Abstract
Context
Many patients with unresectable pancreatic and peripancreatic cancer require treatment for malignant biliary obstruction. We performed a meta-analysis of randomized trials comparing immediate biliary stent placement and immediate surgical biliary bypass in patients with unresectable pancreatic and peripancreatic cancer.
Objectives
To conduct a meta-analysis of the English language literature (1985 through 2011) comparing those two treatments and analyze hospital utilization patterns.
Methods
After identifying five randomized controlled trials comparing immediate biliary stent placement and immediate surgical biliary bypass, we performed a meta-analysis for dichotomous outcomes, using a random effects model. We compared resource utilization in terms of the number of hospital days before death, by reviewing high-quality literature.
Results
379 patients were identified. We found no statistically significant differences in success rates between the two treatments (risk ratio [RR], 0.99; 95% confidence interval [CI], 0.93 to 1.05, P = 0.67). Major complications and mortality were not significantly higher after surgical bypass (RR, 1.54; 95% CI, 0.87 to 2.71; P = 0.14). Recurrent biliary obstruction was significantly less frequent after surgical bypass than after stent placement (RR, 0.14; 95% CI, 0.03–0.63; P < 0.01). Despite similar overall survival rates, longer survival was associated with more hospital days before death in stent patients than in surgical patients.
Conclusions
Nearly all patients with unresectable pancreatic cancer benefit from some procedure to manage biliary obstruction. Patients with low surgical risk may benefit more from surgery because the risk of recurrence and subsequent hospital utilization are much lower than for stent patients.
Keywords: pancreatic cancer, biliary bypass, biliary stent
Introduction
Despite the advent of multidisciplinary cancer therapy, pancreatic adenocarcinoma and peripancreatic cancer are still among the most morbid and lethal cancers in the United States. The only potential for cure remains resection, but even after a presumably curative resection, the median survival time is only 25 to 34 months.1–3 The 5-year survival rate of patients whose cancer is deemed unresectable for cure (per radiologic imaging or intraoperative findings) is less than 5%.2, 4
Biliary obstruction is a major potential consequence of pancreatic and peripancreatic cancer. At the time of diagnosis, more than 70% of patients have biliary obstruction and require intervention.5, 6 Since the late 1970s, endoscopic management of biliary obstruction has been increasingly used, rather than open abdominal surgical procedures.7, 8 But the recent literature has suggested that biliary stent placement, followed by a planned surgical biliary bypass for curative resection after metabolic correction, may be associated with worse outcomes than early or immediate surgical biliary bypass.9 Up to 50% of patients with unresectable pancreatic cancer eventually require an intervention for gastric outlet obstruction as well.5, 10
Surgical management of biliary obstruction typically consists of a bypass from the common bile duct or gallbladder to the duodenum or jejunum.6, 11 Endoscopic or percutaneous stent procedures typically include placement of a covered or uncovered metal or plastic stent from the proximal or mid common bile duct into the duodenum.
For our meta-analysis, we examined published randomized trials to determine the ideal treatment for biliary obstruction in patients with unresectable pancreatic or peripancreatic cancer. Specifically, we performed a meta-analysis of randomized controlled trials comparing stent placement and surgical bypass in the palliative management of malignant biliary obstruction due to pancreatic or peripancreatic cancer. We also performed a meta-analysis of inpatient utilization rates to estimate whether those 2 treatments differ in costliness.
Methods
Search strategy
We searched PubMed, CINAHL [Cumulative Index to Nursing and Allied Health Literature], and Web of Science for articles, using combinations of these relevant keywords: biliary bypass, biliary stent, biliary obstruction, and pancreatic/peripancreatic cancer. Limits we placed on the search included English language, randomized controlled trials, and human studies. In addition, we sought referenced articles if they existed outside of those 3 major databases. Publication dates for inclusion were 1985 through 2011. We followed the PRISMA [Preferred Reporting Items for Systematic Reviews and Meta-Analyses] protocol, which evolved from the QUOROM [QUality Of Reporting Of Meta-analyses] protocol for systemic reviews and meta-analyses.12
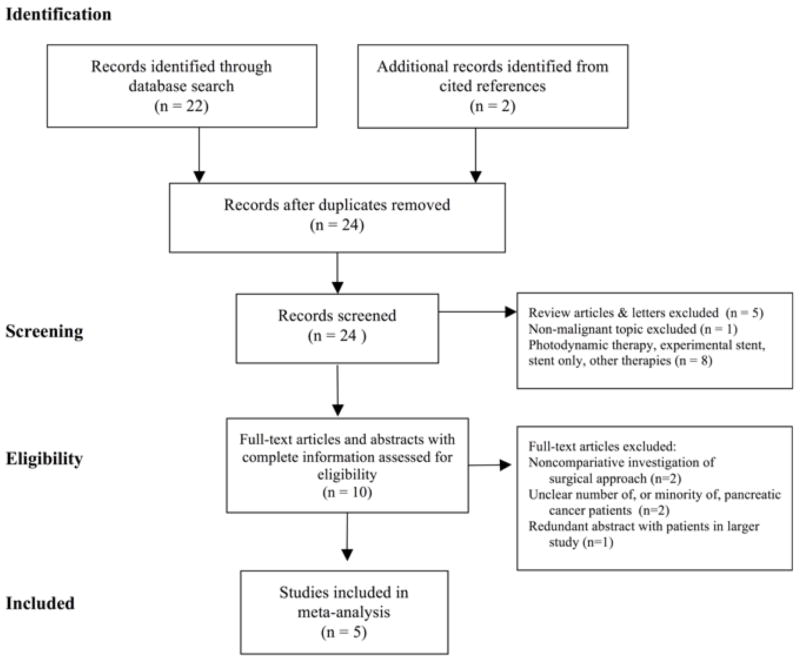
Article selection
Initially, we found 24 publications (Figure 1). We excluded articles with nonrandomized study designs, review articles, articles containing an unspecified number of patients with pancreatic or peripancreatic cancer, and articles comparing only different types of stents or only different types of surgical operations.
Figure 1.
Search Strategy and Article Inclusion/Exclusion Criteria (Modified from Moher et al., PRISMA Statement, 200912)
Major outcomes
The major outcomes that we investigated were technical success of the procedures, major complications of the procedures, mortality, and recurrent biliary obstruction. All of the articles that we included defined technical success and major complications. Major complications included life-threatening hemorrhage, pneumonia, liver failure, pulmonary embolus, cholangitis, severe acute renal failure, and peritonitis. Biliary recurrence was defined by the need for any biliary procedure (surgical or endoscopic), for any reason, after the index procedure.
Statistical analysis
To perform our meta-analysis, we used Review Manager version 5.1.1 (Cochrane Collaboration, Copenhagen, Denmark). We coded outcomes as dichotomous variables and used the Mantel-Haenszel statistical method with a random effects model. We assumed that small random differences between studies were likely due to variations in local practices, variations in patient health patterns across the world, and variation in treatments over time. To compare the median survival time and the total number of hospital days, we used a paired Student t test. Statistical significance was set at α ≤0.05.
Results
Randomized controlled trials
For our meta-analysis, 5 randomized controlled trials13–17 met the final inclusion criteria (Figure 1). The total number of patients was: 191 in the surgical arm and 188 in the stent arm. Variations in the technical aspects of the surgical procedures and in the types of stents are highlighted in Table 1. One of the trials compared surgery to percutaneous stent placement,15 while the other 4 compared surgery to endoscopic stent placement. In addition, the surgical procedures were slightly different in each trial: overall, 60.9% of surgical patients underwent gastric bypass (either simultaneous with or subsequent to biliary bypass), and 27.9% of stent patients underwent gastric bypass.15–17 We do not know whether such procedures were prophylactic or therapeutic. Nor do we know whether endoscopic (i.e., gastroduodenal) maneuvers were performed initially or during follow-up.
Table 1.
Randomized Controlled Trials18–23: Biliary Stent Placement vs. Surgical Biliary Bypass for Obstruction
| Authors | Year | N (surgery) | Surgical Procedure | N (stent) | Stent Type |
|---|---|---|---|---|---|
| Andersen et al. | 1989 | 25 | Choledochoduodenostomy | 25 | Endoscopic Teflon |
| Artifon et al. | 2006 | 15 | Choledochojejunostomy | 15 | Endoscopic Metal |
| Bornman et al. | 1986 | 25 | Cholecystenterostomy or hepaticojejunostomy | 25 | Percutaneous Polyethylene |
| Shepherd et al. | 1988 | 25 | Cholecystostomy or choledochoduodenostomy | 23 | Endoscopic Polyethylene |
| Smith et al. | 1994 | 101 | Choledochoduodenostomy or cholecystojejunostomy | 100 | Endoscopic Teflon |
| Total patients | 191 | 188 |
Technical success
The 2 treatments had essentially equal technical success rates: surgical bypass, 89.5% (171/191) and stent placement, 91.0% (171/188) (P = 0.67, Figure 2). Neither treatment was demonstrated to be technically superior to the other (all 95% CIs included 1.0). The 5 trials were not heterogeneous in terms of technical success (I2 = 0%, P = 0.67).
Figure 2.
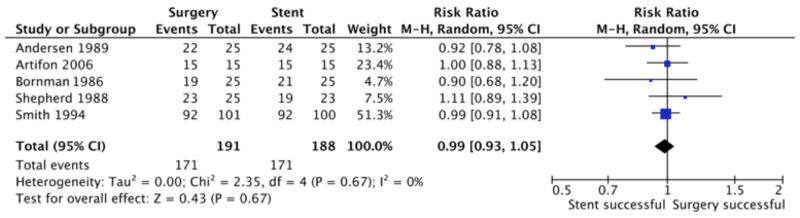
Meta-Analysis of Technical Success
Mortality, morbidity, and survival time
According to our meta-analysis, the 30-day mortality rate for surgical patients was 15%; for stent patients, 12% (P = 0.40). About 39% (75/191) of surgical patients had major complications or died, as compared with 21.2% (40/188) of stent patients (RR, 1.54; 95% CI, 0.87 to 2.71; P = 0.14; Figure 3). The 5 trials were not heterogeneous in terms of major complications or death (I2 = 61%, P = 0.03). We analyzed recurrent biliary obstruction as a separate endpoint, not as a major complication.
Figure 3.
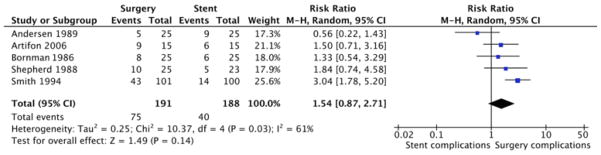
Meta-Analysis of Major Complications and Mortality
The average survival time after randomization was 120 ± 37 days for surgical patients; 129 ± 27 days, for stent patients. The difference was not statistically significant (P = 0.57). Complications in the endoscopic group include gastrointestinal bleeds, cholangitis, renal failure and pneumonia. Complications in the surgical group include gastrointestinal bleeds, wound infections, and gastrointestinal obstructions, pulmonary embolus, and stroke. In one article, bleeding was more common in the endoscopic group while non-cholangitis infections were more common in the surgery group.15
Recurrent obstruction
Of the 5 trials, 4 demonstrated that recurrent biliary obstruction was more frequent after stent placement than after surgical bypass; 1 trial demonstrated no difference (Figure 4). Overall, the RR was 0.14 (95% CI, 0.03 to 0.63) in favor of less frequent obstruction after surgical bypass, as compared with stent placement; the difference was statistically significant (P = 0.01). The 5 trials were heterogeneous (I2 = 63%, P = 0.04) in terms of recurrent obstruction. According to our meta-analysis, only 3.1% (6/191) of surgical patients had recurrent obstruction (requiring re-intervention), as compared with 28.7% (54/188) of stent patients (Figure 4). The incidence was 9 times higher in stent patients.
Figure 4.
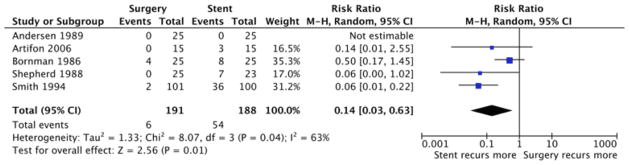
Meta-Analysis of Recurrent Obstruction
Hospital days
The total medical costs of all interventions to treat biliary obstruction are based on multiple factors and thus will vary widely. Nonetheless, the total number of hospital days to treat index and recurrent biliary obstruction can be a useful marker for the relative total costs between the 2 treatment modes (surgical bypass and stent placement), because the number of hospital days represents the most costly category of medical care utilization. In the 5 trials, the average length of stay for the index surgical bypass was 21.8 ± 5.8 days, as compared with 14.6 ± 9.3 days for the index stent placement (P = 0.026). We do not have data regarding the total number of hospital days from the index procedure until death. But in our literature review, we noted a 2-fold increase, for stent patients, in the total number of hospital days from the index procedure until death (Table 2).
Table 2.
Meta-Analysis and Literature Review of Nonrandomized Clinical Trials18–23: Outcomes after Index Procedures for Biliary Obstruction
| Index Procedure | Stent Placement | Surgical Bypass |
|---|---|---|
| Estimated obstruction rate after index procedure until death | 29% (range, 10%–40%) | 3% (range, 0%–10%) |
| Treatment for recurrence | ||
| Surgical | 18% | <1% |
| Endoscopic/percutaneous | 82% | 75% |
| Medical | <1% | 25% |
| Total hospital days from index procedure until death (range) | 30–35 days | 10–22 days |
Discussion
According to our meta-analysis of 5 randomized controlled trials, surgical biliary bypass and biliary stent placement are equivalent in efficacy, with similar average survival times and with statistically equivalent rates of major morbidity and mortality. Currently, in most institutions, stent placement is standard for patients with biliary obstruction due to tumors, even if a curative resection in the future is likely. A 3rd option is medical management, consisting of pain control, symptom control, and palliative care. It is an alternative for patients who are too sick for either stent placement or surgical bypass or for patients who elect to not endure further interventions. Chemotherapy for palliation or down-staging is another option but none of the articles utilized in this meta-analysis investigated this approach.
However, in our meta-analysis, we found that stent patients (as compared with surgical bypass patients) were 9 times more likely to experience recurrent biliary obstruction and to need further procedures, such as endoscopic biliary stent revision. (Figure 4, Table 2). Furthermore, according to our literature review, other studies have shown that the total number of hospital days from the index procedure until death was higher in stent patients (Table 2).18–23
While surgical biliary bypass is clearly an inpatient procedure necessitating a considerable hospital stay (22 days), it is surprising that the length of stay for stent procedures was nearly 15 days. While many current stent procedures may be accomplished on an out-patient or short hospital stay, in these studies the LOS associated with stent placement may also be related to a patient population with cancer-related biliary obstruction. In addition, while LOS may be a surrogate for cost, it has clear limitations that only a prospective trial can delineate.
Of the 5 trials in our meta-analysis, only the most recent trial (Artifon et al., 200614) used metal stents; the other 4 used plastic stents. This represents a limitation of our literature review and meta-analysis. It is possible that the use of modern covered metal endoscopic stents could improve results. According to a meta-analysis by Moss et al. of 13 studies,24 metal stents were associated with a mean patency of 165 days, as compared with only 123 days for plastic stents (P < 0.05). Still, surgical bypass is associated with far fewer recurrent obstructions and increased patency rates; the idea that modern metal stents are far superior to older metal stents (and perhaps to surgical bypass) has not been demonstrated in the literature.
Another limitation of our meta-analysis was that so few trials—only 5— met our strict inclusion criteria. Specifically, we limited our search to full reports of randomized controlled trials, and thus excluded published abstracts and prospective cohort studies. We analyzed only high-quality studies. In addition, the studies analyzed (Table 1) were heterogeneous, with no de facto standard for the biliary bypass procedure. Procedure-specific variations may well be the reality of clinical practice, but they clearly make comparisons difficult. Since the articles utilized were all randomized trials, heterogeneous patient populations and selection bias (i.e., selecting patients with poor fitness for stent over surgery) are an unlikely source of bias. However, the relatively small patient population in this analysis remains a potential limitation.
Pain control as a component of quality life is an important issues that the articles in this meta- analysis did not explore. For patients undergoing surgery, chemical celiac block is an optional adjunct during the operation while post operative celiac blockade can be performed by interventional radiologists or with ionizing radiation therapy.5 In some studies, operative celiac plexus ablation is associated with decreased pain, narcotic use, and increase quality of life for up to 12 months after surgical identification of unresectable pancreatic cancer.5, 25 Furthermore, long-term benefit is seen in up to 90% of patients without major complications.26 Percutaneous and endoscopic approaches performing the above are often complimentary to surgical celiac plexus chemical ablation in that they can be performed postoperatively if inadequate pain relief is initially found or if pain later develops.
Finally, the treatment of gastric outlet obstruction was unclear in the 5 trials investigated. About 20% of patients treated for biliary obstruction will eventually require therapy for gastric outlet obstruction;20, 22, 28, 29 the rate is as high as 50% in some centers.5, 10 Concurrent gastric bypass at the time of biliary bypass could theoretically increase benefits, with minimal increases in operative risk, and could even decrease the total number of hospital days if gastric outlet obstruction is averted.30 While not an endpoint of the studies in this meta-analysis, 45%–85% of patients undergoing biliary bypass had some sort of simultaneous enteric bypass as well in these studies.13, 15–17
Survival time is typically predicted by portents of a poor prognosis: high levels of CA19-9/CEA tumor markers, rapid tumor growth, ascites or peritoneal carcinomatosis, hypoalbuminemia, elevated Ki-67 expression, and elevated VEGF expression.28, 29, 31 Each of those factors independently decreases survival by about 3 to 4 weeks, and these factors may help clinicians recommend specific therapies based on the presence of these factors in a given patient.
Our meta-analysis provides the first evidence that surgical biliary bypass may be more cost-effective than endoscopic biliary stent placement. A significant limitation to this conclusion is the use of LOS as the marker for cost. It is the only marker of costs consistently described in the articles and remains a fairly objective measurement. Our major finding is that recurrent biliary obstruction is significantly more likely after stent placement than after surgical bypass. Importantly, percutaneous transhepatic biliary stent placement may be even more cost-effective than surgery,32 indicating the need for further study to pinpoint the “gold standard” treatment for patients with unresectable pancreatic and peripancreatic cancer.
Conclusion
Even though most patients with pancreatic and peripancreatic cancer present too late for curative resection, nearly all will benefit from procedures to manage biliary obstruction, thereby improving the quality of their remaining life. For pancreatic and peripancreatic cancer patients expected to live at least 6 months, we recommend surgical biliary bypass with a concomitant gastric emptying procedure. However, if survival is expected to be under 4 months, we recommend therapeutic endoscopic stent placement, since such patients are unlikely to outlive a covered metal biliary stent. If survival is expected to be between 4 and 6 months, the appropriate treatment is unclear; covered metal biliary stents might last long enough, but other types of stents likely would not.
Acknowledgments
Funding: ESG was funded by the medical sciences graduate program at the University of Arizona. RSK is partially funded by a National Cancer Institute (NCI) Institutional Core Grant (P01 CA27502). Additional funding was also provided by the University of Arizona Department of Surgery and by Sun Capital Partners Foundation, LLC. MCH was supported by several NCI grants (R01 CA114204 and R01 CA114204-03S; RC2 CA148185; U19 CA79689). Given requirements with National Institutes of Health (NIH) funding, the authors request that the manuscript be submitted to PubMed Central in compliance with the publisher’s embargo.
The authors would like to acknowledge Mary Wagner and Mary Knatterud, PhD, for assistance with manuscript preparation.
Footnotes
Disclosures: All authors declare that they have no conflicts of interest. Portions of this research were presented as a Scientific Paper (oral presentation) at the Annual Clinical Congress of the American College of Surgeons in October 2012.
References
- 1.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009 Jul;16(7):1736–1744. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 2.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008 Jul 20;26(21):3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 3.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fusai G, Warnaar N, Sabin CA, Archibong S, Davidson BR. Outcome of R1 resection in patients undergoing pancreatico-duodenectomy for pancreatic cancer. Eur J Surg Oncol. 2008 Dec;34(12):1309–1315. doi: 10.1016/j.ejso.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Kruse EJ. Palliation in pancreatic cancer. Surg Clin North Am. 2010 Apr;90(2):355–364. doi: 10.1016/j.suc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Scott EN, Garcea G, Doucas H, Steward WP, Dennison AR, Berry DP. Surgical bypass vs. endoscopic stenting for pancreatic ductal adenocarcinoma. HPB (Oxford) 2009 Mar;11(2):118–124. doi: 10.1111/j.1477-2574.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lefebvre AC, Maurel J, Boutreux S, et al. Pancreatic cancer: Incidence, treatment and survival trends-1175 cases in Calvados (France) from 1978 to 2002. Gastroenterologie Clinique Et Biologique. 2009 Oct;33(10–11):1045–1051. doi: 10.1016/j.gcb.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Linder S, Bostrom L, Nilsson B. Pancreatic cancer in sweden 1980–2000: a population-based study of hospitalized patients concerning time trends in curative surgery and other interventional therapies. J Gastrointest Surg. 2006 May;10(5):672–678. doi: 10.1016/j.gassur.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010 Jan 14;362(2):129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 10.Huser N, Michalski CW, Schuster T, Friess H, Kleeff J. Systematic review and meta-analysis of prophylactic gastroenterostomy for unresectable advanced pancreatic cancer. Br J Surg. 2009 Jul;96(7):711–719. doi: 10.1002/bjs.6629. [DOI] [PubMed] [Google Scholar]
- 11.Marrazzo A, Casà L, David M, et al. Surgical palliation for malignant obstructive jaundice: our experience. Supportive & Palliative Cancer Care. 2006;2(2):65–70. [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Medicine. 2009 Jul;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen JR, Sorensen SM, Kruse A, Rokkjaer M, Matzen P. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989 Aug;30(8):1132–1135. doi: 10.1136/gut.30.8.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artifon EL, Sakai P, Cunha JE, et al. Surgery or endoscopy for palliation of biliary obstruction due to metastatic pancreatic cancer. The American journal of gastroenterology. 2006 Sep;101(9):2031–2037. doi: 10.1111/j.1572-0241.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- 15.Bornman PC, Harries-Jones EP, Tobias R, Van Stiegmann G, Terblanche J. Prospective controlled trial of transhepatic biliary endoprosthesis versus bypass surgery for incurable carcinoma of head of pancreas. Lancet. 1986 Jan 11;1(8472):69–71. doi: 10.1016/s0140-6736(86)90719-1. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd HA, Royle G, Ross AP, Diba A, Arthur M, Colin-Jones D. Endoscopic biliary endoprosthesis in the palliation of malignant obstruction of the distal common bile duct: a randomized trial. The British journal of surgery. 1988 Dec;75(12):1166–1168. doi: 10.1002/bjs.1800751207. [DOI] [PubMed] [Google Scholar]
- 17.Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994 Dec 17;344(8938):1655–1660. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 18.Garcia Sanchez MV, Lopez Vallejos P, Perez de Luque D, et al. Biliopancreatic tumors: patient survival and quality of life after palliative treatment. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva. 2004 May;96(5):305–314. doi: 10.4321/s1130-01082004000500003. [DOI] [PubMed] [Google Scholar]
- 19.Kim HO, Hwang SI, Kim H, Shin JH. Quality of survival in patients treated for malignant biliary obstruction caused by unresectable pancreatic head cancer: surgical versus non-surgical palliation. Hepatobiliary & pancreatic diseases international : HBPD INT. 2008 Dec;7(6):643–648. [PubMed] [Google Scholar]
- 20.Kuhlmann KF, van Poll D, de Castro SM, et al. Initial and long-term outcome after palliative surgical drainage of 269 patients with malignant biliary obstruction. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2007 Aug;33(6):757–762. doi: 10.1016/j.ejso.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 21.McGrath PC, McNeill PM, Neifeld JP, et al. Management of biliary obstruction in patients with unresectable carcinoma of the pancreas. Annals of surgery. 1989 Mar;209(3):284–288. doi: 10.1097/00000658-198903000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott EN, Garcea G, Doucas H, Steward WP, Dennison AR, Berry DP. Surgical bypass vs. endoscopic stenting for pancreatic ductal adenocarcinoma. HPB : the official journal of the International Hepato Pancreato Biliary Association. 2009 Mar;11(2):118–124. doi: 10.1111/j.1477-2574.2008.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunpaweravong S, Ovartlarnporn B, Khow-ean U, Soontrapornchai P, Charoonratana V. Endoscopic stenting versus surgical bypass in advanced malignant distal bile duct obstruction: cost-effectiveness analysis. Asian journal of surgery / Asian Surgical Association. 2005 Oct;28(4):262–265. doi: 10.1016/S1015-9584(09)60357-2. [DOI] [PubMed] [Google Scholar]
- 24.Moss AC, Morris E, MacMathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane database of systematic reviews. 2006;(2):CD004200. doi: 10.1002/14651858.CD004200.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercadante S, Catala E, Arcuri E, Casuccio A. Celiac plexus block for pancreatic cancer pain: factors influencing pain, symptoms and quality of life. J Pain Symptom Manage. 2003 Dec;26(6):1140–1147. doi: 10.1016/j.jpainsymman.2003.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Moore JC, Adler DG. Celiac plexus neurolysis for pain relief in pancreatic cancer. J Support Oncol. 2009 May-Jun;7(3):83–87. 90. [PubMed] [Google Scholar]
- 27.Lillemoe KD, Sauter PK, Pitt HA, Yeo CJ, Cameron JL. Current status of surgical palliation of periampullary carcinoma. Surg Gynecol Obstet. 1993 Jan;176(1):1–10. [PubMed] [Google Scholar]
- 28.Ansari D, Rosendahl A, Elebro J, Andersson R. Systematic review of immunohistochemical biomarkers to identify prognostic subgroups of patients with pancreatic cancer. The British journal of surgery. 2011 Aug;98(8):1041–1055. doi: 10.1002/bjs.7574. [DOI] [PubMed] [Google Scholar]
- 29.Mehta J, Prabhu R, Eshpuniyani P, Kantharia C, Supe A. Evaluating the efficacy of tumor markers CA 19–9 and CEA to predict operability and survival in pancreatic malignancies. Tropical gastroenterology : official journal of the Digestive Diseases Foundation. 2010 Jul-Sep;31(3):190–194. [PubMed] [Google Scholar]
- 30.Lillemoe KD, Cameron JL, Hardacre JM, et al. Is prophylactic gastrojejunostomy indicated for unresectable periampullary cancer? A prospective randomized trial. Annals of surgery. 1999 Sep;230(3):322–328. doi: 10.1097/00000658-199909000-00005. discussion 328–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi JH, Lee J, Park SH, et al. A prognostic model to predict clinical outcomes with first-line gemcitabine-based chemotherapy in advanced pancreatic cancer. Oncology. 2011;80(3–4):175–180. doi: 10.1159/000328449. [DOI] [PubMed] [Google Scholar]
- 32.Yao LQ, Tang CW, Zheng YY, Feng WM, Huang SX, Bao Y. Percutaneous Transhepatic Biliary Stenting vs. Surgical Bypass in Advanced Malignant Biliary Obstruction: Cost-Effectiveness Analysis. Hepato-gastroenterology. 2012 May 29;60(121) doi: 10.5754/hge12324. [DOI] [PubMed] [Google Scholar]