Abstract
The optimal approach to postpartum dosing among women treated with methadone maintenance is unclear. We examined doses among 101 methadone-maintained pregnant women 2, 6 and 12 weeks postpartum, and compared the incidence of having doses held for oversedation during pregnancy and postpartum. The average dose at delivery was 83.3 mg, and the mean change from delivery to 12 weeks postpartum was −3.7 mg (95% CI −6.3, −1.1). The incidence of oversedation events per 10,000 days was 2.8 among pregnant women and 5.6 for postpartum women (incidence rate ratio [IRR] 2.04, 95% CI 0.66, 6.28). After adjusting for benzodiazepine prescriptions, the IRR of an oversedation event among postpartum women compared to pregnant women was 1.74 (95% CI 0.56, 5.30). In conclusion, postpartum dose changes were small in a methadone clinic using clinical assessments to determine dose. Although the incidence of oversedation events remained low postpartum, the clinically important but not statistically significant increase in events among postpartum women and those prescribed benzodiazepines requires further research. While there are not yet adequate data to support pre-specified postpartum dose reductions, the findings suggest that more frequent clinical assessments continuing as late as 12 weeks postpartum may be warranted.
Keywords: Pregnancy, postpartum, opioid dependence, methadone maintenance treatment
Introduction
Among opioid dependent women who are pregnant, methadone maintenance treatment (MMT) reduces illicit opioid use, improves women’s access to prenatal care, and improves neonatal outcomes, particularly birth weight (Bell & Harvey-Dodds, 2008; Jones, Martin, et al., 2008). Buprenorphine is used increasingly to treat opioid-dependent women who are pregnant, due to its availability in the office setting and evidence of decreased severity of neonatal abstinence syndrome (NAS) (Jones et al., 2010). Yet for many women, MMT continues to have advantages, including the structured treatment environment and methadone’s properties as a full agonist with no ceiling effect, which may contribute to better retention in care (Jones et al., 2010).
Pregnancy offers powerful motivation for opioid dependent women to seek treatment, including MMT (Daley, Argeriou & McCarty, 1998). The postpartum period is thus a critical crossroads on the path to long-term recovery. Ensuring optimal methadone dosing during this period is important. Methadone doses must be sufficiently high, typically 60 mg or greater, to treat opioid withdrawal, reduce opioid cravings, and block opioid euphoria, all of which lead to reduced illicit opioid use and abstinence in both pregnant and non-pregnant opioid dependent populations (McCarthy, Leamon, Parr & Anania, 2005; Faggiano, Vigna-Taglianti, Versino & Lemma, 2003). Yet, the goal of providing an effective, sufficiently high dose needs to be balanced with concerns about the risks of oversedation.
Achieving this balance can be clinically complex, particularly in the postpartum period. Pregnant women often require increases in methadone dose throughout pregnancy due to factors such as increased intravascular volume, and increased tissue reservoir and hepatic metabolism of the drug (Center for Substance Abuse Treatment, 2005). The optimal approach to methadone dose management in the postpartum period, however, is not well-defined. Federal treatment guidelines state:
“Current treatment practices include continuing methadone after delivery either at dosages similar to those before pregnancy or, for women who began methadone maintenance during pregnancy, at approximately half the dosages they received in the third trimester. However, no empirical data support these approaches, and any decrease should be based on signs of overmedication, withdrawal symptoms, or patient blood plasma levels.” (Center for Substance Abuse Treatment [CSAT], 2005).
Prior observational studies found that women received minimal dose adjustments in the immediate period after delivery (Jones, Johnson et al., 2008; Albright et al., 2011). Postpartum dose reductions to half the third trimester dose as described by CSAT in the quotation above were not described in these studies. However, only the smaller of these two investigations of women taking methadone (n=10), reported on the incidence of overmedication among the women studied (Jones, Johnson et al., 2008). In addition, these studies only followed women until 5 and 6 weeks postpartum, respectively. Hepatic methadone clearance may remain elevated until six weeks post-delivery, and it may take up to 12 weeks or more for intravascular volume and other hemodynamic parameters to return to pre-pregnancy status (Tracy, Venkataramanan, Glover & Caritis, 2005; Silversides & Colman, 2007). Thus, a longer post-delivery observation period of 12 weeks, with data on oversedation from a larger sample, would provide better guidance on dosing safety and effectiveness in this population (Jones, Johnson et al., 2008). In the current study, we sought to describe dosing changes from delivery until 12 weeks postpartum among opioid dependent women in MMT, and to describe the rate of oversedation events in the postpartum period, compared to pregnancy.
Methods
Design
This study was a retrospective cohort study of women who initiated methadone maintenance while pregnant. The current analysis was part of a larger study whose aim was to compare substance use outcomes among pregnant and postpartum women receiving methadone as opioid agonist treatment.
Clinical context
Typically, pregnant women in this MMT program enrolled after an inpatient stay at an affiliated hospital, where they underwent an initial methadone titration. After the women were discharged and began outpatient MMT, the methadone providers, consisting of nursing staff and two supervising physicians, made further, more gradual dosing adjustments based on patients’ clinical signs and symptoms. For example, women who reported ongoing cravings or withdrawal symptoms to providers or to their counselors were eligible for a dose increase, usually 5 mg, followed by an assessment at the window four days later, to ensure the adequacy and safety of the increase. The nurses used a template to assess signs of withdrawal or sedation/intoxication, including pulse, respiratory rate, pupil size, and blood pressure; they also evaluate symptoms such as rhinitis, diaphoresis, lacrimation, nausea/vomiting/diarrhea, gooseflesh and anxiety. In addition, they specifically ask about mid-day sedation when methadone levels typically peak. If the dose was changed again, another assessment took place four days later, and so on. Doses were decreased if women, their counselors, or a medical provider reported sedation, lethargy or other signs of possible overmedication. In addition, women who were observed to be oversedated at the dosing window had their dose for the day held, and the dose was subsequently re-evaluated. In such situations, MMT staff also attempted to contact, with the patient’s permission, any other providers who were prescribing potentially sedating medications such as benzodiazepines to make them aware of the incident and to coordinate care.
After delivery, women had a nursing assessment on the day of their return to the clinic, when in addition to doing a clinical assessment, the nurse confirmed the woman’s hospital methadone dose by telephone with the inpatient nursing staff. Any dose changes made at that visit were typically discussed with the physician by phone, and were followed by assessments following the same protocol as during the pregnant phase. In addition, a methadone physician assessment was scheduled within two weeks postpartum.
Subjects
Women who enrolled in the methadone clinic between February 1, 2006, and January 31, 2010 were eligible for this study if they were pregnant at enrollment. Of note, because of the aims of the larger study, we excluded from data collection all women who miscarried or terminated their pregnancies, or who had two consecutive pregnancies within 18 months of their initial enrollment in the clinic. For the purposes of the current analysis of methadone dose changes, we further excluded women who left MMT before 12 weeks after delivery.
Data collection
We reviewed the electronic medical records of the methadone clinic and the affiliated medical center. The two main outcomes examined were daily methadone dose received and the number of days when women had doses held for oversedation (i.e. oversedation events). Other data included patient demographics, health-related characteristics (i.e., HIV status, receipt of benzodiazepine prescriptions during pregnancy and postpartum, and smoking at enrollment) and MMT-related factors (i.e., previous MMT and take home privileges). Of note, although usually the term postpartum is used for women who have delivered within the last six weeks, we have used the term “postpartum” to refer to women up to 12 weeks after delivery.
Analysis
For demographic and health-related characteristics of the sample, we generated descriptive statistics, including frequencies for categorical variables, and means, medians and standard deviations for continuous variables. In addition, we calculated the mean weekly methadone dose received by the women during the pregnancy and postpartum periods. The time points of the dose are defined relative to delivery (e.g. 20 weeks before delivery, 2 weeks after delivery, etc.). We calculated the mean change in dose between delivery and 2, 6 and 12 weeks postpartum and used paired t-tests to determine whether the differences between methadone doses were statistically significant. Using generalized estimating equations (GEE) Poisson regression, we calculated the incidence rate ratio and associated 95% confidence interval (CI) for oversedation events among postpartum compared to pregnant women across time. Because benzodiazepines were considered to be an important potential confounder, we then calculated an incidence rate ratio and 95% CI for oversedation events among postpartum compared to pregnant women, controlling for benzodiazepine prescriptions. We also calculated an incidence rate ratio and 95% CI comparing women prescribed benzodiazepines to women not prescribed benzodiazepines. The models included an offset for the total number of dosing days in a given time period to account for unequal observation times. All data analyses were generated using SAS/STAT software, Version 9.3 of the SAS System for Windows (SAS Institute, Inc., 2002–2010). Statistical significance was two-tailed and defined as p<0.05.
Human subjects protections
Approval for this study was obtained from the Boston University Medical Campus Institutional Review Board, which represents both the medical center and the MMT program. Because this was a retrospective medical record review in which no identifying information was collected, the requirement for informed consent was waived.
Results
Of the 273 pregnant women who enrolled in the methadone clinic during the time frame of interest, 101 met eligibility criteria for the current study. The other 163 women were not included for the following reasons: ineligible due to miscarriage or pregnancy termination after enrollment (n=39); left the clinic prior to delivery (n=98); had two pregnancies during the first 18 months of MMT treatment (n=10); or left the clinic before 12 weeks postpartum (n=25).
Table 1 illustrates the demographic and health-related characteristics of the women. The mean age was 27 years, the majority was white and 5 were HIV-infected. Of the 44 women who had living children from previous pregnancies, thirteen women (30%) had one or more of these children living with them at the time of delivery. Nineteen percent of women were prescribed benzodiazepines during pregnancy while 28% were prescribed benzodiazepines postpartum.
Table 1.
Characteristics of pregnant and postpartum women enrolled in a methadone maintenance treatment program (N=101)
| Variable | % (n/N1) |
|---|---|
| Mean age in years (SD2) | 27 (5.7) |
| Race / Ethnicity | |
| White, non-Hispanic | 83% (84/101) |
| Black/African-American/non-Hispanic | 2% (2/101) |
| Hispanic | 15% (15/101) |
| HIV-infected | 5% (5/100) |
| Smoker at enrollment | 89% (82/92) |
| Previous MMTP | 41% (41/100) |
| Prior pregnancies | 74% (70/95) |
| Prior deliveries | 48% (45/94) |
| Of those with prior deliveries, lived with their children at enrollment | 30% (13/443) |
| Median number of weeks between enrollment and delivery (IQR4) | 22 (12, 28) |
| Take home privileges achieved by delivery | 21% (21/101) |
| Living situation on delivery | |
| Housed | 63% (64/101) |
| Homeless | 15% (15/101) |
| Residential program | 14% (14/101) |
| Other | 8% (8/101) |
| Benzodiazepine prescription at any point: During pregnancy Up to 12 weeks postpartum |
19% (19/101) 28% (28/101) |
Data for all 101 eligible women were not available for certain variables
Standard deviation
Information available for 44 of the 45 women with prior deliveries
Interquartile range
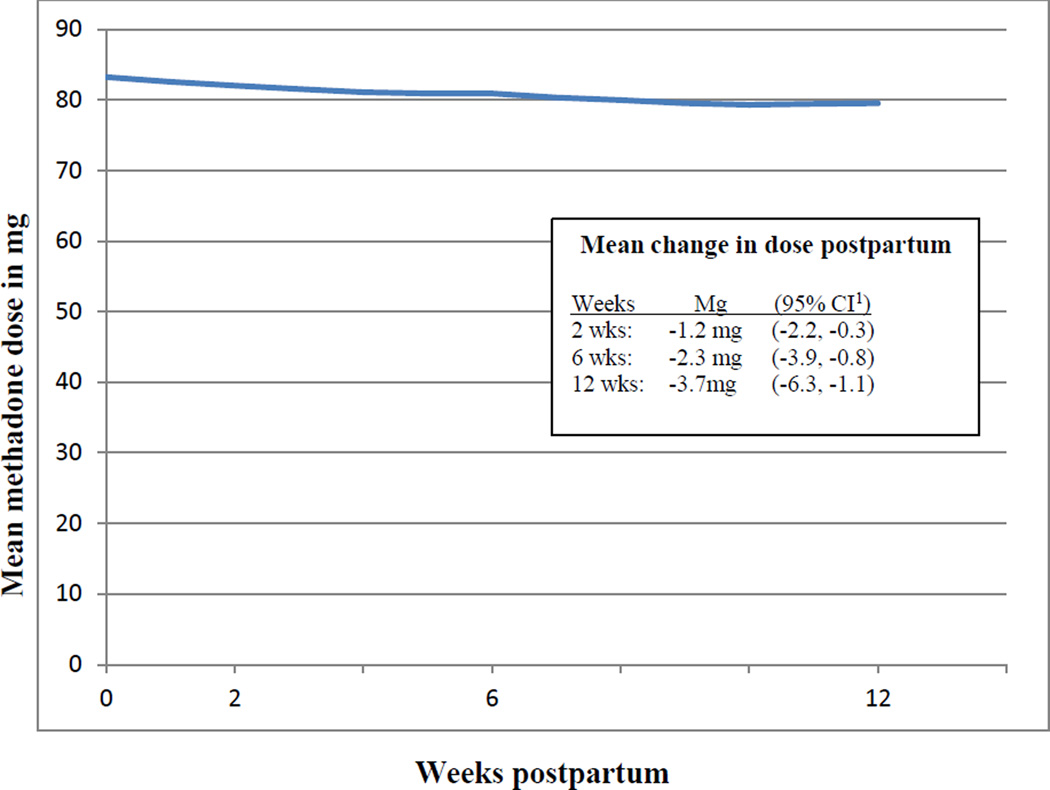
The average dose at delivery was 83.3 mg. Over the first twelve weeks postpartum, 48 women had a net decrease in dose, while 24 women had a net increase and 29 women had no change. As shown in Figure 1, the mean change in dose from delivery to 2 weeks was −1.2 mg (95% confidence interval −2.2, −0.3), delivery to 6 weeks −2.3 mg (95% CI, −3.9, −0.8), and delivery to 12 weeks −3.7 mg (95% CI − 6.3, −1.1).
Figure 1.
Changes in mean methadone dose in relation to delivery date among 101 postpartum women in methadone maintenance treatment.
1CI=confidence interval
Three women had oversedation events during pregnancy only, five women had oversedation events during the twelve-week postpartum period only, and one woman had oversedation events during both pregnancy and the postpartum period. For the postpartum events, the average time from delivery to the event was 60 days (range 51–67, standard deviation 5.9). Among the nine women who had oversedation events, there were 10 total events, such that the rate of events during pregnancy was 2.8 per 10,000 dosing days and the rate during the postpartum period was 5.6 per 10,000 dosing days. Thus, the IRR of an oversedation event among postpartum women compared to pregnant women was 2.04 (95% CI 0.66, 6.28). Of note, 56% (5/9) of women who had oversedation events were prescribed benzodiazepines during the phase (pregnant or up to twelve weeks postpartum) when they had the event. In a model adjusting for benzodiazepine prescriptions, the IRR of an oversedation event among postpartum women compared to pregnant women was 1.74 (95% CI 0.56, 5.30). The IRR for an oversedation event among women prescribed benzodiazepines compared to those not prescribed benzodiazepines was 2.84 (95% CI 0.91, 8.91).
Discussion
At a methadone clinic where postpartum dosing adjustments were made according to clinical signs and symptoms, rather than a pre-specified dose reduction protocol, women experienced only a small reduction in their methadone dose between delivery and 12 weeks postpartum. Despite substantial changes in hepatic methadone clearance and plasma volume during the period between delivery and 12 weeks later, findings from this study indicate most postpartum women did not have signs or symptoms that indicated the need for a large dose reduction on routine assessments. These findings are consistent with prior, limited data from two studies that examined dosing patterns between delivery and 6 weeks postpartum.
Potential explanations for the relatively minor dose adjustments seen include the possibility that some women may not have had their doses titrated to the optimal level during pregnancy, perhaps because they presented late to treatment, leaving less time for such titration, or because they resisted recommended dose increases. In terms of the first possibility, it is noteworthy that postpartum dose adjustments were similarly small in a study that excluded women who presented after 26 weeks gestational age (Albright et al., 2011). With regard as to why women may have resisted dose increases during pregnancy, some may have been concerned that higher methadone doses could increase the likelihood or severity of neonatal abstinence syndrome, even though this connection is not borne out consistently in the medical literature (McCarthy et al., 2005; Cleary et al., 2012). In addition to these possibilities, patients who were indeed on optimal doses while pregnant may have been reluctant to seek lower doses postpartum because of a fear of relapse, even if they began to experience effects of the methadone more strongly. Providers, too, may have failed to initiate dose reductions due either to clinical inertia (Phillips et al., 2001) or because they were reluctant to make a change when a patient’s recovery was going well, so long as they were not markedly oversedated. It is also important to note that methadone providers saw patients in the morning, prior to or shortly after dosing, at nadir methadone levels; if patients did not report sedation or lethargy, providers would have witnessed only the most pronounced cases of overmedication.
In addition to these clinical factors, there are possible physiologic explanations. For example, there may be inter-individual variability in how long hemodynamic parameters take to return to pre-pregnancy levels, leading to some women requiring higher doses even at 12 weeks after delivery. In addition, though the relationship between body weight and methadone dose requires further investigation, obesity is common among patients in MMT (Nolan & Scagnelli, 2007), and it is possible that trouble losing pregnancy-related fat stores could increase dosing requirements in ways that have not been described. Our data do not allow us to distinguish between these or other possible physiologic explanations.
The findings related to oversedation events raise further questions about whether the lack of major dose reductions seen in this study reflects physiology or the limitations of clinical assessment. The number of oversedation events was, importantly, very small relative to the total number of dosing days. Nonetheless, there appeared to be a higher incidence of oversedation events during the postpartum period compared to the pregnant period, although the difference was not statistically significant in this sample. It is striking that first, all the postpartum events occurred between seven and ten weeks postpartum; and second, the difference in oversedation incidence between the postpartum and pregnant periods became smaller, but did not disappear, when the model adjusted for benzodiazepine prescriptions, which were reported by more than half of women with oversedation events. Clearly, further research investigating oversedation is needed, both its incidence among postpartum women, and any role of benzodiazepines. In particular, a prospective study of more aggressive dose reduction (such as that suggested by CSAT) in a larger sample could yield valuable information (CSAT, 2005). In the meantime, it is prudent to ensure that women receive additional clinical assessments postpartum ideally out to 12 weeks. At such assessments, both the adequacy and the safety of patients’ postpartum dose could be evaluated. Women receiving benzodiazepines and other sedating medications may require particular attention, with consideration of the risk/benefit ratio of these medications for each individual.
There are several limitations to this study. The sample size (n=101) may have limited our ability to find a significant difference between the number of oversedation events during the pregnant versus the postpartum periods, as well as our ability to fully discern the relationship between benzodiazepine prescriptions and oversedation events. Yet, our analysis was intended to be primarily descriptive rather than focused on hypothesis testing. Second, oversedation events detected at the dosing window are a very specific, yet insensitive measure of overmedication with methadone. In addition, we could not verify that methadone was the drug causing the signs of sedation in the cases when doses were held; equally likely is the possibility that women were taking other drugs, such as benzodiazepines, resulting in the oversedated presentation. The data allowed us to control only for receipt of benzodiazepine prescriptions in our calculation of the incidence rate ratio of oversedation events among pregnant vs postpartum women, but we could not assess the possible contribution of non-prescribed benzodiazepines, illicit opioids, or other drugs. Notably, postpartum exhaustion and sleep deprivation could play a contributing role in some cases. Finally, this study represents observational data from a single clinical setting and findings from this patient population may not be fully applicable to other groups of opioid dependent pregnant women. However, the two prior studies that have investigated postpartum dosing had notably similar findings, albeit during a shorter period of postpartum observation.
In conclusion, despite concerns that physiologic changes in the postpartum period may necessitate large reductions in methadone dose, postpartum women with opioid dependence in a clinic with an individualized approach to dosing typically received small reductions in methadone dose even at 12 weeks after delivery. Oversedation events remained uncommon, but our data suggest they may be more common among women in the extended postpartum period, and women prescribed benzodiazepines. Larger, prospective studies are needed to guide safe and effective methadone dosing postpartum. For now, given the physiologic changes and psychosocial stressors unique to the postpartum period, it is appropriate for methadone clinics to implement regular postpartum assessments at intervals extending at least up to 12 weeks after delivery, when clinic staff can make appropriate dose adjustments that take into account concomitant benzodiazepine use.
Acknowledgements
Dr. Pace received support from NIDA R25DA13582 (2010–2011) and NIAID T32 AI052074-06A2 (2011–2012) for her work on this study. We wish to acknowledge Eileen Brigandi, Sherry Ceridan, Lisa Sturtz and Mimi Vitale for their contributions to data collection. We thank the women who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albright B, de la Torre L, Skipper B, Price S, Abbott P, Rayburn W. Changes in methadone maintenance therapy during and after pregnancy. Journal of Substance Abuse Treatment. 2011;41:347–353. doi: 10.1016/j.jsat.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bell J, Harvey-Dodds L. Pregnancy and injecting drug use. BMJ. 2008;336:1303–1305. doi: 10.1136/bmj.39514.554375.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opoid Addiction in Opioid Treatment Programs (Treatment Improvement Protocol (TIP) Series 43) Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005. [PubMed] [Google Scholar]
- Cleary B, Eogan M, O'Connell M, Fahey T, Gallagher P, Clarke T, White M, McDermott C, O'Sullivan A, Carmody D, Gleeson J, Murphy D. Methadone and Perinatal Outcomes - a Prospective Cohort Study. Addiction. 2012;107:1482–1492. doi: 10.1111/j.1360-0443.2012.03844.x. [DOI] [PubMed] [Google Scholar]
- Daley M, Argeriou M, McCarty D. Substance abuse treatment for pregnant women: a window of opportunity? Addictive Behaviors. 1998;23:239–249. doi: 10.1016/s0306-4603(97)00029-4. [DOI] [PubMed] [Google Scholar]
- Faggiano F, Vigna-Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Systematic Reviews. 2003;3:CD002208. doi: 10.1002/14651858.CD002208. [DOI] [PubMed] [Google Scholar]
- Jones HE, Johnson RE, O'Grady KE, Jasinski DR, Tuten M, Milio L. Dosing adjustments in postpartum patients maintained on buprenorphine or methadone. Journal of Addiction Medicine. 2008;2:103–107. doi: 10.1097/ADM.0b013e31815ca2c6. [DOI] [PubMed] [Google Scholar]
- Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O'Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. New England Journal of Medicine. 2010;363:2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Martin PR, Heil SH, Kaltenbach K, Selby P, Coyle MG, Stine SM, O'Grady KE, Arria AM, Fischer G. Treatment of opioid-dependent pregnant women: clinical and research issues. Journal of Substance Abuse Treatment. 2008;35:245–259. doi: 10.1016/j.jsat.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Leamon MH, Parr MS, Anania B. High-dose methadone maintenance in pregnancy: maternal and neonatal outcomes. American Journal of Obstetrics and Gynecology. 2005;193:606–610. doi: 10.1016/j.ajog.2005.03.072. [DOI] [PubMed] [Google Scholar]
- Venkataramanan Nolan LJ, Scagnelli LM. Preference for sweet foods and higher body mass index in patients being treated in long-term methadone maintenance. Substance Use and Misuse. 2007;42:1555–1566. doi: 10.1080/10826080701517727. [DOI] [PubMed] [Google Scholar]
- Phillips LS, Branch WT, Cook CB, Doyle JP, El-Kebbi IM, Gallina DL, Miller CD, Ziemer DC, Barnes CS. Clinical inertia. Annals of Internal Medicine. 2001;135:825–834. doi: 10.7326/0003-4819-135-9-200111060-00012. [DOI] [PubMed] [Google Scholar]
- Silversides CK, Colman JM. Physiological Changes in Pregnancy. In: Oakley C, Warnes CA, editors. Heart Disease in Pregnancy. Malden, Massachusetts: Blackwell Publishing; 2007. pp. 6–17. [Google Scholar]
- Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. American Journal of Obstetrics and Gynecology. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]