Abstract
The temporo-parieto-occipital (TPO) junction is a complex brain territory heavily involved in several high-level neurological functions, such as language, visuo-spatial recognition, writing, reading, symbol processing, calculation, self-processing, working memory, musical memory, and face and object recognition. Recent studies indicate that this area is covered by a thick network of white matter (WM) connections, which provide efficient and multimodal integration of information between both local and distant cortical nodes. It is important for neurosurgeons to have good knowledge of the three-dimensional subcortical organisation of this highly connected region to minimise post-operative permanent deficits. The aim of this dissection study was to highlight the subcortical functional anatomy from a topographical surgical perspective. Eight human hemispheres (four left, four right) obtained from four human cadavers were dissected according to Klingler's technique. Proceeding latero-medially, the authors describe the anatomical courses of and the relationships between the main pathways crossing the TPO. The results obtained from dissection were first integrated with diffusion tensor imaging reconstructions and subsequently with functional data obtained from three surgical cases, all resection of infiltrating glial tumours using direct electrical mapping in awake patients. The subcortical limits for performing safe lesionectomies within the TPO region are as follows: within the parietal region, the anterior horizontal part of the superior longitudinal fasciculus and, more deeply, the arcuate fasciculus; dorsally, the vertical projective thalamo-cortical fibres. For lesions located within the temporal and occipital lobes, the resection should be tailored according to the orientation of the horizontal associative pathways (the inferior fronto-occipital fascicle, inferior longitudinal fascicle and optic radiation). The relationships between the WM tracts and the ventricle system were also examined. These results indicate that a detailed anatomo-functional awareness of the WM architecture within the TPO area is mandatory when approaching intrinsic brain lesions to optimise surgical results and to minimise post-operative morbidity.
Keywords: anatomic dissection, awake mapping, eloquent areas, hodotopy, Klingler's technique, temporo-parieto-occipital junction, white matter
Introduction
The temporo-parieto-occipital (TPO) junction is located at the posterior end of the Sylvian fissure, where the temporal, parietal and occipital lobes meet. The TPO is a complex region of the brain through which various white matter (WM) fibres pass. These fibres are involved in several crucial high-level functions, such as language (Duffau et al. 2005; Fehr et al. 2007; Duffau, 2008), visuo-spatial recognition (Duffau et al. 2004; Thiebaut de Schotten et al. 2005; van den Heuvel et al. 2009; Sakurai et al. 2010), writing (Scarone et al. 2009), reading (Mandonnet et al. 2007, 2009), symbol processing (Holloway et al. 2010; Price & Ansari, 2011), calculation (Fehr et al. 2007; Rosenberg-Lee et al. 2011; Zarnhofer et al. 2012), self-processing (Blanke & Arzy, 2005), working memory (Ojemann, 2003; Deprez et al. 2013), musical memory (Platel, 2005), and face and object recognition (Zhen et al. 2013; Tavor et al. 2014).
Over the last decades, neuroscience research has advanced towards a more realistic understanding of the anatomo-functional organisation of the central nervous system, described by the ‘hodotopical’ framework (Ffytche & Catani, 2005; De Benedictis & Duffau, 2011). According to this model, functional output results from the dynamic interaction between cortical epicentres, which are widely integrated through parallel and redundant circuits and subserved by largely distributed long and short WM connections (De Benedictis & Duffau, 2011; Catani et al. 2012a,b). Growing interest in the investigation of the brain network, also called the ‘human brain connectome’ (Sporns, 2011, 2013; Behrens & Sporns, 2012; Bargmann & Marder, 2013; Catani et al. 2013), has revealed consistent structural and functional evidence of its organisation (Bassett & Bullmore, 2006; van den Heuvel et al. 2009; Sampaio et al. 2013).
The TPO region is a good example in which a complex cortico-subcortical substrate has been described. In fact, a mosaic of ‘nodes’ with different functional roles, ranging from essential to modulatory, have been found to establish the cortical organisation. Recent imaging studies have demonstrated that the traditional ‘eloquent’ areas (i.e. Wernicke's and Geschwind's territories) are widely distributed beyond the classical anatomical landmarks [i.e. the posterior part of the superior temporal gyrus and middle temporal gyrus (MTG) and the supramarginal gyrus (SMG), respectively] and have a high degree of inter-individual variability (Mesulam, 1990; Vigneau et al. 2006; Sarubbo et al. 2012a,b). Moreover, these cortical areas can undergo further structural reshaping and provide optimal functional compensation after pathological events, such as ischaemic accidents or infiltrating tumours (Sarubbo et al. 2012a,b, 2013b).
At the subcortical level, the WM of the TPO junction represents a crucial node of intralobar and interlobar connectivity, and provides multimodal integration of information from both local and distant functional hubs (Martino et al. 2011). Various discrete nerve fascicles provide for different eloquent functions via parallel subcomponents (Makris et al. 2005; Martino et al. 2011; Sarubbo et al. 2013a,b); studies of lesion sites have provided strong evidence of this effect. In fact, damage to cortical neurons or their axons has reportedly produced functional changes at both local and distant brain hubs, and the modifications differ based on specific patterns of connectivity (Catani et al. 2013). Note that, contrary to the cortical level, subcortical connectivity does not benefit from the same post-lesional plastic potential, particularly in response to acute accidents (Duffau, 2009) and after surgical damage (Russell et al. 2005).
Traditionally, the anatomy of the brain's connections has been investigated through post mortem observations using Klingler's technique (Klingler, 1935). More recently, the improved resolution attainable using diffusion tensor imaging (DTI) has increased the ability to three-dimensionally organise human dissections by revealing the course of fibres ‘in vivo’ rather than invasively (Catani & Thiebaut de Schotten, 2008). In addition, increasing experience supports the role that direct electrostimulation (DES) plays in detecting the eloquent components of the network that subserves high-level functions within the TPO region (Duffau et al. 2002, 2014; Ojemann, 2003; Duffau, 2006, 2010; Mandonnet et al. 2010; Bartolomeo et al. 2012; Fernández Coello et al. 2013). Moreover, recent studies have reported that DES reliably and significantly decreases post-operative morbidity after resecting infiltrative tumours located within the parietal (Maldonado et al. 2011; Sanai et al. 2012), occipital (Viegas et al. 2011) and TPO (Gras-Combe et al. 2012) regions. However, despite the contribution provided by intraoperative monitoring, a high incidence of post-operative permanent deficits and worsened quality of life has also been described (Sanai et al. 2012).
These results indicate that accurate awareness of the three-dimensional subcortical anatomo-functional organisation of the TPO area is crucial to ensure that neurosurgeons can use the safest and most effective approaches in this highly eloquent region. Interestingly, many studies have focused on characterising the anatomy of single pathways running through the TPO region (Catani & Thiebaut de Schotten, 2008; Martino et al. 2010, 2011), with more comprehensive topographical representations rarely reported (Peltier et al. 2006; Martino et al. 2013).
In the present work, we characterised the subcortical surgical anatomy of the TPO area using a multi-methodological approach. The aims of this investigation are as follows: (i) to use post mortem dissection and DTI to anatomically characterise the courses of and relationships between the main pathways that cross the TPO region; (ii) to correlate cortical and subcortical anatomical evidence based on functional data collected during resections of infiltrative glial tumours that included cortico-subcortical DES; and (iii) to discuss the anatomo-functional topographical implications for different surgical approaches to this region.
Materials and methods
Anatomical dissection
After ethics committee approval, eight hemispheres (four left and four right) obtained from four human cadavers were dissected in the Laboratory for the Human WM Study of the University of Ferrara (Italy) and in the Medicine Anatomy Laboratory of the University of Montpellier (France) between April 2012 and July 2013. The specimens were fixed in a 10% formalin solution for 40 days, and then frozen for 30 days at −20 °C. After gradual defrosting of the specimens, the arachnoid and vessels were gently removed. Blood vessels, the arachnoid mater and the pia mater were removed, and the hemispheres were frozen at −15 °C for at least 15 days, according to a modified Klingler's technique (Klingler, 1935) developed in our laboratory. After carefully studying the anatomy of the cortical surface of the TPO junction, the dissection was performed using wooden spatulas in a manner previously reported by our group (Sarubbo et al. 2013a,b). Sequential pictures were collected during the dissection.
Similar to a previous study of the frontal region (De Benedictis et al. 2012), analysis of the specimens was managed in two stages. In the first stage, we used a multi-step method to dissect the main association and projection pathways, which were identified using subcortical DES during a lateral approach to the TPO junction. We sequentially exposed the U-shaped fibres, the three adjacent components of the superior longitudinal fascicle [SLF II, SLF III and the arcuate fasciculus (AF)], the posterior portion of the inferior fronto-occipital fascicle (IFOF), the inferior longitudinal fascicle (ILF), the optic radiation (OR) and the corona radiata (CR). The integrity of each fascicle was accurately preserved to define the entire course of these tracts and to represent the reciprocal relationships along all their courses. We also thoroughly examined the basal ganglia to better understand the relationships between the association fibres and the projection tracts. Whenever possible, we left in situ the dorsal two-thirds of the central lobule and some portions of the cortical regions traditionally considered the most eloquent language areas [i.e. Wernicke's territories, classically defined as the posterior two-thirds of the STG and MTG, and the angular gyrus (AG) and SMG, constituting Geschwind's territory] and the posterior two-thirds of the inferior frontal gyrus (IFG), called Broca's area, to provide additional reference for identifying relationships among fibres, cortices and deeper structures.
In the second stage, we analysed the subcortical architecture with specimens oriented according to the surgical view of a typical lateral approach to the TPO region. Moreover, we correlated and discussed anatomical images with intraoperative photographs taken during resections of infiltrative low-grade gliomas (LGGs) guided using subcortical DES.
DTI
The TPO junction was analysed in six hemispheres (three left and three right) using 60 direction diffusion-weighted imaging brain tractography performed with a 1.5-T magnetic resonance imaging (MRI) scanner (GE Healthcare, UK) and an eight-channel head coil. DTI was performed using a single-shot multislice spin echo–echo planar sequence with the following attributes: 40 slices; slice thickness: 2.6 mm; matrix 256 × 256; TR: 10 000; TE: 92.7; and flip angle: 90.
After running the BET (Brain Extraction Tool) provided by the FMRIB Software Library (FSL; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/), the diffusion tensor calculation and tracking were performed using Diffusion Toolkit 0.6.1 and TrackVis 0.5.1 (http://trackvis.org;), respectively. The interpolated streamline algorithm was selected for tacking, excluding all voxels with a fractional anisotropy value below 0.05 and setting the angle threshold to 35. The T1-weighted volume was co-registered to the fractional anisotropy map obtained after diffusion tensor calculation. We applied a knowledge-based multiple regions-of-interest inclusion and exclusion approach in which the tracking algorithm was initiated from user-defined seed regions, according to direct visualisation of the stem of the single bundle that was analysed.
Surgical cases
We selected three illustrative cases of patients receiving operations for LGGs located within the TPO area. We considered three different specific regions in particular: the left dominant parieto-temporal junction; the left dominant temporo-occipital (TO) region; and the TPO area within the right non-dominant hemisphere.
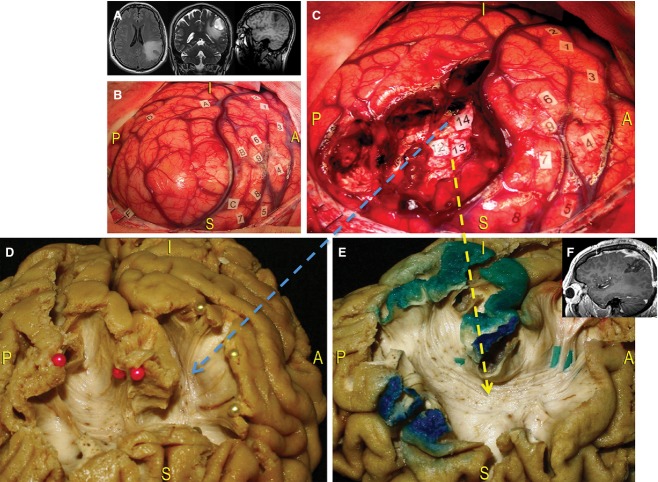
Case 1 (Fig. 11) involved a 42-year-old man with a history of generalised seizures. Magnetic resonance images revealed an intra-axial lesion located within the left dominant inferior parietal lobule (IPL), which infiltrated the periventricular WM. Preoperative neuropsychological examination revealed working memory and attention impairments.
Figure 11.
Case 1. (A) Surgical case involving an infiltrative brain tumour located within the left dominant IPL, visible on the preoperative MRI images. The resection was performed using intraoperative mapping to preserve the cortico-subcortical eloquent structures. (B) Intraoperative photograph, showing the limits of the lesion (letter tags). DES was used to identify the VPMC by eliciting speech arrest during stimulation (tags 1 and 2), as well as the primary area of the face (tags 3–5), the primary somato-sensory area of the face (tags 6 and 9) and the hand (tags 7 and 8). Interestingly, the patient had no trouble with the denomination task. (C) The anterior limit of resection was the retro-CS; the lateral limit was the posterior part of the Sylvian fissure, corresponding to the SMG; the posterior and inferior limits were the STG and the anterior part of the AG; the medial limit was the IPS. Resection was extended at the subcortical level until reaching areas that elicited functional responses to stimulation (articulatory disorders at the level of tag 14 and semantic paraphasias at the level of tags 12 and 13). (D) We correlated the results from DES with post mortem anatomical specimens that had been oriented according to the intraoperative view. In this way, we demonstrated the WM substrates responsible for functional surgical responses. Tag 14 corresponds to the anterior fibres of the lateral component of the SLF (yellow arrow), running from the SMG (yellow pins) to the frontal lobe to form the ‘articulatory loop’. Posterior fibres directed to the posterior part of the STG and the MTG (red pins) were also exposed. (E) The different responses elicited at the level of tags 12 and 13 were dependent upon contact with deeper component of SLF, i.e. the AF (blue arrow). This tract directly connects Wernicke's area (green-coloured cortex) with the VPMC and Broca's territory (green tags, red-coloured cortex), passing under Geschwind's area (red-coloured cortex) around the posterior insula. (F) The resection was quantified as subtotal using post-operative MRI. The patient resumed normal life without permanent deficits. AG, angular gyrus; IPL, inferior parietal lobule; MTG, middle temporal gyrus; retro-CS, retro-central sulcus; SMG, supramarginal gyrus; STG, superior temporal gyrus; VPMC, ventral pre-motor cortex.
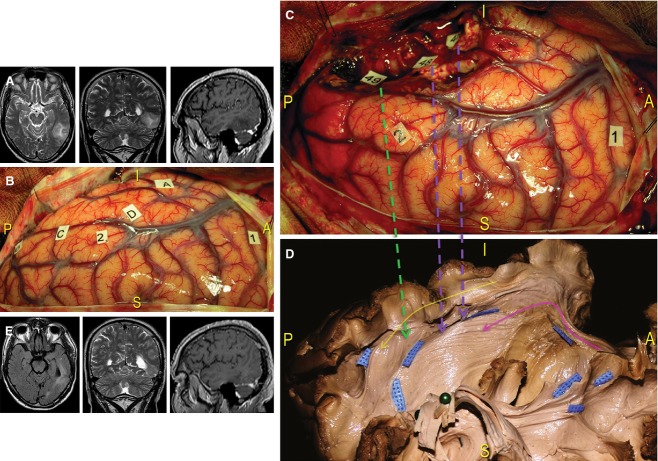
Case 2 (Fig. 12) concerned a 60-year-old right-handed man affected by an intra-axial lesion located at the left dominant TO basal junction. Surgical resection guided by cortico-subcortical awake mapping was proposed due to progressively increasing tumour volume.
Figure 12.
Case 2. (A) Preoperative MRI of this patient revealed a tumour infiltrating the left dominant TO region. (B) After identifying the limits of the lesion using ultrasonography (letter tags), cortical awake mapping induced reproducible speech arrests at the level of the VPMC (tag 1) and anomias at the level of the posterior part of the STG, corresponding to Wernicke's area. (C) The resection included the middle and posterior part of the MTG and the IFG. More ventrally, under the fusiform gyrus, semantic paraphasias and anomia episodes were elicited using DES at tag 46, not visible in the intraoperative photograph, and tag 48. Specific reading troubles were induced in areas more posterior and laterally (tag 49). (D) We performed a dissection of the TO region and oriented the specimen according to the intraoperative perspective. We demonstrated the anatomical substrate responsible for functional intraoperative responses. Tags 46 and 48 correspond to the IFOF (violet arrows), which was exposed from the limen insulae to the parietal and occipital regions passing along the lateral ventricular wall (pink arrow). The location of tag 49 indicates stimulation of the ILF, which connects the temporal and occipital basal regions (yellow arrow). The intraoperative result was most likely dependent upon stimulation where the ILF and IFOF fibres crossed (green arrow, blue tags). (E) Post-operative MRI revealed subtotal resection that produced only left inferior quadrantanopia. IFG, inferior frontal gyrus; STG, superior temporal gyrus; VPMC, ventral pre-motor cortex.
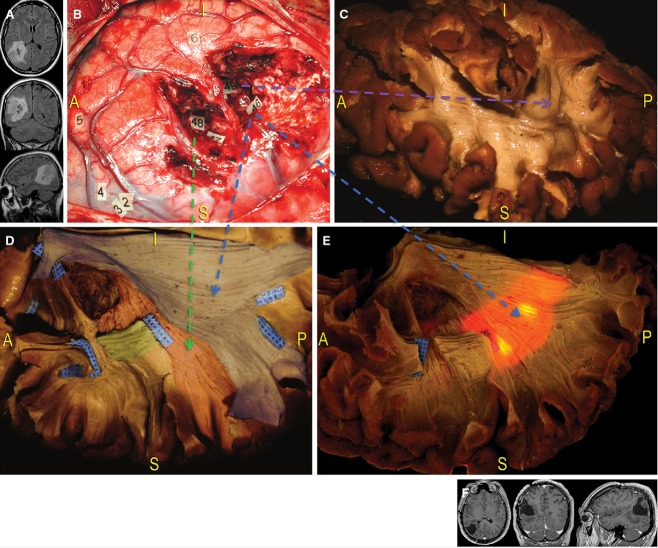
Case 3 (Fig. 13) involved a 42-year-old right-handed man with a history of seizures secondary to an infiltrative lesion of the right TPO junction; MRI follow-up indicated a tendency for progressive growth. During the preoperative evaluation, no impairments related to sensory-motor, visuo-spatial or language function were demonstrated.
Figure 13.
Case 3. (A) The third case concerned a diffuse infiltrative glioma located within the right not-dominant TPO junction, indicated by preoperative MRI. (B) Lesion resection was performed while guided by direct cortico-subcortical mapping in awake condition. This intraoperative photograph shows the locations functional responses were elicited using DES. At the cortical level, tag 1 corresponds to reproducible speech arrest episodes; paraesthesia was induced at the lip (tag 5), the thumb and index fingers (tag 4), and the fingers (tag 2). Interestingly, the patient experienced movements of the left hand in response to stimulation of a retro-central area (tag 3) near the region of sensory responses, indicating that there is overlap in areas responsible for sensory and motor functions. A significant and reproducible deviation of the bisection task occurred when stimulating the posterior portion of the AG (tag 6). At the subcortical level, resection extended up to the areas identified as eloquent WM boundaries, which constituted the real limits of surgery. Paraesthesia of the superior leg was induced at tags 47 and 48; deviation at the bisection line task was reproduced at tag 49; mesially, the resection reached the IPS; more posterior and deeply, complete speech arrest was induced during the denomination task (tag 46). (C–E) We correlated the functional intraoperative results with anatomical photographs revealing the WM pathways. Tags 47 and 48 correspond to the thalamo-cortical sensory fibres, occupying the posterior part of the IC and running directly up to the retro-central cortex (green arrow, red-coloured area; D); tag 49 designates the indirect temporo-parietal portion of the SLF, which is involved in visuo-spatial cognition (violet arrow; C); the visual and repetition deficits produced at tag 46 correspond with the location of the SS (blue-coloured area; D), which runs in an anterior-to-posterior direction along the lateral wall of the occipital horn and the trigone (blue arrows; D and E). Transillumination better revealed the close relationship between eloquent connectivity and the ventricular system, making it too dangerous to open the ventricle from this direction. (F) Post-operative MRI revealed a subtotal resection and the patient experienced only left inferior quadrantanopia, which did not compromise his socio-professional life. AG, angular gyrus; IC, internal capsule.
All patients underwent resection of tumours guided by cortico-subcortical DES performed according to the ‘asleep–awake–asleep’ protocol (De Benedictis et al. 2012). In the first stage, the tumour and the cerebral sulci and gyri were identified using ultrasonography. Cortical mapping was then performed to detect the eloquent areas, with stimulation applied with a bipolar electrode spacing of 5 mm (Nimbus; Newmedic) and delivering a biphasic current (pulse frequency 60 Hz, single pulse phase duration 1 ms, amplitude 1.5–4 mA). The stimulation threshold was determined after inducing complete speech arrest by stimulating the ventral pre-motor cortex (VPMC) during the counting test (regularly from 1 to 10 mA). Sensori-motor mapping was then performed to confirm the stimulation threshold with a positive response (paraesthesia or movement). Then, cortical mapping proceeded with the picture-naming test (DO.80) to identify essential cortical language sites. A speech therapist collected and warned the neurosurgeon of any language disturbances (i.e. speech arrest, anomia, phonetic paraphasia, phonemic paraphasia, semantic paraphasia, slowness with initiation disturbances and perseverations) during the cortico-subcortical mapping and LGG resection. Sterile number tags on the brain surface marked all the eloquent sites. An intraoperative picture of the cortical map was routinely collected before resection.
During the second surgical stage, the glioma was removed by alternating resection and subcortical DES, using the same stimulation parameters. The functional pathways were followed progressively from the cortical eloquent sites already mapped to the depth of the resection.
Intraoperative tasks were selected according to specific tumour locations or to portions of the same surgical cavity that bordered different or multimodal bundles. Visuo-spatial ability was tested using the line-bisection test; reading was evaluated by projecting a word sequence on a screen; the visual pathways were tested using a modified picture-naming task with two pictures placed diagonally on screen (in the case selected, on the left inferior and right superior quadrants). During the picture-naming task, the patient was asked to stare at a red cross at the centre of the screen and, at the same time, to name both items. In this way, both language and overall visual field could be tested. Visual field deficits were distinguished from language disorders when the patient experienced subjective transient visual disturbances within the contralateral visual hemifield (i.e. blurred vision, phosphenes, shadows), which prevented the patient from naming the picture situated in the contralateral quadrant but not the picture situated in the ipsilateral quadrant. Eventual saccades or other eye movements were checked by the speech therapist throughout the task.
Patients continued the selected tasks until resection approached the subcortical eloquent structures. Thus, the WM tracts involved in motor, somato-sensory, language, reading and visuo-spatial functions were detected. All resections continued until eloquent structures were encountered around the surgical cavity, allowing optimal tumour removal while preserving all functions. Thus, resection was performed with attention to the functional cortical and subcortical boundaries.
Results
Cortical dissection
The sulco-gyral anatomy of each specimen was carefully analysed. Special attention was given to the lateral aspect of the junction between the parietal, temporal and occipital lobes (Fig. 1). At the cortical level, the following structures were recognisable: the ascendant parietal gyrus, which is anteriorly separated from the pre-central gyrus (PreCG) by the central sulcus (CS) and, posteriorly, from the parietal lobe by the post-central sulcus (PostCS). The infra-parietal sulcus, which originates from the middle portion of the PostCS, divides the supero-lateral surface of the parietal region into two portions, the superior parietal lobule (SPL) and IPL. The IPL is divided into two gyri, the SMG and the AG, which are separated by an intermediate sulcus originating from the infra-parietal sulcus. The SMG surrounds the distal extremity of the superficial portion of the Sylvian fissure and inferiorly borders the most posterior portion of the superior temporal gyrus (STG). The AG ventrally borders the most posterior portion of the MTG. Finally, the infra-parietal sulcus continues within the occipital lobe with the intra-occipital sulcus, dividing the superior occipital gyrus (SOG) from the middle occipital gyrus (MOG). The latter is separated from the inferior occipital sulcus by the inferior occipital gyrus (IOG; Fig. 1).
Figure 1.
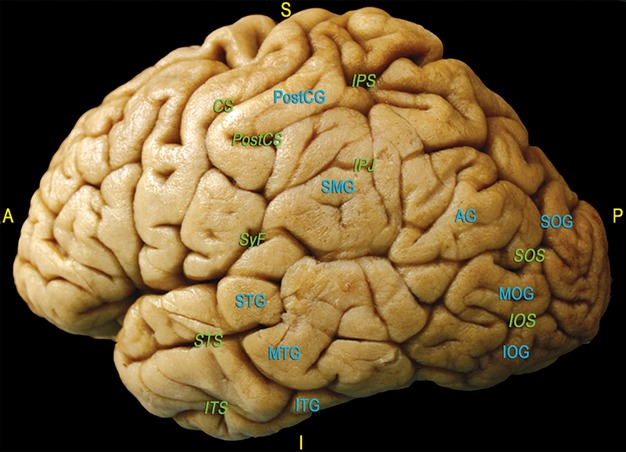
The main sulci and gyri of the lateral TPO region of a left hemisphere are shown in green and blue characters, respectively, after removal of vessels, pia mater and arachnoid membranes. CS, central sulcus; IOG, inferior occipital gyrus; IOS, inferior occipital sulcus; IPJ, intraparietal junction; IPS, intraparietal sulcus; ITS, inferior temporal sulcus; MOG, middle occipital gyrus; PostCG, post-central gyrus; PostCS, post-central sulcus; SMG, supramarginal gyrus; STS, superior temporal sulcus; MTG, middle temporal gyrus; ITS, inferior temporal gyrus; SyF, Sylvian fissure; AG, angular gyrus; SOG, superior occipital gyrus; SOS, superior occipital sulcus. A, anterior; P, posterior; I, inferior; S, superior.
Pathway dissection
U-fibres
We removed the cortical grey matter, revealing the short inter-gyral U-shaped fibres. Only the grey matter on the top of the gyri was preserved to facilitate identification of the superficial and deeper fibre terminations.
SLF
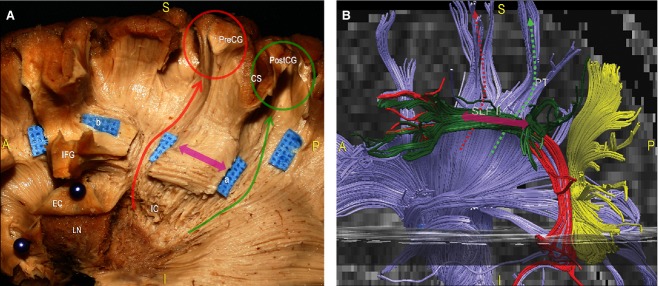
The first and more superficial pathway we exposed below the U-fibres was the SLF (Fig. 2). As demonstrated previously (Catani et al. 2005), this fascicle is composed of a direct and an indirect component, and provides connectivity between Wernicke's, Geschwind's and Broca's territories (Fig. 3).
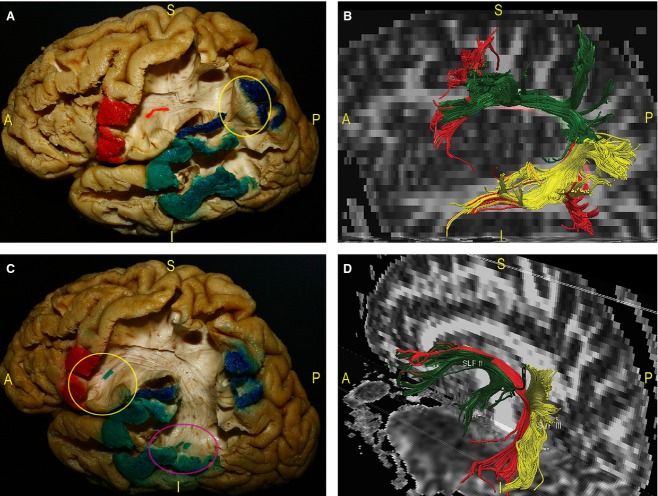
Figure 2.
This panel describes the most superficial long associative fascicle running through the TPO area, the SLF. Proceeding in a latero-medial direction during a left-hemisphere dissection, we located the indirect and direct components of the SLF, which is divided into one direct (AF) and two indirect anterior and posterior components (respectively, the SLF II and III). (A) Starting at the tempo-parietal junction, we identified a posterior group of temporo-parietal fibres (blue arrow) connecting the posterior third of the STG and MTG (pink circle) with the ventral part of the IPL (blue circle, red pin). (B) After removal of the lateral third of the central lobule, we followed the complete course of an anterior group of fibres (blue arrows) between the dorsal part of the IPL (green circle) and the posterior part of the STG (yellow circle) to the IFG. (C) We gently separated and lifted the posterior indirect component of the SLF from the deeper fibres of the AF (pink circle, pink pin). (D) We completed the exposure of the AF. These fibres directly connect the posterior part of the STG and MTG to the frontal lobe with a C-shaped course around the posterior insula beneath the IPL (red arrow). Within the frontal region we isolated the AF terminations (blue tags) directed to the Pop (green circle) and the pars triangularis (yellow circle) of the IFG and the MFG (violet circle). CS, central sulcus; IFG, inferior frontal gyrus; MTG, middle temporal gyrus; Op, opercularis pars of the IFG; PostCG, post-central gyrus; PreCG, pre-central gyrus; STG, superior temporal gyrus; Tr, pars triangularis of the IFG.
Figure 3.
We exposed the indirect and direct components of SLF during a dissection of a left hemisphere. The respective cortical termination territories have been coloured. Dissection pictures were also correlated with DTI reconstruction of the SLF. (A) Geschwind's territory corresponds to the IPL (blue-coloured cortex). It constitutes the ‘kissing’ point (yellow circle) between the posterior indirect SLF fibres, connecting Wernicke's territory (posterior part of the STG and MTG – green-coloured cortex), and the anterior indirect SLF component, running to Broca's territories (IFG – red-coloured cortex and red tag). (B) Fibre tracking of the SLF. The three components were distinguished by different colours: posterior indirect fibres (yellow); anterior indirect part (green); and direct component, that is, the AF (red). (C) The AF directly connects the temporal Wernicke's region (pink circle) to the frontal Broca's area (yellow circle), passing below Geschwind's territory (blue-coloured cortex). (D) The DTI reconstruction of the SLF was oriented to evaluate the relationships among the three components. The indirect fibres (SLF II and SLF III) are more superficial than the AF. AF, arcuate fasciculus; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; MTG, middle temporal gyrus; STG, superior temporal gyrus.
We started dissection of the more lateral indirect portion by removing the posterior third of the STG. Here, a group of fibres forming the SLF III and directly connecting the STG and MTG to the SMG and AG were identified (Figs 2A and 3B). Further resection of the ventral and dorsal cortices of the IPL revealed a separate bundle, running directly from the SMG to the ventro-posterior frontal territories and taking a more horizontal course (i.e. the SLF II; Figs 2B and 3B). To better follow this anterior indirect component of the SLF, we removed the ventral third of the central lobule to demonstrate the terminations of these fibres within the VPMC and at the level of the pars opercularis (Pop) of the IFG (Fig. 3A). We then removed these indirect layers starting at the temporo-parietal junction, demonstrating the deeper direct component of the SLF, the AF (Fig. 2C). This tract runs from the posterior and middle temporal lobe, arching around the posterior portion of the circular sulcus of the insula and underneath the IPL, to reach the posterior cortices of the IFG (Pop and VPMC) and middle frontal gyrus [MFG; the postero-inferior portion of the dorso-lateral prefrontal cortex (DLPFC); Figs 2D and 3C,D].
Basal ganglia, cortico-spinal tract and somato-sensorial thalamic radiation
We carefully analysed the basal ganglia region using stepwise dissection (Fig. 4). After removing the insular cortex and the extreme capsule, we exposed the grey matter of the claustrum (cls) and the WM underlying the external capsule (EC; Fig. 4A,B). We divided this region into three anatomical parts (the anterior, middle and posterior trapezoid; Fig. 4A) and analysed each subregion at different depths. Anteriorly (anterior trapezoid), we exposed the arch-shaped fibres of the uncinate fascicle (UF), connecting the temporal and the frontal poles (Fig. 4B). Postero-superiorly, we identified the IFOF stem. In the middle part (middle trapezoid), we left in situ the EC (Fig. 4C). At the level of the posterior part (posterior trapezoid), after removing the cls and the EC, we exposed the lenticular or lentiforn nucleus (LN), including the putamen laterally and the globus pallidus medially. This structure has a biconvex lens shape that appears like a wedge with the apex directed medially and the base directed laterally when viewed coronally. The LN is completely sunk into the deep hemispheric WM, separating the lateral EC from the medial internal capsule (IC; Fig. 4D). In this posterior dissection area, we removed the grey matter, exposing the fibres running on the superior margin of the LN into the genu and the posterior limb of the IC. Proceeding in a caudal-to-cranial direction, we followed these projection fibres related to the CR. We opened two ‘windows’ along the course of the SLF within the IFG to expose the CR fibres running to the PreCG and post-central gyrus (PostCG; Figs 4E and 5).
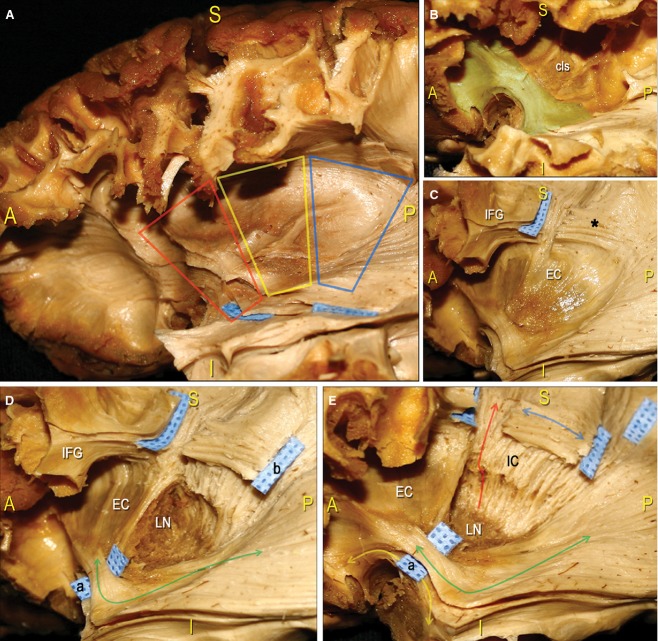
Figure 4.
In this series of pictures we performed a step-by-step dissection of the basal ganglia region of a left hemisphere, proceeding in a latero-medial direction. (A) After removal of the insular cortex, we considered three trapezoid-shaped regions within the subinsular area: anterior (red trapezoid); middle (yellow trapezoid); and posterior (blue trapezoid). (B) Within the anterior third, at the level of the limen insulae, we removed the grey matter of the cls and, on a deeper layer, exposed a thick WM stem corresponding to the point where two fascicles (i.e. UF and IFOF), which connect the frontal lobe to the other brain regions, converge (green-coloured WM area). (C) Within the middle third, we completely exposed the EC. More dorsally, we removed the posterior third of the frontal cortex and exposed the SLF fibres underneath the IFG (blue tag, black asterisk). (D) Within the posterior third, the EC was removed, exposing the grey matter of the LN (for a detailed analysis of the anatomy of the basal ganglia region, see Heimer, 2000 and Tamraz & Comair, 2006). In this panel, we also defined the posterior course of the IFOF (green arrow) from the limen insulae region (blue tag a) and of the SLF (blue tag b). (E) Finally, we exposed IC fibres, forming the CR, which take a vertical course (red arrow) at a deeper level than the horizontally oriented SLF (blue arrow; see also Fig. 8 and Chowdhury et al. 2010 for a more detailed analysis of IC connectivity). At the level of the limen insulae, we separated the IFOF (blue tag a), which runs from the frontal region to the posterior brain territories (green arrow), from the antero-ventral UF (yellow arrow), which connects the frontal and temporal poles. EC, external capsule; cls, claustrum; IC, internal capsule; IFG, inferior frontal gyrus; LN, lenticular/lentiform nucleus; UF, uncinate fascicle.
Figure 5.
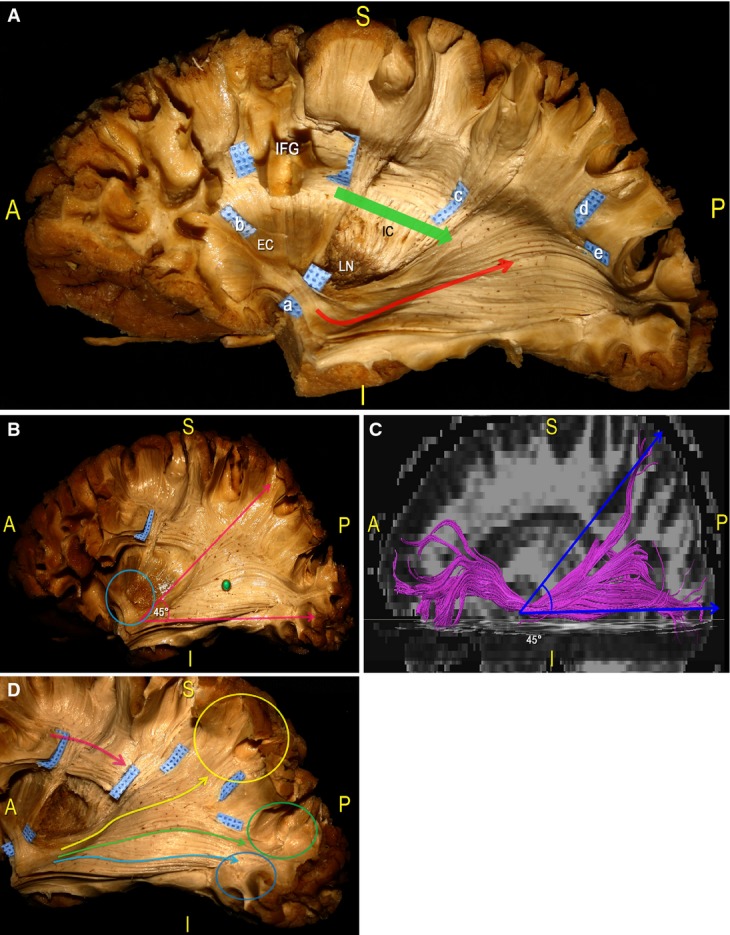
Dissection of a left hemisphere. (A) We started by identifying the IFOF at the level of the limen insulae, where this fascicle occupies the posterior two-thirds of the ventral EC (blue tag a). The fibres then take a horizontal, anterior-to-posterior course (red arrow) from the frontal lobe (blue tag b) to the parietal (blue tag d) and occipital (blue tag e) cortical terminations. The SLF, identified at the base of the IFG, has a superior-to-inferior direction to the TPO region. We cut the SLF at the level of the underlying IC fibres and before it crosses the IFOF (blue tag c). (B) In this panel we emphasised the IFOF posterior course. Starting from the limen insulae (blue circle), these fibres radiate, forming an angle of approximately 45 ° (pink arrows), and run along the lateral wall of the atrium (green pin) and the occipital horn of the lateral ventricle in the context of the SS. (C) DTI reconstruction of the IFOF, confirming the 45 ° angle formed by posterior fibres running directly to the parietal and occipital cortices. (D) Here, we showed the posterior course and terminations of the IFOF. Based on the report of Martino et al. (2010), we identified three groups of fibres, which ran directly to the parietal lobe (yellow arrow and circle), the SOG (green arrow and circle) and the MOG (blue arrow and circle). The SLF lies on a more superficial layer (pink arrow) than the IC fibres and the IFOF. EC, external capsule; cls, claustrum; IC, internal capsule; IFG, inferior frontal gyrus; LN, lenticular/lentiform nucleus; MOG, middle occipital gyrus; SOG, superior occipital gyrus.
IFOF
After removing the insular cortex, we identified the IFOF at the level of the limen insulae, within the antero-ventral portion of the EC.
We separated the IFOF stem from the UF stem, which is located antero-inferiorly, and proceeded with the dissection from the IFOF stem in the direction of the TPO region. To follow the course of the IFOF, we removed the temporal, parietal and occipital cortices as well as the WM of the SLF, which was interrupted at the level of the central lobule (Fig. 5A), to show the relationship with the deeper CR and IC fibres. From the limen insulae, the fibres run in an infero-lateral direction. The bundle passes over the anterior portion of the temporal isthmus to run posterior along the roof of the temporal horn and along the lateral surface of the atrium and the occipital horn of the lateral ventricle, within the stratum sagittalis (SS). Running from the anterior-to-posterior direction, the IFOF fibres progressively enlarge to describe a 45 ° angle, which leads to a wide cortical distribution within the parietal, occipital and temporal cortices (Fig. 5B). We identified the IFOF terminations, in fact, within the SPL, SOG, MOG and IOG, and within the temporo-basal region (Fig. 5C).
ILF
At the level of the temporal and occipital subcortical regions, we identified a consistent component of fibres with a cortico-cortical distribution, corresponding to the short fibres of the ILF (Fig. 6A). More deeply, we identified the long ILF fibres running along a horizontal postero-lateral course from the anterior and basal temporal cortices (strictly related to the temporal portion of the UF at this level), along the infero-lateral wall of the temporal horn of the lateral ventricle (Fig. 6B). At the level of the posterior third of their course, we revealed a consistent group of fibres running in an infero-superior and latero-medial direction to reach the dorso-lateral cortex of the occipital lobe (Fig. 6C).
Figure 6.
ILF dissection within a left hemisphere. The SLF (green arrows) has been lifted at the level of the TPO area (green pin) to expose the course of associative bundles underneath. (A) In this specimen we identified and separated from the SS (blue tags) the short fibres of the ILF, locating the cortico-cortical connections (pink arrows) between the temporo-basal (yellow circle) and occipital regions (blue circle). (B) The long ILF component (blue tag, white asterisk) has a parallel, but deeper and more ventral course than the IFOF (green arrow), and connects the temporal pole to the occipital cortex (pink arrows, asterisk, blue tag a). The roof of the temporal horn of the lateral ventricle has been opened and lifted (red circle, red pin), revealing the ILF course along the lateral ventricle wall. (C) In this picture we identified a group of ILF fibres turning in a ventro-dorsal, medio-lateral and antero-posterior direction (pink arrow), crossing the posterior third of the IFOF (green arrow, blue tags a and b) to terminate within the dorso-lateral cortex of the occipital lobe (pink circle). (D) DTI of the ILF, showing the fibres running from the temporal pole to the occipital lobe.
OR
We exposed the WM fibres of the OR, which represent the inferior and ventro-basal portion of the posterior thalamic radiation (Fig. 7). These fibres run along an anterior-to-posterior course from the medial temporal region to the cortices bordering the calcarine fissure on the medial surface of the occipital lobe, and along the lateral ventricular surface within the SS. At the level of the inferior margin of the ventricular trigone, we identified in all specimens a progressively divergent supero-inferior orientation of the fibres. The superior component terminates within the superior calcarine cortex (Fig. 7A). The inferior fibres have a more basal course and reach the cortex of the inferior calcarine sulcus. More anteriorly, these fibres surround the temporal tip and the basal part of the most anterior portion of the temporal horn and converge antero-medially to the lateral geniculate body, forming the so-called Meyer's loop (Fig. 7B,C).
Figure 7.
In this series the OR of a right hemisphere has been exposed. (A) The whole cortex of the lateral frontal, the insular, the TPO and the temporal areas has been removed, and the LN and the IC are shown along with the CR fibres. The IFOF stem (green area) has been preserved and cut at the level of the OR underneath. These fibres originate from the temporo-mesial area (the lateral geniculate body, not observed in this picture), have an antero-posterior course along the lateral ventricle surface, and terminate within the calcarine cortex on the medial occipital lobe (blue arrow). (B) We opened the temporal horn of the ventricle, exposing the CP. The OR runs along the ventricle roof (blue arrows). (C) After removal of the temporal horn of the lateral ventricle, we exposed the more ventral and basal OR fibres. In the posterior region, these fibres cover both the lateral part of the temporal horn and the inferior portions of the trigone, and are medially directed to the inferior calcarine cortex (blue arrow, blue circle). CP, choroidal plexus; IC: internal capsule; IFOF, inferior fronto-occipital fascicle; LN, lenticular/lentiform nucleus; OR, optic radiation.
Pathways orientation analysis
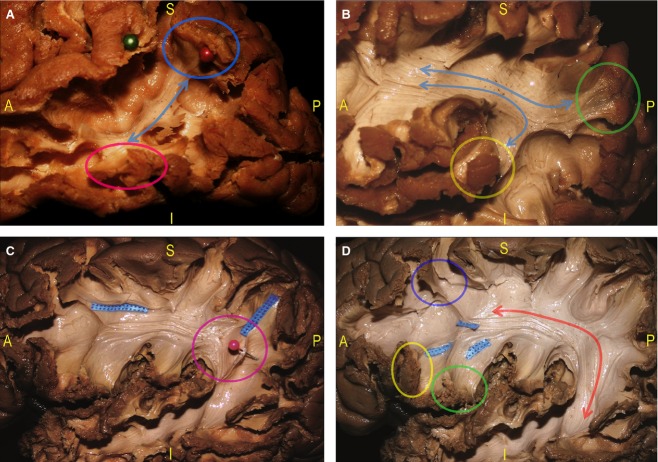
We analysed the multilayer relationships among the different nerve fascicles dissected within the TPO junction (Figs 3,9 and 10). Proceeding in a lateral-to-medial direction, we recognized: the SLF II and SLF III, the AF, the ILF, the IFOF, the OR, the CR and the IC. The indirect temporo-parietal (SLF III) and parieto-frontal fibres (SLF II) both have a parallel course with the direct component (AF; Fig. 3). Underneath the IPL and the temporo-parietal junction, we showed the superior-to-inferior vertical orientations compared with the antero-posterior horizontal position of the IFOF (Fig. 9).
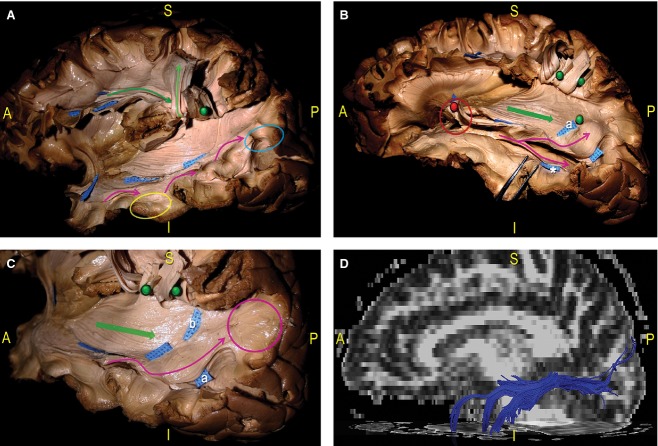
Figure 9.
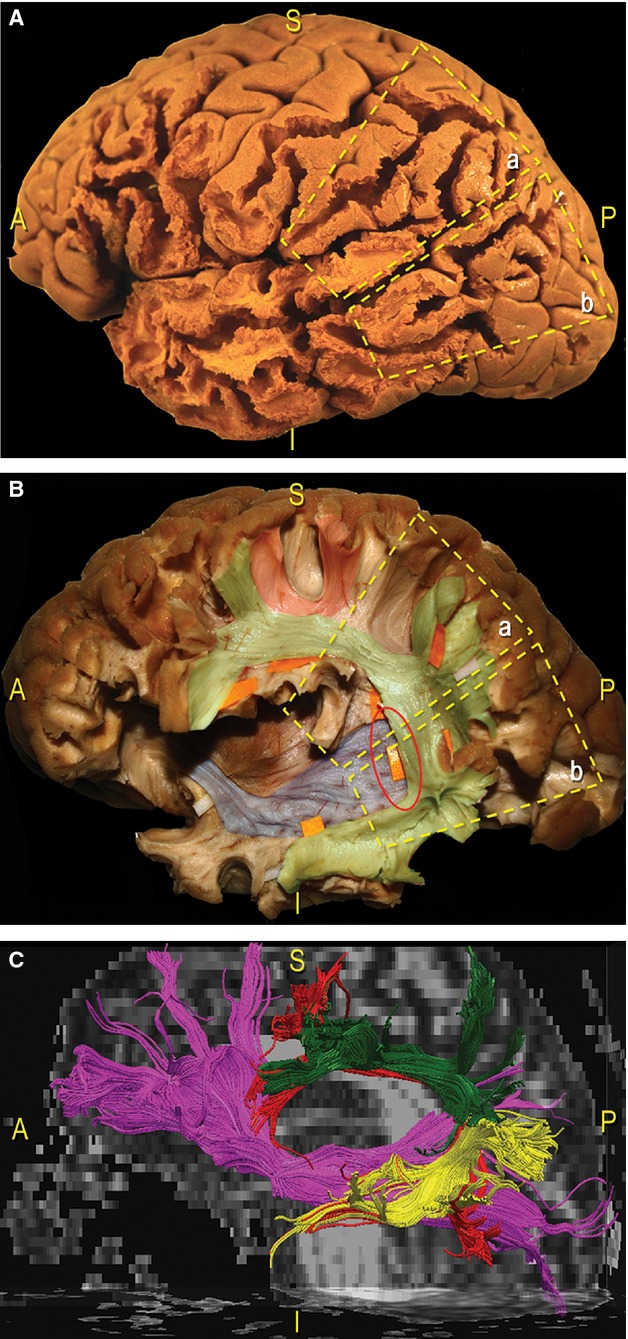
Schematic and coloured correlation between the lateral cortical surface and the subcortical organisation of the TPO region in a dissected left hemisphere. (A) We divided the cortices of the TPO junction into two trapezoidal areas: a, the SMG, posterior part of the STG; b, junction between the posterior MTG and ITG and AG and MOG, IOG. (B) We exposed the subcortical pathways underneath the same two trapezoidal areas we selected on the cortical surface. In particular, the dissection was focused on two main tracts: the SLF (green, orange tags), which connects the frontal, parietal and temporal regions along a vertical, C-shaped course around the posterior insular profile; the IFOF fibres (blue), which have a horizontal orientation running anterior-to-posterior, cross the SLF on a more medial layer (red circle). Loco-regional ‘U’ intergyral fibres were also revealed (red). (C) Fibre tracking of the IFOF (pink fibres) and the SLF (green, yellow: indirect component; red fibres: direct portion). AG, angular gyrus; MTG, middle temporal gyrus; TPO, temporo-parieto-occipital; IOG, inferior occipital gyrus; ITG, inferior temporal gyrus.
Figure 10.
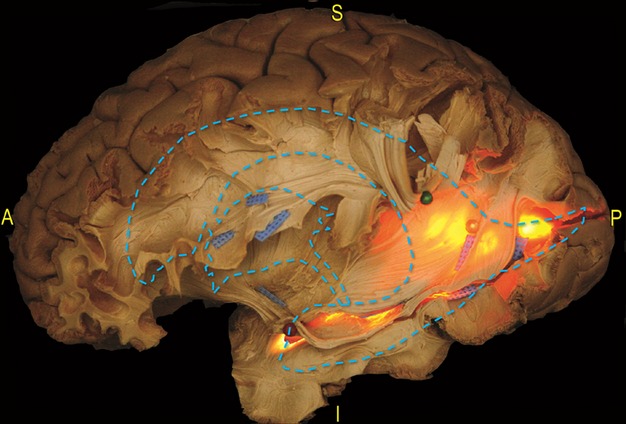
Dissection of a left hemisphere. This picture indicates the relationship between the main WM pathways and the ventricular system at the level of the TPO region. To reveal the ventricle cavity, a light source was applied at the atrium from the medial side of the hemisphere. A schematic profile of the ventricle system has also been superimposed onto the dissection photograph. The SLF and the AF have been lifted at the level of the SMG (green pin). This exposed the course of the SS from the temporal to the occipital region. The posterior portions of the IFOF and OR overlap the occipital horn (yellow pin) and the trigone and cross the posterior component of the ILF (blued tags) before the cortical terminations. The temporal horn tip has been opened and the Meyer's loop fibres, occupying the dorso-lateral wall of the temporal horn, have been lifted (red pin). AF, arcuate fasciculus; IFOF, inferior fronto-occipital fascicle; ILF, inferior longitudinal fascicle; OR, optic radiation; SLF, superior longitudinal fascicle; SMG, supramarginal gyrus.
We revealed that the fibres of the optic tract run just posterior to the main course of the UF, deeper than the IFOF fibres, in a posterior direction along the superior and lateral wall of the temporal horn. The OR and the IFOF run closely related courses. The OR, in fact, runs medially and in parallel to the IFOF along the entire course of both tracts (Fig. 10). In particular, the IFOF completely overlaps the OR fibres coming from the superior calcarine cortex and the two-thirds of the OR coming from the inferior calcarine cortex. At the level of the more posterior part of the SS, these tracts clearly diverge to reach their respective cortical termination areas.
Dissection indicated that the ILF runs inferiorly and laterally to the OR and the IFOF (Figs 6 and 7). At the level of the junction between the occipital, temporal and parietal lobes, the ILF fibres partially overlap and cross the two bundles (the OR and the IFOF; Fig. 6C). More anteriorly, these three tracts have divergent courses: the OR and the IFOF proceed in parallel, with the inferior OR fibres more ventral and basal in respect to the IFOF to reach the tip of the temporal horn of the lateral ventricle; the ILF, however, runs in an anterior and inferior direction to the temporo-basal cortices (Fig. 7C).
To demonstrate the location of the associative pathways with respect to the ventricular system, we applied a light source at the level of the atrium of the ventricle and observed the WM anatomy using a transillumination effect (Fig. 10). This revealed that the posterior course of the OR is more compact than the anterior fibres, which enlarge to completely envelop the temporal horn from the roof to the floor and around the tip. The IFOF fibres have a larger distribution within the occipital and parietal lobes. Within the most anterior and ventral portion of the EC, the IFOF fibres occupy the roof of the temporal horn up to the IFOF stem. In particular, the roof of the temporal horn and the lateral wall of the trigone and the occipital horn are covered by the IFOF, which at this spot overlaps the entire superior OR and the superior third of the inferior OR. The inferior portion of the lateral wall, the floor and the tip of the temporal horn of the ventricle are enveloped by the inferior part of the OR fibres, turning antero-medially forming the Meyer's loop (Fig. 7).
The projection fibres are located deeply with respect to the associative tracts. We exposed the course of these fibres from the IC backward in a superior direction, up to the PreCG and PostCG, showing that they run more perpendicular courses than the more superficial SLF (Figs 4E, 5 and 8).
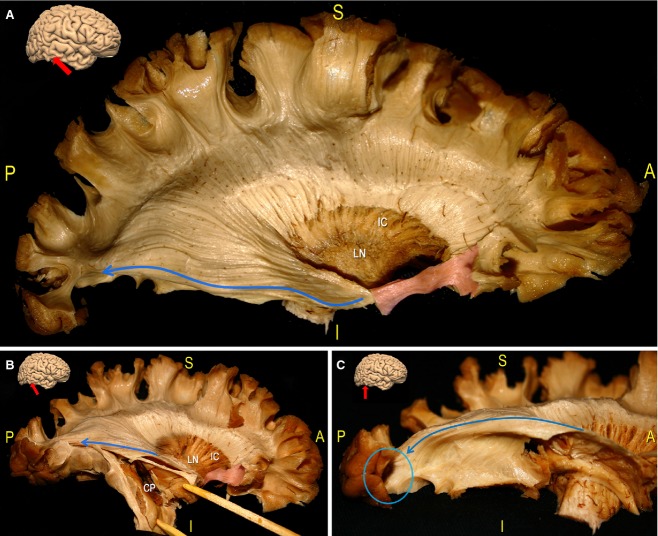
Figure 8.
Dissection of the CR of a left hemisphere. (A) According to the previous description of the subinsular area (see Fig. 4), we opened and lifted, using two green pins, the EC layer within the anterior third, exposing the LN. Posterior to that location, we removed LN grey matter and identified the posterior limb of the IC. In this way, the anatomical layer-by-layer organisation of the basal ganglia region was clearly summarised. We proceeded in a caudal-to-cranial direction, exposing the fibres of the CR coming from the superior margin of the LN. Then, we removed the IFG cortex at the level of the middle and posterior third, isolating the SLF (blue tag a and b, pink arrow). We opened two windows along the SLF to expose the course of fibres directly through the CR to the pre-central (red arrow) and post-central (green arrow) cortices. The posterior limb of the CR is a crucial node for projective connectivity; it contains ascending fibres of the posterior thalamic radiation to the cortex, cortico-spinal fibres to the motor nuclei of the upper and lower extremity and trunk, as well as cortico-rubral and cortico-pontine fibres. (For a more detailed analysis of CR anatomy, see Chowdhury et al. 2010.) (B) Fibre tracking of the CR (blue fibres), and the indirect (green, yellow fibres) and direct (red fibres) components of the SLF. As demonstrated by our dissections, the perpendicular orientation of the vertical projective pre-central (red arrows) and post-central (green arrow) fibres with respect to the supero-inferior and antero-posterior oriented SLF (pink arrow) was clearly indicated. CS, central sulcus; EC, external capsule; IC, internal capsule; IFG, inferior frontal gyrus; LN, lenticular/lentiform nucleus; PreCG, pre-central gyrus; PostCG, post-central gyrus.
DTI
We integrated the WM descriptions and routes revealed by post mortem Klingler's dissections with the in vivo main pathway fibre tracking information obtained using 60 direction DTI. We characterised the indirect (posterior and anterior) and direct (AF) components of the SLF (Fig. 3), the IFOF (Fig. 5) and the ILF (Fig. 6). The anatomical relationships between cortico-spinal and thalamo-cortical fibres and the SLF, and between the IFOF and the SLF (Figs 8 and 9, respectively) were highlighted. The anatomical relationships between the different pathways identified using post mortem dissection were confirmed by DTI reconstructions; no relevant inter-individual differences were detected between the six analysed hemispheres.
Surgical cases
Case 1
Resection boundaries corresponding to the cortico-subcortical eloquent structures identified using DES are shown in Fig. 11 and summarised in Table 1. Post-operative MRI indicated subtotal resection (Fig. 11F). Post-operative neurological examination was normal.
Table 1.
Summary of anatomical surgical boundaries and functional results provided by intraoperative mapping observed in three illustrative cases
| Cortical eloquent sites | Subcortical WM boundaries | ||||||
|---|---|---|---|---|---|---|---|
| Case no. | Age (years), sex | Tumour location | Surgical limits | Functional responses | Corresponding structures | Functional responses | Corresponding structures |
| 1 | M, 42 | Left parieto-temporal junction | Ant: RCS Lat: posterior part SMG Post, inf: STG, AG Med: IPS | 1,2 = speech arrest 3,4 = facial contraction 5 = hand contraction 6,9 = lips disesthesias 7,8 = first finger disesthesias | VPMC Primary motor area of the face Primary motor area of the hand Primary S-S area of the face Primary S-S area of the hand | 14 = dysphasia 12,13 = semantic paraphasias | Anterior indirect SLF AF |
| 2 | M, 60 | Left TO junction | Ant: MTG, STG Sup: STG Inf: FG | 1 = speech arrest 2 = anomia | VPMC Posterior STG | 46, 48 = semantic paraphasias, anomias 49 = reading trouble | IFOF ILF |
| 3 | M, 42 | Right TPO junction | Ant: RCG Inf: AG Mes: IPS Lat: MTG | 1 = speech arrest 2 = fingers disesthesias 3 = hand movement 4 = 1st and 2nd finger disesthesias 5 = lip disesthesias 6 = deviation at the line-bisection task | VPMC Primary somato-sensory area of the fingers Primary motor area of the hand Primary somato-sensory area of the fingers Primary somato-sensory area of the mouth Post STG | 46 = quadrantopsia, phosphenes, anomia 47 = inferior leg disesthesias 48 = superior leg disesthesia 49 = deviation at the line-bisection task | OR Thalamo-cortical fibres Thalamo-cortical fibres SLF |
AF, arcuate fasciculus; AG, angular gyrus; FG, fusiform gyrus; IFOF, inferior fronto-occipital fascicle; ILF, inferior longitudinal fascicle; IPS: intraparietal sulcus; MTG, medial temporal gyrus; OR, optic radiation; RCG, retro-central gyrus; RCS, retro-central sulcus; SLF, superior longitudinal fascicle; SMG, supramarginal gyrus; S-S, somato-sensory; STG, superior temporal gyrus; TPO, temporo-parieto-occipital; VPMC, ventral premotor cortex; WM, white matter.
Case 2
Resection boundaries corresponding to the cortico-subcortical eloquent structures detected using DES are shown in Fig. 12 and summarised in Table 1. MRI performed 3 months after surgery showed subtotal resection (Fig. 12E). The patient did not report post-operative deficits.
Case 3
Resection boundaries corresponding to the cortico-subcortical eloquent structures detected using DES are shown in Fig. 13 and summarised in Table 1. Post-operative MRI indicated subtotal resection (Fig. 13F). After surgery, the patient experienced left inferior quadrantanopia.
Discussion
Several studies indicate that, despite technical advances and the increasing use of intraoperative mapping in neurosurgical practice, a detailed understanding of WM organisation is mandatory to correctly interpret the functional responses and, consequently, to modulate resection according to a patient's specific pattern of connectivity. This is particularly true in cases of infiltrating lesions located within eloquent regions of the brain (De Benedictis et al. 2010; Duffau, 2013).
In a recent study, our team characterised the subcortical surgical anatomy of the lateral frontal region by correlating structural post mortem evidence with functional results of DES performed during approaches to the dominant frontal lobe (De Benedictis et al. 2012). Interestingly, although the TPO area is crucially involved in brain processing, a similar approach has rarely been used to study this region. Recently, Martino et al. used post mortem dissections and DTI reconstructions to identify WM fascicles running between the IPL and the posterior temporal lobe (Martino et al. 2013). Similarly, Peltier et al. performed a dissection analysis of the OR and its relationships with neighbouring WM tracts and the lateral ventricle (Peltier et al. 2006). However, in these studies less attention was devoted to providing a surgical perspective of anatomical findings.
This is, to our knowledge, the first work in which an integrated methodology has been applied to supply a detailed representation of the complex subcortical anatomo-functional architecture of the TPO junction, with special emphasis on WM organisation from a surgical perspective.
In the first part of the study, we performed high-quality dissections of different WM pathways and identified reciprocal relationships. Proceeding step-by-step from the lateral convexity to the depth, we recognised three layers of connectivity: (i) superficial U-fibres, which mediate loco-regional functional interactions; (ii) the intermediate associative fascicles (SLF, IFOF, ILF and OR), which horizontally integrate functional processing with more distant regions (Figs 5, 6 and 9); and (iii) deep ventral vertical fibres that converge on the IC and subserve projective extra-cerebral connectivity (Figs 4E, 5 and 8).
In the second part of the study, we outlined the complex subcortical organisation identified during lateral surgical resection of infiltrating gliomas in the TPO area. We considered three sub-regions: the IPL, the TO junction and the right non-dominant TPO junction. For each case, the functional data collected intraoperatively were related to anatomical data from the cadaveric dissections.
Left parieto-temporal junction
Recently, Maldonado et al. reported results of subcortical mapping performed during the resection of 14 gliomas infiltrating the IPL (Maldonado et al. 2011). The authors detected different, transitory, highly reproducible language disturbances that were induced by deactivating various eloquent bundles using DES. Our dissections offer clear evidence that WM components are implicated in these functional results, providing both systematic and surgical perspectives (Figs 2,3 and 1). Stimulation of the superficial WM of the antero-lateral part of the surgical cavity induced dysarthria or anarthria. As indicated in Fig. 11, this subcortical site corresponds to fibres running from the SMG to the VPMC and to the Pop of the IFG (Fig. 11D). The tract corresponds to the anterior part of the lateral indirect portion of the SLF (SLF II), and connects Wernicke's and Broca's territories. In this manner, it forms the ‘articulatory loop’, which is involved in verbal working memory and oro-facial motor control (Duffau et al. 2003; Makris et al. 2005).
At a deeper level, we elicited phonemic paraphasia and found that the effect resulted from direct stimulation of the supero-posterior portion of the AF (Fig. 11E). This fibre pathway directly connects the posterior and middle portions of the STG and MTG with the Pop and the pars triangularis of the IFG (Broca's area), and represents the dorsal phonological stream of language processing within the dominant hemisphere (Duffau et al. 2014). These results confirm the anatomo-functional segregation of the perisylvian WM network in parallel pathways, which subserve different components of language production (vocalisation vs. phonological processing); additionally, they are consistent with past DTI studies (Makris et al. 2005) and intraoperative evidence (Maldonado et al. 2011; Martino et al. 2012).
Left TO junction
When working in the left dominant TO area (Fig. 12), subcortical DES produced transient semantic disturbance sometimes associated with anomia (Fig. 12C, tags 46 and 48). Subcortical analysis of this region revealed that the stimulation occurred along a ventral section of the IFOF, running within the inferior portion of the SS to reach the inferior occipital region, the IOG and the basal temporal territory (Martino et al. 2010). A growing number of anatomical and clinical studies have confirmed the role this pathway plays in semantic language processing (Duffau et al. 2005; Martino et al. 2010; Sarubbo et al. 2013a).
Dissection of our specimens clearly indicated that the IFOF is strictly correlated with ILF fibres (Fig. 12D). Stimulation of the ILF systematically impaired reading ability at the level of the posterior surgical area. This tract is involved in both the direct and indirect transfer of information between the occipital visual territory and temporal limbic and memory areas, and subserves several aspects of visual input processing, such as face recognition, reading, visual perception and memory (Mandonnet et al. 2007, 2009; Catani & Thiebaut de Schotten, 2008). Moreover, it has been suggested that the ILF also plays a role in elaborating semantic aspects of language by providing an indirect connection between the occipital and the frontal language cortices, in association with the UF (Catani & Thiebaut de Schotten, 2008; Duffau et al. 2008; Martino et al. 2011).
Right non-dominant TPO area
We considered the resection of a glioma infiltrating the TPO junction of the non-dominant right hemisphere (Fig. 13A). Cortical mapping allowed identification of sensory-motor responses according to a medio-lateral somatotopic distribution (Fig. 13B). Interestingly, overlap between the cortical representation of sensitive and motor areas was detected, which confirms that anatomo-functional segregation around the CS is not rigid (Duffau et al. 2000). These responses occurred at a location that constituted the anterior resection limit at the subcortical level, where we encountered thalamo-cortical somato-sensorial fibres corresponding to the upper (tag 47) and inferior (tag 48) limb.
Stimulation of the posterior part of the STG at its junction with the AG generated a significant reproducible deviation during the line bisection test (tag 6). Our dissections (Fig. 13C) indicate that the infero-lateral limit of resection was the SLF (tag 49), which connects the AG and the DLPFC and the MFG/IFG. As previously demonstrated, these fibres are crucially involved in a wide fronto-parietal network, mainly lateralised on the right non-dominant hemisphere, and are associated with visuo-spatial and attentional processing (Makris et al. 2005; Thiebaut de Schotten et al. 2005; Bartolomeo et al. 2007; Doricchi et al. 2008; Tavor et al. 2014).
Medially, tumour removal advanced until bounding the intraparietal sulcus (IPS). Transient left phosphenes in association with complete anomia (tag 46) were systematically elicited during the visuo-verbal tests at the deep and posterior edges of resection. Our dissection indicated (Fig. 13D), along with trans-ventricular illumination (Fig. 13E), that this location corresponds to the lateral part of the OR, which runs in an antero-posterior direction along the wall of the lateral ventricle medially to the SLF and the IFOF. Stimulation not only impaired visual transmission but also affected language elaboration. Such a result depends on the close relationship between visual and language processing pathways, particularly the IFOF, which runs at this level (Duffau et al. 2008; Martino et al. 2011; Gras-Combe et al. 2012).
Conclusions
In this anatomical and functional study, we demonstrated the implications that a detailed understanding of the subcortical WM architecture within the TPO area has for neurosurgical practice.
Our data confirm that standard post mortem dissections, when combined with tractographic results, can be used both to accurately characterise the course of individual fibre pathways and to more specifically investigate complex reciprocal relationships with other fibre tracts. Using these techniques, the complex multilayer connectivity that subserves the TPO region has been showed.
The second part of the study highlighted the anatomical features responsible for our functional data, which was produced while directly mapping the TPO during surgical resection of infiltrating lesions. We provided comprehensive pictures that reveal the sites of those functional responses and the corresponding WM structures, primarily at the subcortical level.
In fact, the cortical limits may show inter-individual variability and depend on the natural history of the disease (particularly in cases of slowly progressing intra-axial tumours), as well as on potential compensatory reshaping. On the other hand, the limited plastic potential of WM requires that subcortical fibres be carefully preserved and considered the true surgical boundaries. In summary, we revealed that the anterior limit of resections within the parietal region is the anterior horizontal part of the SLF and, more deeply, the AF. More deeply and dorsally, these fibres cross the vertical projective thalamo-cortical fibres. When approaching more ventral lesions that involve the temporal and occipital territories, we demonstrated that resection should be tailored according to the orientation of horizontal associative pathways, including the IFOF, the ILF and the OR. These tracts run along parallel and partially overlapping courses.
We suggest that the results of the current study refine and extend the understanding of subcortical functional anatomy related to the TPO junction. This improved knowledge will be of particular benefit for neurosurgeons, allowing them to optimise the quality of resection of tumours located within the TPO region, and to reduce the risk of post-operative functional deficiencies. Moreover, we provide evidence for selecting the most appropriate functional task for brain mapping and for correctly interpreting intraoperative functional data based on the expected pattern of connectivity. Finally, these findings indicate that resection should be extended and stopped only when encountering the eloquent boundaries (i.e. at the functional limit) to increase surgical quality and minimise the risk of permanent deficits.
Further studies, based on an integrated methodological approach, including anatomical post mortem observations, in vivo fibre tracking and functional intraoperative mapping, will continue increasing three-dimensional awareness of anatomo-functional WM organisation in other districts of the brain, optimising the quality of neurosurgical management.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Duffau H. Mapping of visuospatial functions during brain surgery: a new tool to prevent unilateral spatial neglect. Neurosurgery. 2007;61:E1340. doi: 10.1227/01.neu.0000306126.46657.79. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Chica AB. Brain networks of visuospatial attention and their disruption in visual neglect. Front Hum Neurosci. 2012;4:110. doi: 10.3389/fnhum.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Sporns O. Human connectomics. Curr Opin Neurobiol. 2012;72:144–153. doi: 10.1016/j.conb.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O, Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. The Neuroscientist. 2005;11:16–24. doi: 10.1177/1073858404270885. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH, Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Bizzi A, et al. Beyond cortical localization in clinico-anatomical correlation. Cortex. 2012a;48:1262–1287. doi: 10.1016/j.cortex.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Vergani F, et al. Short frontal lobe connections of the human brain. Cortex. 2012b;48:273–291. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M, Slater D, et al. Connectomic approaches before the connectome. Neuroimage. 2013;80:2–13. doi: 10.1016/j.neuroimage.2013.05.109. [DOI] [PubMed] [Google Scholar]
- Chowdhury F, Haque M, Sarkar M, et al. White fiber dissection; the internal capsule: a cadaveric study. Turkish Neurosurgery. 2010;20:314–322. doi: 10.5137/1019-5149.JTN.3052-10.2. [DOI] [PubMed] [Google Scholar]
- De Benedictis A, Duffau H. Brain hodotopy: from esoteric concept to practical surgical applications. Neurosurgery. 2011;68:1709–1723. doi: 10.1227/NEU.0b013e3182124690. [DOI] [PubMed] [Google Scholar]
- De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery. 2010;66:1074–1084. doi: 10.1227/01.NEU.0000369514.74284.78. [DOI] [PubMed] [Google Scholar]
- De Benedictis A, Sarubbo S, Duffau H. Subcortical surgical anatomy of the lateral frontal region: human white matter dissection and correlations with functional insights provided by intraoperative direct brain stimulation: laboratory investigation. J Neurosurg. 2012;117:1053–1069. doi: 10.3171/2012.7.JNS12628. [DOI] [PubMed] [Google Scholar]
- Deprez S, Vandenbulcke M, Peeters R, et al. The functional neuroanatomy of multitasking: combining dual tasking with a short term memory task. Neuropsychologia. 2013;51:2251–2260. doi: 10.1016/j.neuropsychologia.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, et al. White matter (dis)connections and gray matter (dys)functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex. 2008;44:983–995. doi: 10.1016/j.cortex.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Duffau H. New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity – a review. J Neurooncol. 2006;79:77–115. doi: 10.1007/s11060-005-9109-6. [DOI] [PubMed] [Google Scholar]
- Duffau H. The anatomo-functional connectivity of language revisited: new insights provided by electrostimulation and tractography. Neuropsychologia. 2008;46:927–934. doi: 10.1016/j.neuropsychologia.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Duffau H. Does post-lesional subcortical plasticity exist in the human brain? Neurosci Res. 2009;65:131–135. doi: 10.1016/j.neures.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Duffau H. Awake surgery for nonlanguage mapping. Neurosurgery. 2010;66:523–529. doi: 10.1227/01.NEU.0000364996.97762.73. [DOI] [PubMed] [Google Scholar]
- Duffau H. Surgical neurooncology is a brain networks surgery: a ‘connectomic’ perspective. World Neurosurg. 2013;14 doi: 10.1016/j.wneu.2013.02.051. doi: 10.1016/j.wneu.2013.02.051. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Duffau H, Sichez JP, Lehéricy S. Intraoperative unmasking of brain redundant motor sites during resection of a precentral angioma. Evidence using direct cortical stimulations. Ann Neurol. 2000;47:132–135. [PubMed] [Google Scholar]
- Duffau H, Denvil D, Lopes M, et al. Intraoperative mapping of the cortical areas involved in multiplication and subtraction: an electrostimulation study in a patient with a left parietal glioma. J Neurol Neurosurg Psychiatry. 2002;73:733–738. doi: 10.1136/jnnp.73.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Denvil D, et al. The articulatory loop: study of the subcortical connectivity by electrostimulation. NeuroReport. 2003;14:2005–2008. doi: 10.1097/00001756-200310270-00026. [DOI] [PubMed] [Google Scholar]
- Duffau H, Velut S, Mitchell MC, et al. Intra-operative mapping of the subcortical visual pathways using direct electrical stimulations. Acta Neurochir (Wien) 2004;146:265–270. doi: 10.1007/s00701-003-0199-7. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, et al. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Duffau H, Thiebaut de Schotten M, Mandonnet E. White matter functional connectivity as an additional landmark for dominant temporal lobectomy. J Neurol Neurosurg Psychiatry. 2008;79:492–495. doi: 10.1136/jnnp.2007.121004. [DOI] [PubMed] [Google Scholar]
- Duffau H, Moritz-Gasser S, Mandonnet E. A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 2014;131:1–10. doi: 10.1016/j.bandl.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Fehr T, Code C, Herrmann M. Common brain regions underlying different arithmetic operations as revealed by conjunct fMRI-BOLD activation. Brain Res. 2007;1172:93–102. doi: 10.1016/j.brainres.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Fernández Coello A, Duvaux S, De Benedictis A, et al. Involvement of the right inferior longitudinal fascicle in visual hemiagnosia: a brain stimulation mapping study. J Neurosurg. 2013;118:202–205. doi: 10.3171/2012.10.JNS12527. [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Catani M. Beyond localization: from hodology to function. Philos Trans R Soc Lond B Biol Sci. 2005;360:767–779. doi: 10.1098/rstb.2005.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras-Combe G, Moritz-Gasser S, Herbet G, et al. Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J Neurosurg. 2012;117:466–473. doi: 10.3171/2012.6.JNS111981. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway ID, Price GR, Ansari D. Common and segregated neural pathways for the processing of symbolic and nonsymbolic numerical magnitude: an fMRI study. Neuroimage. 2010;49:1006–1017. doi: 10.1016/j.neuroimage.2009.07.071. [DOI] [PubMed] [Google Scholar]
- Klingler J. Erleichterung der makroskopischen praeparation des gehirns durch den gefrierprozess. Schweiz Arch Neurol Psychiatr. 1935;36:247–256. [Google Scholar]
- Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Maldonado IL, Moritz-Gasser S, de Champfleur NM, et al. Surgery for gliomas involving the left inferior parietal lobule: new insights into the functional anatomy provided by stimulation mapping in awake patients. J Neurosurg. 2011;115:770–779. doi: 10.3171/2011.5.JNS112. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, et al. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Gatignol P, Duffau H. Evidence for an occipito-temporal tract underlying visual recognition in picture naming. Clin Neurol Neurosurg. 2009;111:601–605. doi: 10.1016/j.clineuro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Winkler PA, Duffau H. Direct electrical stimulation as an input gate into brain functional networks: principles, advantages and limitations. Acta Neurochir (Wien) 2010;152:185–193. doi: 10.1007/s00701-009-0469-0. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, et al. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Vergani F, et al. Cortex-sparing fiber dissection: an improved method for the study of white matter anatomy in the human brain. J Anat. 2011;219:531–541. doi: 10.1111/j.1469-7580.2011.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Berger MS, et al. Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct. 2012;218:105–121. doi: 10.1007/s00429-012-0386-5. [DOI] [PubMed] [Google Scholar]
- Martino J, da Silva-Freitas R, Caballero H, et al. Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Neurosurgery. 2013;72:87–97. doi: 10.1227/NEU.0b013e318274294b. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. The neurobiology of language and verbal memory: observations from awake neurosurgery. Int J Psychophysiol. 2003;48:141–146. doi: 10.1016/s0167-8760(03)00051-5. [DOI] [PubMed] [Google Scholar]
- Peltier J, Travers N, Destrieux C, et al. Optic radiations: a microsurgical anatomical study. J Neurosurg. 2006;105:294–300. doi: 10.3171/jns.2006.105.2.294. [DOI] [PubMed] [Google Scholar]
- Platel H. Functional neuroimaging of semantic and episodic musical memory. Ann N Y Acad Sci. 2005;1060:136–147. doi: 10.1196/annals.1360.010. [DOI] [PubMed] [Google Scholar]
- Price GR, Ansari D. Symbol processing in the left angular gyrus: evidence from passive perception of digits. Neuroimage. 2011;57:1205–1211. doi: 10.1016/j.neuroimage.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Lee M, Chang TT, Young CB, et al. Functional dissociations between four basic arithmetic operations in the human posterior parietal cortex: a cytoarchitectonic mapping study. Neuropsychologia. 2011;49:2592–2608. doi: 10.1016/j.neuropsychologia.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SM, Elliott R, Forshaw D, et al. Resection of parietal lobe gliomas: incidence and evolution of neurological deficits in 28 consecutive patients correlated to the location and morphological characteristics of the tumor. J Neurosurg. 2005;103:1010–1017. doi: 10.3171/jns.2005.103.6.1010. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Asami M, Mannen T. Alexia and agraphia with lesions of the angular and supramarginal gyri: evidence for the disruption of sequential processing. J Neurol Sci. 2010;288:25–33. doi: 10.1016/j.jns.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Sampaio A, Soares JM, Coutinho J, et al. The Big Five default brain: functional evidence. Brain Struct Funct. 2013;24 doi: 10.1007/s00429-013-0610-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sanai N, Martino J, Berger MS. Morbidity profile following aggressive resection of parietal lobe gliomas. J Neurosurg. 2012;116:1182–1186. doi: 10.3171/2012.2.JNS111228. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Latini F, Sette E, et al. Is the resection of gliomas in Wernicke's area reliable?: Wernicke's area resection. Acta Neurochir (Wien) 2012a;154:1653–1662. doi: 10.1007/s00701-012-1416-z. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Le Bars E, Moritz-Gasser S, et al. Complete recovery after surgical resection of left Wernicke's area in awake patient: a brain stimulation and functional MRI study. Neurosurg Rev. 2012b;35:287–292. doi: 10.1007/s10143-011-0351-4. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado I, et al. Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013a;218:21–37. doi: 10.1007/s00429-011-0372-3. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Basso G, Chioffi F, et al. Technical, anatomical, and functional study after removal of a symptomatic cavernous angioma located in deep Wernicke's territories with cortico-subcortical awake mapping. Case Rep Neurol Med. 2013b;2013:835029. doi: 10.1155/2013/835029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarone P, Gatignol P, Guillaume S, et al. Agraphia after awake surgery for brain tumor: new insights into the anatomo-functional network of writing. Surg Neurol. 2009;72:223–241. doi: 10.1016/j.surneu.2008.10.074. [DOI] [PubMed] [Google Scholar]
- Sporns O. Network of the Brain. Cambridge, MA: The MIT Press; 2011. pp. 31–73. [Google Scholar]
- Sporns O. Structure and function of complex brain networks. Dialogues Clin Neurosci. 2013;15:247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamraz JC, Comair YG. Atlas of Regional Anatomy of the Brain Using MRI: with Functional Correlations. Berlin Heidelberg: Springer; 2006. pp. 207–209. [Google Scholar]
- Tavor I, Yablonski M, Mezer A, et al. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. Neuroimage. 2014;86:123–130. doi: 10.1016/j.neuroimage.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226–2228. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Viegas C, Moritz-Gasser S, Rigau V, et al. Occipital WHO grade II gliomas: oncological, surgical and functional considerations. Acta Neurochir (Wien) 2011;153:1907–1917. doi: 10.1007/s00701-011-1125-z. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Zarnhofer S, Braunstein V, Ebner F, et al. The influence of verbalization on the pattern of cortical activation during mental arithmetic. Behav Brain Funct. 2012;12(8):13. doi: 10.1186/1744-9081-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Z, Fang H, Liu J. The hierarchical brain network for face recognition. PLoS ONE. 2013;8:e59886. doi: 10.1371/journal.pone.0059886. [DOI] [PMC free article] [PubMed] [Google Scholar]