has received consulting fees or honorarium from GlaxoSmithKline Biologicals SA. F. Y. A. has received payment for board membership from GlaxoSmithKline Biologicals SA. B. R. and W. A. J. P. have been paid for expert testimony. A. S., N. S. D. C., and W. A. J. P. have received payment for board membership. J. C. T., S. M. G., and S. R. S. have received payment for board membership from GlaxoSmithKline Biologicals SA and S. M. G. also from CSL. W. A. A. T. and X. C. have received payment for lectures including service on speakers’ bureaus from GlaxoSmithKline Biologicals SA, MSD, and Sanofi Pasteur and W. A. A. T. also for the development of educational presentations. A. S. and S. M. G. have received payment for lectures including service on speakers’ bureaus from GlaxoSmithKline Biologicals SA and MSD, and S. M. G. also from CSL. S. M. G. has received fees for participation in review activities from GlaxoSmithKline Biologicals SA and CSL. A. S., J. C. T., M. J. V. G., and P. N. have received payment from GlaxoSmithKline Biologicals SA for lectures including service on speakers’ bureaus and A. S. and M. J. V. G. also for the development of educational presentations. F. Y. A., N. S. D. C., B. R., and T. F. S. have received payment for lectures including service on speakers’ bureaus and T. F. S., F. Y. A., N. S. D. C., and W. A. J. P. for the development of educational presentations. S. M. G. has received payment from GlaxoSmithKline Biologicals SA for the development of educational presentations. A. S., C. M. W., F. Y. A., J. C. T., M. J. V. G., P. N., and S. R. S. have received travel reimbursements from GlaxoSmithKline Biologicals SA. K. P., W. A. J. P., A. S., C. M. W., F. Y. A., J. C. T., M. J. V. G., P. N., and S. R. S. have received travel reimbursements from GlaxoSmithKline Biologicals SA. N. S. D. C report privately funded travel support. A. S. has received travel reimbursements from GlaxoSmithKline Biologicals SA and MSD. D. A. has received support for travel from Väestöliitto. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.de Sanjosé S, Quint WGV, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional survey. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 2.The GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–85. doi: 10.1016/S0140-6736(09)61567-1. [DOI] [PubMed] [Google Scholar]
- 3.Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 4.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 5.The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1916–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102:325–39. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- 7.Kirnbauer R, Hubbert NL, Wheeler CM, Becker TM, Lowy DR, Schiller JT. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type 16. J Natl Cancer Inst. 1994;86:494–9. doi: 10.1093/jnci/86.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viscidi RP, Kotloff KL, Clayman B, Russ K, Shapiro S, Shah KV. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV infection among college women. Clin Diagn Lab Immunol. 1997;4:122–6. doi: 10.1128/cdli.4.2.122-126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faust H, Jelen MM, Poljak M, Klavs I, Učakar V, Dillner J. Serum antibodies to human papillomavirus (HPV) pseudovirions correlate with natural infection for 13 genital HPV types. J Clin Virol. 2013;4:336–41. doi: 10.1016/j.jcv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Porras C, Bennett C, Safaeian M, et al. Determinants of seropositivity among HPV-16/18 DNA positive young women. BMC Infect Dis. 2010;10:238. doi: 10.1186/1471-2334-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong Y, Ermel A, Tu W, Shew M, Brown DR. Association of HPV types 6, 11, 16, and 18 DNA detection and serological response in unvaccinated adolescent women. J Med Virol. 2013;85:1786–93. doi: 10.1002/jmv.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjellberg L, Wang Z, Wiklund F, et al. Sexual behaviour and papillomavirus exposure in cervical intraepithelial neoplasia: a population-based case-control study. J Gen Virol. 1999;80:391–8. doi: 10.1099/0022-1317-80-2-391. [DOI] [PubMed] [Google Scholar]
- 13.Carter JJ, Koutsky LA, Hughes JP, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–9. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 14.Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis. 1998;177:1710–4. doi: 10.1086/517428. [DOI] [PubMed] [Google Scholar]
- 15.Viscidi RP, Schiffman M, Hildesheim A, et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:324–7. doi: 10.1158/1055-9965.epi-03-0166. [DOI] [PubMed] [Google Scholar]
- 16.Viscidi RP, Snyder B, Cu-Uvin S, et al. Human papillomavirus capsid antibody response to natural infection and risk of subsequent HPV infection in HIV-positive and HIV-negative women. Cancer Epidemiol Biomarkers Prev. 2005;14:283–8. [PubMed] [Google Scholar]
- 17.Olsson SE, Kjaer S, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5:696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 18.Palmroth J, Namujju P, Simen-Kapeu A, et al. Natural seroconversion to high-risk human papillomaviruses (hrHPVs) is not protective against related HPV genotypes. Scand J Infect Dis. 2010;42:379–84. doi: 10.3109/00365540903501608. [DOI] [PubMed] [Google Scholar]
- 19.Ho GYF, Studentsov Y, Hall CB, et al. Risk factors for subsequent cervicovaginal human papillomavirus (HPV) infection and the protective role of antibodies to HPV-16 virus-like particles. J Infect Dis. 2002;186:737–42. doi: 10.1086/342972. [DOI] [PubMed] [Google Scholar]
- 20.Safaeian M, Porras C, Schiffman M, et al. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst. 2010;102:1653–62. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wentzensen N, Rodriguez AC, Viscidi R, et al. A competitive serological assay shows naturally acquired immunity to human papillomavirus infections in the Guanacaste natural history study. J Infect Dis. 2011;204:94–102. doi: 10.1093/infdis/jir209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 23.Lehtinen M, Paavonen J, Wheeler CM, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler CM, Castellsagué X, Garland SM, et al. Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13:100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 25.van Doorn L-J, Molijn A, Kleter B, Quint W, Colau B. Highly effective detection of human papillomavirus 16 and 18 DNA by a testing algorithm combining broad-spectrum and type-specific PCR. J Clin Microbiol. 2006;44:3292–8. doi: 10.1128/JCM.00539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dessy FJ, Giannini SL, Bougelet CA, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Human Vacc. 2008;4:425–34. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 27.Roset Bahmanyar E, Paavonen J, Salmerón J, et al. Prevalence and risk factors for cervical HPV infection and abnormalities in young adult women at enrolment in the multinational PATRICIA trial. Gynecol Oncol. 2012;127:440–50. doi: 10.1016/j.ygyno.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Merikukka M, Kaasila M, Namujju PB, et al. Differences in incidence and co-occurrence of vaccine and nonvaccine human papillomavirus types in Finnish population before human papillomavirus mass vaccination suggest competitive advantage for HPV33. Int J Cancer. 2011;128:1114–9. doi: 10.1002/ijc.25675. [DOI] [PubMed] [Google Scholar]
- 29.Safaeian M, Porras C, Pan Y, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res. 2013;6:1242–50. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roteli-Martins C, Naud P, Borba P, et al. Sustained immunogenicity and efficacy of the HPV-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother. 2012;8:390–7. doi: 10.4161/hv.18865. [DOI] [PubMed] [Google Scholar]
- 31.Coseo S, Porras C, Hildesheim A, et al. for the Costa Rica HPV Vaccine Trial (CVT) Group. Seroprevalence and correlates of human papillomavirus 16/18 seropositivity among young women in Costa Rica. Sex Transm Dis. 2010;37:706–10. doi: 10.1097/OLQ.0b013e3181e1a2c5. [DOI] [PubMed] [Google Scholar]
- 32.Carter JJ, Koutsky LA, Wipf GC, et al. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J Infect Dis. 1996;174:927–36. doi: 10.1093/infdis/174.5.927. [DOI] [PubMed] [Google Scholar]
- 33.Insinga RP, Perez G, Wheeler CM, et al. Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev. 2010;19:1585–94. doi: 10.1158/1055-9965.EPI-09-1235. [DOI] [PubMed] [Google Scholar]
- 34.Schiller JT, Lowy DR. Immunogenicity testing in human papillomavirus virus-like-particle vaccine trials. J Infect Dis. 2009;200:166–71. doi: 10.1086/599988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Safaeian M, Ghosh A, Porras C, et al. Direct comparison of HPV16 serological assays used to define HPV-naive women in HPV vaccine trials. Cancer Epidemiol Biomarkers Prev. 2012;21:1547–54. doi: 10.1158/1055-9965.EPI-12-0558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.