Abstract
Objective
To characterize the implementation of hypothermia for neonatal hypoxic ischemic encephalopathy in a population-based cohort.
Study design
Using the California Perinatal Quality Care Collaborative and California Perinatal Transport System linked 2010–2012 datasets; infants >/= to 36 weeks gestation with hypoxic ischemic encephalopathy were categorized as receiving hypothermia or normothermia. Socio-demographic and clinical factors were compared and multivariable logistic regression was used to determine factors associated with hypothermia therapy.
Results
There were 238 reported encephalopathy cases in 2010, 280 in 2011 and 311 in 2012. Hypothermia therapy use in newborns with hypoxic ischemic encephalopathy increased from 59% to 73% across the study period, mainly occurring in newborns with mild or moderate encephalopathy. A total of 36 centers provided hypothermia and cared for 94% of infants, the remaining 6% being cared for at one of 25 other centers. Of the centers providing hypothermia, 12 centers performed hypothermia therapy to over 20 patients during the three-year study period, and 24 centers cared for < 20 patients receiving hypothermia. In-hospital mortality was 13%, primarily associated with the severity of encephalopathy.
Conclusions
Our findings highlight an opportunity to explore practice site variation and to develop quality improvement interventions to assure consistent evidence-based care of term infants with hypoxic ischemic encephalopathy and appropriate application of hypothermia therapy for eligible newborns.
Keywords: sociodemographic characteristics, practice variation, mortality rate
Neonatal hypoxic ischemic encephalopathy (HIE) has an incidence of 1.5 per 1000 live births.1, 2 Randomized trials have demonstrated the benefit of hypothermia treatment initiated within 6 hours of life for moderate to severe HIE on the outcome of death and/or moderate to severe neurodevelopmental impairment at 18 months of age.3–9 Reduction in the relative risk of death or neurodevelopmental impairment of 0.75 (95% CI, 0.68–0.83) and a number needed to treat of 7 (95% CI, 5–10)9–12 have established hypothermia as the only effective treatment available.
When new medical therapies are introduced, treatment may not be uniformly applied to eligible infants, may change patterns of disparity in care, or may be applied to patient populations outside of those targeted in the original trial designs. There are limited studies documenting the treatment patterns, socio-demographic characteristics, and mortality rates of term infants with HIE during this era of implementation of therapeutic hypothermia. In the United Kingdom, the UK TOBY Cooling Registry has tracked implementation of hypothermia treatment for HIE since December 2006 prior to widespread implementation with the intention to audit uptake and implementation.13, 14 Although they have observed a steady increase over time in registrations for patients treated with hypothermia, they did not collect data on patients with HIE who did not receive hypothermia.
In this population-based study we examined the socio-demographic and clinical characteristics of infants with HIE in California, characterized the utilization of hypothermia, and compared in-hospital outcomes of infants that received treatment with hypothermia versus those who did not.
METHODS
We linked the California Perinatal Transport System (CPeTS) and the California Perinatal Quality Care Collaborative (CPQCC) datasets. These quality improvement organizations provide benchmarks, site-specific data reports, and conduct performance improvement activities in perinatal care.15 CPeTS collects information from 53 transport systems in California, and completes a neonatal data transport form for each transported infant. CPQCC collects maternal and neonatal data across 132 Neonatal Intensive Care Units (NICUs), representing greater than 90% of NICUs in California, and estimated to cover greater than 95% of NICU admissions.
We examined the outcome of death prior to discharge by treatment type hypothermia vs. normothermia, and risk factors including HIE severity. We also examined the hypothermia rates for centers by level of encephalopathy. Institutional review board approval was obtained from Stanford University Administrative Panel for the Protection of Human Subjects.
All CPQCC member hospitals participate in standardized data collection via a comprehensive web-based data entry system.16 Training sessions promote accuracy and uniformity in data abstraction. Each record has range and logic checks at the time of data collection and closeout, with auditing of records with excessive missing data.
The CPQCC database collects data on infants less than 28 days old with a birth weight greater than 1,500 grams in the event of death, acute transfer, intubated and non-intubated ventilation with a back-up rate for greater than 4 continuous hours, culture proven early bacterial sepsis, major surgery requiring general anesthesia, and readmission for hyperbilirubinemia greater than 25 mg/dl or requiring exchange transfusion.
Eligibility criteria were: gestational age >/= to 36 weeks, birth weight >/= to 2,000 grams, HIE diagnosis, birth in the State of California between January 1, 2010 and December 31, 2012, admission to a CPQCC member NICU, and absence of congenital anomalies.
HIE was defined in this study based on the Vermont Oxford Network specifications,16 as occurring in infants with a gestational age >/= to 36 weeks, with recognized encephalopathy within 72 hours of birth, and three or more of the following: arterial cord pH less than 7.00, Apgar score at 5 minutes </= to 5, multi-organ system dysfunction, fetal distress on antepartum monitoring, brain imaging scan within 7 days of birth with diffuse or multifocal ischemia or cerebral edema, and/or abnormal electroencephalogram; and the absence of an infectious cause, congenital cerebral malformation, or inborn error of metabolism, which could explain the encephalopathy.
The encephalopathy level was categorized as mild, moderate, or severe based on the neurological state of alert or hyperalert (normal or exaggerated responsiveness), lethargy or mild stupor (diminished responsiveness), and deep coma or stupor (not arousable), respectively, during the first 7 postnatal days and ascertained by CPQCC coders after chart review. Regardless of the severity, a patient would have been noted to be encephalopathic in the first 72 hours to meet the definition of HIE.
The main outcome measure was the receipt of hypothermia, defined as an intervention using head cooling devices, or body cooling blankets and/or ice packs to reduce the core body temperature to 33–34°C for whole body hypothermia or to 34–35°C for selective head hypothermia. Centers that provided hypothermia to at least one patient over the study period were designated as cooling centers.
Treatment type was characterized by socio-demographic and clinical factors, delivery room interventions, and respiratory management in the NICU. We assessed potential differences in receipt of hypothermia by the following characteristics: sex, race, ethnicity, prenatal care, maternal age, maternal hypertension, maternal diabetes, perinatal hemorrhage, chorioamnionitis, fetal distress, malpresentation, multiple birth, delivery mode, meconium stained amniotic fluid, birth weight, gestational age, Apgar scores, and early onset sepsis. Fetal distress was defined based on the presence of documentation in the medical record of fetal distress, poor biophysical profile, or non-reassuring (abnormal) stress test on fetal monitoring or fetal status. Detailed definitions are available in the CPQCC data specification manual.16
Statistical analyses
For bivariate comparisons, we used chi-square or Student t-test. The dependent variables of hypothermia treatment and in-hospital death were assessed by stepwise multivariable logistic regression to determine independent risk factors, with estimation of odds ratios and 95% confidence intervals. Socio-demographic, maternal, and neonatal clinical variables were considered in the models. For the multivariable model for in-hospital death, the main independent variable of interest was level of encephalopathy (mild, moderate, or severe). Records with missing data were incorporated into the crude analyses, but were excluded from the regression models (1% of records). Analyses were performed using Stata/SE 13.0 (College Station, Texas). Because these were exploratory analyses, no corrections were made for multiple comparisons.
RESULTS
Hypothermia Treatment
During the study period there were 829 infants with HIE diagnosis and without congenital anomalies, with 238 (29%) neonatal HIE cases in 2010, 280 (34%) cases in 2011, and 311 (37%) cases in 2012. We observed an increase in the number of HIE cases, as well as hypothermia treatment over the study period, primarily occurring in infants with mild or moderate HIE, with an 17% absolute rate increase in infants with mild HIE, 15% increase in infants with moderate HIE and 12% increase in infants with severe HIE (Table I).
Table 1.
HIE severity by year and by treatment group
| Normothermia n (%) | Hypothermia n (%) | Total n | P | |
|---|---|---|---|---|
| Total HIE severity 2010–2012 | 266 | 563 | 829 | < 0.0001 |
| Mild | 119 (50) | 118 (50) | 237 | |
| Moderate | 86 (24) | 270 (76) | 356 | |
| Severe | 61 (26) | 175 (74) | 236 | |
| Total HIE severity 2010 | 238 | < 0.0001 | ||
| Mild | 41 (62) | 25 (38) | 66 | |
| Moderate | 34 (34) | 66 (66) | 100 | |
| Severe | 23 (32) | 49 (68) | 72 | |
| Total HIE severity 2011 | 280 | 0.001 | ||
| Mild | 37 (47) | 42 (53) | 79 | |
| Moderate | 27 (22) | 96 (78) | 123 | |
| Severe | 21 (27) | 57 (73) | 78 | |
| Total HIE severity 2012 | 311 | < 0.0001 | ||
| Mild | 41 (45) | 51 (55) | 92 | |
| Moderate | 25 (19) | 108 (81) | 133 | |
| Severe | 17 (20) | 69 (80) | 86 |
Percents in parentheses reflect row percentages.
There were no differences between the hypothermia vs. normothermia groups in terms of sex, race and ethnicity, gestational hypertension, gestational diabetes, perinatal hemorrhage, chorioamnionitis, malpresentation, meconium stained amniotic fluid, fetal distress, birth weight or gestational age. Maternal age was more advanced in the hypothermia group, which also had higher rates of prenatal care. The majority of infants were delivered via cesarean in both groups. Infants in the hypothermia group had a higher rate of lower Apgar scores at 5 and 10 minutes. There was a trend toward higher rate of multiple births in the normothermia group, though not statistically significant (Table II).
Table 2.
Patient characteristics by normothermia or hypothermia treatment
| Normothermia n (%) | Hypothermia n (%) | Total n (%) | P | |
|---|---|---|---|---|
| Total | 266 | 563 | 829 | |
| Sex | 0.5 | |||
| Female | 124 (47) | 275 (49) | 399 (48) | |
| Male | 142 (53) | 288 (51) | 430 (52) | |
| Race/Ethnicity | 0.1 | |||
| Black | 26 (10) | 33 (6) | 59 (7) | |
| Hispanic | 100 (38) | 227 (40) | 327 (40) | |
| White - Non Hispanic | 60 (23) | 118 (21) | 178 (21) | |
| Asian | 21 (8) | 30 (5) | 51 (6) | |
| Other | 56 (21) | 150 (27) | 206 (25) | |
| Prenatal care | 251 (94) | 550 (98) | 801 (97) | 0.02 |
| Maternal age [mean, (+/−SD)]* | 28 (+/− 6.9) | 29.2 (+/− 7.3) | 28.8 (+/− 7.2) | 0.03 |
| Multiple births | 9 (3) | 8 (1) | 17 (2) | 0.06 |
| Gestational hypertension | 27 (10) | 73 (13) | 100 (12) | 0.6 |
| Gestational diabetes | 29 (11) | 71 (13) | 100 (12) | 0.8 |
| Perinatal hemorrhage | 42 (16) | 92 (16) | 134 (16) | 0.5 |
| Chorioamnionitis | 28 (11) | 50 (9) | 78 (9) | 0.8 |
| Malpresentation | 6 (2) | 23 (4) | 29 (4) | 0.3 |
| Meconium | 43 (16) | 87 (15) | 130 (16) | 0.8 |
| Fetal distress | 130 (49) | 311 (55) | 441 (53) | 0.1 |
| Mode of delivery | 0.02 | |||
| Vaginal | 110 (41) | 186 (33) | 296 (36) | |
| Cesarean | 156 (59) | 377 (67) | 533 (64) | |
| Birth weight in kilograms [mean, (+/−SD)] | 3.3 (+/− 0.6) | 3.3 (+/− 0.6) | 3.3 (+/− 0.6) | 0.2 |
| Gestational age in weeks [mean, (+/−SD)] | 38.8 (+/− 1.5) | 38.9 (+/− 1.5) | 38.9 (+/− 1.5) | 0.3 |
| 5 minute Apgar score </= 5 | 150 (56) | 444 (79) | 593 (72) | < 0.0001 |
| 10 minute Apgar score </= 5** | 79 (30) | 280 (50) | 359 (43) | < 0.0001 |
Percents in parentheses reflect column percentages.
Apgar score at 10 minutes available for 705 infants
Infants in the hypothermia group had higher rates of delivery room interventions, including oxygen supplementation (93% vs. 88%, p = 0.04), bag and mask ventilation (89% vs. 77%, p = < 0.0001), endotracheal intubation (70% vs. 51%, p = < 0.0001), cardiac compressions (42% vs. 26%, p = < 0.0001), and epinephrine administration (26% vs. 12%, p = < 0.0001). Respiratory support in the NICU was more prevalent in the hypothermia group, including oxygen supplementation (94% vs. 84%, p = < 0.0001), and mechanical ventilation (82% vs. 63%, p = < 0.0001). There was no difference in use of inhaled nitric oxide (10% vs. 7%, p = 0.2), high frequency ventilation (13% vs. 10%, p = 0.3) or extracorporeal membrane oxygenation (1% vs. 2%, p = 0.6).
In stepwise multivariable logistic regression analysis with the variables in Table II considered for inclusion, there were higher odds of hypothermia therapy with increasing HIE severity: moderate HIE OR 3.2, 95% CI 2.2–4.6, and severe HIE OR 3.0, 95% CI 2.0–4.5, compared with mild HIE. Hypothermia was applied more often in infants with other or unknown race compared with non-Hispanic Whites: OR 1.5, 95% CI 1.1–2.2, and in those born to mothers who received prenatal care: OR 3.3, 95% CI 1.3–8.3.
Hospital Variation in Practice
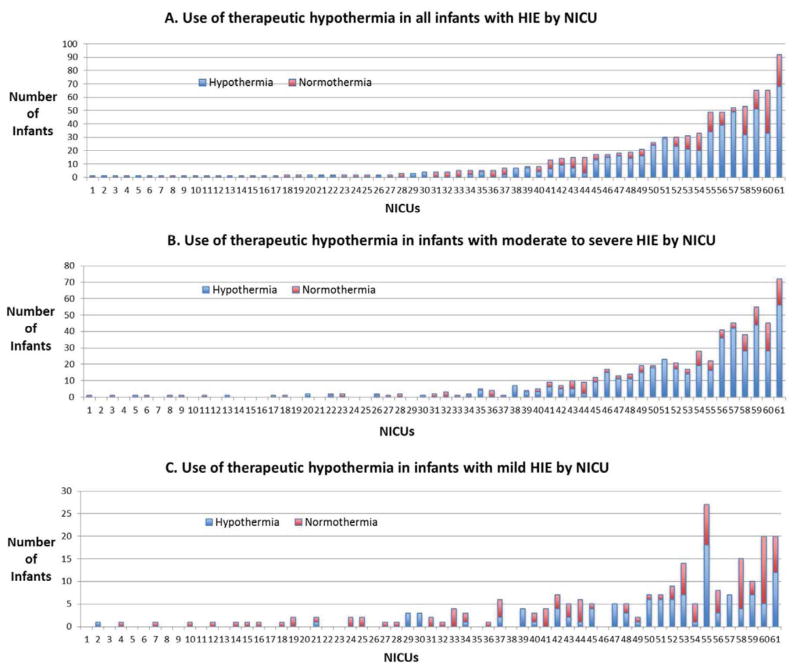
There were 61 NICUs that were the final NICU of care for patients with HIE, of which 36 (59%) centers provided hypothermia treatment to at least one patient during the study period and were designated for this analysis as a “cooling center”. Of the 829 patients with HIE, 781 (94%) were cared for in a cooling center. The remaining 25 NICUs cared for 48 (6%) patients, and did not provide hypothermia therapy to any of the patients who remained in that NICU. In the cooling centers, the hypothermia treatment rate for all infants with HIE varied from 20% to 100% with a mean of 73% and median of 7 patients over the study period (Figure).
Figure. Hospital Variation in Practice.
Each individual NICU is represented in the X-axis. Infants with HIE are represented in the Y-axis. Each number on the X-axis corresponds to the same NICU.
The total number of patients receiving hypothermia at an individual center ranged from 1 to 68, with 12 centers providing hypothermia therapy to more than 20 patients over the study period. The hypothermia treatment rate for infants with mild HIE varied from 0 to 100%, with a range of 1 to 18 infants. There were 6 centers that treated 100% of their infants with mild HIE (range of 1–7 infants per center). For patients with moderate or severe HIE, 569 (96%) infants were cared for at a cooling center, of which 445 (78%) infants received hypothermia therapy. The hypothermia treatment rate for infants with moderate or severe HIE per center varied from 0 to 100% (rage of 1–56 infants per center), with 16 centers not providing therapy to any of their infants (range of 1 to 4 infants per center).
In-hospital Mortality
In the overall group of infants there were 110 (13%) deaths; 103 (94%) occurred in infants with severe HIE, 5 (5%) in moderate and 2 (2%) in mild, p = < 0.0001, with no difference between hypothermia (17%) or normothermia (21%) groups for infants with moderate to severe HIE (p = 0.3). There was a higher death rate in infants with lower gestational ages, with 30 (17%) deaths in infants of 36–37 weeks gestation, 49 (15%) in infants of 38–39 weeks, 28 (9%) in infants of 40–41 weeks, and 2 (29%) in infants of 42–43 weeks (p = 0.001).
In stepwise multivariable logistic regression with the variables in Table II considered for inclusion, the odds of death were higher with greater HIE severity: severe HIE OR 71, 95% CI 32–157 compared with mild HIE, and reduced in infants with more advanced gestational age (OR 0.7, 95% CI 0.50–0.97 for every week increase), in infants born to Asian mothers (OR 0.27, 95% CI 0.08–0.86) and in infants born to mothers with chorioamnionitis (OR 0.29, 95% CI 0.09–0.89). The odds of death were not significant for any other factor in the model, including hypothermia vs. normothermia.
DISCUSSION
In this analysis of 829 infants with HIE admitted to NICUs in California during 2010–2012, over two thirds (68%) of infants with HIE of any severity received hypothermia therapy, with an increase in hypothermia use from 2010 to 2012. In comparison with the reported UK TOBY Cooling Register, in which over 80% of infants had Thompson encephalopathy scores in the moderate to severe range,13, 14 our observed increase in HIE treated cases mainly consisted of infants with mild or moderate encephalopathy. The increase in hypothermia for infants with moderate HIE is encouraging. Nevertheless, improved implementation and dissemination of this effective therapy is needed, as half of the untreated infants had moderate or severe HIE and potentially may have benefited from hypothermia treatment.
As clinical regionalization of care becomes more prevalent, it is relevant to note that the majority of infants with HIE, even those not treated with hypothermia, received care at what we termed a “cooling center”. There were 12 high volume cooling centers performing hypothermia therapy to over 20 patients during the 3-year study period, 5 moderate volume centers performing therapy on 10–19 patients, and 19 low volume centers performing therapy on 9 or fewer patients. The use of hypothermia for infants with moderate or serve HIE did not vary by high volume vs. low volume centers, with all of the centers with the exception of two performing therapy on over 50% of their patients. At the start of the study period there were 25 centers performing hypothermia therapy, with 5 additional centers for year two and 6 for year three of the study, with all the centers included in years two and three being low volume centers with exception of one which was a moderate volume center.
The UK TOBY Cooling Register provides another view of hospital implementation of hypothermia treatment. In the first 18 months of the Register, only the hospitals who participated in the UK TOBY cooling trial (35 centers) were registering hypothermia patients, with the addition of other hospitals after the results of the TOBY trial had been published.13, 14 Ultimately, with 74 NICUs notifying the Registry of patients receiving hypothermia treatment, 54 were classed as Level 3 and 11 as Level 2, with the remainder being indeterminate. The median number of infants cooled at Level 3 NICUs was 29 (20–52) vs. 3 (0–11) at Level 2 NICUs.
The question of whether hypothermia should be regionalized to high volume dedicated centers vs. more widespread implementation to lower volume centers is worth further exploration, as this highly specialized therapy requires advanced multidisciplinary clinical practice protocols. Presumably, care for these patients would benefit from close support from pediatric neurology, electroencephalography, neonatal brain magnetic resonance imaging, and coordination with outpatient high-risk infant follow-up. Centers providing hypothermia infrequently may not be able to maintain consistent expertise and resources in all of these areas.
Of the 25 centers that cared for patients with HIE and did not provide hypothermia therapy, 15 centers cared for patients with moderate or severe HIE. We speculate that the potential reasons for not providing hypothermia for patients with moderate or severe HIE could include lack of timely identification of symptoms consistent with moderate or severe HIE, inability to transport the infant within the therapeutic window, or an active decision not to offer intensive care. Ongoing studies from the National Institute of Health and Human Development (NICHD) on the benefit of hypothermia therapy between 6 and 24 hours of life in infants with moderate to serve HIE may aid in expanding its application to currently non-qualifying infants (NCT00614744).
A large number of infants with mild HIE received hypothermia, with significant variability in utilization by center. As described in the NICHD executive summary workshops,17, 18 hypothermia should be offered to infants meeting the criteria of published trials focusing on infants with moderate or severe HIE. Currently, treatment of infants with mild HIE is not routinely recommended, as there is no evidence that these infants benefit from therapy. A study by DuPont et al found that 20% of infants with perinatal acidemia and a neurologic examination revealing only mild encephalopathy had abnormal short-term outcomes including feeding difficulties, abnormal MRI, seizures, abnormal neurologic exam at discharge or death.19 An ongoing observational study may provide further information regarding the natural history and outcomes of children with mild HIE (NCT01747863).
The overall death rate of 13% was lower than that observed in clinical trials (~25%) or in the UK TOBY Cooling Registry (20%), which may represent a greater representation of milder cases in our cohort. The reduced odds of death for infants born to mothers with chorioamnionitis may be secondary to early antenatal and postnatal monitoring and management. There were no differences in hypothermia, gestational age, or HIE severity in infants with maternal chorioamnionitis.
There was a clear relationship between the severity of encephalopathy and the risk for death, with 94% of deaths occurring in infants with severe HIE. Clinical factors commonly associated with higher severity of illness were more common among infants who received hypothermia including lower Apgar scores, and a greater need for intensive delivery room and NICU interventions. As such, the lack of difference in mortality between hypothermia and normothermia should not be interpreted as lack of efficacy of this treatment. Furthermore, the severity of HIE had an overwhelming impact on mortality, with very few fatalities in infants with mild or moderate HIE.
It is worth noting that prior to 2012, HIE alone was not an inclusion diagnosis for CPQCC data collection. Although many infants with moderate and severe HIE would likely have qualified for inclusion based on mechanical ventilation and neonatal transport, infants with HIE in the milder spectrum may have been omitted if they did not meet other criteria. As such, patients with mild HIE in our analysis may have been on the more acutely ill range. There is also the potential for differences in categorization of level of encephalopathy compared with clinical trials, as the diagnostic criteria for HIE was adopted from the Vermont Oxford Network.
The socio-demographic characteristics of infants with HIE did not differ between hypothermia and normothermia groups, which is encouraging as new and evidence-based practices may sometimes find faster uptake in higher socio-demographic strata, thereby leading to disparities in health at a large population based level.20, 21
In order to maximize the effectiveness of hypothermia in the community, there is benefit in developing screening protocols of eligible infants with moderate or severe HIE at birthing hospitals across both urban and rural centers. Based on an annual term birth rate of over 500,000 infants in California, and with an HIE incidence rate of 1.5 per 1,000 live births reported in the literature, one would expect about 750 total HIE cases per year in California. Clinical protocols that address the close surveillance needed to identify cooling candidates with less dramatic onset of illness or evolution of encephalopathy following birth (“the quiet baby”), and that assist in the streamlining of communication at a local and regional level, will enhance the large-scale population based implementation of hypothermia therapy.
Efforts are underway in establishing regional guidelines for the systematic conduct of hypothermia therapy in California. Regional conferences of cooling centers have been occurring and a CPQCC hypothermia toolkit for birthing hospitals and regional NICUs is being developed. As part of its quality care initiative, CPQCC has broadened its eligibility criteria for data collection to include hypothermia treatment, the diagnosis of HIE, and a broader diagnosis of suspected asphyxia.
This is a statewide database study in California characterizing treatment patterns and outcomes for term infants with HIE in the emerging era of hypothermia therapy. Many infants with moderate to severe HIE still do not receive hypothermia treatment despite its proven benefit; many infants with mild HIE are receiving hypothermia despite the lack of evidence supporting its use in this population. The severity of HIE remains the strongest predictor of mortality. Our findings highlight an opportunity to identify practice site variation and develop quality improvement interventions, to assure consistent evidence-based care of term infants with HIE, and appropriate application of hypothermia therapy for eligible infants.
Acknowledgments
Supported by the Department of Health and Human Services Health Resources and Services Administration Centers of Excellence (D34HP16047), from the National Institute of Health Developmental & Neonatal Biology Training Program (HD07249), the Cooperative Multicenter Neonatal Research Network Research Supplement to promote Diversity in Health-Related Research from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (3U10HD02788019S1), and the Eunice Kennedy Shriver National Institute of Child Health & Human Development Grant Number K23HD068400.
The authors thank the participating CPQCC members.
ABBREVIATIONS
- HIE
Hypoxic ischemic encephalopathy
- CPeTS
California Perinatal Transport System
- CPQCC
California Perinatal Quality Care Collaborative
- NICU
Neonatal intensive care Unit
- NICHD
National Institute of Health and Human Development
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shankaran S. Hypoxic-ischemic encephalopathy and novel strategies for neuroprotection. Clinics in perinatology. 2012;39:919–29. doi: 10.1016/j.clp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early human development. 2010;86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 4.Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 5.Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72. 72 e1–3. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- 9.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66. doi: 10.1001/archpediatrics.2011.1772. [DOI] [PubMed] [Google Scholar]
- 10.Edwards AD, Brocklehurst P, Gunn AJ, Halliday H, Juszczak E, Levene M, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:c363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Seminars in fetal & neonatal medicine. 2010;15:238–46. doi: 10.1016/j.siny.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azzopardi D, Strohm B, Edwards AD, Halliday H, Juszczak E, Levene M, et al. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Archives of disease in childhood Fetal and neonatal edition. 2009;94:F260–4. doi: 10.1136/adc.2008.146977. [DOI] [PubMed] [Google Scholar]
- 14.Azzopardi D, Strohm B, Linsell L, Hobson A, Juszczak E, Kurinczuk JJ, et al. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK--analysis of national data. PloS one. 2012;7:e38504. doi: 10.1371/journal.pone.0038504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould JB. The role of regional collaboratives: the California Perinatal Quality Care Collaborative model. Clinics in perinatology. 2010;37:71–86. doi: 10.1016/j.clp.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 16. [Accessed February 22, 2014];CPQCC Network Database Manual of Definitions for Infants Born in 2013. Available at: http://cpqcc.org/data/cpqcc_downloads.
- 17.Higgins RD, Raju TN, Perlman J, Azzopardi DV, Blackmon LR, Clark RH, et al. Hypothermia and perinatal asphyxia: executive summary of the National Institute of Child Health and Human Development workshop. J Pediatr. 2006;148:170–5. doi: 10.1016/j.jpeds.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Higgins RD, Raju T, Edwards AD, Azzopardi DV, Bose CL, Clark RH, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159:851–8.e1. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuPont TL, Chalak LF, Morriss MC, Burchfield PJ, Christie L, Sanchez PJ. Short-term outcomes of newborns with perinatal acidemia who are not eligible for systemic hypothermia therapy. J Pediatr. 2013;162:35–41. doi: 10.1016/j.jpeds.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bain LC, Dudley RA, Gould JB, Lee HC. Factors Associated with Failure to Screen Newborns for Retinopathy of Prematurity. J Pediatr. 2012 doi: 10.1016/j.jpeds.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HC, Lyndon A, Blumenfeld YJ, Dudley RA, Gould JB. Antenatal steroid administration for premature neonates in California. Obstetrics and gynecology. 2011;117:603–9. doi: 10.1097/aog.0b013e31820c3c9b. [DOI] [PMC free article] [PubMed] [Google Scholar]