Abstract
Objectives
The aim of this study was to investigate the longitudinal effect of work-related stress, sleep deficiency and physical activity on 10-year cardiometabolic risk among an all-female worker population.
Methods
Data on patient care workers (n=99) was collected two years apart. Baseline measures included: job stress, physical activity, night work and sleep deficiency. Biomarkers and objective measurements were used to estimate 10-year cardiometabolic risk at follow-up. Significant associations (P<0.05) from baseline analyses were used to build a multivariable linear regression model.
Results
The participants were mostly white nurses with a mean age of 41 years. Adjusted linear regression showed that having sleep maintenance problems, a different occupation than nurse, and/or not exercising at recommended levels at baseline increased the 10-year cardiometabolic risk at follow-up.
Conclusions
In female workers prone to work-related stress and sleep deficiency, maintaining sleep and exercise patterns had a strong impact on modifiable 10-year cardiometabolic risk.
Keywords: Cardiometabolic risk, Nurses, Sleep maintenance, Physical activity, Follow-up, work-family conflict, sleep
Introduction
Cardiovascular diseases are the leading cause of adult death in the world, with approximately 32 % and 27 % of adult female and male fatalities in 2004 attributed to cardiovascular diseases [Mathers, et al. 2008]. In the U.S., recent statistics show that heart diseases and strokes caused 34 % of all deaths and represented a total annual cost of 475.3 billion dollars; making cardiovascular disease the foremost cause of death and the third-most expensive disease in America [Roger, et al. 2011]. The last decade has seen both increased awareness and reduction of the incidence and prevalence of cardiovascular disease, but it remains a major burden [Backe, et al. 2012].
Recent research has identified sleep quality and sleep duration as important factors in cardiovascular disease risk [Buxton and Marcelli 2010, Laugsand, et al. 2013]. Indeed, insufficient sleep duration and poor sleep quality appear to contribute to increased cardiovascular disease risk [Buxton and Marcelli 2010, Cappuccio, et al. 2010], and have been linked to elevated body mass index [Hasler, et al. 2004] [Kohatsu, et al. 2006], weight gain [Patel, et al. 2004, Patel, et al. 2008], obesity [Buxton, et al. 2013, Cizza, et al. 2005, Gangwisch, et al. 2005, Taheri, et al. 2004], and diabetes mellitus [Ayas, et al. 2003, Buxton, et al. 2013, Gottlieb, et al. 2005]. Additionally, modulations of cardiovascular function by sleep duration have been demonstrated showing elevations in blood pressure [Lusardi, et al. 1999, Meier-Ewert, et al. 2004], which is consistent with observed associations of sleep duration with blood pressure [Gottlieb, et al. 2006, Stranges, et al. 2010]. Perhaps most importantly, past research has shown that short sleep (<6 hours per day) predicts premature mortality [Dew, et al. 2003, Grandner, et al. 2010, Kripke, et al. 2002, Patel, et al. 2004, Wingard and Berkman 1983]. Long sleep duration (>8 hours per day) may also be associated with cardiovascular risk [Gallicchio and Kalesan 2009] [Buxton and Marcelli 2010, Hale and Do 2007].
Shift work, particularly night shift work, has been associated with sleep deficiency and has shown a dose-response relationship with coronary heart disease [Kawachi, et al. 1995, Åkerstedt 2003]. A vulnerable population with regards to both sleep deficiency and shift work are patient care workers [Buxton, et al. 2012, Kawachi, et al. 1995]. A recent study demonstrated that 57 % of a large patient care worker cohort reported sleep deficiency, specifically a lack of quality, duration and/or perceived sufficiency of sleep [Buxton, et al. 2012]. The Nurses Health Study showed a 4 % increase in the hazard ratio for ischemic heart disease with every 5 years nurses worked rotating night shifts [Brown, et al. 2009]. Furthermore, a large-scale, prospective study on the same population of married nurses demonstrated a significant increase in mortality risk in those who reported either short or long sleep duration [Patel, et al. 2004]. This increased risk was associated with hypertension [Patel, et al. 2004], and other studies have shown the independent association of short and long sleep duration with coronary events in this cohort [Ayas, et al. 2003].
Patient care workers also report significantly higher psychosocial stress than other occupational groups [Evans and Steptoe 2002]. When compared with other workers, nurses report more job strain due to higher demands and lower skill utilization [Evans and Steptoe 2002]. In addition, patient care workers report high levels of work-family conflict, an important cause of perceived stress [Kim, et al. 2012].
There is a well-established association between work-related stress and risk of cardiovascular disease [Backe, et al. 2012, Eller, et al. 2009, Schnall, et al. 2009], but the effect has been claimed to be significantly less than that of traditional risk factors like amount of physical activity [Kivimaki, et al. 2012, Steptoe and Kivimäki 2012].
The concept of work-related stress is constantly evolving. A recent review highlights the need for a broader understanding of how it relates to cardiovascular risk [Backe, et al. 2012]. Work-family conflict has been identified as a major cause of work-related stress in several studies, and has been implicated as a contributing factor to cardiovascular risk [Bellavia and Frone 2005, Berkman, et al. 2010]. The conflict of roles at work and in one’s personal life is recognized as an independent factor from job strain and effort-reward imbalance [Bhui, et al. 2012, Netemeyer, et al. 1996], and might contribute to our understanding of the psychosocial work-environment in cardiovascular risk research [Frone, et al. 2011]. Two recent systematic reviews on cardiovascular risk and workplace stressors also underline the need for more longitudinal studies on female populations [Backe, et al. 2012, Eller, et al. 2009]. The association between high demands, low control and low co-worker support (iso-strain) at work and cardiovascular risk is consistently found in men [Belkic, et al. 2004], but the evidence in women is less conclusive [Backe, et al. 2012]. To address this, we focus the current analysis on a sample of women.
A study on a patient worker population combining several different measures of work stress controlled by other confounding factors such as physical activity [Hublin, et al. 2010], sleep duration [Chandola, et al. 2010] and night-work hours [Yang, et al. 2006].
In the current study we test the hypothesis that work-related stress, sleep deficiency and physical activity over time influences modifiable 10-year cardiometabolic risk in a female worker population. More specifically, we investigate the predictive associations of baseline characteristics on modifiable cardiometabolic risk factors in a female worker population, and investigate the longitudinal effects of iso-strain, work-family conflict, night work, physical activity and sleep deficiency on a non-self reported, modifiable cardiometabolic risk score [Marino, et al. 2014], in a female patient care worker population.
Materials and Methods
Study Design
This study had a longitudinal design where data was collected at two time-points from a group of patient care workers at a large hospital in Boston. Baseline collection spanned from October 2009 to January 2010 and the second was a follow-up from August to November 2011 (Figure 1). All participants provided written informed consent. Research protocols were approved by the designated institutional review board and consistent with the principles in the Declaration of Helsinki.
Figure 1.
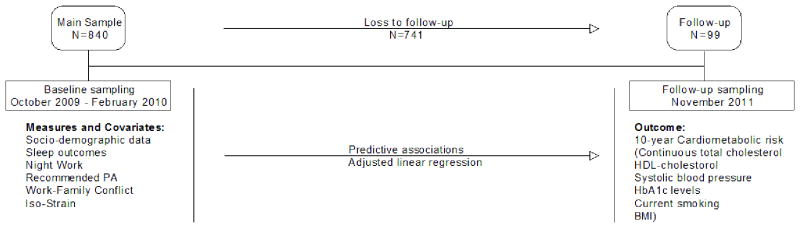
Study Sample and Data collection
Data collection at baseline has been described in previous articles [Buxton, et al. 2012] [Sorensen, et al. 2011] [Kim, et al. 2012]. In brief, the data presented at baseline was collected through a cross-sectional survey of patient care workers at two major hospitals in 2009. In this initial data collection, we randomly selected 2000 eligible workers and invited them via e-mail to participate in the survey on-line. Of these patient care workers initially contacted, 1572 participants completed at least 50 % of the survey. When collecting follow-up data, only employees from one of the two hospitals were contacted. Employees who completed the initial survey at this hospital (n=840) were randomized and divided into six equal groups. Each group received an initial contact via e-mail, reminding employees of their participation in the original survey and asking about their interest in the second phase of the study. If not responsive after the initial contact, employees received up to 7 additional contacts via email. A small number of subjects who expressed interest but subsequently became unresponsive were re-contacted by phone. Subjects who completed phase two of the study were compensated $100. Out of 840 employees who were re-contacted after completing the survey, a total of 99 (11.8 %) females completed the phase-two visit and blood draw. A low participation rate at time two was expected as this was a much more comprehensive sampling with in-person collection of both biomarkers and self-report data. Test statistics (frequency distributions and mean differences) showed that these responders were similar to the overall sample on important baseline socio-demographic characteristics, with race/ethnicity (described below) being the only significant difference.
Measures
Registered nurses measured height, weight, and blood pressure, and collected blood samples. Blood pressure was taken using a calibrated, clinical-standard arm cuff (Welch Allyn Spot Vital Signs monitor model# 4200B-E1) and systolic blood pressure (SBP) was used as a continuous measure. Body Mass Index (BMI) was calculated as weight (kg) per meter squared (m2) of height. Subjects were given a smoking status as current smoker if they answered yes to the following question “Have you smoked a cigarette, even a puff, in the last 7 days? (Yes; No)”. Venous blood samples provided by all 99 subjects were assayed to determine cholesterol and glycosylated hemoglobin values.
Assay details
All assays at follow-up were performed by CLIA certified laboratories. Glycosylated hemoglobin (Hb A1C) was assayed using a Roche P-Modular Tina-Quant Immunoassay, with an intra-assay precision coefficient of variation (CV) of 0.8–1.5%, an inter-assay CV of 1.3–2.0%, and a lower limit of detection of 2.9%. Total cholesterol was measured enzymatically in serum using a Roche/Hitachi analyzer with an intra-assay CV of 0.8%, an inter-assay CV of 1.7%, and a lower limit of detection of 3mg/dL. HDL cholesterol was measured enzymatically in serum via a Roche/Hitachi analyzer with an intra-assay CV of 0.60–0.95%, an inter-assay CV of 1.2–1.3%, and a lower limit of detection of 3mg/dL.
Assessment of 10-year cardiometabolic risk
Cardiometabolic 10-year risk was assessed based on five non-self report modifiable cardiometabolic risk factors, initially developed in the Framingham Study [Wilson, et al. 1998] modified by D’Agostino [D’Agostino, et al. 2008], and further developed by Marino et al. in 2013 using the Framingham offspring study to focus solely on modifiable factors [Marino, et al. 2014]. The most recent modification evaluates risk through the addition of glycosylated hemoglobin (Hb A1C) systolic blood pressure and BMI. In brief, while controlling for age and in gender-stratified risk models, the model assessed current smoking, and continuous total cholesterol, HDL-cholestorol, systolic blood pressure, HbA1c levels, and BMI. Details of the model are described elsewhere [Marino, et al. 2014]. Each of the items in the component score was kept continuous to keep them sensitive to small changes and to reflect the potential impact of interventions. Some (n=2) of the participants had missing data on more than one of these risk factors and were excluded from analyses.
Covariates
The covariates were determined a priori and reported at baseline. We selected covariates that have been associated with cardiovascular health, sleep and psychosocial stress. All covariates have been described in previous studies [Buxton, et al. 2012, Kim, et al. 2012, Sorensen, et al. 2011].
Socio-demographic factors were obtained through participants reporting their age (years), gender, race/ethnicity (Hispanic, White, Black and mixed race/others), occupation (staff nurse, patient care associate (PCA) and others), education (GED or less; Some College; College Degree; Graduate School), ability to pay bills (great deal of difficulty; some difficulty; a little difficulty; no difficulty; don’t know; refused), height (inches) and weight (pounds). Body mass index (BMI) was measured by a nurse at follow-up using weight and height (kilograms per square meter).
Night work was quantified from detailed administrative payroll data and calculated as average night work-hours per month (between 10 PM and 6 AM), calculated from October 2008 until August 2009, making the assessment a year before initiating the survey for all workers. Excluding shifts shorter than 4 hours, the variable was trichotomized into 0–6 hours, 6–72 hours and more than 72 hours per month, over the past year, during months worked, as described previously [Buxton, et al. 2012].
Work-related stress was assessed by self-reported job demands, decision latitude, coworker/supervisor support and work-family conflict. A modified version of the Job Content Questionnaire [Karasek Jr 1979, Karasek, et al. 1998, Lusardi, et al. 1999] measured job demands, decision latitude and co-worker/supervisor support. Job demands were assessed through 5 items that were weighted and summed yielding a scale from 12 to 48 [Karasek, et al. 1998]. Decision latitude was assessed through 9 items and created as a weighted sum of decision authority and skill discretion from the Job Content Questionnaire [Karasek, et al. 1998]. Co-worker/supervisor support was assessed through 5 items (3 and 2 respectively) with 5 response categories summed, yielding a scale from 2 to 10 [Karasek, et al. 1998]. Supervisor support was summed and scaled giving a scale of 3 to 15 [Karasek, et al. 1998]. Social support was defined as the sum of co-worker and supervisor support. Iso-strain was a composite variable assessed by combining the risk categories job demands, decision latitude and social support and dichotomizing these three variables into low/high categories [Bhui, et al. 2012, Harvey, et al. 2011]. Iso-strain was defined as the combination of high job demands, low control and low social support [Bhui, et al. 2012].
Work-family conflict was assessed by the work-family conflict scale [Netemeyer, et al. 1996] consisting of five items with response categories from 1–5 summed, scaled and giving a range of 5–25: (1) “The demands of my work interfere with my family or personal time”. (2) “The amount of time my job takes up makes it difficult to fulfill family or personal responsibilities”. (3) “Things I want to do at home do not get done because of the demands my job puts on me”. (4) “My job produces strain that makes it difficult to fulfill my family or personal duties”. (5) “Due to work-related duties, I have to make changes to my plans for family or personal activities”. Response categories ranged from 5 = “Strongly Agree” to 1 = “Strongly Disagree”, making a higher score an indicator of more work-family conflict.
Physical Activity (PA) outside of work was assessed with a measure adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor and Surveillance System Physical Activity measure [Centers for Disease Control and Prevention 2009]. Respondents were asked about their participation in vigorous and moderate PA of at least 10 minutes’ duration outside of work. Each activity was defined by number of days per week and the total duration (hours and minutes) per day. Recommended physical activity was defined as reporting at least 30 minutes of moderate or vigorous activity on at least 5 days a week or at least 20 minutes of vigorous activity on at least 3 days a week [US Department of Health and Human Services].
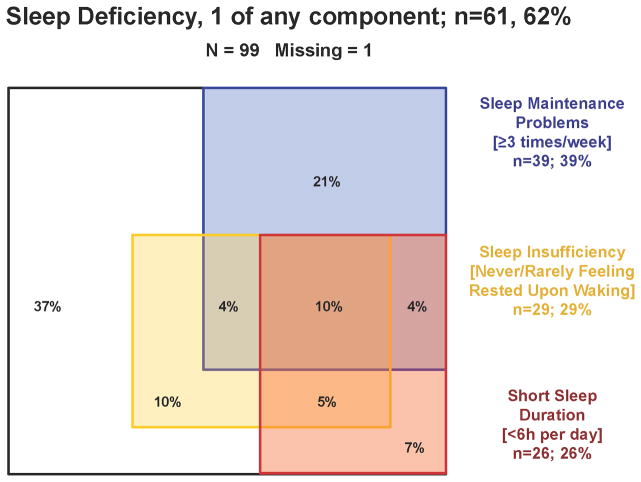
Sleep deficiency [Luyster, et al. 2012] in the last 4 weeks was assessed by self-report. Sleep was measured using three self-reported variables: sleep duration, sleep quality and sleep insufficiency. Short sleep duration was assessed by asking subjects how many hours of sleep they got each night over the past four weeks [Buxton, et al. 2012, Sorensen, et al. 2011]. Sleep quality was determined based on the frequency of sleep maintenance problems, assessed by asking subjects how often they woke up in the middle of the night or early morning, with four response categories: “not during the past month”; “Less than once per week”; “Once or twice per week”; or “three or more times a week” [Buysse, et al. 1989]. Sleep insufficiency was determined based on subject response on a five-component scale ranging from “never” to “always” to the question “how often during the past 4 weeks did you get enough sleep to feel rested upon waking up?” [Sorensen, et al. 2011], similar to the Centers for Disease Control measures used in the BRFSS study to assess U.S. national sleep sufficiency by state [McKnight-Eily, et al. 2008]. Sleep outcomes were dichotomized into “Yes/No” based on participant response on the component scales. In brief, a response of <6 hours per night yielded a “Yes” on short sleep duration, a response of “three or more times a week” for interrupted sleep yielded a “Yes” on sleep maintenance problems and a response of “always” or “sometimes” yielded a “Yes” on sleep insufficiency. Sleep deficiency was defined as having responded “Yes” to one or more of these sleep outcomes. The distribution and overlap of the sleep variables are illustrated in figure 2.
Figure 2.
Data analysis
In initial analyses, the characteristics of workers were compared on 10-year cardiometabolic risk score (%) treated continuously for analysis purposes. For dichotomous variables we used the independent sample t-test to compare means. For ordinal and continuously measured characteristics we used simple linear regression or a one-way ANOVA. Significant variables from baseline analyses were entered into a multivariable linear regression. As baseline assessments only had self-report measures, no measure of risk change is included in the analyses.
Results
Our participants (n=99) were predominantly white (91 %), female (100 %), nurses (68 %) with a college degree (65 %) and a mean age of 40.8 (SD 11.9, range 21–62) years. Fourteen (13.7 %) of total respondents had iso-strain, 61 (59 %) met recommended levels of physical activity and 64 (63 %) had sleep deficiency. The participants’ score on the individual cardiometabolic risk factors demonstrated means and standard deviations of 112.1 mmHg (SD 12.1) on systolic blood pressure, 5.6 % (SD 0.68) on Hb A1C, 26.2 kg/m2 (SD 5.7, range 17.6 – 46.1) on BMI and 67.6 mg/dL (SD 16.7) on HDL cholesterol. The participant characteristics measured by cardiometabolic risk (range 1 – 46 %) are presented in table I.
Table 1.
Mean (SD), correlation (r), variance (r2/F) of participant characteristics on 10-year cardiometabolic risk score within covariates. Covariates are either dichotomous (yes; no) or ordinal/continuous (range). Categorical variables are shown with both test statistic and mean (SD). Reference categories are bolded. All characteristics may not add up to n=99, because of missing data.
| Work Conditions/Demographics |
Cardiometabolic risk score
(Range 0–100 %) |
P-Value | |
|---|---|---|---|
| Independent Variables | |||
| Sleep Deficiency | Yes (n=63) | No (n=36) | |
| 9.29 % (10.5 %) | 6.03 % (6.2 %) | 0.058 | |
| Sleep Duration | < 6 hours (n=26) | >= 6 hours (n=73) | |
| 9.5 % (9.0 %) | 7.54 % (9.3 %) | 0.36 | |
| Insufficient Sleep | Yes (n=29) | No (n=69) | |
| 6.13 % (8.8 %) | 8.86 % (9.3 %) | 1.000 | |
| Sleep Maintenance Problems | Yes (n=39) | No (n=59) | |
| 10.51 % (10.6 %) | 6.5 % (7.8 %) | 0.048 | |
| Recommended Physical Activity | Yes (n=61) | No (n=38) | |
| 5.6 % (6.0 %) | 11.9 % (11.7 %) | 0.003 | |
| Iso-strain | Yes (n=14) | No (n=74) | |
| 8.82 % (9.2 %) | 8.36 % (9.7 %) | 0.87 | |
| Job Demands (12–48) | r= 0.09 r2=0.008 | 0.37 | |
| Decision Latitude (2–10) | r= 0.36 r2=0.001 | 0.73 | |
| Coworker Support (2–10) | r= 0.14 r2=0.02 | 0.17 | |
| Supervisor Support (3–15) | r= 0.09 r2=0.009 | 0.36 | |
| Work-Family Conflict (5–25) | r= 0.04 r2=0.002 | 0.68 | |
| Occupation (Staff Nurse; Patient Care Associate (PCA); Other) | (Welch’s F) 28.2 (2, 50.2) | <0.001 | |
| Staff Nurse | 6.5 % (7 %) | ||
| PCA | 1.2 % (0.6 %) | ||
| Other | 13 % (12.3 %) | ||
| Race\Ethnicity (White; Hispanic; Black) | F(2, 92) 0.14 | 0.87 | |
| White | 8.2 % (9.2 %) | ||
| Hispanic | 5.4 % (5.1 %) | ||
| Black | 8.1 % (12.9 %) | ||
| Education (GED or Less; Some College; Degree; Graduate School) | (Welch’s F) 1.3 (3, 4.6) | 0.38 | |
| GED or Less | 17.4 % (20.5 %) | ||
| Some College | 7.8 % (9 %) | ||
| Degree | 6.6 % (8.1 %) | ||
| Graduate School | 13 % (11.0 %) | ||
| Economic Status (Difficulty paying bills?) (Little difficulty w/ bills; … At least some difficulty w/ bills) | F(4, 91) 0.6 | 0.7 | |
| A Great Deal of Difficulty w/ Bills | 5.2 % (3.6 %) | ||
| At Least Some Difficulty w/ Bills | 8.4 % (8.8 %) | ||
| A Little Difficulty | 10 % (10.2 %) | ||
| No Difficulty | 7.6 % (9.3 %) | ||
| Refused to Answer | 2.9 % (2.9 %) | ||
| Night work (0–6 hrs monthly; >6 hrs but < 72; 72 +) | (Welch’s F) 6.1 (2, 25.6) | 0.007 | |
| 0–6 hrs monthly | 8.8 % (9.6 %) | ||
| >6 hrs but < 72 | 3.9 % (4.8 %) | ||
| 72 + | 10.6 % (9.7 %) | ||
Significant P-values (<0.05) are bolded
Multivariable associations
Significant variables from baseline analyses were included in a multi-linear model in order to control for covariation. Table II reports the unadjusted regression coefficient (B). The adjusted regression coefficient (β) reported in the following paragraph, represents the relation between X1 and Y averaged over all values of X2, hence showing the most influential predictor of the significant covariates. Sleep maintenance problems at baseline significantly predicted cardiometabolic risk score at follow-up and this association remained after adjusting for covariates (β=0.26). In addition, whether or not the patient care worker reported doing the recommended level of physical activity at baseline, predicted increased cardiometabolic risk at follow-up in the adjusted model (β= 0.29), as did the occupational category the worker belonged to (β= 0.24). The overall model fit was (X2red=0.23) and a Durbin-Watson test yielded d=1.59, which is within the critical value standards for non-autocorrelation, meaning the unobserved error terms are not correlated (see table II for details of model results).
Table II.
Multivariable linear regression showing associations of baseline characteristics on 10-year cardiometabolic risk at follow-up. Categories are listed in parentheses.
| Independent Variables | Cardiometabolic risk model | |
|---|---|---|
| Unstandardized Coefficient (95 % CI) | P-value | |
|
|
||
| Sleep Maintenance Problems (yes; no) | 0.046 (0.01, 0.048) | .01 |
| Occupation (Staff nurse; PCA; Other) | 0.027 (0.006, 0.48) | .01 |
| Recommended Physical Activity (yes; no) | 0.054 (0.019, 0.09) | .003 |
| Night work Categories (<=6, 6–72, 72+) | 0.015 (−.0.012, 0.041) | .27 |
Significant P-values are bolded. The interpretation of the coefficient is the expected change in y for a one-unit change in x when the other covariates are held fixed—that is, the expected value of the partial derivative in y for a one unit change in x when other covariates are held fixed. This is sometimes called the unique effect of x on y.
Discussion
In this longitudinal study of 99 female patient care workers, we investigated how baseline measures of sleep deficiency, physical activity, work stress and night work predicted 10-year cardiometabolic risk from biomarkers at 2-year follow-up. Sleep maintenance problems, occupational category, and not meeting the CDC’s recommended amount of physical activity at baseline, significantly predicted increased 10-year cardiometabolic risk. These predictive associations remained when controlled for covariates.
Our finding that sleep maintenance problems independently predict increased cardiometabolic 10-year risk is consistent with a recent large-scale prospective study on reduced sleep quality and incidence of heart failure which identified a dose-response relationship between cumulative increase in insomnia symptoms and risk of heart failure [Laugsand, et al. 2013]. Earlier studies on insomnia symptoms and cardiovascular risk further support a link to heart failure as well as cardiovascular disease [Chien, et al. 2010, Newman, et al. 2000]. A recent study of insomnia and heart failure [Laugsand, et al. 2013] describes an increased relative risk of heart failure for women compared to men, which was attributed to either a higher prevalence of insomnia symptoms in females [Ohayon 2002] or the lower baseline risk for heart failure in women [Laugsand, et al. 2013]. Our results support further investigation into whether insomnia symptoms pose a greater threat to women than men when assessing cardiometabolic risk.
Short sleep duration has previously been linked to increased blood pressure [Buxton and Marcelli 2010, Gottlieb, et al. 2006]. Recent findings from a large American cohort also demonstrated that insomnia symptoms led to a 5-fold elevated risk for hypertension when combined with short sleep duration (≤5 hours), whereas insomnia symptoms and adequate sleep duration did not show significant associations with hypertension [Vgontzas, et al. 2009]. Our results support a predictive link between sleep maintenance problems and cardiometabolic risk, but we did not find evidence for such a “combination effect” when investigating sleep deficiency. However, this could be due to our small sample or all female population; future studies should include combination/composite variables to further the understanding of this link.
Our results failed to show a link between night/shift work hours per month and 10-year cardiometabolic risk, when controlling for covariates. Rotating night shifts has previously been associated with increased risk for ischemic heart disease [Brown, et al. 2009] and exhibits a dose-response relationship with coronary heart disease in the Nurses Health Study [Brown, et al. 2009, Kawachi, et al. 1995]. However, there are other studies showing no association between night shifts and ischemic heart disease [Frost, et al. 2009]. A recent review on night work and ischemic heart disease looked at 16 studies with a relative risk varying from 0.64 to 2.25 [Frost, et al. 2009]. However, overlap between sleep maintenance problems and working night shifts could perhaps be expected as sleep deficiency has been suggested as a mechanism explaining earlier findings [Brown, et al. 2009]. In the current study, there was no significant correlation between working night shifts and reporting sleep maintenance problems. Our results are in line with a recent review showing only a marginally increased relative risk when obtaining night work hours from company records, compared to when night work is self-reported [Frost, et al. 2009].
Failing to exercise moderately or vigorously for 150 minutes or more during a week is a well-established risk factor in cardiovascular disease [Sattelmair, et al. 2011, Wen, et al. 2011]. The results from our study are in accordance with numerous prospective cohort studies and meta-analyses done in several countries [Sattelmair, et al. 2011, Wen, et al. 2011]. Our occupational group, patient care workers, have been investigated through the Nurses Health Study, demonstrating that even light physical activity was associated with lower cardiovascular risk [Lee, et al. 2001].
Interestingly, several studies have documented a link between short sleep duration and reduced physical activity [Briones, et al. 1996, Weaver, et al. 1997]. A recent laboratory study demonstrated how induced short sleep duration (≤5.5) in 18 healthy subjects led to a 31 % decrease in daily activity and 24 % reduction in moderate and vigorous activity [Bromley, et al. 2012]. When looking at this in our population, though not significant, we observed a trend that a lower percentage of the participants’ reporting short sleep duration met the recommended amounts of physical activity than participants reporting adequate sleep duration. Such a link between sleep and physical activity underlines the need for a specific focus on sleep adequacy, duration and maintenance in occupational health interventions.
No measures of job stress were significant in the adjusted analyses in our study. A review on psychosocial stress and ischemic heart disease risk highlights the contradictory evidence in female populations when it comes to job stress [Eller, et al. 2009].
This study has limitations, especially that the sample size is small and was not chosen randomly, which limits the generalizability of our results. However, an all female population is not common in cardiometabolic risk studies. These results should be considered a contribution to a growing research base on female cardiometabolic risk. Another limitation is that we have only two time points approximately two years apart that does not allow for investigations of mediating mechanisms or directionality. But, the extensive questionnaires at baseline, administrative payroll data on night work status, and lengthy follow-up with biomarkers still make a strong case for the relevance of variables showing significant predictive value. Another limitation of note is that our prospective design does not preclude reverse causality. We do not have information on events before the beginning of the study and cannot account for other factors influencing causality. Moreover, we do not have a measure or question addressing postmenopausal status. The average menopausal age in American women is 51 years, and the range is 40–61 years, and about half of our sample is within this range, which is an important risk factor.
The relationship between sleep maintenance problems, physical activity and cardiometabolic risk is a widely studied connection. Yet, there is less evidence in female patient care workers, a population with very high stress levels and high prevalence of sleep deficiency. The Nurses Health Study is perhaps the largest cohort with the most extensive follow-up done on a nurse population. However, its participants consist only of married nurses and do not include patient care associates or single nurses, who are vulnerable workers with regards to both social support and workload. This study also implements biomarker data in a high priority population providing objective measurements to motivate change in occupational health practices. In addition, our data includes non self-report variables such as administrative payroll data on night work status, and measured cardiometabolic risk [Marino, et al. 2014], a much more sensitive outcome than cardiovascular disease or death as registered by death certificates, self-report or hospitalization due to modest accuracy of diagnoses and referral biases [Frost, et al. 2009]. Sleep has previously been suggested as a third pillar of health, alongside nutrition and exercise, in cardiometabolic disease risk [Buxton, et al. 2013]. Our results support this view and highlight the need for sleep deficiency to be prioritized in occupational health programs, especially those targeting cardiovascular health in women.
Acknowledgments
This work was supported by a grant (Grant sponsor: National Institute for Occupational Safety and Health. Grant number: U19 OH008861) for the Harvard School of Public Health Center for Work, Health and Well-being, for the Harvard Clinical and Translational Science Center, (Grant sponsor: National Center for Research Resources. Grant number: UL1 RR025758-04), the Work, Family, and Health Network (Grant sponsor: Grant number: U01 AG5186989), (Grant sponsor: National Heart, Lung, and Blood Institute. Grant number: R01HL107240), and additional support was provided by Dean Hashimoto and Partners Occupational Health Service. This study would not have been accomplished without the participation of Partners HealthCare System and leadership from Dennis Colling, Sree Chaguturu, and Kurt Westerman. The authors would like to thank Partners Occupational Health Services including Marlene Freeley for her guidance, as well as Elizabeth Taylor, Elizabeth Tucker O’Day, and Terry Orechia. We also thank individuals at each of the hospitals including Jeanette Ives Erickson and Jacqueline Somerville in Patient Care Services leadership, and Jeff Davis and Julie Celano in Human Resources. Additionally, we wish to thank Charlene Feilteau, Mimi O’Connor, Margaret Shaw, Eddie Tan and Shari Weingarten for assistance with supporting databases. We also thank Chris Kenwood of NERI for his statistical and programming support.
Footnotes
Conflict of interest: None
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
References
- Ayas NT, White DP, Al-Delaimy WK, Manson JAE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- Ayas NT, White DP, Manson JE, Stampfer MJ, Speizer FE, Malhotra A, Hu FB. A prospective study of sleep duration and coronary heart disease in women. Archives of internal medicine. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- Backe EM, Seidler A, Latza U, Rossnagel K, Schumann B. The role of psychosocial stress at work for the development of cardiovascular diseases: a systematic review. International Archives of Occupational and Environmental Health. 2012;85:67–79. doi: 10.1007/s00420-011-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkic KL, Landsbergis PA, Schnall PL, Baker D. Is job strain a major source of cardiovascular disease risk? Scandinavian journal of work, environment & health. 2004;30:85–128. doi: 10.5271/sjweh.769. [DOI] [PubMed] [Google Scholar]
- Bellavia GM, Frone MR. Work-family conflict. Handbook of work stress. 2005:113–147. [Google Scholar]
- Berkman LF, Buxton O, Ertel K, Okechukwu C. Managers’ practices related to work–family balance predict employee cardiovascular risk and sleep duration in extended care settings. Journal of occupational health psychology. 2010;15:316–329. doi: 10.1037/a0019721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhui KS, Dinos S, Stansfeld SA, White PD. A Synthesis of the Evidence for Managing Stress at Work: A Review of the Reviews Reporting on Anxiety, Depression, and Absenteeism. Journal of Environmental and Public Health. 2012;2012 doi: 10.1155/2012/515874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones B, Adams N, Strauss M, Rosenberg C. Relationship between sleepiness and general health status. Sleep: Journal of Sleep Research & Sleep Medicine. 1996;19:583–588. doi: 10.1093/sleep/19.7.583. [DOI] [PubMed] [Google Scholar]
- Bromley LE, Booth JN, III, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep. 2012;35:977–984. doi: 10.5665/sleep.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DL, Feskanich D, Sánchez BN, Rexrode KM, Schernhammer ES, Lisabeth LD. Rotating night shift work and the risk of ischemic stroke. American journal of epidemiology. 2009;169:1370–1377. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Broussard JL, Zahl AK, Hall M. Effects of Sleep Deficiency on Hormones, Cytokines, and Metabolism. In: Redline SB, NA, editors. Energy Balance and Cancer Volume 8: Impact of Sleep and Sleep Disturbances on Obesity and Cancer. New York: Springer; 2013. [Google Scholar]
- Buxton OM, Hopcia K, Sembajwe G, Porter JH, Dennerlein JT, Kenwood C, Stoddard AM, Hashimoto D, Sorensen G. Relationship of Sleep Deficiency to Perceived Pain and Functional Limitations in Hospital Patient Care Workers. Journal of Occupational and Environmental Medicine. 2012;54:851–858. doi: 10.1097/JOM.0b013e31824e6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Social Science & Medicine. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- Chandola T, Ferrie JE, Perski A, Akbaraly T, Marmot MG. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep. 2010;33:739–744. doi: 10.1093/sleep/33.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K-L, Chen P-C, Hsu H-C, Su T-C, Sung F-C, Chen M-F, Lee Y-T. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33:177–184. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizza G, Skarulis M, Mignot E. A link between short sleep and obesity: building the evidence for causation. Sleep. 2005;28:1217–1220. doi: 10.1093/sleep/28.10.1217. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, Hall M, Kupfer DJ, Reynolds CF., III Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosomatic Medicine. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Eller NH, Netterstrom B, Gyntelberg F, Kristensen TS, Nielsen F, Steptoe A, Theorell T. Work-related psychosocial factors and the development of ischemic heart disease: a systematic review. Cardiology in Review. 2009;17:83–97. doi: 10.1097/CRD.0b013e318198c8e9. [DOI] [PubMed] [Google Scholar]
- Evans O, Steptoe A. The contribution of gender-role orientation, work factors and home stressors to psychological well-being and sickness absence in male-and female-dominated occupational groups. Social Science & Medicine. 2002;54:481–492. doi: 10.1016/s0277-9536(01)00044-2. [DOI] [PubMed] [Google Scholar]
- Frone MR, Russell M, Cooper ML. Relation of work-family conflict to health outcomes: a four-year longitudinal study of employed parents. Journal of Occupational and Organizational Psychology. 2011;70:325–335. [Google Scholar]
- Frost P, Kolstad HA, Bonde JP. Shift work and the risk of ischemic heart disease-a systematic review of the epidemiologic evidence. Scandinavian journal of work, environment & health. 2009:163–179. doi: 10.5271/sjweh.1319. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. Journal of sleep research. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Archives of internal medicine. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, Punjabi NM. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep medicine reviews. 2010;14:191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–1103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Murray G, Chandler RA, Soehner A. Sleep disturbance as transdiagnostic: Consideration of neurobiological mechanisms. Clinical Psychology Review. 2011;31:225–235. doi: 10.1016/j.cpr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rössler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- Hublin C, Partinen M, Koskenvuo K, Silventoinen K, Koskenvuo M, Kaprio J. Shift-work and cardiovascular disease: a population-based 22-year follow-up study. European journal of epidemiology. 2010;25:315–323. doi: 10.1007/s10654-010-9439-3. [DOI] [PubMed] [Google Scholar]
- Karasek RA., Jr Job demands, job decision latitude, and mental strain: Implications for job redesign. Administrative Science Quarterly. 1979:285–308. [Google Scholar]
- Karasek R, Brisson C, Kawakami N, Houtman I, Bongers P, Amick B. The Job Content Questionnaire (JCQ): an instrument for internationally comparative assessments of psychosocial job characteristics. Journal of Occupational Health Psychology. 1998;3:322–355. doi: 10.1037//1076-8998.3.4.322. [DOI] [PubMed] [Google Scholar]
- Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JAE, Speizer FE, Hennekens CH. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- Kim SS, Okechukwu CA, Buxton OM, Dennerlein JT, Boden LI, Hashimoto DM, Sorensen G. Association between work-family conflict and musculoskeletal pain among hospital patient care workers. American Journal of Industrial Medicine. 2012;56:488–495. doi: 10.1002/ajim.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivimaki M, Nyberg ST, Batty GD, Fransson EI, Heikkila K, Alfredsson L, Bjorner JB, Borritz M, Burr H, Casini A, Clays E, De Bacquer D, Dragano N, Ferrie JE, Geuskens GA, Goldberg M, Hamer M, Hooftman WE, Houtman IL, Joensuu M, Jokela M, Kittel F, Knutsson A, Koskenvuo M, Koskinen A, Kouvonen A, Kumari M, Madsen IE, Marmot MG, Nielsen ML, Nordin M, Oksanen T, Pentti J, Rugulies R, Salo P, Siegrist J, Singh-Manoux A, Suominen SB, Vaananen A, Vahtera J, Virtanen M, Westerholm PJ, Westerlund H, Zins M, Steptoe A, Theorell T Consortium IP-W. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380:1491–1497. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohatsu ND, Tsai R, Young T, VanGilder R, Burmeister LF, Stromquist AM, Merchant JA. Sleep duration and body mass index in a rural population. Archives of internal medicine. 2006;166:1701–1705. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Archives of general psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- Laugsand LE, Strand LB, Platou C, Vatten LJ, Janszky I. Insomnia and the risk of incident heart failure: a population study. European Heart Journal. 2013 doi: 10.1093/eurheartj/eht019. [DOI] [PubMed] [Google Scholar]
- Lee IM, Rexrode KM, Cook NR, Manson JAE, Buring JE. Physical activity and coronary heart disease in women. JAMA: the journal of the American Medical Association. 2001;285:1447–1454. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24-h study. American journal of hypertension. 1999;12:63–68. doi: 10.1016/s0895-7061(98)00200-3. [DOI] [PubMed] [Google Scholar]
- Luyster FS, Strollo PJ, Jr, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. 2012;35:727–734. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Li Y, Pencina MJ, D’Agostino RB, Berkman L, Buxton OM. Quantifying Cardiometabolic Risk Using Modifiable Non-self-reported Risk Factors. American Journal of Preventive Medicine. 2014 doi: 10.1016/j.amepre.2014.03.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C, Fat DM, Boerma J. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- McKnight-Eily L, Presley-Cantrell L, Strine T, Chapman D, Perry G, Croft J. Perceived insufficient rest or sleep-four states, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:200–203. [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Mullington JM, Dinges DF, Price NJ, Regan MM, Rifai N. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Netemeyer RG, Boles JS, McMurrian R. Development and validation of work-family conflict and family-work conflict scales. Journal of Applied Psychology. 1996;81:400–410. [Google Scholar]
- Newman AB, Spiekerman CF, Enright P, Lefkowitz D, Manolio T, Reynolds CF, Robbins J. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. Journal of the American Geriatrics Society. 2000;48:115–123. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep medicine reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, Stampfer MJ, Hu FB. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- Patel SR, Blackwell T, Redline S, Ancoli-Israel S, Cauley JA, Hillier TA, Lewis CE, Orwoll ES, Stefanick ML, Taylor BC. The association between sleep duration and obesity in older adults. International Journal of Obesity. 2008;32:1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES. Heart Disease and Stroke Statistics-2011 Update. Circulation. 2011;123:18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelmair J, Pertman J, Ding EL, Kohl HW, III, Haskell W, Lee IM. Dose Response Between Physical Activity and Risk of Coronary Heart DiseaseClinical Perspective A Meta-Analysis. Circulation. 2011;124:789–795. doi: 10.1161/CIRCULATIONAHA.110.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnall PL, Dobson M, Rosskam E. Unhealthy Work: causes, consequences, cures. New York: Baywood Publishing Company; 2009. [Google Scholar]
- Sorensen G, Stoddard AM, Stoffel S, Buxton O, Sembajwe G, Hashimoto D, Dennerlein JT, Hopcia K. The role of the work context in multiple wellness outcomes for hospital patient care workers. Journal of Occupational and Environmental Medicine. 2011;53:899–910. doi: 10.1097/JOM.0b013e318226a74a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- Stranges S, Dorn JM, Cappuccio FP, Donahue RP, Rafalson LB, Hovey KM, Freudenheim JL, Kandala N-B, Miller MA, Trevisan M. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. Journal of hypertension. 2010;28:896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Medicine. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Focus area 7: educational and community-based programs—worksite setting. Healthy People. 2010 [Google Scholar]
- Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–843. [PubMed] [Google Scholar]
- Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. The Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wingard DL, Berkman LF. Mortality risk associated with sleeping patterns among adults. Sleep. 1983;6:102–107. doi: 10.1093/sleep/6.2.102. [DOI] [PubMed] [Google Scholar]
- Yang H, Schnall PL, Jauregui M, Su TC, Baker D. Work hours and self-reported hypertension among working people in California. Hypertension. 2006;48:744–750. doi: 10.1161/01.HYP.0000238327.41911.52. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T. Shift work and disturbed sleep/wakefulness. Occupational Medicine. 2003;53:89–94. doi: 10.1093/occmed/kqg046. [DOI] [PubMed] [Google Scholar]