Abstract
Neutrophils are rapidly recruited to the site of Leishmania infection and play an active role in capturing and killing parasites. They are the main source of leukotriene B4 (LTB4), a potent proinflammatory lipid mediator. However, the role of LTB4 in neutrophil infection by Leishmania amazonensis is not clear. In this study, we show that L. amazonensis or its lipophosphoglycan can induce neutrophil activation, degranulation, and LTB4 production. Using pharmacological inhibitors of leukotriene synthesis, our findings reveal an LTB4-driven autocrine/paracrine regulatory effect. In particular, neutrophil-derived LTB4 controls L. amazonensis killing, degranulation, and reactive oxygen species production. In addition, L. amazonensis infection induces an early increase in Toll-like receptor 2 expression, which facilitates parasite internalization. Nuclear factor kappa B (NFkB) pathway activation represents a required upstream event for L. amazonensis–induced LTB4 synthesis. These leishmanicidal mechanisms mediated by neutrophil-derived LTB4 act through activation of its receptor, B leukotriene receptor 1 (BLT1).
Keywords: Leishmania amazonensis, LPG, human neutrophils, leukotriene B4, TLR2; NFkB
Leishmaniasis represents a group of diseases that are a health problem in 90 countries, with 2 million new cases per year [1]. Leishmania amazonensis belongs to the Leishmania mexicana complex, which comprises species of cutaneous forms in the New World. In South America, L. amazonensis can cause severe cutaneous leishmaniasis that is characterized by multiple nodules, known as diffuse cutaneous leishmaniasis [2].
Leishmania sp. interact with and infect different cell types [3]. Studies in mice showed that neutrophils are predominantly recruited and infected at early stages of infection in the skin [4]. Intravital imaging provides a visual demonstration of the rapidity of infection, with neutrophils descending on Leishmania major after transmission [5]. The engulfment of L. major by human neutrophils prevents fusion with primary and tertiary granules, which contain the machinery necessary for reactive oxygen species (ROS) generation and acidification, which are critical processes for parasite survival [6].
The major function of neutrophils is phagocytosis and killing of microorganisms through ROS generation and release of granule enzymes [7]. As neutrophils proceed through the endothelium, tertiary granules are mobilized and metalloproteases are released, which help neutrophils traverse the basement membrane and extracellular matrix [8]. At the inflammatory site, complete activation of neutrophils prompts the initiation of the oxidative burst and the mobilization of primary granules, which contain myeloperoxidase (MPO), a critical enzyme for the oxidative burst [9].
Lipid mediators are important players during the early immune response against Leishmania [10, 11] and contribute to parasite establishment or destruction. Products derived from 5-lipoxygenase (5-LO) are released during the infection of mouse macrophages by Leishmania donovani [12] and L. amazonensis, and leishmanicidal activity induced by leukotriene B4 (LTB4) in mouse macrophages is dependent on nitric oxide production [10]. Signaling through LTB4 induces leukocyte accumulation [13], microbial ingestion [14], and killing [15]. LTB4 also plays a critical role in human neutrophil migration induced by Toll-like receptor (TLR) stimulation [16]. TLR ligands prime LTB4 production and neutrophil migration [17], and the LTB4 receptor, B leukotriene receptor 1 (BLT1), is responsible for mediating these effects [18].
Although the effect of LTB4 on the leishmanicidal activity of macrophages was previously described, this effect in human neutrophils, which is the major source of this lipid mediator, has not been addressed. Therefore, here, we characterize the profile and function of human neutrophils infected with L. amazonensis. We also investigate the role of TLR and BLT1 signaling in LTB4 production, including the pathways involved and their role in parasite control.
METHODS
Ethics
Blood sample collection from subjects was performed after obtaining informed consent, in accordance with the Declaration of Helsinki, and approval from the ethical review board.
Neutrophil Culture and Infection
Human blood was obtained from healthy donors at the Hemocentro do Estado da Bahia, BA, Brazil. Neutrophils were isolated by centrifugation using Polymorphprep medium according to the manufacturer's instructions (AXIS-SHIELD). Polymorphonuclear cells were collected and washed 3 times at 4°C at 200 g. These cells were then labeled with human anti-CD16 antibody and analyzed by flow cytometry (see Supplementary Figure 1). Neutrophils were infected or not with L. amazonensis–Green fluorescent protein (GFP) (MHOM/BR/87/BA125, a stable transfected line expressing GFP, at a 1 cell to 10 L. amazonensis ratio) or its lipophosphoglycan (LPG; 5 µg/mL) for 3 hours. The infection rate was evaluated by optical microscopy after hematoxylin-eosin staining or by flow cytometry. Representative images were obtained using optical or confocal microscopy (see Supplementary Figure 2) after diamidino-2-phenylindole staining (Vector Laboratories).
Parasite Culture and Viability
Leishmania amazonensis metacyclic promastigotes were obtained from stationary-phase cultures (5–7 days). After 3 hours of culture with neutrophils, cells were washed twice for 5 minutes at 100 g and fed with supplemented Schneider's medium. The cells were then cultured at 23°C for an additional 24 hours. The relative intracellular load of L. amazonensis was measured by assessing the number of extracellular motile promastigotes produced [19]. The profiles of relative parasite loads closely followed the microscopic assessment of the number of internalized parasites per 100 neutrophils and the percentage of infected cells.
LPG Extraction and Purification
LPG was extracted from stationary promastigotes in solvent E (water/ethanol/diethyl ether/pyridine/ammonium hydroxide; 15:15:5:1:0.017). The extract was dried by nitrogen gas evaporation, resuspended in 0.1 N acetic acid/0.1 M sodium chloride, and applied to a column of phenyl-Sepharose (2 mL). LPG was eluted using solvent E [20]. The purity of LPG was previously tested using Chinese hamster ovary cells transfected with TLR2 or TLR4 and CD25 as a reporter protein [21].
Antibodies and Reagents
Single-cell suspensions were stained with phycoerythrin-conjugated CD11b, CD18, CD62L, CD16, TLR2, TLR4, and isotype control antibodies (BD Biosciences). Dihydroethidium was used to detect ROS (10 µM; Invitrogen). In some cases, neutrophils were treated for 15 minutes with inhibitors and washed twice (5 minutes at 100 g) prior to infection. These inhibitors included Zileuton (1 µM, 10 µM, and 100 µM; Cayman), tissue inhibitor of metalloproteinase 1 (TIMP-1; 30 ng/mL; Calbiochem), anti-MPO (1 µg/mL; R&D), antineutrophil elastase (anti-NE; 50 µg/mL; Calbiochem), anti-TLR2 and anti-TLR4 (100 µg/mL; InvivoGen), PI3k inhibitor (LY294002; 10 µM; Cayman), extracellular signal-regulated kinase (ERK) kinase inhibitor (PD98059; 50 µM; Promega), protein kinase C (PKC) inhibitor bisindolylmaleimide (BIS; 20 nM; Cell Signaling Technology), five lypoxygenase activating protein (FLAP) inhibitor MK886 (10 µM; Cayman), I kappa B alpha kinase phosphorylation inhibitor 3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile (BAY)-11-7082 (10 µM; Calbiochem), BLT1 antagonist CP105969 (100 nM, 1 µM, and 10 µM; Pfizer), and IkB kinase complex inhibitor Wedelolactone (80 µM; Sigma). The concentrations used were based on previous studies [22]. For add-back experiments, exogenous LTB4 (10 pg/mL and 50 pg/mL; Cayman) was added to the culture 30 minutes prior to infection, according to the method described in previous studies [15]. These concentrations were used based on the level of LTB4 production by resting and infected neutrophils. Control cells were treated with medium containing the vehicle dimethyl sulfoxide (DMSO; ACROS).
Measurement of Mediator Production
After 3 hours of culture, the supernatants were collected and immediately used to measure MPO and NE activity following a previously published protocol [19]. After 10–20 minutes, the reaction was stopped with 50 μL 8 N sulfuric acid. MPO activity was measured by reading the absorbance at 492 nm. For NE activity, the absorbance was measured at 410 nm, using a dose curve of serial dilutions of purified human NE starting at 1 U/mL. LTB4 and prostaglandin E2 were measured using enzyme-linked immunoassay kits (Cayman). Tumor necrosis factor-alpha (TNF-α) was measured by sandwich enzyme-linked immunosorbent assay (BD Bioscience).
Statistical Analysis
Data were analyzed using GraphPad Prism 5.0 software. The results were analyzed using the Mann–Whitney t test or Kruskal–Wallis test with Dunns post test and are expressed as median values.
RESULTS
L. amazonensis Infection Induces the Expression of Surface Markers and ROS Production in Human Neutrophils
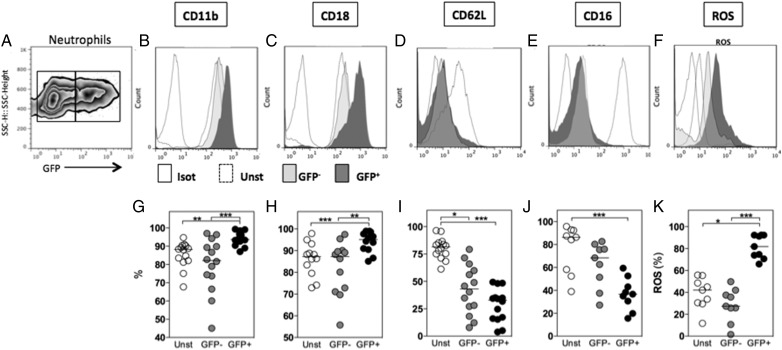
Human neutrophils were infected with L. amazonensis–GFP; 3 hours later, the infection rate was evaluated. No differences were observed in the frequency of infected cells as evaluated by optical microscopy and flow cytometry (see Supplementary Figure 2A). Cells cultured in medium alone (resting neutrophils) were termed “unstimulated.” Cells cultured with Leishmania could be infected or not. GFP− cells were cultured with Leishmania–GFP but were not fluorescent by flow cytometry analysis. GFP− cells were assumed to be uninfected, whereas GFP+ cells were considered infected (Figure 1A). The mean fluorescence intensity (MFI) and the frequency of cells expressing surface markers were compared among unstimulated, GFP– (bystander), and GFP+ (infected) cells. The MFI of CD11b (Figure 1B) and CD18 (Figure 1C) was upregulated in GFP+ cells, and similar results were obtained for the percentage of cells expressing these markers (Figure 1G and 1H). In comparison to unstimulated cells, L. amazonensis infection decreased the MFI and frequency of GFP+ cells expressing CD62L (Figure 1D and 1I) and CD16 (Figure 1E and 1J), although no differences were observed between GFP+ and GFP− cells, indicating that bystander cells presented a similar degree of activation. Cells were also evaluated for ROS production, and the MFI was increased in GFP+ cells (Figure 1F). Approximately 80% of GFP+ cells produced ROS compared with 38% of unstimulated or GFP− cells (Figure 1K). These results indicate that L. amazonensis infection induces the activation of human neutrophils.
Figure 1.
The interaction between human neutrophils and Leishmania amazonensis induces the differential expression of surface markers and reactive oxygen species (ROS) production. Human neutrophils were cultured alone (unstimulated) or infected with L. amazonensis–Green fluorescent protein (GFP) (1 neutrophil to 10 L. amazonensis) for 3 hours. The mean intensity fluorescence (B–F) and the frequency of cells expressing surface markers (G–K) were compared among unstimulated, noninfected bystander (GFP−), and infected (GFP+) cells (A). The expression of surface markers, such as CD11b (B and G), CD18 (C and H), CD62L (D and I), and CD16 (E and J), as well as ROS production (F and K) were evaluated by flow cytometry. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Lipid Mediator Production and Neutrophil Degranulation Are Induced Following Exposure to L. amazonensis or LPG
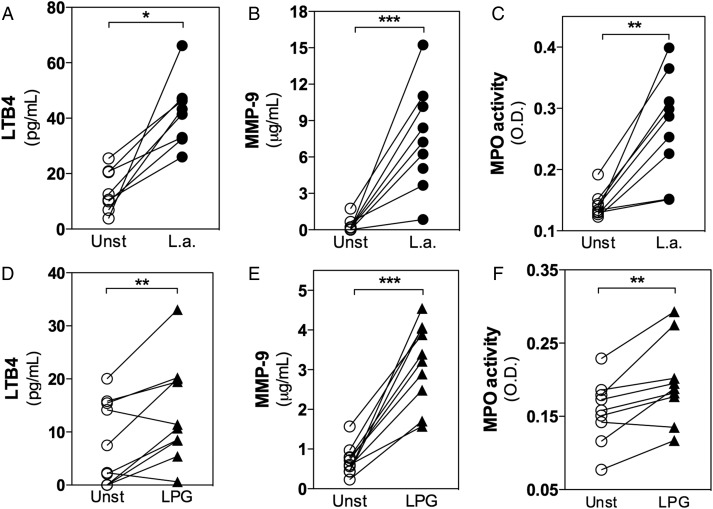
Following L. amazonensis infection, a significant increase in LTB4 production by human neutrophils was observed (Figure 2A). However, no differences in the production of TNF-α or prostaglandin E2 were observed between unstimulated and infected neutrophils (data not shown). To evaluate degranulation, the metaloprotease-9 (MMP-9) concentration was measured in order to evaluate the release of tertiary granules. The activity of enzymes present in primary granules, such as MPO and NE, was also evaluated. The levels of these enzymes were significantly increased in supernatants from infected neutrophils, indicating that L. amazonensis infection induced the release of tertiary (Figure 2B) and primary granules (Figure 2C).
Figure 2.
Leishmania amazonensis infection or lipophosphoglycan (LPG) triggers leukotriene B4 (LTB4) production and the release of granule content from human neutrophils. Neutrophils were cultured alone (unstimulated [unst]), infected with L. amazonensis–GFP (1 neutrophil to 10 L. amazonensis; A–C), or treated with LPG (5 μg/mL) for 3 hours (D–F). Supernatants from the cultures were used to measure LTB4 production by competition enzyme-linked immunosorbent assay (ELISA; A and D) and metaloprotease-9 (MMP-9) by sandwich ELISA (B and E). Myeloperoxidase (MPO) was evaluated according to its activity for a specific substrate (C and F). *P ≤ .05; **P ≤ .01; ***P ≤ .001.
LPG represents a main virulence factor associated with Leishmania infection [21], and neutrophil treatment with L. amazonensis LPG induced a response that was similar to that observed for the whole parasite. LTB4 production (Figure 2D), MMP-9 release (Figure 2E), and MPO activity (Figure 2F) were also increased in neutrophils treated with LPG. These data support the activation of human neutrophils by L. amazonensis, as observed through the expression of surface molecules, ROS production (Figure 1), and degranulation (Figure 2).
TLR2 Plays an Active Role in L. amazonensis Internalization by Human Neutrophils
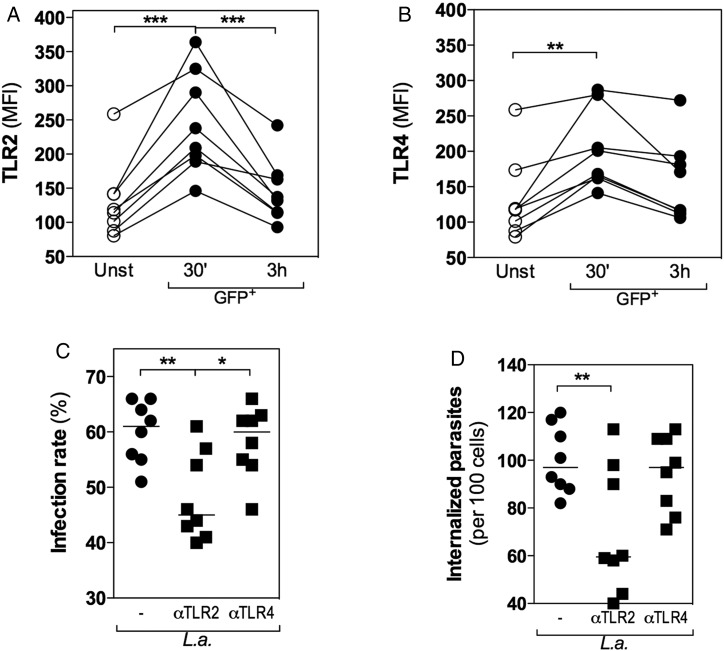
TLRs detect pathogen-associated molecular patterns, such as lipoproteins, from viruses, bacteria, fungi, and parasites [23]. Since L. amazonensis and LPG induced similar responses in neutrophils (Figure 2), the expression of TLRs was evaluated. There was an increase in the expression of TLR2 (Figure 3A) and TLR4 (Figure 3B) at 30 minutes post-infection in GFP+ cells. However, at 3 hours post-infection, only TLR2 expression was significantly reduced to a basal level (Figure 3A). These data suggest that TLR2 is internalized along with L. amazonensis and that this process may be mediated by LPG recognition. In contrast, no difference in TLR4 expression was observed between 30 minutes and 3 hours post-infection (Figure 3B), suggesting that TLR4 was not involved.
Figure 3.
Toll-like receptor 2 (TLR2) plays an active role in Leishmania amazonensis internalization by human neutrophils. Neutrophils were cultured alone (unstimulated [unst]) or treated with neutralizing antibodies against TLR2 and TLR4 (100 µg/mL) for 15 minutes prior to infection with L. amazonensis–GFP (1 neutrophil to 10 L. amazonensis). The kinetics of TLR2 (A) and TLR4 (B) expression were compared by flow cytometry for unstimulated and GFP+ cells after 30 minutes and 3 hours of infection. The percentage of infected cells (C) and the number of internalized parasites (D) were quantified by optical microscopy after 3 hours of infection. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
To confirm whether TLR2 was internalized with the parasite, we quantified the infection rate and the number of internalized parasites in neutrophils treated with neutralizing antibodies against TLR2 and TLR4. In the absence of TLR2, the infection rate (Figure 3C) and the number of internalized parasites (Figure 3D) were reduced, whereas no differences were observed following treatment with anti-TLR4 antibody (Figure 3C and 3D).
Inhibition of 5-LO Increases Human Neutrophil Susceptibility to L. amazonensis Infection
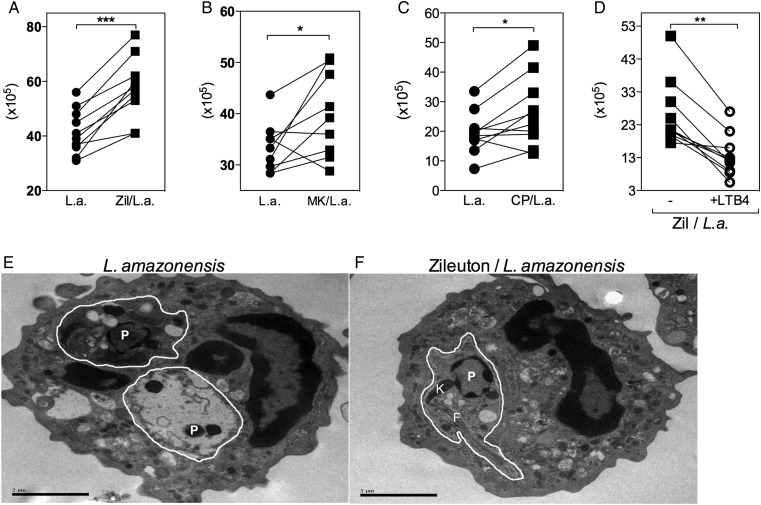
The role of lipid mediators in the control of L. amazonensis was investigated by treating neutrophils with pharmacological inhibitors of 5-LO prior to infection. The following concentrations of Zileuton, a 5-LO specific inhibitor, were tested: 1 µM, 10 µM, and 100 µM. Treatment with Zileuton at 1 µM did not produce any effect on parasite viability in infected neutrophils. The number of live Leishmania 24 hours after treatment was similar to that of control cells cultured in the absence of Zileuton. However, treatment of infected neutrophils with 10 μM and 100 μM Zileuton significantly increased parasite viability with similar magnitude (data not shown). Based on these data, 10 µM was chosen as the optimal concentration to evaluate 5-LO inhibition. Zileuton (Figure 4A) and MK886, a FLAP inhibitor (Figure 4B), induced a significant increase in parasite viability, and similar results were obtained with MMP-9 and MPO inhibitors (TIMP-1 and αMPO, respectively). However, the NE inhibitor had no effect on parasite viability (see Supplementary Figure 3).
Figure 4.
Inhibition of 5-lipoxygenase increases human neutrophil susceptibility to Leishmania amazonensis infection through the high-affinity leukotriene B4 (LTB4) receptor B leukotriene receptor 1 (BLT1). Human neutrophils were treated with 10 µM Zileuton (A), 10 µM MK886 (B), or 100 nM CP105969 (C) for 15 minutes prior to infection with L. amazonensis–GFP (1 neutrophil to 10 L. amazonensis). Exogenous LTB4 was added to selected cultures of Zileuton-treated neutrophils (D). After 3 hours of infection, cells were washed, fed with supplemented Schneider's medium, and cultured at 23°C for an additional 24 hours. Parasite viability was measured by assessing the number of extracellular motile promastigotes produced. Transmission electron microscopy was used to investigate ultrastructural morphological alterations in infected (E) and Zileuton-treated infected neutrophils (F). White lines delimit parasitophorous vacuoles. Parasites (P), kinetoplastids (K), and flagella (F) are indicated. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Effects of LTB4 Are Mediated Through Its High-Affinity Receptor BLT1
To evaluate the role of LTB4/BLT1 signaling in the control of L. amazonensis infection, an antagonist of BLT1, CP105696 (CP), was used. The increased parasite viability observed following CP treatment (Figure 4C) confirmed the importance of this pathway for parasite killing. Furthermore, the addition of exogenous LTB4 to cultures containing Zileuton-treated neutrophils restored their ability to control L. amazonensis (Figure 4D).
By transmission electron microscopy, unstimulated neutrophils presented a normal shape, with cytosolic granules and multilobulated nuclei (data not shown). In Figure 4E, the Leishmania inside the parasitophorous vacules (delimited by white lines) presents ultrastructural abnormalities, such as condensed chromatin, vacuole-like structures, and poorly preserved cytoplasm and organelles, indicating parasite killing. Conversely, in Zileuton-treated infected neutrophils, the parasites appeared viable, with intact kinetoplastids and flagella (Figure 4F), suggesting that leukotrienes play a significant role in Leishmania elimination.
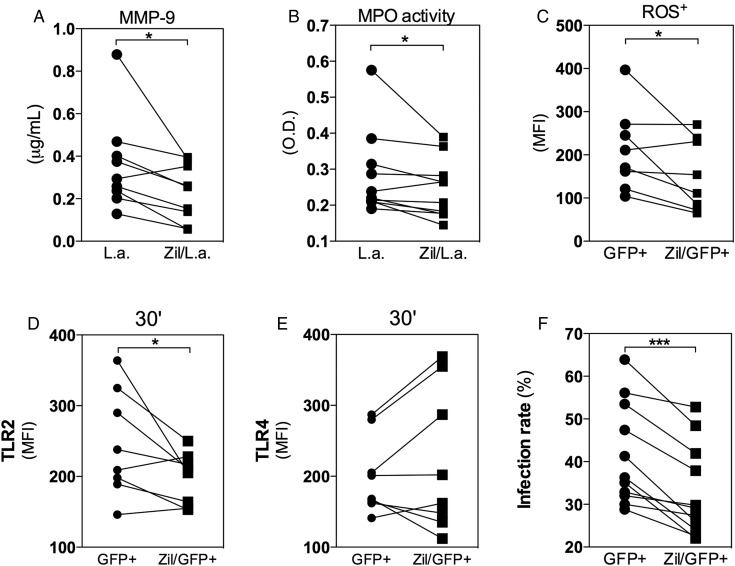
Degranulation and TLR2 Expression in Human Neutrophils Infected With L. amazonensis Are Partially Dependent on LTB4
LTB4 can lead to neutrophil degranulation [18], and significant reductions in MMP-9 concentration and MPO activity were observed when cells were treated with Zileuton (Figure 5A and 5B). These results suggest that LTB4 production in neutrophils is induced following L. amazonensis infection, leading to degranulation. ROS represent a well-established mediator of leishmanicidal mechanisms, and their level of production was also reduced in GFP+ cells pretreated with Zileuton (Figure 5C). These results reinforce the essential role of leukotrienes in the early events of degranulation cascade, including the release and activity of several mediators crucial for parasite control.
Figure 5.
Degranulation and Toll-like receptor 2 (TLR2) expression in human neutrophils infected with Leishmania amazonensis are dependent on leukotriene B4. Human neutrophils were treated with Zileuton for 15 minutes prior to infection with L. amazonensis–GFP (1 neutrophil to 10 L. amazonensis). After 3 hours of infection, the supernatants were collected and evaluated for degranulation by measuring metaloprotease-9 (MMP-9) release (A) and myeloperoxidase (MPO) activity (B). Cells were harvested for flow cytometry analysis of reactive oxygen species production (C) and TLR2 (D) and TLR4 (E) expression as well as the infection rate (F). *P ≤ .05; **P ≤ .01; ***P ≤ .001.
The expression of TLR2 and TLR4 in GFP+ neutrophils treated with Zileuton was also compared, and this treatment significantly reduced the expression of TLR2 (Figure 5D), but not TLR4 (Figure 5E), after 30 minutes in GFP+ cells. Furthermore, our results showed a significant reduction in the internalization of L. amazonensis by Zileuton-treated neutrophils (Figure 5F). These data reinforce the role of LTB4 in TLR expression and its recognition of pathogen-associated molecules. In addition, parasite killing was increased when infected neutrophils were treated with Pam3CSK4 (a TLR2 agonist) during the infection (data not shown), indicating that further activation of TLR signaling likely stimulates the mechanisms involved in parasite control.
Intracellular Signaling Pathways Regulate LTB4-Mediated Leishmanicidal Activity in Human Neutrophils
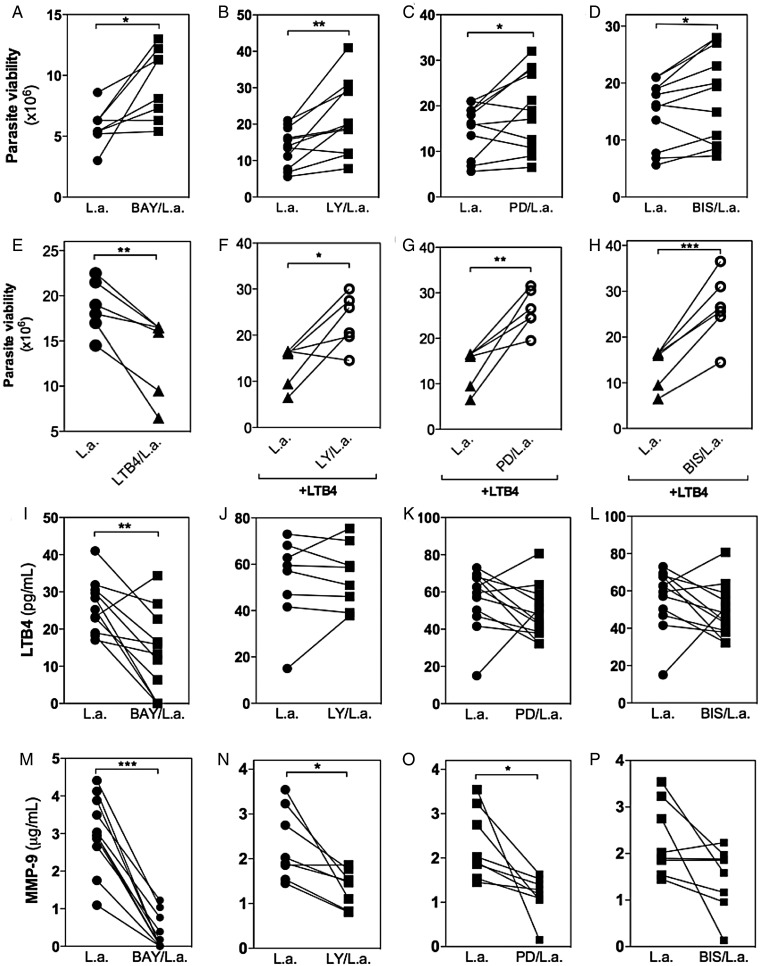
The responses initiated by TLRs culminate in Nuclear factor kappa B (NFkB) and mitogen-activated protein kinase (MAPK) activation [24]. BAY, a specific inhibitor of NFkB, which prevents the phosphorylation of IkB, significantly increased the number of viable parasites, suggesting a reduction in leishmanicidal activity (Figure 6A). Similar results were obtained with Wedelolactone, a nonspecific NFkB inhibitor (data not shown). The number of viable parasites was also increased in cultures of infected neutrophils treated with LY294002 (LY), a PI3k inhibitor (Figure 6B); PD98059 (PD), an ERK inhibitor (Figure 6C); and BIS, an adenosine triphosphate-competitive PKC inhibitor (Figure 6D). In contrast, incubation of neutrophils with the solvent DMSO did not alter parasite viability (data not shown).
Figure 6.
Signaling pathways involved in Leishmania amazonensis killing, leukotriene B4 (LTB4) production, and degranulation in human neutrophils. Neutrophils were treated or not with 3-[(4-methylphenyl)sulfonyl]-(2E)-propenenitrile (BAY) (10 µM), LY294002 (LY; 10 µM), PD98059 (PD; 50 µM), or bisindolylmaleimide (BIS; 20 nM) for 15 minutes prior to infection with L. amazonensis–GFP (1 neutrophil to 10 L. amazonensis). In some conditions, exogenous LTB4 (10 pg/mL) was added back in the culture (F–H). After 3 hours of infection, the cells were washed, fed with supplemented Schneider's medium, and cultured at 23°C for an additional 24 hours. Parasite viability was measured by assessing the number of extracellular motile promastigotes produced (A–H). LTB4 production (I–L) and metaloprotease-9 (MMP-9) release (M–P) were measured by enzyme-linked immunosorbent assay in the culture supernatants. *P ≤ .05; **P ≤ .01; ***P ≤ .001.
Treatment of neutrophils with exogenous LTB4 prior to infection significantly reduced the number of viable parasites (Figure 6E). To characterize the signaling pathways induced by LTB4, we pretreated infected neutrophils with different kinase inhibitors and then added back LTB4 prior to infection. PI3k (Figure 6F), ERK (Figure 6G), and PKC (Figure 6H) were each important for L. amazonensis killing, as the addition of LTB4 did not reverse the effect observed using only the kinase inhibitors.
Inhibition of NFkB with the specific inhibitor BAY (Figure 6I) or the unspecific inhibitor Wedelolactone (data not shown) reduced LTB4 production. However, the kinesis inhibitors LY (Figure 6J), PD (Figure 6K), and BIS (Figure 6L) did not alter LTB4 production. These results suggest that NFkB activation plays a relevant role in lipid mediator production. Moreover, it seems that LTB4 contributes to parasite destruction through PI3k, ERK, and PKC signaling.
Additionally, MMP-9 release was reduced following inhibition of NFkB (Figure 6M), PI3K (Figure 6N), ERK (Figure 6O), and BIS (Figure 6P). These data indicate that LTB4 signaling can lead to degranulation mediated by PI3k, ERK, and PKC signaling, as previously demonstrated [22]. Thus, our results highlight the participation of NFkB in LTB4 production and degranulation, which are both crucial for L. amazonensis control.
DISCUSSION
This study reveals the essential role played by neutrophil-derived LTB4 in the control of L. amazonensis infection. In particular, the production of LTB4 by infected neutrophils and its paracrine/autocrine effects mediate degranulation and parasite killing through the PI3k, ERK, and PKC signaling pathways. In addition, L. amazonensis and LPG interact with TLR2, which activates NFkB signaling. This transcription factor seems to be involved in LTB4 production and the leishmanicidal activity of neutrophils.
The interaction between L. amazonensis and human neutrophils induces cellular activation, characterized by an increase in Mac-1 and a decrease in CD62L and CD16 expression at the cell surface. Our results are similar to data previously published in the literature. Resting neutrophils obtained from the peripheral blood of healthy donors were shown to express high levels of Mac-1, which was further upregulated upon stimulation [25]. In addition, ROS production also indicates the activated phenotype of infected cells, and it has been shown that ROS are highly toxic and may be responsible for parasite destruction [26]. The activation of human neutrophils by LPS induced similar alterations in surface markers and ROS production [27].
Leishmania amazonensis–infected neutrophils produced LTB4, which led to degranulation, MMP-9 release, and MPO activity. It was previously reported that LTB4 can trigger degranulation [28] and the killing of microorganisms [10, 29, 30]. In fact, treatment of infected neutrophils with Zileuton led to an increase in L. amazonensis viability and a decrease in degranulation. The inhibition of parasite killing was confirmed by the ultrastructure of Zileuton-treated L. amazonensis–infected neutrophils, showing the crucial role of LTB4 in the control of infection.
The interaction between Leishmania LPG and host cells has been extensively described [31]. We observed that LPG also induced LTB4 production and degranulation by human neutrophils. Lipoproteins and peptidoglycans, including LPG, are recognized by TLR2 [32]. Our results showed that L. amazonensis–infected neutrophils presented an increase in TLR2 expression at an early time post-infection, whereas after 3 hours, there was a significant reduction. Although the role of TLRs in the engulfment of pathogens remains controversial, it has been reported that TLR2 enhances phagocytosis of Listeria monocytogenes by macrophages [33] and plays an important role in phagocytosis of Streptococcus pneumonia by mouse neutrophils [34]. Furthermore, it was recently demonstrated that TLR2 could mediate the phagocytosis of Staphylococcus aureus in a mouse macrophage cell line through the MAPK signaling pathway [35]. Although the infection rate and number of internalized parasites were reduced by anti-TLR2–neutralizing antibody, based on our results, we cannot unequivocally state that TLR2 is a phagocytic receptor for Leishmania. Moreover, signaling through TLRs activates different pathways that mediate phagocytosis [24]. In this way, the conclusion from our findings is that TLR2 could participate in Leishmania internalization through PI3k and/or MAPK activation or be internalized with other phagocytic receptors. Nevertheless, we cannot exclude the possibility that during Leishmania phagocytosis by neutrophils, TLR2 may be included in a lipid raft that contains different phagocytic receptors already described for Leishmania [36]. Future studies should be conducted to address this issue.
Previous studies have shown an association between TLR signaling and lipid mediators [16, 37]. Our results further indicate that this interaction may lead to NFkB activation, possibly through TLR2, and LTB4 production. The mechanisms involved in the production of eicosanoids following TLR ligand stimulation remain incompletely defined and may involve various processes. LPS increases arachidonic acid availability and induces the expression of enzymes responsible for eicosanoid production [38, 39]. Similarly, a β-glucan from the Histoplasma capsulatum cell wall induces lipid body formation through TLR2 [40]. Based on our findings, we suggest the existence of a positive loop between NFkB and 5-LO. In particular, TLR2 signaling culminates in NFkB activation, which induces expression of the enzymes responsible for eicosanoid production, such as 5-LO. Therefore, this increased production of 5-LO may produce higher levels of LTB4 that, via a paracrine/autocrine mechanism, may amplify NFkB activation.
Furthermore, the synthesis of LTB4 is necessary for optimal MyD88 expression and NFkB activation in mouse macrophages [41]. Additionally, treatment of human neutrophils with LTB4 in vitro potentiates the TLR response [42]. Similar results were also obtained for the pattern recognition receptor dectin-1, a C-type lectin receptor that recognizes β-glucans [43] and whose expression is controlled by LTB4 in mouse macrophages [44].
LTB4 is the agonist for 2 membrane G protein–coupled receptors, BLT1 and BLT2 [45]. Our use of a BLT1 antagonist confirmed its role in parasite killing by human neutrophils. Similar results were obtained with mouse macrophages infected with L. amazonensis and treated with antagonists for BLT1 receptor [10]. However, an antagonist for the cysteinyl leukotriene receptors (CysTL1 and CysTL2) demonstrated no effect on the leishmanicidal activity of macrophages [10].
The role of PI3K, MEK, and PKC signaling in neutrophil degranulation and LTB4 production during L. amazonensis infection was demonstrated in the current study. Degranulation by human neutrophils and eosinophils was previously shown to be PI3k dependent [46]. Our results showed that LTB4 production was not affected by treatment with kinase inhibitors. However, a decrease in MMP-9 release and MPO activity was observed along with an increase in parasite viability. Together, these data imply a role for these enzymes downstream of LTB4 production. These factors may also be necessary for neutrophil degranulation, as previously shown [47]. The role of LTB4 as an activator of MAPK family members has been established [48], and PKC is directly involved in multiple steps of TLR signaling pathways [49].
In this study, we uncovered a novel role for neutrophil-derived LTB4. Our results indicate that L. amazonensis may be recognized by TLR2, which stimulates neutrophil activation. This activation then leads to neutrophil degranulation via a LTB4-dependent autocrine/paracrine mechanism. NFkB activation is also involved in LTB4 production, and its effects, via BLT1 receptor, contribute to parasite killing. Furthermore, these inflammatory mechanisms may also be responsible for the resolution of other infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Manoel Barral-Netto and Dr Marcello Bozza for helpful discussions; Adriana Rangel, Cláudio Figueira, and Lúcia Moreno from the electron microscopy facility at Gonçalo Moniz Research Center (CPqGM)–Oswaldo Cruz Foundation (FIOCRUZ); and Bruno Solano for help with confocal microscopy at São Rafael Hospital.
Financial support. This work was supported by the National Council of Research (CNPq–Universal) grants 476926/2011-4 for C. B. and 478480/2013-0 for V. M. B. N. M. T., T. A. S., L. A., and P. M. N. receive fellowship support from CNPq. U. G. L., R. P. S., P. T. B., C. B. M., V. M. B., and C. B. are senior investigators from CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi: 10.1038/nrmicro981. [DOI] [PubMed] [Google Scholar]
- 2.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–9. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 3.Charmoy M, Auderset F, Allenbach C, Tacchini-Cottier F. The prominent role of neutrophils during the initial phase of infection by Leishmania parasites. J Biomed Biotechnol. 2010;2010:719361. doi: 10.1155/2010/719361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tacchini-Cottier F, Zweifel C, Belkaid Y, et al. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:2628–36. doi: 10.4049/jimmunol.165.5.2628. [DOI] [PubMed] [Google Scholar]
- 5.Peters NC, Egen JG, Secundino N, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–4. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mollinedo F, Janssen H, de la Iglesia-Vicente J, Villa-Pulgarin JA, Calafat J. Selective fusion of azurophilic granules with Leishmania-containing phagosomes in human neutrophils. J Biol Chem. 2010;285:34528–36. doi: 10.1074/jbc.M110.125302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy AD, DeLeo FR. Neutrophil apoptosis and the resolution of infection. Immunol Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 8.Delclaux C, Delacourt C, D'Ortho MP, Boyer V, Lafuma C, Harf A. Role of gelatinase B and elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol. 1996;14:288–95. doi: 10.1165/ajrcmb.14.3.8845180. [DOI] [PubMed] [Google Scholar]
- 9.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–70. doi: 10.1016/j.immuni.2010.11.011. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 10.Serezani CH, Perrela JH, Russo M, Peters-Golden M, Jancar S. Leukotrienes are essential for the control of Leishmania amazonensis infection and contribute to strain variation in susceptibility. J Immunol. 2006;177:3201–8. doi: 10.4049/jimmunol.177.5.3201. [DOI] [PubMed] [Google Scholar]
- 11.Araújo-Santos T, Prates DB, Andrade BB, et al. Lutzomyia longipalpis saliva triggers lipid body formation and prostaglandin E(2) production in murine macrophages. PLoS Negl Trop Dis. 2010;4:e873. doi: 10.1371/journal.pntd.0000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiner NE, Malemud CJ. Arachidonic acid metabolism by murine peritoneal macrophages infected with Leishmania donovani: in vitro evidence for parasite-induced alterations in cyclooxygenase and lipoxygenase pathways. J Immunol. 1985;134:556–63. [PubMed] [Google Scholar]
- 13.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–4. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 14.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun. 1998;66:5140–6. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serezani CHC, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–75. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefebvre JS, Marleau S, Milot V, et al. Toll-like receptor ligands induce polymorphonuclear leukocyte migration: key roles for leukotriene B4 and platelet-activating factor. FASEB J. 2010;24:637–47. doi: 10.1096/fj.09-135624. [DOI] [PubMed] [Google Scholar]
- 17.Lefebvre JS, Lévesque T, Picard S, et al. Extra domain A of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of Toll-like receptor 4. Arthritis Rheum. 2011;63:1527–33. doi: 10.1002/art.30308. [DOI] [PubMed] [Google Scholar]
- 18.Gaudreault E, Thompson C, Stankova J, Rola-Pleszczynski M. Involvement of BLT1 endocytosis and Yes kinase activation in leukotriene B4-induced neutrophil degranulation. J Immunol. 2005;174:3617–25. doi: 10.4049/jimmunol.174.6.3617. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro-Gomes FL, Moniz-de-Souza MC, Alexandre-Moreira MS, et al. Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. Cell. 2010;179:3988–94. doi: 10.4049/jimmunol.179.6.3988. [DOI] [PubMed] [Google Scholar]
- 20.Coelho-Finamore JM, Freitas VC, Assis RR, et al. Leishmania infantum: Lipophosphoglycan intraspecific variation and interaction with vertebrate and invertebrate hosts. Int J Parasitol. 2011;41:333–42. doi: 10.1016/j.ijpara.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Ibraim IC, Assis RR, de, Pessoa NL, et al. Two biochemically distinct lipophosphoglycans from Leishmania braziliensis and Leishmania infantum trigger different innate immune responses in murine macrophages. Parasit Vectors. 2013;6:54. doi: 10.1186/1756-3305-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos MRM, Serezani CH, Peters-Golden M, Jancar S. Differential kinase requirement for enhancement of Fc gammaR-mediated phagocytosis in alveolar macrophages by leukotriene B4 vs. D4. Mol Immunol. 2009;46:1204–11. doi: 10.1016/j.molimm.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Akira S, Hoshino K, Kaisho T. The role of Toll-like receptors and MyD88 in innate immune responses. J Endotoxin Res. 2000;6:383–7. [PubMed] [Google Scholar]
- 24.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–50. doi: 10.1016/j.immuni.2011.05.006. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 25.Carrigan SO, Weppler AL, Issekutz AC, Stadnyk AW. Neutrophil differentiated HL-60 cells model Mac-1 (CD11b/CD18)-independent neutrophil transepithelial migration. Immunology. 2005;115:108–17. doi: 10.1111/j.1365-2567.2005.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novais FO, Nguyen BT, Daniel P, Carvalho LP, Nelson D. Human classical monocytes control the intracellular stage of Leishmania braziliensis by reactive oxygen species. J Infect Dis. 2014;209:1288–96. doi: 10.1093/infdis/jiu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillay J, Kamp VM, Van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lärfars G, Lantoine F, Devynck MA, Palmblad J, Gyllenhammar H. Activation of nitric oxide release and oxidative metabolism by leukotrienes B4, C4, and D4 in human polymorphonuclear leukocytes. Blood. 1999;93:1399–405. [PubMed] [Google Scholar]
- 29.Gaudreault E, Gosselin J. Leukotriene B4-mediated release of antimicrobial peptides against cytomegalovirus is BLT1 dependent. Viral Immunol. 2007;20:407–20. doi: 10.1089/vim.2006.0099. [DOI] [PubMed] [Google Scholar]
- 30.Secatto A, Rodrigues LC, Serezani CH, et al. 5-Lipoxygenase deficiency impairs innate and adaptive immune responses during fungal infection. PLoS One. 2012;7:e31701. doi: 10.1371/journal.pone.0031701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Assis RR, Ibraim IC, Nogueira PM, Soares RP, Turco SJ. Glycoconjugates in New World species of Leishmania: Polymorphisms in lipophosphoglycan and glycoinositolphospholipids and interaction with hosts. Biochim Biophys Acta. 2012;1820:1354–65. doi: 10.1016/j.bbagen.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Kawamura I, Nomura T, et al. Toll-like receptor 2- and MyD88-dependent phosphatidylinositol 3-kinase and Rac1 activation facilitates the phagocytosis of Listeria monocytogenes by murine macrophages. Infect Immun. 2010;78:2857–67. doi: 10.1128/IAI.01138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letiembre M, Echchannaoui H, Ferracin F, et al. Toll-like receptor 2 deficiency delays pneumococcal phagocytosis and impairs oxidative killing by granulocytes. Infect Immun. 2005;73:8397. doi: 10.1128/IAI.73.12.8397-8401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang L, Wu H-M, Ding P-S, Liu R-Y. TLR2 mediates phagocytosis and autophagy through JNK signaling pathway in Staphylococcus aureus-stimulated RAW264.7 cells. Cell Signal. 2014;26:806–14. doi: 10.1016/j.cellsig.2013.12.016. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 36.Ferracini M, Martins JO, Campos MR, Anger DB, Jancar S. Impaired phagocytosis by alveolar macrophages from diabetic rats is related to the deficient coupling of LTs to the Fc gamma R signaling cascade. Mol Immunol. 2010;47:1974–80. doi: 10.1016/j.molimm.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Kogut MH, He H, Genovese KJ. Bacterial toll-like receptor agonists induce sequential NF-κB-mediated leukotriene B(4) and prostaglandin E(2) production in chicken heterophils. Vet Immunol Immunopathol. 2012;145:159–70. doi: 10.1016/j.vetimm.2011.11.003. Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- 38.Lin WW. Priming effects of lipopolysaccharide on UTP-induced arachidonic acid release in RAW 264.7 macrophages. Eur J Pharmacol. 1997;321:121–7. doi: 10.1016/s0014-2999(96)00930-2. [DOI] [PubMed] [Google Scholar]
- 39.Surette ME, Dallaire N, Jean N, Picard S, Borgeat P. Mechanisms of the priming effect of lipopolysaccharides on the biosynthesis of leukotriene B4 in chemotactic peptide-stimulated human neutrophils. FASEB J. 1998;12:1521–31. doi: 10.1096/fasebj.12.14.1521. [DOI] [PubMed] [Google Scholar]
- 40.Sorgi CA, Secatto A, Fontanari C, et al. Histoplasma capsulatum cell wall {beta}-glucan induces lipid body formation through CD18, TLR2, and dectin-1 receptors: correlation with leukotriene B4 generation and role in HIV-1 infection. J Immunol. 2009;182:4025–35. doi: 10.4049/jimmunol.0801795. [DOI] [PubMed] [Google Scholar]
- 41.Serezani CH, Lewis C, Jancar S, Peters-Golden M. Leukotriene B 4 amplifies NF-κB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J Clin Invest. 2011;121:671–82. doi: 10.1172/JCI43302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaudreault E, Paquet-Bouchard C, Fiola S, et al. TAK1 contributes to the enhanced responsiveness of LTB4-treated neutrophils to Toll-like receptor ligands. Int Immunol. 2012;24:693–704. doi: 10.1093/intimm/dxs074. [DOI] [PubMed] [Google Scholar]
- 43.Brown GD. Macrophage receptors and innate immunity: insights from dectin-1. Novartis Found Symp. 2006;279:114–23. discussion 123–6, 216–9. [PubMed] [Google Scholar]
- 44.Serezani CH, Kane S, Collins L, Morato-Marques M, Osterholzer JJ, Peters-Golden M. Macrophage dectin-1 expression is controlled by leukotriene B4 via a GM-CSF/PU.1 axis. J Immunol. 2012;189:906–15. doi: 10.4049/jimmunol.1200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tryselius Y, Nilsson NE, Kotarsky K, Olde B, Owman C. Cloning and characterization of cDNA encoding a novel human leukotriene B(4) receptor. Biochem Biophys Res Commun. 2000;274:377–82. doi: 10.1006/bbrc.2000.3152. [DOI] [PubMed] [Google Scholar]
- 46.Kämpe M, Lampinen M, Stolt I, Janson C, Stålenheim G, Carlson M. PI3-kinase regulates eosinophil and neutrophil degranulation in patients with allergic rhinitis and allergic asthma irrespective of allergen challenge model. Inflammation. 2012;35:230–9. doi: 10.1007/s10753-011-9309-5. [DOI] [PubMed] [Google Scholar]
- 47.Xiao H, Bai X-H, Wang Y, Kim H, Mak AS, Liu M. MEK/ERK pathway mediates PKC activation-induced recruitment of PKCζ and MMP-9 to podosomes. J Cell Physiol. 2013;228:416–27. doi: 10.1002/jcp.24146. [DOI] [PubMed] [Google Scholar]
- 48.Peters-Golden M, Henderson WR. Leukotrienes. N Engl J Med. 2007;357:1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X, Yang W, Li J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-kappaB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-alpha production in lipopolysaccharide-stimulated rat peritoneal macrophages. J Biol Chem. 2006;281:31337–47. doi: 10.1074/jbc.M602739200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.