Abstract
Objective
To explore the early childhood pulmonary outcomes of infants who participated in the NICHD SUPPORT Trial, using a factorial design that randomized extremely preterm infants to lower vs. higher oxygen saturation targets and delivery room CPAP vs. intubation/surfactant, found no significant difference in the primary composite outcome of death or BPD.
Study design
The Breathing Outcomes Study, a prospective secondary to SUPPORT, assessed respiratory morbidity at 6 month intervals from hospital discharge to 18–22 months corrected age (CA). Two pre-specified primary outcomes, wheezing more than twice per week during the worst 2 week period and cough longer than 3 days without a cold were compared between each randomized intervention.
Results
One or more interviews were completed for 918 of 922 eligible infants. The incidence of wheezing and cough were 47.9% and 31.0%, respectively, and did not differ between study arms of either randomized intervention. Infants randomized to lower vs. higher oxygen saturation targets had similar risks of death or respiratory morbidities (except for croup, treatment with oxygen or diuretics at home). Infants randomized to CPAP vs. intubation/surfactant had fewer episodes of wheezing without a cold (28.9% vs. 36.5%, p<0.05), respiratory illnesses diagnosed by a doctor (47.7% vs. 55.2%, p<0.05) and physician or emergency room visits for breathing problems (68.0% vs. 72.9%, p<0.05) by 18–22 months CA.
Conclusion
Treatment with early CPAP rather than intubation/surfactant is associated with less respiratory morbidity by 18–22 months CA. Longitudinal assessment of pulmonary morbidity is necessary to fully evaluate the potential benefits of respiratory interventions for neonates.
MeSH terms: Bronchopulmonary Dysplasia; Infant, Newborn; Infant, Low Birth Weight; Infant, Extremely Low Birth Weight; Infant, Premature; Infant, Extremely Low Gestational Age; Infant mortality; Respiratory morbidity; Intensive care, neonatal; Hospital Readmission; Oximetry; Randomized controlled trial; Retinopathy of prematurity (ROP); Continuous Positive Airway Pressure; Intubation, endotracheal; Pulmonary surfactants/therapeutic use; Oxygen inhalation therapy/methods; Oxygen administration & dosage; Follow-up studies
Extremely preterm infants are at greater risk of respiratory morbidity and need for pulmonary care in early childhood than later preterm or term infants (1–7) and contribute substantially to the public health burden of childhood respiratory disease in the United States.(8) Lung injury, which may result from mechanical ventilation and supplemental oxygen exposure in the early neonatal period, has been identified as a risk factor for development of Bronchopulmonary Dysplasia (BPD) and pulmonary morbidity in infancy, childhood and beyond.(1, 2, 9, 10) Though infants with BPD are at highest risk for poor pulmonary outcome, neonates without BPD are also at risk for airway dysfunction and pulmonary morbidity during infancy.(4, 11)
The Surfactant Positive Airway Pressure and Pulse Oximetry Randomized Trial (SUPPORT) conducted by the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) studied infants 24 0/7th – 27 6/7th weeks’ gestation treated with each of two respiratory strategies designed to minimize mechanical ventilation and supplemental oxygen exposure lower (85–89%) compared with higher (91–95%) oxygen saturation targets and early non-invasive continuous positive airway pressure (CPAP) compared with early intubation and early surfactant administration (intubation/surfactant). Our Network previously reported results of SUPPORT demonstrating no significant differences in the composite outcomes of death or BPD and death or neurodevelopmental impairment between infants randomized to either of the two respiratory interventions.(12–14) It is important to note that although the composite incidence of death or BPD was similar, infants randomized to lower rather than higher oxygen saturation targets had significantly lower incidences of retinopathy of prematurity but significantly greater mortality at discharge.
We now report on The Breathing Outcomes Study, a sub study to the SUPPORT Trial, which compared respiratory morbidities among extremely preterm infants treated with the SUPPORT interventions as neonates. It was hypothesized that infants randomized to lower rather than higher oxygen saturation targets or CPAP, rather than intubation and surfactant, would have a lower incidence of wheezing more than twice per week during their worst 2 week period, a lower incidence of cough lasting more than 3 days without a cold, and as a secondary outcome, less need for outpatient pulmonary care between discharge and 18–22 months’ corrected age (CA, age in months following the expected date of full term delivery).
METHODS
Infants eligible for The Breathing Outcomes Study were infants enrolled in SUPPORT who survived to hospital discharge and consented for enrollment into the study. Infants (n=1316) from 20 centers across the United States were enrolled into SUPPORT between February 2005 and February 2009 and seen in follow-up between 2006 and 2011. As a sub study to SUPPORT, Breathing Outcomes gained approval and began recruitment after SUPPORT began enrollment. As a result not all SUPPORT patients were successfully recruited into Breathing Outcomes. Written informed consent to participate in Breathing Outcomes was obtained either at the time of enrollment into SUPPORT or separately for those patients already enrolled in SUPPORT but not yet discharged from the hospital. The study was approved by the institutional review boards at all participating Network centers.(12, 13)
Interventions of the SUPPORT Trial
Subjects enrolled in SUPPORT were randomly assigned prior to delivery to receive CPAP after birth, followed by a limited ventilation strategy if intubation was needed or to intubation in the delivery room and receipt of prophylactic surfactant by 1 hour of age (intubation/surfactant). Using a 2×2 factorial design, SUPPORT subjects were also randomly assigned to treatment with either an oxygen saturation target of 85% to 89% (lower saturation group) or with a target of 91% to 95% (higher saturation group). Research methods for study enrollment, intervention, data collection and primary analyses have been previously reported.(13) Primary outcomes of SUPPORT included the incidence of death or meeting criteria for the physiologic definition of BPD and death or meeting criteria for traditional BPD, defined as receipt of supplemental oxygen at 36 weeks PMA. (15)
Assessments of the Breathing Outcomes Study
For subjects enrolled in Breathing Outcomes, a parent or primary caregiver was interviewed by research staff either in person or by telephone using structured questionnaires and interview scripts at each of 4 time points; at or near the time of hospital discharge and at or near 6, 12 and 18–22 months CA. To standardize administration of the interview, a lead interviewer at each participating center underwent training consisting of a teleconference with 1 of 2 project trainers (Rochester site) to discuss each study question and review the manual of operations (MOP) which included a written interview script. Interview trainees then interviewed a standardized patient simulated by the project trainers. With the aid of the MOP, lead interviewers at each center were then able to train additional interviewers at their sites as needed. To minimize misinterpretation of other respiratory sounds as wheezing, a verbal description of wheezing and a brief audio clip of wheezing were played for the interviewee at the beginning of the interview. Questionnaires originally written in English were translated into Spanish using a certified translation service (Cornell Translation Service, Ithaca, NY). Interviews were conducted in either English or Spanish as appropriate.
To minimize loss of recall over time, four interviews were conducted at approximately 6 month intervals beginning at the time of hospital discharge.(16) Study personnel conducted the first parent interview using a questionnaire designed to collect information on family history of respiratory diseases and atopy, home environment including tobacco and pet exposures, and diet at discharge from the hospital. Based upon the preference of each participating center, the 6, 12 and 18–22 month interviews were conducted either by trained staff at the local center (15 centers) or by long distance telephone interview from the Rochester center (5 centers). At each of the 6, 12 and 18–22 month interviews, the parent or caregiver was asked to base their responses on the 6 month interval since the last interview. If an interview at one time point was not completed, parents were asked to base their responses during the next interview upon the interval history since the last completed interview. Taken together, the four questionnaire series was designed to provide a complete respiratory history over the first 18–22 months’ CA. In addition to reporting interview responses during the first 18–22 months CA (defined as the combined responses to the 6, 12, 18–22 month interviews and listed as 6–22 months in Table IV), we report responses from the 6 month interview because preterm infants are at especially high risk of respiratory morbidity during the first 6 months of age.(17)
Table 4.
Respiratory outcomes for lower vs. higher oxygen saturation and early CPAP vs. intubation and surfactant cohorts at the 6 month interview and for the first 18–22 months corrected age (combined responses to the 6, 12 and 18–22 month interviews).
| Primary Outcomes | Low Sat | High Sat | ARR (95% CI) | p-value | CPAP | Intubation/Surfactant | ARR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Has your child’s chest sounded wheezy or whistling more than twice in one week? | ||||||||
| 6 months | 94 (22.0) | 129 (27.7) | 0.73 (0.53, 1.01) | 0.06 | 107 (23.2) | 116 (26.9) | 0.79 (0.58, 1.09) | 0.16 |
| 6–22 months | 203 (46.7) | 233 (49.1) | 0.92 (0.70, 1.22) | 0.57 | 224 (47.7) | 212 (48.2) | 0.90 (0.68, 1.19) | 0.47 |
| Has your child had a cough for more than 3 days without a cold? | ||||||||
| 6 months | 63 (16.9) | 76 (19.3) | 0.84 (0.57, 1.22) | 0.35 | 63 (16.2) | 76 (20.2) | 0.77 (0.53, 1.12) | 0.17 |
| 6–22 months | 127 (30.8) | 141 (31.1) | 1.01 (0.75, 1.37) | 0.93 | 127 (28.4) | 141 (33.7) | 0.81 (0.60, 1.10) | 0.18 |
| Secondary Outcomes | ||||||||
| Symptoms | ||||||||
| Wheezing/whistling more than twice in one week or cough more than 3 days | ||||||||
| 6 months† | 162 (43.5) | 195 (49.5) | 0.78 (0.58, 1.05) | 0.10 | 178 (45.8) | 179 (47.5) | 0.95 (0.70, 1.28) | 0.72 |
| 6–22 months | 276 (66.8) | 316 (69.5) | 0.87 (0.65, 1.18) | 0.37 | 303 (67.8) | 289 (68.7) | 0.95 (0.70, 1.29) | 0.74 |
| Has your child’s chest sounded wheezy or whistling? | ||||||||
| 6 months | 135 (36.3) | 171 (43.4) | 0.73 (0.54, 1.00) | <0.05 | 151 (38.8) | 155 (41.1) | 0.89 (0.66, 1.21) | 0.47 |
| 6–22 months | 245 (59.3) | 286 (62.9) | 0.85 (0.64, 1.13) | 0.27 | 269 (60.2) | 262 (62.2) | 0.86 (0.64, 1.15) | 0.31 |
| Has your baby’s chest sounded wheezy or whistling apart from colds? | ||||||||
| 6 months | 61 (16.4) | 84 (21.3) | 0.73 (0.50, 1.06) | 0.10 | 66 (17.0) | 79 (21.0) | 0.77 (0.53, 1.11) | 0.16 |
| 6–22 months | 117 (28.4) | 165 (36.3) | 0.67 (0.49, 0.91) | 0.01 | 129 (28.9) | 153 (36.5) | 0.68 (0.50, 0.92) | 0.01 |
| Illnesses | ||||||||
| Has your child had asthma, reactive airway disease or BPD exacerbation or flare-up diagnosed by a doctor? | ||||||||
| 6 months† | 51 (13.7) | 62 (15.7) | 0.84 (0.56, 1.27) | 0.41 | 48 (12.3) | 65 (17.2) | 0.66 (0.44, 1.00) | <0.05 |
| 6–22 months† | 140 (33.9) | 158 (35.0) | 1.01 (0.75, 1.37) | 0.93 | 144 (32.2) | 154 (36.8) | 0.81 (0.60, 1.09) | 0.16 |
| Has your child had bronchiolitis, bronchitis or pneumonia diagnosed by a doctor? | ||||||||
| 6 months | 72 (19.4) | 78 (19.8) | 0.98 (0.67, 1.41) | 0.90 | 70 (18.0) | 80 (21.2) | 0.82 (0.57, 1.19) | 0.30 |
| 6–22 months | 161 (39.0) | 183 (40.4) | 0.96 (0.72, 1.28) | 0.79 | 167 (37.4) | 177 (42.2) | 0.81 (0.61, 1.09) | 0.17 |
| Any of asthma, reactive airway disease, BPD exacerbation or flare-up or bronchiolitis, bronchitis, or pneumonia diagnosed by a doctor | ||||||||
| 6 months | 95 (25.5) | 109 (27.7) | 0.91 (0.65, 1.27) | 0.58 | 96 (24.7) | 108 (28.7) | 0.81 (0.58, 1.13) | 0.22 |
| 6–22 months | 204 (49.4) | 241 (53.1) | 0.91 (0.69, 1.21) | 0.52 | 213 (47.7) | 232 (55.2) | 0.71 (0.53, 0.95) | 0.02 |
| Has your child had croup diagnosed by a doctor? | ||||||||
| 6 months†† | 9 (2.4) | 11 (2.8) | 0.87 (0.36, 2.08) | 0.75 | 7 (1.8) | 13 (3.5) | 0.52 (0.21, 1.30) | 0.16 |
| 6–22 months† | 46 (11.2) | 39 (8.6) | 1.35 (0.84, 2.16) | 0.21 | 40 (9.0) | 45 (10.8) | 0.77 (0.49, 1.23) | 0.28 |
| Health Services | ||||||||
| Has your child ever had to visit the doctor or Emergency Room for breathing or wheezing problems? | ||||||||
| 6 months | 167 (44.9) | 188 (47.8) | 0.82 (0.60, 1.11) | 0.20 | 173 (44.6) | 182 (48.3) | 0.81 (0.60, 1.10) | 0.18 |
| 6–22 months | 292 (70.1) | 319 (70.1) | 0.98 (0.72, 1.34) | 0.89 | 304 (68.0) | 307 (72.9) | 0.73 (0.53, 1.00) | <0.05 |
| Has your child had to stay in a hospital overnight? | ||||||||
| 6 months | 105 (28.2) | 118 (30.0) | 0.89 (0.64, 1.23) | 0.47 | 106 (27.3) | 117 (31.0) | 0.79 (0.57, 1.10) | 0.17 |
| 6–22 months | 169 (41.0) | 199 (43.7) | 0.90 (0.68, 1.20) | 0.48 | 182 (40.7) | 186 (44.3) | 0.87 (0.66, 1.16) | 0.35 |
| Has your child had to stay in a hospital overnight for wheezing/breathing problems? | ||||||||
| 6 months | 69 (18.6) | 73 (18.6) | 0.98 (0.67, 1.44) | 0.93 | 64 (16.5) | 78 (20.7) | 0.72 (0.49, 1.05) | 0.09 |
| 6–22 months | 129 (31.3) | 140 (30.8) | 1.04 (0.77, 1.40) | 0.80 | 130 (29.1) | 139 (33.1) | 0.82 (0.61, 1.11) | 0.21 |
| Medications | ||||||||
| Treated with a diuretic medication? | ||||||||
| 6 months† | 27 (6.3) | 24 (5.2) | 1.29 (0.72, 2.32) | 0.39 | 23 (5.0) | 28 (6.5) | 0.72 (0.40, 1.29) | 0.27 |
| 6–22 months† | 31 (7.1) | 24 (5.0) | 1.50 (0.85, 2.64) | 0.16 | 24 (5.1) | 31 (7.0) | 0.68 (0.39, 1.20) | 0.18 |
| Treated with an inhaled steroid medication? | ||||||||
| 6 months | 51 (11.9) | 53 (11.4) | 1.12 (0.73, 1.71) | 0.61 | 54 (11.7) | 50 (11.6) | 1.00 (0.66, 1.53) | 0.99 |
| 6–22 months | 112 (25.6) | 129 (26.9) | 0.97 (0.71, 1.32) | 0.82 | 128 (27.1) | 113 (25.5) | 1.10 (0.80, 1.50) | 0.56 |
| Treated with a nebulized medication? | ||||||||
| 6 months†† | 5 (1.2) | 18 (3.9) | 0.30 (0.11, 0.81) | 0.02 | 13 (2.8) | 10 (2.3) | 1.22 (0.54, 2.75) | 0.63 |
| 6–22 months† | 29 (6.6) | 42 (8.8) | 0.73 (0.44, 1.22) | 0.23 | 39 (8.3) | 32 (7.2) | 1.11 (0.67, 1.84) | 0.69 |
| Treated with a systemic steroid medication? | ||||||||
| 6 months†† | 11 (2.6) | 8 (1.7) | 1.50 (0.61, 3.70) | 0.38 | 12 (2.6) | 7 (1.6) | 1.61 (0.64, 4.04) | 0.31 |
| 6–22 months | 44 (10.1) | 42 (8.8) | 1.13 (0.71, 1.80) | 0.62 | 48 (10.2) | 38 (8.6) | 1.22 (0.77, 1.95) | 0.40 |
| Treated with oxygen at home? | ||||||||
| 6 months† | 90 (24.3) | 80 (20.3) | 1.22 (0.83, 1.79) | 0.31 | 80 (20.6) | 90 (23.9) | 0.82 (0.56, 1.21) | 0.32 |
| 6–22 months | 104 (25.2) | 96 (21.1) | 1.31 (0.92, 1.88) | 0.14 | 94 (21.0) | 106 (25.3) | 0.80 (0.56, 1.15) | 0.23 |
| Family | ||||||||
| Have you had to change your plans because of your child’s breathing problems? | ||||||||
| 6 months | 58 (15.5) | 69 (17.5) | 0.85 (0.57, 1.27) | 0.43 | 50 (12.9) | 77 (20.4) | 0.58 (0.39, 0.87) | <0.01 |
| 6–22 months | 139 (33.7) | 170 (37.4) | 0.87 (0.65, 1.17) | 0.36 | 145 (32.4) | 164 (39.0) | 0.74 (0.55, 1.00) | <0.05 |
Results presented as number/total number (%); ARR – adjusted relative risk with adjustments for stratification factors (study center and gestational age group) and familial clustering. Where models did not converge, adjustments are limited to center and gestational age (†). If the two adjustment model failed to converge, unadjusted relative risks are reported (††).
Respiratory Questionnaires
Questionnaires developed, validated and used with permission of the Tucson Children’s Respiratory Study were used to elicit the frequency and characteristics of respiratory signs, including wheezing and cough; incidence of physician-diagnosed asthma or allergy, presence of pets in home, siblings, reactive airway disease; incidence of bronchiolitis, bronchitis or pneumonia, croup; use of medications to treat respiratory illnesses including diuretics, nebulized bronchodilators, inhaled steroids, systemic steroids or oxygen; use of health services including respiratory related physician visits, emergency room visits and hospitalizations.(18, 19)
Outcomes
Primary Outcomes: Because preterm infants with or without BPD are at risk for altered airway function and greater risk of wheezing in infancy and later childhood (20–24), we chose to assess respiratory symptoms as a measure of pulmonary morbidity in infancy. Some authors have used incidence of recurrent wheezing as a primary measure of pulmonary morbidity (8, 23, 25), and others have used a combined outcome of either recurrent wheezing or chronic cough as a measure of occult wheezing in preterm infants. (1, 2, 26) To best capture overt and occult wheezing, two primary outcomes were assessed by parental report: the incidence of wheezing more than twice per week during the worst 2 week period and incidence of cough lasting more than 3 days without a cold.
The incidence of wheezing was ascertained using the primary question used and validated in the Tucson Study (a large prospective birth cohort study of term infants), “Has his/her chest sounded wheezy or whistling?”. (18) The outcome for wheezing more than twice per week during the worst 2 week period was considered positive if the parent selected “More than two times a week” in response to the question, “during the worst 2 week period, how often has your child’s chest sounded wheezy or whistling”. The incidence of cough lasting more than 3 days without a cold was ascertained using the Tucson question, “Has your child had a cough for 3 days or more when he/she did not have a cold?”.(18)
Secondary outcomes and covariates: Secondary outcomes included incidence of any wheezing and incidence of the combined outcome, wheezing more than twice per week during the worst 2 week period or cough lasting more than 3 days without a cold. Also assessed were parental report of respiratory signs, physician diagnosed respiratory diseases, medication use, health services use and impact on the family. To assure that follow up cohorts were comparable, questions not validated prior to this study were added to the Tucson questionnaires to more fully elicit use of preventive therapies including palivizumab and influenza immunization; attendance at daycare, frequency of BPD exacerbation or flare-up and impact on the family including whether the parent or caregiver needed to change their plans due to their child’s breathing; parental report of at least some breast milk intake on any of the 6, 12 or 18–22 month questionnaires; family history of inhaled allergies, food allergies, asthma, COPD or emphysema, other chronic respiratory illness; environmental exposure to tobacco smoke, daycare, children under 12 years old and pets; and use of preventive therapies as outlined above. In addition, each patient’s outcomes from SUPPORT were available to the Breathing Outcomes Study analysis.
Statistical Analyses
For Breathing Outcomes, a sample size of 817 subjects was calculated as necessary to detect an absolute risk difference of 0.1 in the incidence of the primary outcome of wheezing more than twice per week between groups with 90% power and alpha of 0.05 assuming an 80% minimum follow-up rate and baseline incidence of wheezing more than twice per week of 29%.(24) Sample size calculations for SUPPORT have been reported. (12, 13) Based upon SUPPORT’s target enrollment of 1310 patients and assuming a 22% mortality (NICHD historical data for calendar year 2000), we anticipated 1021 patients potentially eligible for the Breathing Outcomes Study.
The two primary analyses used the number of patients with either wheezing more than twice per week during their worst 2 week period or cough lasting more than 3 days without a cold as the numerator and the number of infants for whom that outcome was known as the denominator. Secondary responses were tabulated similarly. To assess the robustness of our findings, we calculated respiratory outcomes as a composite outcome with death and also calculated respiratory outcomes for patients with and without BPD. Unadjusted comparisons of neonatal and demographic characteristics between treatment groups were conducted using chi-square tests for categorical variables. Using Poisson regression models to adjust for gestational age stratum, study center and familial clustering, adjusted relative risk (ARR) values and 95% confidence intervals were calculated and are reported. When Poisson models did not converge, relative risk adjusted for gestational age and center is reported. When the two adjustment models failed to converge due to low prevalence (<5%), unadjusted relative risks are reported. Results were considered statistically significant if the two-sided p value was less than 0.05; a trend towards significance was considered if the two sided p value was between 0.05 and 0.10 inclusive.
Given the 2×2 factorial design of our randomized trial, we considered the potential for interactions between primary outcomes of one arm on the other (CPAP vs. surfactant and lower vs higher saturation targets). Analysis by robust Poisson regression implemented in Generalized Estimating Equation (GEE) models conducted for the primary outcomes of the main trial did not identify significant interactions between the two treatment arms (p-value for interaction terms all > 0.05). For this reason, only marginal (main) effects of each randomization are reported. No adjustments have been made for multiple comparisons. All calculations were performed using SAS software, version 9.3 (Cary, NC).
RESULTS
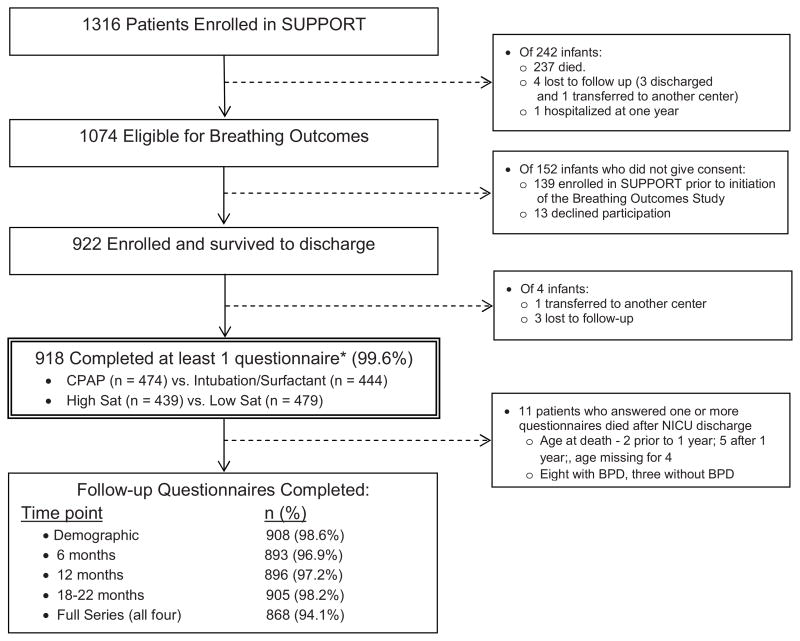
Of the 1316 patients enrolled in SUPPORT, 922 were eligible and gave consent to participate in the Breathing Outcomes Study. The 918 subjects with at least one completed questionnaire were considered the study cohort (Figure; available at www.jpeds.com). Follow up rates at each time point are listed in the Figure.
Figure.
CONSORT diagram including follow up rates.
* Follow-up Cohort
Among the follow up cohort, the group randomized to lower compared with higher oxygen saturation targets had fewer non-Hispanic white patients and a lower proportion of patients with BPD defined using the traditional criteria of supplemental oxygen use at 36 weeks’ PMA. The group randomized to CPAP and limited ventilation had similar demographics and neonatal outcomes as the group randomized to intubation/surfactant (Table I). Family history and environmental exposure histories were similar between the lower and higher oxygen saturation target groups and the CPAP and intubation/surfactant groups (Table II; available at www.jpeds.com). Subjects with responses to all four questionnaires were similar in demographic characteristics, neonatal outcomes and home environmental exposures with the exception that those with less than four responses were more apt to have been discharged on respiratory medications (Table III; available at www.jpeds.com).
Table 1.
Demographic and neonatal characteristics of follow-up cohorts.
| Low Sat N=439 |
High Sat N=479 |
CPAP N=474 |
Intubation/Surfactant N=444 |
|
|---|---|---|---|---|
| Birth Weight (g, mean ± s.d.) | 858 ± 186 | 844 ± 190 | 850 ± 184 | 851 ± 193 |
| Gestational Age (w, mean ± s.d.) | 25.9 ± 1.0 | 25.9 ± 1.0 | 25.9 ± 1.0 | 25.9 ± 1.0 |
| 24 wks 0 days - 25 wks 6 dys - no. (%) | 158 (35.5) | 184 (37.5) | 183 (37.7) | 159 (35.3) |
| 25 wks 0 days - 27 wks 6 dys - no. (%) | 287 (64.5) | 307 (62.5) | 303 (62.4) | 291 (64.7) |
| Male - no. (%) | 222 (49.7) | 264 (53.8) | 238 (49.0) | 248 (54.9) |
| Non-Hispanic Black - no. (%) | 168 (37.6) | 157 (32.0) | 173 (35.6) | 152 (33.6) |
| Non-Hispanic White - no. (%) | 176 (39.4) * | 226 (46.0) | 196 (40.3) | 206 (45.6) |
| Hispanic - no. (%) | 88 (19.7) | 91 (18.5) | 98 (20.2) | 81 (17.9) |
| Other/unknown - no. (%) | 15 (3.4) | 17 (3.5) | 19 (3.9) | 13 (2.9) |
| Length of NICU Hospitalization (median (min-max)) | 90 (39 – 365) | 93 (46 – 366) | 91 (44 – 366) | 93 (39 – 365) |
| BPD (traditional definition) - no. (%) | 160 (36.3) ** | 221 (45.8) | 187 (39.1) | 194 (43.5) |
| BPD (physiologic definition) - no. (%) | 165 (37.4) | 193 (40.0) | 183 (38.3) | 175 (39.2) |
| Discharged home on oxygen - no. (%) | 105 (24.0) | 111 (23.2) | 108 (22.8) | 108 (24.4) |
| Discharged home on respiratory medications - no. (%) | 101 (27.3) | 106 (27.1) | 110 (27.8) | 97 (26.6) |
| Discharged home October - March - no. (%) | 232 (52.9) | 227 (47.5) | 232 (48.8) | 227 (51.4) |
Low sat vs. high sat, p < 0.05
Low sat vs. high sat, p < 0.01
Table 2.
Family and environmental exposure history of follow-up cohorts.
| Low Sat N=439 |
High Sat N=479 |
CPAP N=474 |
Intubation/Surfactant N=444 |
|
|---|---|---|---|---|
| First degree relative with asthma - no. (%) | 142 (31.8) | 159 (32.4) | 152 (31.3) | 149 (33.0) |
| Family history of | ||||
| COPD, emphysema, etc - no. (%) | 48 (10.7) | 43 (8.8) | 53 (10.9) | 38 (8.4) |
| Food allergies - no. (%) | 60 (14.4) | 52 (11.3) | 61 (13.5) | 51 (11.9) |
| Inhaled allergies – no. (%) | 140 (30.4) | 129 (30.9) | 136 (30.1) | 133 (31.2) |
| Chronic Respiratory Disease no. (%) | 7 (1.7) | 4 (0.9) | 1 (0.2) | 10 (2.5) |
| Any breast milk - no. (%) | 167 (37.4) | 148 (30.1) | 166 (34.2) | 149 (33.0) |
| Smoking in house - no. (%) | 189 (44.1) | 186 (39.3) | 189 (40.6) | 186 (42.7) |
| Spent time at daycare - no. (%) | 163 (41.5) | 142 (33.2) | 163 (38.4) | 142 (35.8) |
| Living with children under 12 - no. (%) | 241 (61.3) | 264 (61.7) | 255 (60.1) | 250 (63.0) |
| Pets in home- no. (%) | 181 (40.5) | 177 (36.1) | 187 (38.5) | 171 (37.8) |
| Flu vaccination- no. (%) | 307 (78.1) | 342 (80.1) | 335 (79.0) | 314 (79.3) |
| RSV prophylaxis - no. (%) | 281 (71.5) | 313 (73.1) | 308 (72.6) | 286 (72.0) |
Overall in the Breathing Outcomes cohort during the first 18–22 months CA, wheezing more than twice per week during the worst 2 week period was reported in 47.9% of patients, cough lasting more than 3 days without a cold in 31.0% and either wheezing more than twice per week or cough more than 3 days without a cold in 68.2%. Among cohort subjects, use of inhaled (26.3%) and/or systemic steroids (9.4%) was common. Cohort subjects also had high use of physician visits (63.8%), emergency room visits (46.6%) and hospitalizations for wheezing or breathing problems (31.0%).
Primary Outcomes
There was no difference in incidence of the two primary outcomes, wheezing more than twice per week during the worst 2 week period and cough lasting more than 3 days without a cold, between infants randomized to lower compared with higher oxygen saturation targets nor between infants randomized to treatment with CPAP rather than intubation/surfactant (Table IV). Analyzed as a combined outcome, the incidence of death or cough more than 3 days without a cold trended (p=0.05) lower among patients in the CPAP compared with intubation/surfactant study arms. The combined outcome of episodes of wheezing more than twice per week during the worst 2 week period or cough lasting more than 3 days without a cold for the overall cohort was 64.6% and did not differ significantly between infants randomized to lower rather than higher oxygen saturation target or CPAP rather than intubation/surfactant when analyzed alone or as a combined outcome with death (Tables IV and V).
Table 6.
Combined outcomes of death or respiratory morbidity for lower vs. higher oxygen saturation and early CPAP vs. intubation and surfactant cohorts for the first 18–22 months corrected age (combined responses to the 6, 12 and 18–22 month interviews).
| Outcomes with Death | Low Sat N=586 |
High Sat N=569 |
ARR (95% CI) | p-value | CPAP N=583 |
Intubation/Surfactant N=572 |
ARR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Primary Outcomes | ||||||||
| Has your child’s chest sounded wheezy or whistling more than twice in one week? | 337 (59.5) | 344 (59.0) | 1.06 (0.83, 1.37) | 0.62 | 337 (58.0) | 344 (60.6) | 0.86 (0.67, 1.11) | 0.26 |
| Has your child had a cough for more than 3 days without a cold | 262 (47.9) | 254 (45.0) | 1.18 (0.91, 1.51) | 0.21 | 240 (42.9) | 276 (49.9) | 0.78 (0.60, 1.00) | 0.05 |
| Secondary Outcomes | ||||||||
| Wheezing/whistling more than twice in one week or cough more than 3 days | 410 (75.0) | 425 (75.2) | 0.99 (0.74, 1.32) | 0.96 | 414(74.1) | 421 (76.1) | 0.92 (0.69, 1.23) | 0.56 |
| Has your child’s chest sounded wheezy or whistling? | 379 (69.3) | 395 (69.9) | 0.98 (0.75, 1.29) | 0.9 | 380 (68.0) | 394 (71.2) | 0.84 (0.64, 1.10) | 0.21 |
| Has your baby’s chest sounded wheezy or whistling apart from colds? | 252 (46.1) | 275 (48.7) | 0.91 (0.71, 1.17) | 0.48 | 241 (43.1) | 286 (51.7) | 0.70 (0.54, 0.90) | <0.01 * |
| Has your child had asthma, reactive airway disease or BPD exacerbation or flare-up diagnosed by a doctor? | 274 (50.1) | 270 (47.9) | 1.17 (0.91, 1.50) | 0.23 | 256 (45.8) | 288 (52.2) | 0.76 (0.59, 0.98) | 0.03* |
| Has your child had bronchiolitis, bronchitis or pneumonia diagnosed by a doctor? | 295 (53.9) | 295 (52.3) | 1.12 (0.87, 1.44) | 0.39 | 279 (49.9) | 311 (56.3) | 0.78 (0.60, 1.00) | 0.05 |
| Any of asthma, reactive airway disease, BPD exacerbation or flare-up or bronchiolitis, bronchitis, or pneumonia diagnosed by a doctor | 339 (62.0) | 351 (62.2) | 1.05 (0.81, 1.36) | 0.71 | 326 (58.3) | 364 (65.9) | 0.71 (0.54,0.92) | <0.01 * |
| Has your child had croup diagnosed by a doctor? | 180 (32.9) | 153 (27.1) | 1.35 (1.03, 1.77) | 0.03* | 154 (27.5) | 179 (32.4) | 0.78 (0.60, 1.03) | 0.08 |
| Has your child ever had to visit the doctor or Emergency Room for breathing or wheezing problems? | 427 (78.1) | 430 (76.1) | 1.11 (0.82, 1.50) | 0.49 | 417 (74.6) | 440 (79.6) | 0.73 (0.54,0.99) | <0.05* |
| Has your child had to stay in a hospital overnight? | 303 (55.5) | 310 (54.9) | 1.06 (0.82, 1.37) | 0.64 | 294 (52.6) | 319 (57.8) | 0.84 (0.65, 1.08) | 0.17 |
| Has your child had to stay in a hospital overnight for wheezing/breathing problems? | 263 (48.2) | 252 (44.6) | 1.20 (0.93, 1.54) | 0.17 | 242 (43.3) | 273 (49.5) | 0.78 (0.61, 1.01) | 0.06 |
| Treated with a diuretic medication? | 165 (29.0) | 137 (23.4) | 1.42 (1.07, 1.88) | 0.02* | 137 (23.5) | 165 (28.8) | 0.74 (0.56, 0.98) | 0.04* |
| Treated with an inhaled steroid medication? | 246 (43.2) | 240 (41.0) | 1.15 (0.89, 1.47) | 0.29 | 241 (41.3) | 245 (42.8) | 0.96 (0.75, 1.24) | 0.76 |
| Treated with a nebulized medication? | 163(28.6) | 155 (26.5) | 1.15 (0.87, 1.52) | 0.33 | 152 (26.1) | 166 (29.0) | 0.85 (0.64, 1.13) | 0.27 |
| Treated with a systemic steroid medication? | 178 (31.3) | 156 (26.6) | 1.28 (0.98, 1.68) | 0.07 | 162 (27.8) | 172 (30.1) | 0.88 (0.68, 1.16) | 0.38 |
| Treated with oxygen at home? | 238 (43.5) | 206 (36.5) | 1.46 (1.11, 1.91) | <0.01 * | 206 (36.9) | 238 (43.1) | 0.76 (0.58, 1.00) | 0.05* |
| Have you had to change your plans because of your child’s breathing problems? | 273 (49.9) | 281 (49.7) | 1.04 (0.81, 1.34) | 0.74 | 257 (46.0) | 297 (53.7) | 0.72 (0.56,0.92) | 0.01* |
P <0.05
Secondary Outcomes
Oxygen Saturation Targeting Intervention
At 6 months CA, infants randomized to lower compared with higher oxygen saturation targets had a lower incidence of wheezing and use of nebulized medications following NICU discharge (Table IV). Over the first 18–22 months CA, infants treated with lower rather than higher oxygen saturation targets were less likely to have episodes of wheezing without a cold (Table IV). When analyzed as composite outcomes, the lower compared with higher saturation group had a similar incidence of death or respiratory morbidities except for croup diagnosed by a doctor or treatment with a diuretic or oxygen at home) (Table V).
Early CPAP Intervention
At 6 months CA, infants randomized to treatment with CPAP and a limited ventilation strategy rather than intubation/surfactant were reported to have fewer asthma, reactive airway disease or BPD exacerbation or flare-up episodes diagnosed by a doctor since NICU discharge and a trend toward fewer hospitalizations for wheezing or breathing problems. Perhaps related to these differences, parents or primary caregivers of infants randomized to CPAP were less likely at 6 months CA to report changing their plans due to their child’s breathing problems (Table V).
During the first 18–22 months CA, infants randomized to early CPAP versus intubation/surfactant were significantly less likely to have wheezing episodes occurring without a cold (28.9% vs. 36.5%, p=0.01), respiratory illnesses diagnosed by a doctor (one or more episodes of asthma, reactive airway disease or BPD exacerbation or flare up or bronchiolitis, bronchitis or pneumonia) (47.7% vs. 55.2%, p=0.02), or wheezing or breathing problems that prompted a physician or emergency room visit (68.0% vs. 72.9%, p<0.05). Compared with those of infants in the intubation/surfactant group, parents or guardians of infants in the CPAP group were also less likely to report changing their plans due to their child’s breathing problems (32.4% vs. 39.0%, p<0.05). When outcomes were analyzed as composite outcomes with death, similar findings were observed with additional differences noted in incidence of treatment with oxygen or diuretics at home and a trend towards lower incidence of overnight hospitalization for breathing problems.
As expected, our study questionnaires were able to detect significant differences in respiratory outcomes for infants with versus without BPD (Table VI). Although the incidence of wheezing more than twice per week was different between infants with and without BPD, there was no difference in incidence of cough lasting more than 3 days as an indicator of occult wheezing. Taken together, the combined incidence of either overt (wheezing more than twice per week) or potential occult (cough lasting more than 3 days) wheezing was significantly different between infants with BPD and those without (Table VI).
Table 5.
Respiratory outcomes for infants with traditional BPD (oxygen requirement at 36 weeks post-menstrual age) for the first 18–22 months corrected age (combined responses to the 6, 12 and 18–22 month interviews).
| Primary Outcomes | Traditional BPD | Not Traditional BPD | ARR (95% CI) | P-Value |
|---|---|---|---|---|
| N=377 | N=539 | |||
| Has your child’s chest sounded wheezy or whistling more than twice in one week? | 194 (52.0) | 242 (45.1) | 1.52 (1.15, 2.01) | <0.01 |
| Has your child had a cough for more than 3 days without a cold? | 119 (33.7) | 149 (29.0) | 1.17 (0.86, 1.60) | 0.314 |
| Secondary Outcomes Symptoms | ||||
| Wheezing/whistling more than twice in one week or cough more than 3 days | 262 (74.2) | 330 (64.1) | 1.76 (1.27, 2.43) | <0.01 |
| Has your child’s chest sounded wheezy or whistling? | 231 (65.4) | 300 (58.3) | 1.61 (1.17, 2.21) | <0.01 |
| Has your baby’s chest sounded wheezy or whistling apart from colds? | 129 (36.8) | 153 (29.7) | 1.57 (1.16, 2.13) | <0.01 |
| Illnesses | ||||
| Has your child had asthma, reactive airway disease or BPD flare-up diagnosed by a doctor? | 133 (38.0) | 165 (32.0) | 1.58 (1.17, 2.13) | <0.01 |
| Has your child had bronchiolitis, bronchitis or pneumonia diagnosed by a doctor? | 152 (43.2) | 192 (37.4) | 1.34 (1.00, 1.80) | 0.05 |
| Any of asthma, reactive airway disease, BPD flare-up or bronchiolitis, bronchitis, or pneumonia diagnosed by a doctor? | 195 (55.4) | 250 (48.5) | 1.47 (1.08, 2.00) | 0.01 |
| Has your child had croup diagnosed by a doctor? | 29 (8.2) | 56 (10.9) | 0.78 (0.46, 1.33) | 0.36 |
| Health Services | ||||
| Has your child ever had to visit the doctor or Emergency Room for breathing or wheezing problems? | 267 (75.6) | 344 (66.8) | 1.56 (1.08, 2.25) | 0.02 |
| Has your child had to stay in a hospital overnight? | 186 (52.7) | 182 (35.4) | 2.22 (1.64, 3.02) | <.0001 |
| Has your child had to stay in a hospital overnight for wheezing/breathing problems? | 136 (38.5) | 133 (25.9) | 1.89 (1.40, 2.57) | <.0001 |
| Medications | ||||
| Treated with a diuretic medication?† | 47 (12.5) | 8 (1.5) | 11.86 (5.28, 26.62) | <.0001 |
| Treated with an inhaled steroid medication? | 135 (35.8) | 106 (19.7) | 2.40 (1.75, 3.29) | <.0001 |
| Treated with a nebulized medication?† | 35 (9.3) | 36 (6.7) | 1.53 (0.88, 2.67) | 0.14 |
| Treated with a systemic steroid medication? | 40 (10.6) | 46 (8.5) | 1.45 (0.93, 2.26) | 0.10 |
| Treated with oxygen at home? | 164 (46.5) | 36 (7.0) | 9.18 (5.81, 14.52) | <.0001 |
| Family | ||||
| Have you had to change your plans because of your child’s breathing problems? | 143 (40.5) | 166 (32.2) | 1.34 (1.00, 1.79) | < 0.05 |
Where models did not converge, adjustments are limited to center and gestational age (†).
DISCUSSION
We report results of the Breathing Outcomes Study, a sub study to SUPPORT, which sought to quantify respiratory morbidity by 18–22 months corrected age for extremely premature children born 24–27 weeks gestation. We found no significant differences at 18–22 months CA in the incidence of either of the two primary outcomes, wheezing more than twice per week during the worst 2 week period or cough lasting more than 3 days without a cold, between patients randomized to lower versus higher oxygen saturation targets or randomized to CPAP versus intubation/surfactant.
In secondary analyses, although extremely preterm infants randomized to low compared with high oxygen saturation targets were less likely to have wheezing or use a home nebulizer at 6 months CA and to have wheezing apart from a cold between discharge and 18–22 months CA, these differences were not seen when respiratory outcomes were analyzed as composite outcomes with death. In fact, analyzed this way, the incidence of death or adverse respiratory outcome for some measures of morbidity were worse for patients in the low saturation group. Several pulmonary outcome studies have found an association between neonatal oxygen exposure and expiratory flow dysfunction and airway hyperreactivity among infants with or without BPD. (2, 7, 27–29) Though patients treated with lower compared with higher saturation targets in SUPPORT had a shorter duration of oxygen exposure, they had greater mortality, similar incidence of BPD, and based on results of the Breathing Outcomes Study, survivors had a similar use of outpatient services for respiratory care and only minor differences in the incidence of respiratory signs. Based on these findings, if oxygen related pulmonary morbidity is to be minimized, strategies of reducing oxygen exposure and oxidant lung injury other than targeting lower oxygen saturations will be needed. (24, 30)
Though the primary outcomes were similar, patients in the first 18–22 months CA who were randomized to CPAP and limited ventilation rather than intubation followed by surfactant administration within 1 hour had a lower incidence of several important respiratory morbidities including respiratory illnesses diagnosed by a doctor, treatment with oxygen or diuretics at home and a trend towards lower incidence of overnight hospitalization for breathing problems. Likely related to these findings was a significant reduction in the proportion of parents reporting that they needed to change their daily plans due to their child’s breathing difficulties. These differences persisted whether the outcome was analyzed among survivors only or as composite outcomes with death.
Respiratory benefits of CPAP and a limited ventilation strategy were found in spite of the fact that the proportion of children with BPD, defined using either the traditional or physiologic criteria(15), was similar between CPAP and intubation/surfactant arms in the SUPPORT study and in the Breathing Outcomes’ follow-up cohort. Our data are consistent with follow up data from The COIN Trial, which despite finding no difference in the incidence of death or BPD among 610 infants randomized to either CPAP or conventional management, found better pulmonary function at 8 weeks corrected age among a 39 patient single-center sub cohort of study infants randomized to CPAP. (31, 32) These observations suggest that treatment of infants 25–27 6/7 weeks gestation at risk for RDS with a limited ventilation strategy is associated with respiratory benefits that are unapparent or underestimated by the incidence of BPD alone. As confirmed in our analysis of respiratory morbidity, BPD has proven to be useful surrogate to identify infants at highest risk of later morbidity. However, based upon the high incidence of respiratory morbidity among infants without BPD, it is likely, though not proven in this study, that the prevalence of respiratory morbidity in former preterm infants may be under recognized. Given the potential for respiratory therapies to improve pulmonary outcomes for infants with and without BPD, longitudinal assessment of pulmonary morbidity is necessary to fully evaluate the potential benefits of respiratory interventions in randomized clinical trials.
We found that regardless of treatment arm, respiratory signs and use of health care are common among infants 24–27 6/7th weeks’ gestation during the first 18–22 months CA. Over two-thirds of subjects in the Breathing Outcomes Study cohort reported wheezing more than twice per week during their worst 2 week period or a cough lasting more than 3 days without a cold. Treatment of these respiratory signs was not only associated with frequent use of both inhaled and systemic steroids, medications that have potential long term effects on growth and development, (33, 34) but also with frequent physician and emergency room visits and hospitalizations, health services which contribute greatly to health care costs.(8)
The strengths of this study include the large number of extremely preterm infants enrolled. Other strengths include the high follow up rates for enrolled patients and use of comprehensive respiratory questionnaires administered in a scripted interview by trained personnel. Though not as objective as pulmonary function testing, respiratory history was used to assess outcome measures due to clinical and financial concerns associated with the use of invasive pulmonary testing and the potential complications of sedation in former preterm infants. In addition, parental report of wheezing has been shown to correlate with pulmonary function testing and data extracted from office records and provides an estimate of the burden of respiratory morbidity to the patient and family as well as the health care system. (16, 35)
Among potential weaknesses, respiratory history data were taken by parental report, which has the potential for classification and recall bias. To minimize classification bias, all primary and follow up study data of this randomized trial were collected in a blinded manner. Hence, though it may affect the precision of point estimates, classification bias is unlikely to have introduced systematic bias into our study that favors one study arm over another. To reduce recall bias, parent interviews were conducted at 6 month intervals.(16) As has been previously reported, the results of SUPPORT and thereby potentially the follow up studies associated with it may not be fully generalizable to all extremely preterm infants because the need for antenatal consent resulted in a trial cohort with higher socioeconomic status and more common use of antenatal steroids than the entire eligible cohort. (36)
In summary, we found no significant differences in the incidence of wheezing more than twice per week during the worst 2 week period or cough lasting more than 3 days without a cold at 18–22 months CA between extremely preterm survivors who were randomized at delivery to either lower versus higher oxygen saturation targets or early CPAP and a limited ventilation strategy versus intubation/surfactant. In secondary analyses, we found minor reductions in the incidence of wheezing and nebulizer use at 6 months and wheezing without a cold at 18–22 months CA, but an overall increase in the risk of death or respiratory morbidity (except for croup and treatment with oxygen or diuretics at home) for infants randomized to lower vs. higher oxygen saturation targets. Also in secondary analyses, we report less respiratory morbidity among survivors and lower incidence or respiratory morbidity or death among infants randomized to CPAP rather than intubation/surfactant administration. Results of SUPPORT and neurodevelopmental follow up of SUPPORT patients found no deleterious effects of CPAP over intubation/surfactant.(12–14) Those findings coupled with the respiratory outcomes reported here suggest that treatment of extremely premature infants with CPAP and limited ventilation rather than intubation and surfactant within 1 hour is safe and may result in less respiratory morbidity during the first 18–22 months CA. Lastly, our findings demonstrate a high risk of post-discharge respiratory morbidities among preterm infants 24–27 6/7 weeks gestation (with or without BPD) that not only require close medical monitoring but also pose potential burdens to families as well as to society by increasing health care costs.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Heart, Lung, and Blood Institute (recruitment 2004–2009; follow-up 2006–2011). T.S. supported by NICHD (SUPPORT Breathing Outcomes Secondary Protocol K23 HD50646). Data collected at participating sites of the NICHD Neonatal Research Network were transmitted to RTI International, the data coordinating center for the network, which stored, managed, and analyzed the data for this study.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. We acknowledge the Tucson Children’s Respiratory Study (Marilyn Lindell, RN), University of Arizona, Tucson, Arizona, for support of this project by sharing respiratory symptom questionnaires that were adapted for use in this study. We also acknowledge Jill Halterman, MD, University of Rochester Medical Center, Rochester, NY for her contributions to this study, especially to the development of the respiratory symptom questionnaires.
Abbreviations
- BPD
Bronchopulmonary Dysplasia
- CA
Corrected Age
- CPAP
Continuous Positive Airway Pressure
- NICHD
National Institute of Child Health and Human Development
- NRN
NICHD Neonatal Research Network
- PMA
Postmenstrual Age
- ROP
Retinopathy of Prematurity
- SUPPORT
Surfactant Positive Airway Pressure and Pulse Oximetry Randomized Trial
Appendix
The following investigators, in addition to those listed as authors, are members of the SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network:
Dr Abhik Das (DCC Principal Investigator) and Dr Marie Gantz (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003–2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011).
NRN Participating Centers
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – William Oh, MD; Angelita M. Hensman, RN BSN; Bonnie E. Stephens, MD; Barbara Alksninis, PNP; Dawn Andrews, RN; Kristen Angela, RN; Melinda Caskey, MD; Kim Francis, RN; Regina A. Gargus, MD FAAP; Dan Gingras. RRT; Katharine Johnson, MD; Shabnam Lainwala, MD; Suzy Ventura; Rachel V. Walden.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Bonnie S. Siner, RN; Arlene Zadell RN; Julie DiFiore, BS; Monika Bhola, MD; Harriet G. Friedman, MA; Gulgun Yalcinkaya, MD.
Cincinnati Children’s Hospital Medical Center, University of Cincinnati Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) –Edward F. Donovan, MD; Vivek Narendran, MD MRCP; Kate Bridges, MD; Barbara Alexander, RN; Estelle E. Fischer, MHSA MBA; Teresa L. Gratton, PA; Cathy Grisby, BSN CCRC; Jody Hessling, RN; Lenora Jackson, CRC; Kristin Kirker, CRC; Holly L. Mincey, RN BSN; Marcia Worley Mersmann, RN CCRC.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Patricia Ashley, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Katherine A. Foy, RN; Sharon F. Freedman, MD; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; William F. Malcolm, MD; David K. Wallace, MD MPH.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, RR25008, M01 RR39) – Barbara J. Stoll, MD; Susie Buchter, MD; Anthony J. Piazza, MD; David P. Carlton, MD; Ellen C. Hale, RN BS CCRC.
Eunice Kennedy Shriver National Institute of Child Health and Human Development –Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) –Brenda B. Poindexter, MD MS; James A. Lemons, MD; Leslie D. Wilson, BSN CCRC; Faithe Hamer, BS; Dianne E. Herron, RN; Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP, Anna M. Dusick MD (deceased).
National Heart, Lung, and Blood Institute – Mary Anne Berberich, PhD; Carol J. Blaisdell, MD; Dorothy B. Gail, PhD; James P. Kiley, PhD.
RTI International (U10 HD36790) –W. Kenneth Poole, PhD; Betty K. Hastings; Jeanette O’Donnell Auman, BS; Carolyn Petrie Huitema, MS; James W. Pickett II, BS; Dennis Wallace, PhD; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University, Lucile Packard Children’s Hospital (U10 HD27880, UL1 RR25744, M01 RR70) –Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS CCRC; Elizabeth F. Bruno, PhD; Alexis S. Davis, MD MS; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Lynne C. Huffman, MD; Jean G. Kohn, MD MPH; Melinda S. Proud, RCP; Nicholas H. St. John, PhD; Hali E. Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Anne Furey, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Ana Brussa, MS OTR/L; Cecelia Sibley, PT MHA.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) –Namasivayam Ambalavanan, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN. Vivien A. Phillips, RN BSN; Kirstin J. Bailey, PhD; Fred J. Biasini, PhD; Maria Hopkins, PhD; Kristen C. Johnston, MSN CRNP; Sara Krzywanski, MS; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Richard V. Rector, PhD; Leslie Rodriguez, PhD; Amanda Soong, MD; Sally Whitley, MA OTR-L FAOTA; Sheree York, PT DPT MS PCS.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461) –Maynard R. Rasmussen, MD; Paul R. Wozniak, MD; Kathy Arnell, RNC; Rene Barbieri-Welge; Ayala Ben-Tall; Renee Bridge, RN; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Elaine Ito; Meghan Lukasik; Deborah Pontillo; Donna Posin, OTR/L MPA; Cheryl Runyan; James Wilkes; Paul Zlotnik.
University of Iowa Children’s Hospital (U10 HD53109, UL1 RR24979, M01 RR59) –Edward F. Bell, MD; John A. Widness, MD; Jonathan M. Klein, MD; Tarah T. Colaizy, MD MPH; Karen J. Johnson, RN BSN; Diane L. Eastman, RN CPNP MA.
University of Miami, Holtz Children’s Hospital (U10 HD21397, M01 RR16587) –Shahnaz Duara, MD; Ruth Everett-Thomas, RN MSN; Maria Calejo, MEd; Alexis N. Diaz, BA; Silvia M. Frade Eguaras, BA; Andrea Garcia, MA; Kasey Hamlin-Smith, PhD; Michelle Harwood Berkowits, PhD; Sylvia Hiriart-Fajardo, MD; Elaine O. Mathews, RN; Helina Pierre, BA; Arielle Riguard, MD; Alexandra Stroerger, BA.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997) –Kristi L. Watterberg, MD; Robin K. Ohls, MD; Julie Rohr, MSN RNC CNS; Conra Backstrom Lacy, RN; Jean Lowe, PhD; Rebecca Montman, BSN.
University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44) –Nirupama Laroia, MD; Gary Myers, MD; Gary David Markowitz, MD; Linda J. Reubens, RN CCRC; Caryn Graff Havens, MPH MBA; Diane Hust, MS RN CS; Julie Babish Johnson, MSW; Erica Burnell, RN; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Kelley Yost, PhD; Lauren Zwetsch, RN MS PNP.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) –Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Luc P. Brion, MD; Sally S. Adams, MS RN CPNP; James Allen, RRT; Laura Grau, RN; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T. Heyne, PsyD PA-C; Melissa H. Lepps, RN; Linda A. Madden, RN CPNP; Melissa Martin, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Araceli Solis, RRT; Lizette E. Torres, RN; Catherine Twell Boatman, MS CIMI; Diana M Vasil, RNC-NIC; Kerry Wilder, RN.
University of Texas Health Science Center at Houston Medical School and Children’s Memorial Hermann Hospital (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Esther G. Akpa, RN BSN; Nora I. Alaniz, BS; Susan E. Dieterich, PhD; Patricia W. Evans, MD; Charles E. Green, PhD; Beverly Foley Harris, RN, BSN; Margarita Jiminez, MD MPH; Anna E. Lis, RN BSN; Sara C. Martin, RN BSN; Georgia E. McDavid, RN; Brenda H. Morris, MD; M. Layne Poundstone, RN BSN; Stacey Reddoch, BA; Saba Khan Siddiki, MD; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64) –Shawna Baker, RN; Karie Bird, RN; Jill Burnett, RN; Laura Cole, RN; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC; Mike Steffens, PhD; Kimberlee Weaver-Lewis, RN BSN; Karen Zanetti, RN.
Wake Forest Baptist Medical Center, Brenner Children’s Hospital, and Forsyth Medical Center (U10 HD40498, M01 RR7122) –Robert G. Dillard, MD; Lisa K. Washburn, MD; Nancy J. Peters, RN CCRP; Barbara G. Jackson, RN BSN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Beena G. Sood, MD MS;; Lilia DeJesus MD; Rebecca Bara, RN BSN; Elizabeth Billian, RN MBA; Laura A. Goldston, MA; Mary Johnson, RN BSN.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 RR24139, MO1 RR125) –Vineet Bhandari, MD DM; Harris C. Jacobs, MD; Pat Cervone, RN; Patricia Gettner, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Christine G. Butler, MD; Nancy Close, PhD; Walter Gilliam, PhD; Sheila Greisman, RN; Elaine Romano, MSN; Joanne Williams, RN BSN.
Data and Safety Monitoring Committee – Gordon Avery, MD, chair, Children’s National Medical Center; Christine A. Gleason, MD, chair, University of Washington; Marilee C. Allen, MD, Johns Hopkins University School of Medicine; Shrikant I. Bangdiwala, PhD, University of North Carolina; Carol J. Blaisdell, MD, National Heart, Lung, and Blood Institute; Robert J. Boyle, MD, University of Virginia Health System; Traci Clemons, PhD, The EMMES Corporation; Mary E. D’Alton, MD, Columbia University; Abhik Das (ex officio), PhD, RTI International; Dorothy B. Gail, PhD, National Heart, Lung, and Blood Institute; Carl Hunt, MD, National Heart, Lung, and Blood Institute; Martin Keszler, MD, Georgetown University Hospital; W. Kenneth Poole (ex officio), PhD, RTI International; Carol K. Redmond, ScD, University of Pittsburg; Michael G. Ross, MD, MPH; UCLA School of Medicine and Public Health; Merran A. Thomson, MD, Hammersmith Hospital, UK; Steven J. Weiner, MS, The George Washington University; Marian Willinger (ex officio), PhD, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
The authors declare no conflicts of interest.
Registered with ClinicalTrials.gov: NCT00233324
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenough A, Giffin FJ, Yuksel B. Respiratory morbidity in preschool children born prematurely. Relationship to adverse neonatal events. Acta Paediatr. 1996;85:772–7. doi: 10.1111/j.1651-2227.1996.tb14150.x. [DOI] [PubMed] [Google Scholar]
- 2.Thomas M, Greenough A, Johnson A, Limb E, Marlow N, Peacock JL, et al. Frequent wheeze at follow up of very preterm infants: which factors are predictive? Arch Dis Child Fetal Neonatal Ed. 2003;88:F329–F32. doi: 10.1136/fn.88.4.F329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004;144:799–803. doi: 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 5.Palta M, Sadek-Badawi M, Madden K, Green C. Pulmonary testing using peak flow meters of very low birth weight children born in the perisurfactant era and school controls at age 10 years. Pediatr Pulmonol. 2007;42:819–28. doi: 10.1002/ppul.20662. [DOI] [PubMed] [Google Scholar]
- 6.McLeod A, Ross P, Mitchell S, Tay D, Hunter L, Hall A, et al. Respiratory health in a total very low birthweight cohort and their classroom controls. Arch Dis Child. 1996;74:188–94. doi: 10.1136/adc.74.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mai XM, Gaddlin PO, Nilsson L, Finnstrom O, Bjorksten B, Jenmalm MC, et al. Asthma, lung function and allergy in 12-year-old children with very low birth weight: a prospective study. Pediatr Allergy Immunol. 2003;14:184–92. doi: 10.1034/j.1399-3038.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 8.Brooks AM, Byrd RS, Weitzman M, Auinger P, McBride JT. Impact of low birth weight on early childhood asthma in the United States. Arch Pediatr Adolesc Med. 2001;155:401–6. doi: 10.1001/archpedi.155.3.401. [DOI] [PubMed] [Google Scholar]
- 9.Hilgendorff A, Parai K, Ertsey R, Jain N, Navarro EF, Peterson JL, et al. Inhibiting lung elastase activity enables lung growth in mechanically ventilated newborn mice. American journal of respiratory and critical care medicine. 2011;184:537–46. doi: 10.1164/rccm.201012-2010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevens TP, Dylag A, Panthagani I, Pryhuber G, Halterman J. Effect of cumulative oxygen exposure on respiratory symptoms during infancy among VLBW infants without bronchopulmonary dysplasia. Pediatric pulmonology. 2010;45:371–9. doi: 10.1002/ppul.21199. [DOI] [PubMed] [Google Scholar]
- 11.Doyle LW, Cheung MM, Ford GW, Olinsky A, Davis NM, Callanan C. Birth weight <1501 g and respiratory health at age 14. Arch Dis Child. 2001;84:40–4. doi: 10.1136/adc.84.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaucher YE, Peralta-Carcelen M, Finer NN, Carlo WA, Gantz MG, Walsh MC, et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367:2495–504. doi: 10.1056/NEJMoa1208506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–6. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 16.Pless CE, Pless IB. How well they remember. The accuracy of parent reports. Arch Pediatr Adolesc Med. 1995;149:553–8. doi: 10.1001/archpedi.1995.02170180083016. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham CK, McMillan JA, Gross SJ. Rehospitalization for respiratory illness in infants of less than 32 weeks’ gestation. Pediatrics. 1991;88:527–32. [PubMed] [Google Scholar]
- 18.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 19.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children’s Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–75. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 20.Merth IT, de Winter JP, Zonderland HM, Borsboom GJ, Quanjer PH. Pulmonary function in infants with neonatal chronic lung disease with or without hyaline membrane disease at birth. The European respiratory journal. 1997;10:1606–13. doi: 10.1183/09031936.97.10071606. [DOI] [PubMed] [Google Scholar]
- 21.Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. American journal of respiratory and critical care medicine. 2002;165:83–7. doi: 10.1164/ajrccm.165.1.2107093. [DOI] [PubMed] [Google Scholar]
- 22.Friedrich L, Stein RT, Pitrez PM, Corso AL, Jones MH. Reduced lung function in healthy preterm infants in the first months of life. American journal of respiratory and critical care medicine. 2006;173:442–7. doi: 10.1164/rccm.200503-444OC. [DOI] [PubMed] [Google Scholar]
- 23.Sell M, Cotton R, Hirata T, Guthrie R, LeBlanc M, Mammel M, et al. One-year follow-up of 273 infants with birth weights of 700 to 1100 grams after prophylactic treatment of respiratory distress syndrome with synthetic surfactant or air placebo. American Exosurf Neonatal Study Group I. J Pediatr. 1995;126:S20–S5. doi: 10.1016/s0022-3476(95)70004-8. [DOI] [PubMed] [Google Scholar]
- 24.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. 2003;111:469–76. doi: 10.1542/peds.111.3.469. [DOI] [PubMed] [Google Scholar]
- 25.Koopman LP, Brunekreef B, de Jongste JC, Neijens HJ. Definition of respiratory symptoms and disease in early childhood in large prospective birth cohort studies that predict the development of asthma. Pediatr Allergy Immunol. 2001;12:118–24. doi: 10.1034/j.1399-3038.2001.012003118.x. [DOI] [PubMed] [Google Scholar]
- 26.Greenough A, Giffin FJ, Yuksel B, Dimitriou G. Respiratory morbidity in young school children born prematurely--chronic lung disease is not a risk factor? Eur J Pediatr. 1996;155:823–6. [PubMed] [Google Scholar]
- 27.Baraldi E, Filippone M, Trevisanuto D, Zanardo V, Zacchello F. Pulmonary function until two years of life in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1997;155:149–55. doi: 10.1164/ajrccm.155.1.9001304. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand JM, Riley SP, Popkin J, Coates AL. The long-term pulmonary sequelae of prematurity: the role of familial airway hyperreactivity and the respiratory distress syndrome. N Engl J Med. 1985;312:742–5. doi: 10.1056/NEJM198503213121202. [DOI] [PubMed] [Google Scholar]
- 29.Evans M, Palta M, Sadek M, Weinstein MR, Peters ME. Associations between family history of asthma, bronchopulmonary dysplasia, and childhood asthma in very low birth weight children. Am J Epidemiol. 1998;148:460–6. doi: 10.1093/oxfordjournals.aje.a009671. [DOI] [PubMed] [Google Scholar]
- 30.Hibbs AM, Walsh MC, Martin RJ, Truog WE, Lorch SA, Alessandrini E, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. The Journal of pediatrics. 2008;153:525–9. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB, et al. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–8. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]
- 32.Roehr CC, Proquitte H, Hammer H, Wauer RR, Morley CJ, Schmalisch G. Positive effects of early continuous positive airway pressure on pulmonary function in extremely premature infants: results of a subgroup analysis of the COIN trial. Archives of disease in childhood Fetal and neonatal edition. 2011;96:F371–3. doi: 10.1136/adc.2009.181008. [DOI] [PubMed] [Google Scholar]
- 33.Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367:904–12. doi: 10.1056/NEJMoa1203229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gedalia A, Shetty AK. Chronic steroid and immunosuppressant therapy in children. Pediatrics in review/American Academy of Pediatrics. 2004;25:425–34. [PubMed] [Google Scholar]
- 35.Robin B, Kim YJ, Huth J, Klocksieben J, Torres M, Tepper RS, et al. Pulmonary function in bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;37:236–42. doi: 10.1002/ppul.10424. [DOI] [PubMed] [Google Scholar]
- 36.Rich WD, Auten KJ, Gantz MG, Hale EC, Hensman AM, Newman NS, et al. Antenatal consent in the SUPPORT trial: challenges, costs, and representative enrollment. Pediatrics. 2010;126:e215–21. doi: 10.1542/peds.2009-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.