Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) has had little improvement in mortality rates in decades. A clearer understanding of the HNSCC tumor microenvironment will aid in finding more effective targeted therapies for this disease. Tumor associated fibroblasts (TAFs) are the largest stromal cellular components of the tumor microenvironment in HNSCC.
Methods
We isolated TAFs from clinical HNSCC cases and propagated in vitro. The effects of TAF secreted paracrine factors on in vitro HNSCC migration, invasion and proliferation was assessed. The effect of TAFs on HNSCC growth and metastases was determined in an orthotopic floor of mouth tumor model.
Results
TAF conditioned media increased HNSCC cell migration, invasion and proliferation. TAFs increased HNSCC tumor growth and metastases in vivo.
Conclusions
TAFs play a major role in increasing tumor growth and metastasis in HNSCC. Targeting the tumor stroma may be important to reduce the rate of HNSCC metastasis.
Keywords: Head and neck cancer, tumor associated fibroblasts, invasion, metastasis, tumor microenvironment
Introduction
Every year 40,000 people are diagnosed with head and neck squamous cell carcinoma (HNSCC) in the United States. HNSCC is associated with severe disease- and treatment-related morbidity, with 5-year survival rates of approximately 50%(1). Locoregional invasion and lymph node metastasis occurs with a high frequency (66%) in HNSCC patients(2,3). Patients with metastatic HNSCC have less than a 20% 5-year survival rate(4). Currently, there are no treatment modalities to prevent tumor invasion and metastasis of HNSCC.
The tumor microenvironment is composed of many cell types, among the most abundant are tumor associated fibroblasts (TAFs)(5). TAFs account for up to 50-70% of tumor volume in several epithelial cancers including pancreatic, gastric and breast(6). It is likely that existing fibroblasts in the area of tumor growth are converted into myofibroblasts, also known as TAFs and CAFs(7). It is also postulated that these TAFs may arise from fibrocytes that circulate in the blood(8,9). Tumor associated fibroblasts (TAFs) are a crucial component of the tumor microenvironment that promote tumor growth and metastasis in several epithelial tumor types(10-16). TAFs exist in close proximity with tumors and co-evolve along with the tumor actively contributing to tumor progression(17).
HNSCC tumors are frequently associated with desmoplastic stromal fibroblasts(18). TAFs have been shown to contribute to increased invasion and migration of oral SCC cells(19) as well as proliferation in a lingual carcinoma cell line(20). The stromal component of oral SCC has also been found to be prognostic of disease mortality(21). We have previously shown that HNSCC derived TAFs can increase HNSCC cell invasion (in vitro) via HGF(22). In order to develop therapeutic strategies to mitigate invasion and metastasis, a better understanding between the tumor and its associated stroma is necessary.
We undertook this study to more fully characterize the effects of TAF secreted factors on HNSCC cells in vitro and the effect of TAFs on HNSCC cells in vivo. We present here the effects of TAF conditioned medium on HNSCC proliferation, invasion and metastasis and the first in vivo evidence of increased local and distant metastasis with TAFs and HNSCC cells compared to HNSCC alone. This is also the first report to our knowledge to demonstrate the bidirectional interaction between TAFs and HNSCC.
Methods
Tissue Culture
HNSCC and tonsil or uvulopalatoplasty explants from cancer-free patients were collected with written consent from patients, using protocols approved by the Institutional Review Boards at the University of Pittsburgh. To establish primary fibroblast cultures, tissue explants were immersed in antibiotic/antimycotic solution for a minimum of 10 minutes and minced under sterile conditions with surgical scissors as previously described(23). Tumor pieces were placed in uncoated plastic tissue culture flasks and allowed to adhere for 2-3 mins. Dulbelcco's Modification of Eagle's Medium (Mediatech Inc., Herndon, VA, USA) supplemented with 10% head-inactivated FBS was then added to the flasks. Flasks were placed at 37°C in a 5% CO2 incubator. Media was replaced the next day and changed once per week subsequently. Fibroblasts grew out of explants that stuck to the bottom of the flasks. Confluent flasks were trypsinized gently without disturbing the tissue explants stuck to the flask. Trypsinized cells were transferred to new flasks and grown out for experiments. All TAFs used for experiments were passaged fewer than 10 times. Due to the finite nature of primary lines (passage <10) TAF lines were used as growth and passage number allowed for experiments and were not screened for any parameters except cell type homogeneity.
NIH3T3 and Cal27 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). UMSCC1 cells were a kind gift from Dr. Thomas E. Carey (University of Michigan, Michigan, USA). OSC19 cells were a kind gift from Dr. Theresa Whiteside at the University of Pittsburgh Medical Center (Pittsburgh, PA, USA). All cells were maintained in Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). Cells were incubated at 37°C in the presence of 5% CO2. All HNSCC cell lines were genotyped by STR profiling using the AmpFℓSTR® Profiler PCR Amplification Kit (Applied Biosystems, Carlsbad, CA, USA).
Fluorescence microscopy
Primary fibroblast candidate cells, NIH3T3, and the HNSCC cell line Cal27 were seeded in the Lab-Tek II 8 Chamber Slides (Lab-Tek, Thermo Fisher Scientific Inc., Rochester, NY, USA) at a density of 2×104 cells/well (Cal27 were seeded at a density of 3×104 cells/well in wells pretreated with 1 μg/ml fibronectin for 20 min) and cultured in a 5% CO2 incubator at 37 °C for 48 hours. Following 48 hours of incubation cells were washed three times with 1xPBS, fixed with 2% Paraformaldehyde for 20 minutes, washed three times with 1xPBS and permeabilized with 0.1% Triton X-100 for 20 minutes. Blocking was performed in 2% BSA for 45 minutes and cells were subsequently stained in 0.5% BSA containing Cy3-anti alpha Smooth Muscle Actin (1:4000; Sigma-Aldrich, St. Louis, MO, USA) and FITC-anti cytokeratin-14 (1:400; Abcam, Cambridge, MA, USA) for 2 hours. Slides were counterstained with DAPI (Life Technologies, Grand Island, NY) to visualize the nucleus. Olympus Provis III fluorescent microscope and Olympus Fluoview 1000 I Confocal Microscope (Waltham, MA, USA) were used to image fluorescently labeled cells at 400x magnification.
Conditioned Media
UMSCC-1 cells or TAFs were grown to confluence. Growth media on confluent cultures was replaced with serum-free DMEM for 24 hours in case of HNSCC cells and 72 hours for fibroblast cultures. Supernatants were clarified by centrifugation at 5000 rpm for 5 min, aliquoted and stored at −80°C.
HNSCC Invasion Assay
Cell invasion was evaluated in vitro using Matrigel-coated semipermeable modified Boyden inserts with a pore size of 8μm (Becton Dickinson/Biocoat, Bedford, MA, USA). HNSCC cells were plated in triplicate at a density of 1.25 × 104 UMSCC1 cells per well in serum-free media or TAF conditioned media in the insert. Outer wells contained TAF conditioned media or serum free media. At the same time, HNSCC cells were plated in 96-well plates to serve as loading controls. After 24 hours at 37°C in a 5% CO2 incubator, the cells in the insert were removed by wiping gently with a cotton swab. Cells on the reverse side of the insert were fixed and stained with Hema 3 (Fisher Scientific, Hampton, NH, USA) according to the manufacturer's instructions. Cells plated in 96-well plates were subjected to Cell Titer-Glo Luminescent Cell Viability Assays (Promega, Madison, WI, USA) and the cell numbers across the groups were normalized. The number of invading cells was adjusted accordingly. Fold change in invasion relative to the vehicle control (DMEM) was determined.
Proliferation and Migration
Cell proliferation and migration were assessed in real-time of the xCELLigence DP system from Roche (Indianapolis, IN, USA) as previously described(24). Briefly, cell proliferation was assessed by plating 2000 cells in 200 μl DMEM + 10% FBS in quadruplicates into 16-well E-plates, Roche (Indianapolis, IN, USA) after baseline measurements. Media (175 μl) in the wells was replaced with DMEM or conditioned media the next day. Cell index (difference between the impedance at a given time point and the background, divided average resistance across the plate in media only using 10 kHz frequency of current) measurements were taken every 15 minutes for up to 72 hours. For migration assays, 60,000 cells were plated per well in quadruplicates into uncoated 16-well CIM plates (Roche, Indianapolis, IN, USA). Treatments (DMEM or TAF conditioned media with and without DMSO were added at the time of plating. Cell index measurements were taken every 15 mins for 17-24 h.
HGF ELISA and RT-PCR
To determine the effect of HNSCC conditioned medium on TAF-secreted HGF, 0.1 × 106 TAF cells were plated in 6-well plates. Media was replaced with 2 ml DMEM or HNSCC conditioned media the next day. After 48 h, the supernatant was collected and clarified by centrifugation at 5000 rpm for 5 min. The Quantikine Human HGF kit (R&D Systems, Minneapolis, MN, USA) was used to assess HGF levels in 50 μl of supernatant according to the manufacturer's instructions. The optical density of each well was obtained on the μQuant microplate reader (Bio-Tek Instruments Inc, Winooski, VT, USA) set to 450 nm and reference wavelength at 540nm.
To determine if HNSCC secreted factors regulate HGF mRNA levels in TAFs, TAFs were plated at 4 × 105 cells/well in 6-well plates. Media was replaced with 1 ml DMEM or HNSCC conditioned media the next day. Cells were washed with 1× PBS twice and lysed at 1, 4 and 24 h. Lysates were subjected to RNA extraction using the RNeasy kit (QIAGEN, Germantown, MD, USA) according to the manufacturer's instructions. RNA was subjected to DNAse digestion to ensure removal of genomic DNA.
To determine if HNSCC secreted factors regulate HGF mRNA levels in TAFs, TAFs were plated at 1.6 × 104 cells/well in 6-well plates. Media was replaced with 1 ml DMEM or HNSCC conditioned media the next day. Cells were washed with 1× PBS twice and lysed at 1, 4 and 24 h. Lysates were subjected to RNA extraction using the RNeasy kit (QIAGEN, Germantown, MD, USA) according to the manufacturer's instructions. RNA was subjected to DNAse (QIAGEN, RNase-free DNase Set) digestion to ensure removal of genomic DNA. Total RNA was subjected to cDNA synthesis using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. PCR was performed using primers to HGF and GAPDH as previously described(25). Briefly, cDNA was denatured at 94C for 15s, annealed at 55-60C for 30s and extended at 72C for 30s for 35 cycles. PCR product was fractionated on an agarose gel for 45min at 76V and imaged on the Kodak Gel Logic 2200 Imaging System.
Orthotopic HNSCC metastasis in mice
Luciferase-expressing OSC19 HNSCC cells (3 × 106 cells) mixed with control media (n=5), TAFs (0.5 × 106 cells, n=5), or normal fibroblasts (NFs) from cancer free subjects (0.5 × 106 cells, n=5) were injected into the floor-of-the-mouth of mice and imaged using the IVIS 200 imaging system post intraperitoneal injection of 40 mg/kg luciferin (OZ Biosciences, Marseille, France) under anesthesia with inhalant Isofluorane. Tumor volumes were measured at day 5, 7, 11 and 13 using a vernier caliper in 2 dimensions. Tumors were allowed to grow for 2 weeks post implantation. Mice then were sacrificed and dissected for primary tumors and lungs. In addition, the superficial cervical lymph nodes in close proximity to the submandibular salivary glands were dissected. The draining cervical lymph nodes are located close to the neck of the mouse near the salivary glands and are at a sufficient distance and from the primary tumor in the floor-of-the-mouth allowing for efficient excision without tumor contamination. The organs were visualized ex vivo using the IVIS 200 imaging system (Caliper Life Sciences, Hopkinton, MA, USA) and quantifying the photons of light emitted. Lymph nodes were fixed in 10% formalin and paraffin embedded. Hematoxylin and eosin stained sections were assessed for presence of tumor metastasis. All animal use and care was in strict compliance with institutional guidelines established by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Statistical Analysis
Samples were compared using the one-sided Wilcoxon Mann Whitney non-parametric test with Cytel Studio 9 (Cytel, Inc., Cambridge, MA, USA) and PRISM 6 Graphpad software (GraphPad Software, Inc. La Jolla, CA USA).
Results
Tumor associated fibroblasts express α-smooth muscle actin
Patient specimens were obtained fresh from the University of Pittsburgh Medical Center, Department of Pathology. Patients undergoing surgical excision of HNSCC gave informed consent and samples were screened by pathology and de-identified by an honest broker. Tumor site, stage and other pertinent information for all samples that yielded tumor associated fibroblasts (TAFs) is listed in Supplemental Table 1. The majority of samples were late stage oral cavity HNSCC from current smokers.
Several studies have demonstrated that fibroblasts isolated from tumors express α-smooth muscle actin(26,27). Further, epithelial cells express cytokeratin-14(28). Since fibroblasts do not express cytokeratin-14, we used this marker to determine if primary fibroblasts grown from human tissue explants were pure populations devoid of epithelial cells. We verified the purity of fibroblast cultures, (passage # 2-3) by immunofluorescence (Supplemental Figure 1). The well characterized fibroblast line NIH3T3 was used as a positive control for α-smooth muscle actin (red), the HNSCC cell line Cal27 was used as a positive control for cytokeratin-14 (green), and nuclei were visualized using DAPI (blue). Cytokeratin-14 positive cells were not detected in the fibroblast cultures, while all the fibroblast cultures were positive for α-smooth muscle actin. In addition, we were unable to detect cytokeratin-14 positive cells in the fibroblast cultures by flow cytometric analyses (data not shown).
HNSCC cells and TAFs secrete paracrine factors that facilitate cellular proliferation
Human embryonic lung fibroblasts preferentially migrate towards tumor cells instead of normal epithelial cells(29). Soluble factors secreted by tumors induce fibroblast transdifferentiation into activated TAFs, which in turn facilitate tumor progression(29-32). In order to examine the effect of HNSCC cells on TAF proliferation, serum deprived TAFs were treated with DMEM alone or HNSCC conditioned medium (Figure 1A). In all five TAF lines tested HNSCC conditioned medium increased the proliferation of TAFs compared to DMEM alone (p=0.004), indicating that soluble factors secreted by HNSCC can increase TAF proliferation.
Figure 1. Conditioned media from each cell type induces proliferation in the opposite cell type.
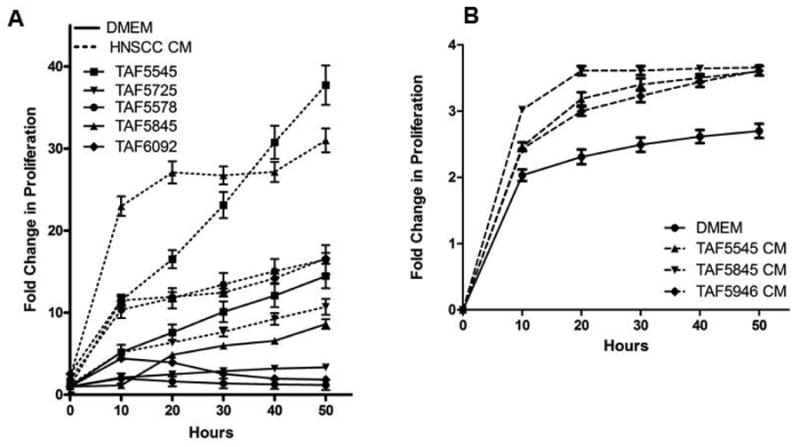
(A) Five TAF lines were treated with DMEM alone (solid lines) or SCC1 conditioned media (HNSCC conditioned media, dashed lines) and assayed for proliferation using the xCELLigence System. HNSCC conditioned media increased proliferation in all five TAF lines tested. n=5 p=0.004 at 50 hours. (B) UMSCC1 cells were treated with DMEM alone or conditioned media from one of three TAF lines and assayed for proliferation over 60 hours. Data is presented as fold change in proliferation compared to the vehicle control p = 0.029 at 50 hours.
In order to determine the effect of TAFs on HNSCC cells, UMSCC1 cells were treated with DMEM alone or TAF conditioned media. Conditioned media from TAFs derived from 3 patient tumors significantly increased the proliferation of UMSCC1 cells compared to DMEM alone, indicating soluble factors secreted by TAFs can increase HNSCC proliferation (Figure 1B, p=0.029). The sum of these proliferation results indicates that HNSCC cells and TAFs produce soluble factors that participate in intercellular signaling to increase proliferation.
Soluble factors from TAFs increase migration and invasion of HNSCC cells in vitro
HNSCC currently has no treatment for control of metastatic spread and patients with metastases have a 5 year survival rate of <20%(4). We previously published that the presence of TAFs can increase the invasion of HNSCC cells in vitro(22). To determine if TAFs influence migration of HNSCC cells, UMSCC1 cells were treated with DMEM alone or conditioned media from one of three TAF lines and assayed for migration as described in the materials and methods section. UMSCC1 cells showed increased migration when treated with TAF conditioned media compared to DMEM alone after just 9 hours (p=0.029) (Figure 2A). UMSCC1 cells were treated with DMEM alone or TAF conditioned media from 5 TAF lines and assayed for cell invasion (Figure 2B). There was a significant increase in UMSCC1 invasion with TAF conditioned media compared to DMEM alone after 24 hours (p<0.0004).
Figure 2. TAF conditioned media increases migration and invasion in HNSCC cells.
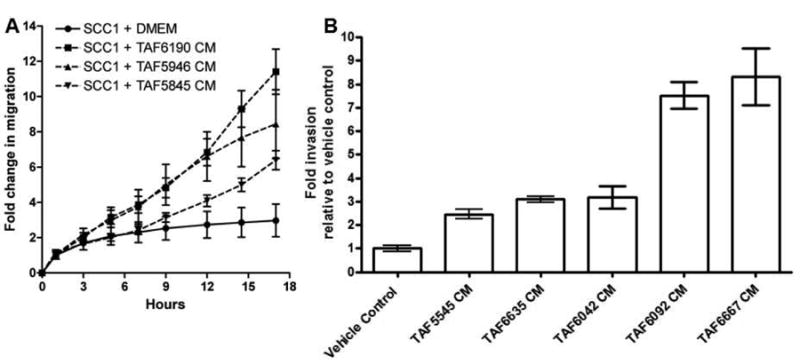
(A) UMSCC1 cells were treated in quadruplicates with TAF conditioned media (TAF CM) from three different TAF lines and assayed in real-time for migration on the xCELLigence System. TAF conditioned media significantly increased HNSCC migration (p= 0.029). The experiment was repeated three times with similar results. (B) UMSCC1 cells were plated in Boyden invasion chambers, treated in triplicates with conditioned media from 5-TAF lines or DMEM and assayed for invasion after 24 hours. TAF conditioned media significantly increased HNSCC invasion in vitro (p<0.0004). The experiment was repeated 4 times with similar results.
HNSCC conditioned media increases HGF production in TAFs
HNSCC associated fibroblasts have been reported to secrete HGF(33,34). Further, HGF secreted by TAFs increased HNSCC invasion(33). HGF induces signaling through its receptor c-Met that results in increased motility of several carcinomas(35). Both HGF and c-Met are reported to be deregulated or over-expressed in HNSCC(36). HNSCC cells do not secrete detectable levels of HGF in conditioned media(22). However, HGF concentrations of 1-8 ng/ml were detected in medium conditioned by stromal fibroblasts for 24 hr, which is comparable to levels measured in human serum(22). TAFs secrete 1-8 ng/ml of HGF(22). We and others have demonstrated the role of the c-Met signaling axis in HNSCC invasion(22,37). We have previously published that an HGF neutralizing antibody can abrogate the increased invasion in HNSCC cells caused by the presence of TAFs(22). In order to elucidate if HNSCC cells facilitate secretion of HGF from TAFs, TAFs treated with HNSCC conditioned media were assayed for production of HGF by ELISA. Cumulative results from 6 separate TAF lines demonstrate a significant increase in HGF production upon exposure to HNSCC conditioned media over 24 h (p=0.001) (Figure 3A). There was no detectable HGF in HNSCC conditioned media (without TAFs) or in DMEM. In order to determine if HGF is regulated at the mRNA level, HGF mRNA levels were measured in TAFs stimulated with vehicle control (DMEM) or HNSCC conditioned media for 1, 4 and 24 h. HNSCC conditioned media increased HGF mRNA levels in TAFs at the 1 and 4 h time points (Figure 3B). This indicates that HNSCC-secreted paracrine factors facilitate HGF transcription in TAFs.
Figure 3. HNSCC conditioned media induces HGF secretion from TAFs.
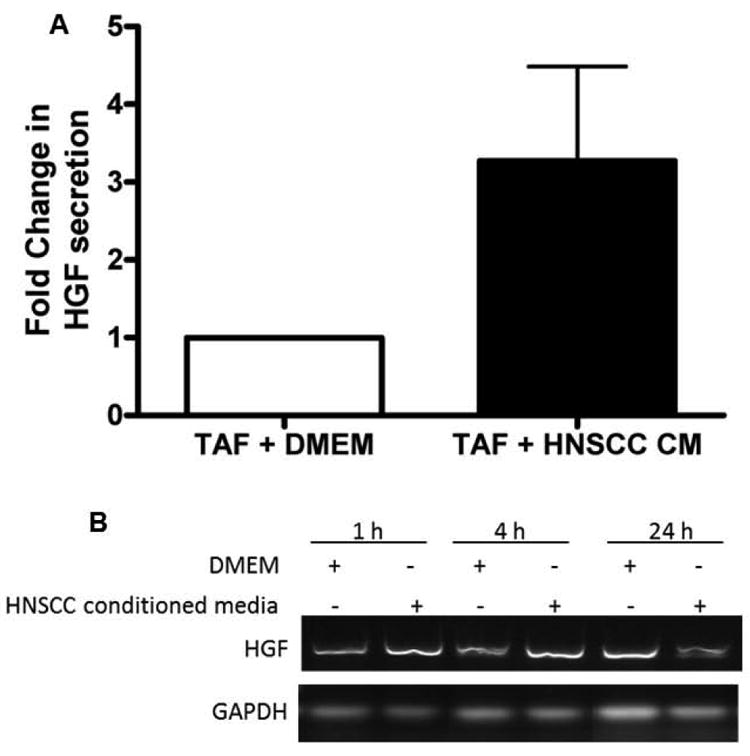
(A) Six TAF lines (TAF5545, TAF5633, TAF5655, TAF5715, TAF5746, TAF5747) were treated with HNSCC cell line UMSCC1 conditioned media or DMEM alone for 48 h. Supernatants were collected and clarified by centrifugation for analysis by HGF ELISA. The average of all 6 TAF lines is shown with error bars (n=6, p=0.001). (B) TAFs (4 × 105 cells/well) were treated with DMEM or HNSCC conditioned medium (CM) for 1h, 4h or 24h. RT-PCR for HGF demonstrates an increase in HGF mRNA at 1 and 4h post treatment with HNSCC CM. The experiment was repeated 3 times with similar results.
TAFs increase growth and metastasis of HNSCC in vivo
In order to determine the effect of stromal fibroblasts on HNSCC metastasis, OSC19 alone or admixed with either TAFs or fibroblasts were inoculated in the floor of the mouth of athymic mice. Tumor volume was measured over 13 days in 2 dimensions using a vernier caliper. Addition of TAFs to HNSCC significantly increased tumor volume over HNSCC alone (p = 0.008) and HNSCC with fibroblasts (p = 0.004) (Figure 4A). The lungs and lymph nodes of mice were excised after 13 days and imaged on the IVIS 200 system for presence of luciferase expressing HNSCC cells. Metastatic lesions in the lung were almost undetectable in HNSCC alone tumor bearing mice, but significant metastases were observed in mice with addition of TAFs (p = 0.008) (Figure 4B). Hematoxylin and eosin stained sections of lymph nodes from HNSCC + TAF tumors demonstrated presence of metastatic tumor in the extra capsular region of the nodes (Figure 4C). Addition of TAFs significantly increased metastasis to the regional lymph nodes compared to HNSCC alone (p = 0.016) and HNSCC with normal fibroblasts (p = 0.047) (Figure 4D). Some metastases were observed in HNSCC with normal fibroblasts but were significantly fewer than HNSCC with TAFs (p = 0.01).
Figure 4. TAFs increase HNSCC tumor volume and metastasis in vivo.
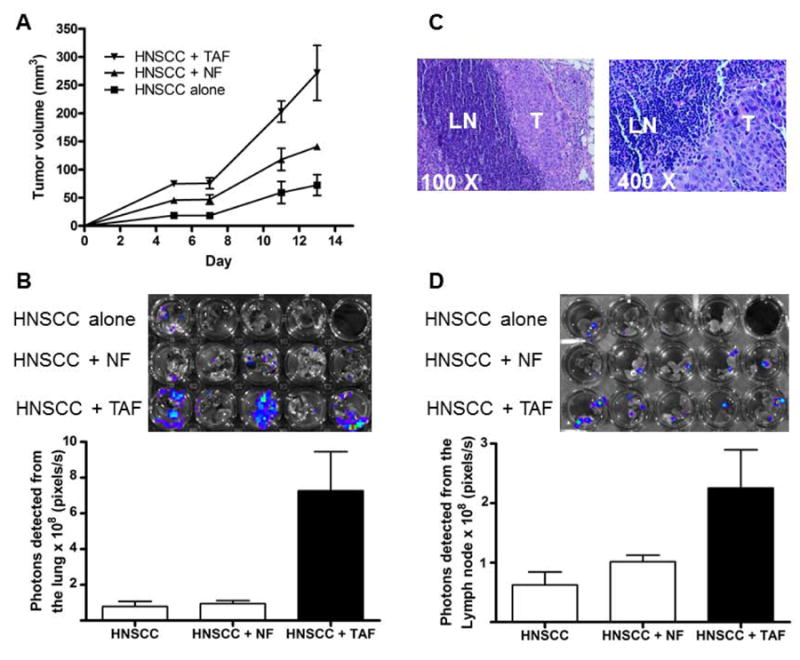
Nude mice were inoculated in the floor-of-the-mouth with 3 × 106 cells luciferase-expressing OSC19 HNSCC cells alone (n = 4) or combined with 0.5 × 106 TAF cells (TAF5545) or 0.5 × 106 normal fibroblasts (NF) from cancer-free patients (n = 5). (A) The graph depicts growth of primary tumor volume measured using vernier calipers. The volume of HNSCC+TAF tumors at day 13 was significantly higher than the HNSCC alone (p = 0.008) and the HNSCC+NF (p = 0.004) tumors. (B) Lungs were isolated and imaged ex-vivo. Photons emitted by lungs were measured and plotted (±SEM). Light emitted from tumors in the HNSCC +TAF group was significantly higher compared to HNSCC alone (p = 0.016) and HNSCC + NF (p = 0.047) groups. (C) Cervical lymph nodes were paraffin embedded and imaged after hematoxylin and eosin staining. HNSCC metastases (T) to the lymph nodes (LN) are depicted. (D) Cervical lymph nodes were imaged ex-vivo. Photons emitted by lymph nodes were measured and plotted (±SEM). Light emitted from tumors in the HNSCC +TAF group was significantly higher compared to HNSCC alone (p = 0.008) and HNSCC + NF (p = 0.01) groups.
Discussion
In the last decade research has made it apparent that the components of tumor stroma once thought to be ‘normal’ (such as fibroblasts, inflammatory cells and endothelial cells) have an active role in promoting tumor progression(38,39). Studies indicate that TAFs are important in epithelial tumor progression(31,40). Here we have developed a method of propagating tumor associated fibroblasts without epithelial cell contamination. We verified that these TAFs expressed α-smooth muscle actin and did not express the epithelial marker cytokeratin-14 (Supplemental Figure 1).
The literature on tumor associated fibroblasts in HNSCC is still developing and we describe here the effects of TAF secreted factors on HNSCC cells in vitro and TAFs on HNSCC cells in vivo. In vitro we found that TAF conditioned media increased the proliferation of HNSCC cells and, interestingly, HNSCC conditioned media also increased the proliferation of TAFs (Figure 1). It is not surprising that both cell types would secrete paracrine factors to affect the overall tumor microenvironment and increase tumor progression(18). Further investigation of the paracrine factors from HNSCC cells that influence TAFs is warranted. We chose to continue our studies looking specifically at the effects of TAFs on HNSCC cells. We found that TAF conditioned media significantly increased in vitro migration of HNSCC cells (Figure 2A). We previously reported that the presence of one TAF line was able to increase HNSCC cell invasion in vitro(22). Here we more rigorously proved this finding using three TAF lines for conditioned media and an independent cell line (Figure 2B) which demonstrated this as a common phenotype not specific to one particular TAF line.
Heterotopic injection of HNSCC cells in mice allows for evaluation of local and distant metastasis in vivo. By utilizing a luciferase expression system we were able, for the first time in HNSCC, to evaluate lymph node and lung metastasis (Figure 4). A limitation of this model is that the luminescent signal is not a precise quantitative measure of metastasis. However, lungs and cervical lymph nodes were dissected, placed in a 24-well plate and imaged ex vivo. Tissue form HNSCC alone, HNSCC +TAF and HNSCC + NF groups were imaged together allowing for comparative assessment of the photons of light emitted across groups. Heterotopic injection of HNSCC cells alone in the floor of the mouth produced locoregional spread to the lymph nodes of mice. Implantation of admixed HNSCC cells and TAFs led to increased primary tumor growth as well as disease spread to the lymph nodes and significant lung metastases. This finding supports strongly the need to target tumor stroma in addition to the cancer epithelial cells, and may provide insight into potential mechanisms of targeted therapy resistance in HNSCC. A recent study in 282 OSCC cases found that the stromal component of the tumor was a better predictor of mortality than any measure of the epithelial cells or disease staging(21), further supporting the need to exhaustively study the tumor microenvironment to fully understand and treat HNSCC.
In many cancer types myofibroblasts promote cell motility through up-regulation of growth factors and matrix-proteins in addition to ECM remodeling(39). TAFs effects on epithelial tumors (perhaps best studied in breast) are mediated by the paracrine secretion of growth factors including HGF, SDF1, TGF-β(14,41,42) and ECM remodeling(43). HGF functions in normal tissue in organogenesis, regeneration and wound healing and in cancer has an important role in the tumor microenvironment. Another recent study has shown that the HGF/Met pathway is a key mediator of increased in vitro invasion in esophageal SCC by TAFs(44). Here we demonstrate that HNSCC paracrine factors induce HGF mRNA and protein production in TAFs. We have previously published that TAF-secreted HGF mediates the increased invasion in HNSCC cells(22). Our cumulative findings underscore the contribution of the tumor associated stroma in tumor progression.
Supplementary Material
Acknowledgments
This work was supported by P50 CA097190 (career development award) and the competitive medical research fund from the Clinical and Translational Science Institute at the University of Pittsburgh (to SMT) and 1F31DE020223 - 01A1 (SW). This project used the UPCI Animal Facility and was supported in part by award P30CA047904.
Abbreviations
- HNSCC
Head and neck squamous cell carcinoma
- TAF
tumor associated fibroblast
References
- 1.Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359(11):1143–54. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- 2.Ozdek A, Sarac S, Akyol MU, Unal OF, Sungur A. Histopathological predictors of occult lymph node metastases in supraglottic squamous cell carcinomas. Eur Arch Otorhinolaryngol. 2000;257(7):389–92. doi: 10.1007/s004050000231. [DOI] [PubMed] [Google Scholar]
- 3.Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63(4):202–7. doi: 10.1159/000055740. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.Gonda TA, Varro A, Wang TC, Tycko B. Molecular biology of cancer-associated fibroblasts: can these cells be targeted in anti-cancer therapy? Semin Cell Dev Biol. 2010;21(1):2–10. doi: 10.1016/j.semcdb.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48(5-6):509–17. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- 7.Ronnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95(2):859–73. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 9.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 10.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66(2):632–7. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 11.Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci. 15:166–79. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Li Q, Yamada T, et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin Cancer Res. 2009;15(21):6630–8. doi: 10.1158/1078-0432.CCR-09-1001. [DOI] [PubMed] [Google Scholar]
- 13.Kaminski A, Hahne JC, Haddouti el M, Florin A, Wellmann A, Wernert N. Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int J Mol Med. 2006;18(5):941–50. [PubMed] [Google Scholar]
- 14.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Hawsawi NM, Ghebeh H, Hendrayani SF, et al. Breast carcinoma-associated fibroblasts and their counterparts display neoplastic-specific changes. Cancer Res. 2008;68(8):2717–25. doi: 10.1158/0008-5472.CAN-08-0192. [DOI] [PubMed] [Google Scholar]
- 16.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68(3):918–26. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 21(1):33–9. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): Active stromal participants in tumor development and progression? Histol Histopathol. 2002;17(2):599–621. doi: 10.14670/HH-17.599. [DOI] [PubMed] [Google Scholar]
- 19.Wu MH, Hong HC, Hong TM, Chiang WF, Jin YT, Chen YL. Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression. Clin Cancer Res. 2011;17(6):1306–16. doi: 10.1158/1078-0432.CCR-10-1824. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Liu C, Ge L, et al. Carcinoma-associated fibroblasts promotes the proliferation of a lingual carcinoma cell line by secreting keratinocyte growth factor. Tumour Biol. 2011;32(3):597–602. doi: 10.1007/s13277-011-0158-5. [DOI] [PubMed] [Google Scholar]
- 21.Marsh D, Suchak K, Moutasim KA, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011;223(4):470–81. doi: 10.1002/path.2830. [DOI] [PubMed] [Google Scholar]
- 22.Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clin Cancer Res. 2009;15(11):3740–50. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koontongkaew S, Amornphimoltham P, Yapong B. Tumor-stroma interactions influence cytokine expression and matrix metalloproteinase activities in paired primary and metastatic head and neck cancer cells. Cell Biol Int. 2009;33(2):165–73. doi: 10.1016/j.cellbi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Hickok JR, Sahni S, Mikhed Y, Bonini MG, Thomas DD. Nitric oxide suppresses tumor cell migration through N-Myc downstream-regulated gene-1 (NDRG1) expression: role of chelatable iron. J Biol Chem. 2011;286(48):41413–24. doi: 10.1074/jbc.M111.287052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang JG, Johnson C, Zarnegar R. Peroxisome proliferator-activated receptor gamma-mediated transcriptional up-regulation of the hepatocyte growth factor gene promoter via a novel composite cis-acting element. J Biol Chem. 2001;276(27):25049–56. doi: 10.1074/jbc.M101611200. [DOI] [PubMed] [Google Scholar]
- 26.Lygoe KA, Wall I, Stephens P, Lewis MP. Role of vitronectin and fibronectin receptors in oral mucosal and dermal myofibroblast differentiation. Biol Cell. 2007;99(11):601–14. doi: 10.1042/BC20070008. [DOI] [PubMed] [Google Scholar]
- 27.Valach J, Fik Z, Strnad H, et al. Smooth muscle actin-expressing stromal fibroblasts in head and neck squamous cell carcinoma: Increased expression of galectin-1 and induction of poor prognosis factors. Int J Cancer. 2012;131(11):2499–508. doi: 10.1002/ijc.27550. [DOI] [PubMed] [Google Scholar]
- 28.Castello-Cros R, Cukierman E. Stromagenesis during tumorigenesis: characterization of tumor-associated fibroblasts and stroma-derived 3D matrices. Methods Mol Biol. 2009;522:275–305. doi: 10.1007/978-1-59745-413-1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma H, Liu T, Qin J, Lin B. Characterization of the interaction between fibroblasts and tumor cells on a microfluidic co-culture device. Electrophoresis. 31(10):1599–605. doi: 10.1002/elps.200900776. [DOI] [PubMed] [Google Scholar]
- 30.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 32.Grose R, Dickson C. Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor Rev. 2005;16(2):179–86. doi: 10.1016/j.cytogfr.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Daly AJ, McIlreavey L, Irwin CR. Regulation of HGF and SDF-1 expression by oral fibroblasts--implications for invasion of oral cancer. Oral Oncol. 2008;44(7):646–51. doi: 10.1016/j.oraloncology.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Rosenthal E, McCrory A, Talbert M, Young G, Murphy-Ullrich J, Gladson C. Elevated expression of TGF-beta1 in head and neck cancer-associated fibroblasts. Mol Carcinog. 2004;40(2):116–21. doi: 10.1002/mc.20024. [DOI] [PubMed] [Google Scholar]
- 35.Desiderio MA. Hepatocyte growth factor in invasive growth of carcinomas. Cell Mol Life Sci. 2007;64(11):1341–54. doi: 10.1007/s00018-007-7050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Herdt MJ, Baatenburg de Jong RJ. HGF and c-MET as potential orchestrators of invasive growth in head and neck squamous cell carcinoma. Front Biosci. 2008;13:2516–26. doi: 10.2741/2863. [DOI] [PubMed] [Google Scholar]
- 37.Seiwert TY, Jagadeeswaran R, Faoro L, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69(7):3021–31. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 39.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123(10):2229–38. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 40.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 41.Kuperwasser C, Chavarria T, Wu M, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101(14):4966–71. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng N, Chytil A, Shyr Y, Joly A, Moses HL. Enhanced hepatocyte growth factor signaling by type II transforming growth factor-beta receptor knockout fibroblasts promotes mammary tumorigenesis. Cancer Res. 2007;67(10):4869–77. doi: 10.1158/0008-5472.CAN-06-3381. [DOI] [PubMed] [Google Scholar]
- 43.Gaggioli C, Hooper S, Hidalgo-Carcedo C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 44.Grugan KD, Miller CG, Yao Y, et al. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci U S A. 2010;107(24):11026–31. doi: 10.1073/pnas.0914295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


