Abstract
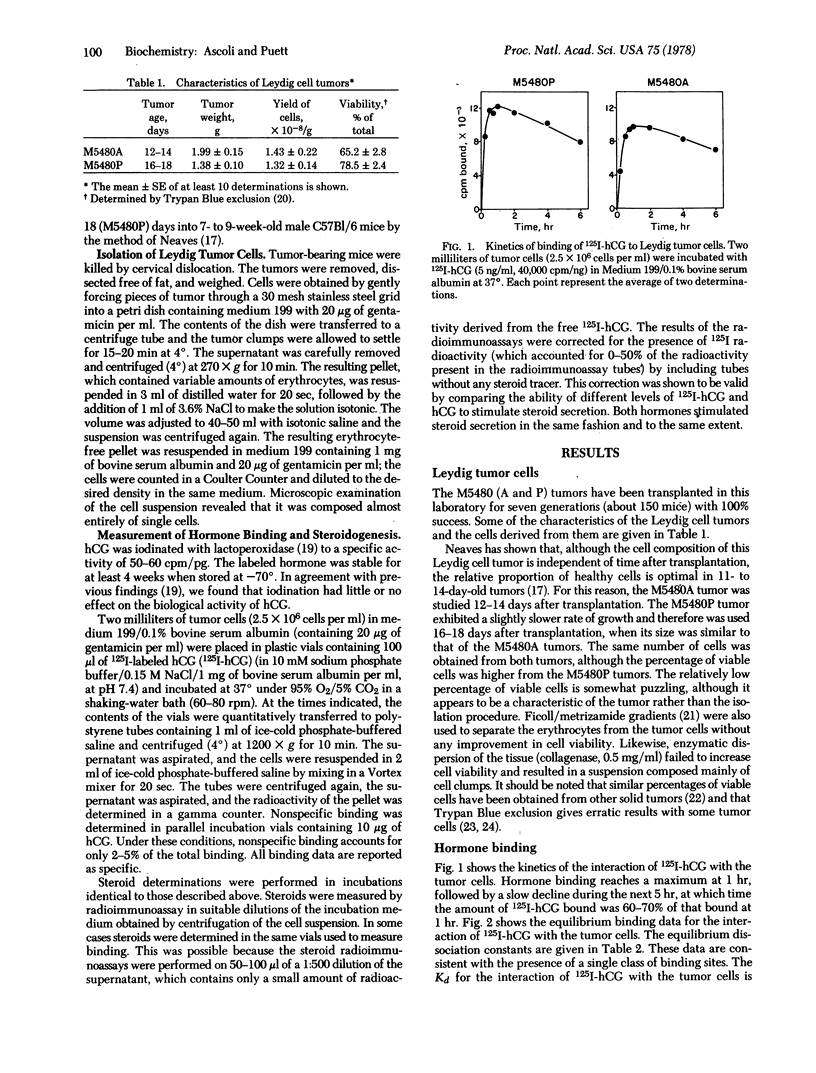
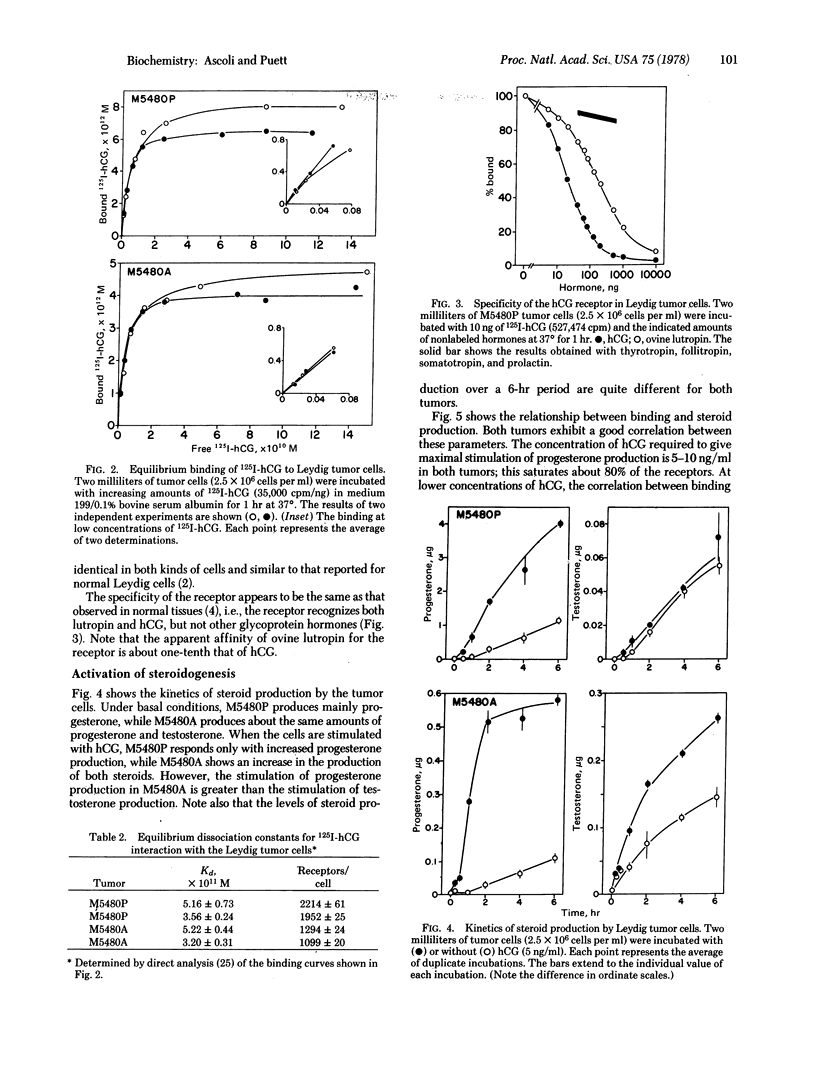
Testicular tumors are generally characterized by a loss of responsiveness to gonadotropins. The M5480 Leydig cell tumor is unusual, if not unique, in that it responds to human choriogonadotropin and to lutropin via increased steroidogenesis. This report describes the identification of two variants of the original M5480 tumor that have altered steroid output both in the basal state and in response to human choriogonadotropin. One of the tumors produces mainly progesterone, which is stimulated by the choriogonadotropin; the other tumor produces about equal amounts of progesterone and testosterone, and the secretion of both is stimulated by the choriogonadotropin. The dissociation constant describing the interaction between Leydig tumor cells and 125I-labeled human choriogonadotropin is between 3 and 5×10-11 M. This agrees with values reported for normal Leydig cells, although the tumor cells appear to have fewer receptors. The differences noted in the two tumors and normal Leydig cells may have arisen from alterations in gene regulation, or in mutations, involving one or more enzymes in the pathway in which progesterone is converted to testosterone. Under the experimental conditions used, all the tumors studied (seven generations) responded to the choriogonadotropin both in binding and in the resultant stimulation of steroidogenesis. This property, together with the characteristic that a homogeneous cell population can be obtained without enzymatic treatment, should qualify the M5480 Leydig cell tumor(s) as a model system for further studies on the mechanism of action of gonadotropin, on hormone receptors, and on hormonally responsive tumors.
Keywords: choriogonadotropin, hormone action, testicular tumor
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bliss C. I., James A. T. Fitting the rectangular hyperbola. Biometrics. 1966 Sep;22(3):573–602. [PubMed] [Google Scholar]
- Cassel W. A. Long-term preservation of the Ehrlich ascites tumor. Methods Cell Biol. 1976;14:181–186. doi: 10.1016/s0091-679x(08)60480-x. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Tsuruhara T., Dufau M., Catt K. J. Isolation of highly purified Leydig cells by density gradient centrifugation. Endocrinology. 1977 Aug;101(2):639–642. doi: 10.1210/endo-101-2-639. [DOI] [PubMed] [Google Scholar]
- Harris R., Ukaejiofo E. O. Tissue typing using a routine one-step lymphocyte separation procedure. Br J Haematol. 1970 Feb;18(2):229–235. doi: 10.1111/j.1365-2141.1970.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Puett D. Gonadotropin and subunit conformation. Arch Biochem Biophys. 1975 Dec;171(2):708–720. doi: 10.1016/0003-9861(75)90083-1. [DOI] [PubMed] [Google Scholar]
- Jacobs B. B., Huseby R. A. Transplantable Leydig cell tumors in Fischer rats: horomone responsivity and hormone production. J Natl Cancer Inst. 1968 Nov;41(5):1141–1153. [PubMed] [Google Scholar]
- Janszen F. H., Cooke B. A., van Driel M. J., van der Molen H. J. Purification and characterization of Leydig cells from rat testes. J Endocrinol. 1976 Sep;70(3):345–359. doi: 10.1677/joe.0.0700345. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Isersky C., Metzger H. The interaction of IgE with rat basophilic leukemia cells. I. Evidence for specific binding of IgE. J Exp Med. 1974 Mar 1;139(3):600–616. doi: 10.1084/jem.139.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975 Nov 25;250(22):8818–8823. [PubMed] [Google Scholar]
- Moore G. E., Ito E., Ulrich K., Sandberg A. A. Culture of human leukemia cells. Cancer. 1966 May;19(5):713–723. doi: 10.1002/1097-0142(196605)19:5<713::aid-cncr2820190518>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Moudgal N. R., Moyle W. R., Greep R. O. Specific binding of luteinizing hormone to Leydig tumor cells. J Biol Chem. 1971 Aug 25;246(16):4983–4986. [PubMed] [Google Scholar]
- Moyle W. R., Armstrong D. T. Stimulation of testosterone biosynthesis by luteinizing hormone in transplantable mouse Leydig cell tumors. Steroids. 1970 May;15(5):681–693. doi: 10.1016/s0039-128x(70)80073-3. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Jungas R. L., Greep R. O. Influence of luteinizing hormone and adenosine 3':5'-cyclic monophosphate on the metabolism of free and esterified cholesterol in mouse Leydig-cell tumours. Biochem J. 1973 Jun;134(2):407–413. doi: 10.1042/bj1340407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle W. R., Jungas R. L., Greep R. O. Metabolism of free and esterified cholesterol by Leydig-cell tumour mitochondria. Biochem J. 1973 Jun;134(2):415–424. doi: 10.1042/bj1340415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyle W. R., Moudgal N. R., Greep R. O. Cessation of steroidogenesis in Leydig cell tumors after removal of luteinizing hormone and adenosine cyclic 3',5'-monophosphate. J Biol Chem. 1971 Aug 25;246(16):4978–4982. [PubMed] [Google Scholar]
- Moyle W. R., Ramachandran J. Effect of LH on steroidogenesis and cyclic AMP accumulation in rat Leydig cell preparations and mouse tumor Leydig cells. Endocrinology. 1973 Jul;93(1):127–134. doi: 10.1210/endo-93-1-127. [DOI] [PubMed] [Google Scholar]
- Neaves W. B. Brief communication: ultrastructural transformation of a murine Leydig-cell tumor after gonadotropin administration. J Natl Cancer Inst. 1973 Apr;50(4):1069–1073. doi: 10.1093/jnci/50.4.1068. [DOI] [PubMed] [Google Scholar]
- Neaves W. B. Gonadotropin-induced proliferation of endoplasmic reticulum in an androgenic tumor and its relation to elevated plasma testosterone levels. Cancer Res. 1975 Oct;35(10):2663–2669. [PubMed] [Google Scholar]
- Neaves W. B. Growth and composition of a transplantable murine Leydig cell tumor. J Natl Cancer Inst. 1975 Sep;55(3):623–631. doi: 10.1093/jnci/55.3.623. [DOI] [PubMed] [Google Scholar]
- Papaionannou S., Gospodarowicz D. Comparison of the binding of human chorionic gonadotropin to isolated bovine luteal cells and bovine luteal plasma membranes. Endocrinology. 1975 Jul;97(1):114–124. doi: 10.1210/endo-97-1-114. [DOI] [PubMed] [Google Scholar]
- Pokel J. D., Moyle W. R., Greep R. O. Depletion of esterified cholesterol in mouse testis and Leydig cell tumors by luteinizing hormone. Endocrinology. 1972 Jul;91(1):323–325. doi: 10.1210/endo-91-1-323. [DOI] [PubMed] [Google Scholar]
- Shin S. I. Studies on interstitial cells in tissue culture: steroid biosynthesis in monolayers of mouse testicular interstitial cells. Endocrinology. 1967 Sep;81(3):440–448. doi: 10.1210/endo-81-3-440. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Yasumura Y., Sato G. H. Studies on interstitial cells in tissue culture. II. Steroid biosynthesis by a clonal line of rat testicular interstitial cells. Endocrinology. 1968 Mar;82(3):614–616. doi: 10.1210/endo-82-3-614. [DOI] [PubMed] [Google Scholar]



