Abstract
Background
To evaluate knee joint contact mechanics and kinematics during the loading response phase of downhill gait in knee osteoarthritis patients with self-reported instability.
Methods
Forty-three subjects, 11 with medial compartment knee osteoarthritis and self-reported instability (unstable), 7 with medial compartment knee osteoarthritis but no reports of instability (stable), and 25 without knee osteoarthritis or instability (control) underwent Dynamic Stereo X-ray analysis during a downhill gait task on a treadmill.
Findings
The medial compartment contact point excursions were longer in the unstable group compared to the stable (p=0.046) and the control groups (p=0.016). The peak medial compartment contact point velocity was also greater for the unstable group compared to the stable (p=0.047) and control groups (p=0.022). Additionally, the unstable group demonstrated a coupled movement pattern of knee extension and external rotation after heel contact which was different than the coupled motion of knee flexion and internal rotation demonstrated by stable and control groups.
Interpretation
Our findings suggest that knee joint contact mechanics and kinematics are altered during the loading response phase of downhill gait in knee osteoarthritis patients with self-reported instability. The observed longer medial compartment contact point excursions and higher velocities represent objective signs of mechanical instability that may place the arthritic knee joint at increased risk for disease progression. Further research is indicated to explore the clinical relevance of altered contact mechanics and kinematics during other common daily activities and to assess the efficacy of rehabilitation programs to improve altered joint biomechanics in knee osteoarthritis patients with self-reported instability.
Keywords: Contact Mechanics, Instability, Kinematics, Gait
Prevalence of episodic knee instability, described as subjective sensation of buckling, shifting, or giving way of the knee joint, is estimated to be as high as 63–80% in patients with knee osteoarthritis (OA) (Fitzgerald et al. 2004; Knoop et al. 2012; Ramsey et al. 2007). Findings from population-based studies further suggest that knee instability is significantly associated with self-reported and performance-based functional deficits in patients with knee OA (Felson et al. 2007; van der Esch et al. 2012). To this end, Fitzgerald and colleagues reported that up to 44% of knee OA patients participating in an observational study complained of instability affecting their ability to function (Fitzgerald et al. 2004). Felson and colleagues also reported that up to 47% of the Framingham Osteoarthritis study participants who experienced knee instability over the previous 3 months were limited in the kind of work they could do (Felson et al. 2007). These findings suggest that self-reported instability is an important and relevant independent variable related to function in patients with knee OA.
To date, little work has been done to evaluate the potential alterations in dynamic knee joint function in knee OA patients with self-reported instability. Previous reports indicate that knee OA patients with self-reported instability demonstrate decreased knee flexion excursions during level and downhill gait compared to volunteers without knee OA or self-report of instability (Farrokhi et al. 2012; Schmitt and Rudolph 2007). However, since reduced knee flexion excursions have also been reported for knee OA patients without self-reported instability (Briem and Snyder-Mackler 2009; Childs et al. 2004), the exact contribution of either knee OA or self-reported instability to the observed alterations in gait kinematics cannot be clearly elucidated from these studies and warrants further investigation. It also stands to reason that the subjective sensation of instability reported by patients with knee OA may be the result of excessive movements of the joint contact surfaces detected by proprioceptive joint receptors (Sharma 1999). However, no previous attempts have been made to evaluate knee joint contact mechanics during a dynamic activity in this patient population.
Current literature is also void of objective measures of functional instability in patients with knee OA. If an objective measure of instability could be identified, mechanism-based interventions to address functional instability in patients with knee OA could be devised and implemented. Van der Esch and colleagues recently hypothesized that increased knee varus/valgus motion during gait may be a potential objective sign of joint instability in patients with knee OA as healthy knees move through minimal amounts of frontal-plane motion (van der Esch et al. 2008). However, their findings suggested that knee varus/valgus motion during gait is not related to biomechanical variables responsible for joint stability such as muscle strength, joint proprioception, laxity or skeletal alignment, and therefore cannot be used as a valid measure of joint instability. This conclusion should be interpreted with caution, however, as major skin-related movement artifacts associated with the video-based optoelectronic gait analysis approach used in this study may have limited accurate quantification of knee varus/valgus motion during gait (Benoit et al. 2006; Leardini et al. 2005). Additionally, only static correlates of joint instability were evaluated which may provide an incomplete picture of how varus/valugs motion may relate to dynamic joint instability. Therefore, additional studies to identify objective signs of knee joint instability in patients with knee OA are needed.
The aim of current study was to evaluate kinematics and contact mechanics of the knee joint during the loading response phase of downhill gait in knee OA patients with self-reported instability compared to knee OA patients without instability and a control group without knee OA or instability. We hypothesized that self-reported instability in patients with knee OA will be associated with biomechanical evidence of joint instability consisting of excessive and higher velocity joint contact excursions and knee rotations.
PATIENTS AND METHODS
Participants
All subjects provided signed informed consent approved by the University of Pittsburgh’s institutional review board. Participants in the study were stratified into one of following three groups: 1) an “unstable” group of knee OA patients with self-reported instability; 2) a “stable” group of knee OA patients without instability; and 3) a “control” group of volunteers without knee OA or self-reported instability. Participants were included in the stable and unstable groups if they: 1) met the American College of Rheumatology classification criteria for knee OA (Altman et al. 1986), and 2) demonstrated primary medial compartment knee OA of grade II or greater according to the Kellgren and Lawrence (KL) radiographic severity rating scale (Kellgren and Lawrence 1957). Knee OA patients with coexisting lateral compartment disease with KL grades less than the involved medial compartment and patients with bilateral knee OA were deemed eligible for the study and were included in the analysis. Participants in the unstable group also had to have a self-reported knee instability rating of ≤3 on the knee stability scale indicating that the patient perceived the symptom of instability to be affecting their ability to perform activities of daily living (Fitzgerald et al. 2004). To be included in the control group, participants had to have no history of knee pain and a KL radiographic grade of ≤1.
Participants were excluded if they had a past history of traumatic knee injury, total joint arthroplasty, cardiovascular disease, or neurological disorders that affected lower extremity function. To ensure safe participation in the study, individual patients were also excluded if they required use of an assistive device for ambulation, reported a history of two or more falls within the previous year, or if they reported lack of confidence in ambulating a distance of 30.5 meters (100 feet) without an assistive device.
Testing Procedures
Dynamic Stereo X-ray (DSX) methods were used to quantify 3D joint kinematics and tibiofemoral contact mechanics from biplane radiographic images acquired during the loading response phase of downhill gait. Loading response was selected as a critical time period associated with high demands on the knee joint and reports of dynamic alignment change in patients with knee OA (Astephen and Deluzio 2005; Schipplein and Andriacchi 1991). Participants were positioned on a treadmill within the biplane X-ray system so that the knee of interest would remain in the system’s 3D imaging volume throughout the loading response phase of gait. For participants with knee OA, the knee in which they reported symptoms or episodes of instability was designated as the test knee. In cases where both knees experienced symptoms, the more problematic knee was designated as the test knee. For control participants, the knee from the dominant lower limb was designated as the test knee.
The biplane X-ray system contained two X-ray gantries that were configured with their beam paths intersecting at 60° in a plane parallel to the floor. Each gantry contained a 100 kW pulsed X-ray generator (CPX 3100CV; EMD Technologies, Quebec, Canada), a 40 cm image intensifier (Thales, Neuilly-sur-Seine, France), and a high-speed 4 megapixel digital video camera (Phantom v10, Vision Research, Wayne, New Jersey, USA). The X-ray generators were customized to provide short-duration pulses at very high repetition rates. For the current study, radiographs were generated with a 1ms pulse width at 100 Hz, with a maximum radiographic protocol of 90 kVp/200 mA and a 1 second collection time (100 ms total x-ray exposure) per trial.
Participants’ knees were imaged during a moderately declined gait condition (7% grade, 0.75 m/s) on an instrumented treadmill (Bertec Corp., Columbus, OH, USA) due to the frequent patient reports of knee instability during this task. To this end, previous reports indicate that downhill gait is more demanding on the knee joint as it leads to significant increases in knee flexion angle, vertical ground reaction force and knee joint moments compared to level gait (Kuster et al. 1995; Lay et al. 2006; McIntosh et al. 2006; Redfern 1997). Data was collected for 3 individual downhill gait trials and averaged for statistical analysis. For each trial, the X-ray system was triggered manually prior to heel contact to record a 200ms time period. The loading response phase was then defined as the first 20% of the stance phase of gait after heel contact determined from the vertical ground reaction force profile (Perry and Burnfield 2010). For safety, participants were attached to an overhead harness system during all gait trials.
Quantification of Knee Joint Kinematics
All participants also underwent computed tomography (CT) imaging of their knee using a GE LightSpeed CT Scanner (LightSpeed Pro 16, GE Medical Systems, Fairfield, Connecticut, USA). The CT field of view was approximately 28 × 28 cm, slice thickness ranged from 0.6 to 1.25 mm, and in-plane resolution was approximately 0.55 mm per pixel. The CT images were reconstructed to create 3D bone models of the distal femur and proximal tibia (Tashman and Anderst 2003). The tibia and femur were manually segmented and feature-based interpolation was performed to create 3D bone models using MIMICS (Materialise Inc, Ann Arbor, Michigan, USA). A model-based tracking algorithm was then employed to determine 3D joint motion by matching the radiographic images with projections through the 3D volumetric bone models (Anderst et al. 2009). Local anatomical coordinate systems were created for both the femur and tibia (Tashman and Anderst 2003). The origins of the femur and tibia coordinate systems were defined as the midpoints between the center of the medial and lateral femoral condyles and the most medial and lateral aspects of the tibia plateau, respectively. This experimental approach has shown to have excellent accuracy in terms of measurement bias of better than 1.0 degrees for rotations and 0.7mm for translations and measurement precision of better than 0.9 degrees for rotations and 0.7mm for translations (Anderst et al. 2009).
Data Analysis
Rotations of the tibia relative to the femur were defined with respect to the bone-fixed coordinate systems, and calculated using body-fixed axes in the order of flexion/extension, adduction/abduction, and internal/external rotation. Neutral rotations (zero values) were defined as the position where the tibia and femoral coordinate systems were aligned. Joint rotation excursions were then computed by subtracting the minimum from maximum joint angles. The knee joint angular velocities, being the first derivatives of the knee joint angular displacement, were derived from the difference in the knee angular displacement over two consecutive data points divided by the corresponding time interval.
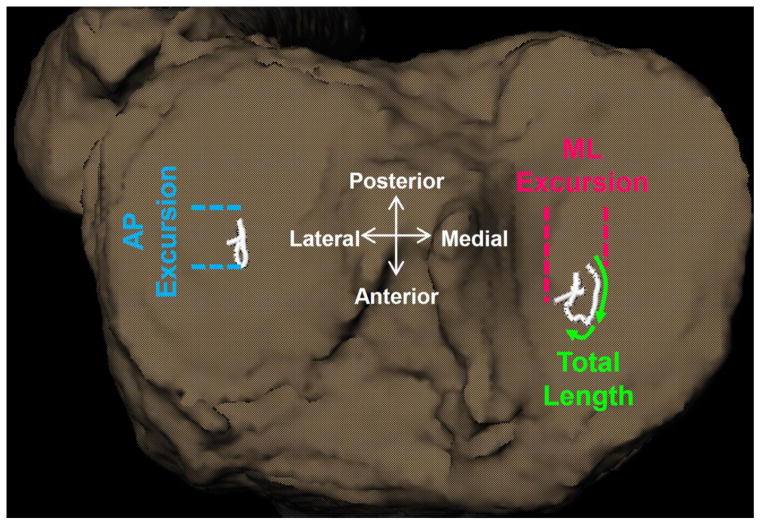
The location of the joint contact point was estimated using the distance-weighted centroid of the region of closest proximity between the bony surfaces in both the medial and lateral tibiofemoral joint compartments (Anderst et al. 2005). Joint contact points for each frame were described for the tibia using a Cartesian coordinate system aligned with the tibial plateau. Anteroposterior (AP) and mediolateral (ML) contact point excursions were computed by subtracting the minimum and maximum contact point position across all frames and the total contact point excursion as the distance along the path (Figure 1). Contact point velocities were derived from the difference of the contact point position between two consecutive data points divided by the corresponding time interval.
Figure 1.
The anterior-posterior (AP) and medial-lateral (ML) contact point excursions were computed by subtracting the minimum from the maximum contact point position across all frames during the loading response phase of downhill gait. The total contact path length was computed as the total distance along the path.
Statistical Analysis
Analysis of variance (ANOVA) and chi-square tests were used to determine group differences in demographics and radiographic knee OA severity. Tibiofemoral joint contact point excursions and kinematics along with their associated velocities were assessed using Analysis of Covariance (ANCOVA), adjusting for group differences in body mass index (BMI) and radiographic severity of knee OA. To account for differences in bone geometry, the AP, ML, and total joint contact point excursions were also adjusted for the AP length, ML width, and the total area of the medial and lateral tibia plateaus, respectively. Post-hoc, paired t-tests with Tukey corrections were then performed to compare group differences for each dependent variable. In addition, Hochberg adjustments for multiple comparisons were applied within each biomechanical category to decrease the probability of type I error (Hochberg 1988). All adjustments were applied to the p-values while the significance levels were kept at <0.05.
RESULTS
Demographics
A total of 43 older adults participated in this study. Knee OA patients were divided into two mutually exclusive groups of unstable [N=11; female/male: 6/5, age: 69.9 (8.2) years, BMI: 31.5 (4.8) Kg/m2] and stable [N=7; female/male: 6/1, mean age: 71.4 (9.1) years, BMI: 30.1 (6.8 Kg/m2)]. The control group consisted of volunteers without knee OA or reports of instability [N=25, female/male: 13/12, mean age 69.6 (7.4), BMI: 24.7 (3.8 Kg/m2)]. There were no statistically significant group differences for gender and age. However, BMI was significantly lower in the control group compared to the stable (mean difference 5.4 Kg/m2; p=0.02) and the unstable groups (mean difference 6.8 Kg/m2; p=0.0006). Radiographic disease severity was also significantly different between groups (P=0.0001) with median and interquartile (IQL) ranges of KL scores for each group as follows: control = 0 (0,0); stable = 3 (3,3), and unstable = 4 (3,4).
Contact Point Excursions and Velocities
The total length of the medial compartment contact point excursion for the unstable group was significantly longer compared to the stable (p=0.046) and the control groups (p=0.016; Table 1). However, no group differences were observed for the medial compartment contact point excursions in the AP or ML directions (Table 1). Average medial compartment contact point velocities for the unstable group were also greater compared to the stable (p=0.047) and the control groups (p=0.022; Table 2). No group differences were observed for the lateral compartment contact point excursions or velocities (Tables 1–2).
Table 1.
Means (standard deviation) for tibiofemoral compartment contact point excursions and knee joint kinematics during the loading response phase of downhill gait. All analyses were adjusted for body mass index and radiographic disease severity.
| Control | Stable | Unstable | Effect Size | |
|---|---|---|---|---|
| (n=25) | (n=7) | (n=11) | (Eta2) | |
| Medial Compartment Contact Path | ||||
| Anterior/Posterior Excursion (mm) | ||||
| 4.1 (2.1) | 3.5 (1.4) | 5.6 (3.4) | 0.14 | |
| Medial/Lateral Excursion (mm) | 1.2 (0.9) | 1.6 (1.7) | 2.4 (1.4) | 0.20 |
| Total Length (mm) | 5.8 (2.6)† | 5.2 (2.0)† | 9.5 (4.6) | 0.25 |
| Lateral Compartment Contact Path | ||||
| Anterior/Posterior Excursion (mm) | 4.9 (2.5) | 3.0 (1.2) | 5.5 (4.4) | 0.13 |
| Medial/Lateral Excursion (mm) | 1.5 (1.3) | 0.9 (0.9) | 1.4 (1.4) | 0.07 |
| Total Length (mm) | 6.2 (3.0) | 4.0 (1.4) | 7.5 (5.5) | 0.18 |
| Knee Extension (−) / Flexion (+) | ||||
| Position @ Heel Contact (degrees) | 2.0 (4.5) | 9.2 (11.3) | 7.4 (9.1) | 0.18 |
| Total Joint Excursion (degrees) | 11.0 (4.1) | 7.2 (3.6) | 7.2 (3.2) | 0.10 |
| Knee Adduction (−) / Abduction (+) | ||||
| Position @ Heel Contact (degrees) | −0.4 (2.9) | −3.7 (6.3) | 0.6 (5.0) | 0.43 |
| Total Joint Excursion (degrees) | −0.9 (0.4) | −2.0 (1.3)* | −1.7 (0.8)* | 0.54 |
| Knee External Rotation (−) / Internal Rotation (+) | ||||
| Position @ Heel Contact (degrees) | −2.6 (7.3) | −4.9 (9.8) | 0.1 (8.1) | 0.37 |
| Total Joint Excursion (degrees) | 4.9 (2.2) | 3.9 (1.7) | 3.6 (1.7) | 0.11 |
The effect size, partial eta2, is interpreted in the same manner as an R2 statistic
Statistically significant differences compared to the “unstable” group
Statistically significant differences compared to the “control” group
Table 2.
Means (standard deviation) for tibiofemoral compartment contact point linear velocities and knee joint angular velocities during the loading response phase of downhill gait.
| Control | Stable | Unstable | Effect Size | |
|---|---|---|---|---|
| (n=25) | (n=7) | (n=11) | (Eta2) | |
| Medial Compartment Contact Point Velocity | ||||
| Heel Contact (mm/s) | 39.1 (27.8) | 74.3 (38.4) | 80.3 (46.0) | 0.10 |
| Peak (mm/s) | 100.6 (60.0) | 99.1 (46.4) | 173.5 (125.9) | 0.28 |
| Average (mm/s) | 41.5 (20.7)† | 36.7 (15.6)† | 71.0 (41.3) | 0.34 |
| Lateral Compartment Contact Point Velocity | ||||
| Heel Contact (mm/s) | 39.4 (18.5) | 40.6 (20.1) | 52.0 (33.4) | 0.21 |
| Peak (mm/s) | 95.9 (56.7) | 59.6 (22.8) | 153.8 (174.0) | 0.11 |
| Average (mm/s) | 44.2 (23.4) | 28.5 (11.4) | 53.1 (30.1) | 0.12 |
| Knee Extension (−) / Flexion (+) Angular Velocity | ||||
| Heel Contact (degrees/s) | 54.9 (59.4)† | 79.8 (36.1)† | −26.2 (50.8) | 0.33 |
| Peak (degrees/s) | 146.2 (64.6) | 104.0 (48.8) | 96.6 (48.9) | 0.07 |
| Average(degrees/s) | 73.3 (33.6)† | 48.1 (34.9) | 29.1 (38.9) | 0.27 |
| Knee Adduction (−) / Abduction (+) Angular Velocity | ||||
| Heel Contact (degrees/s) | −2.9 (9.1) | −26.2 (24.1)†* | −3.0 (11.9) | 0.41 |
| Peak (degrees/s) | −15.2 (7.6) | −40.5 (29.6)* | −26.6 (16.4) | 0.44 |
| Average(degrees/s) | −4.6 (4.2) | −9.6 (8.1) | −5.8 (11.2) | 0.37 |
| Knee External Rotation (−) / Internal Rotation (+) Angular Velocity | ||||
| Heel Contact (degrees/s) | 16.5 (42.2) | 19.1 (40.7) | −11.0 (27.7) | 0.02 |
| Peak (degrees/s) | 82.2 (27.9) | 84.6 (34.1) | 58.9 (35.5) | 0.15 |
| Average (degrees/s) | 27.0 (22.0) | 17.8 (11.2) | 6.9 (21.8) | 0.12 |
All analyses were adjusted for body mass index and radiographic disease severity. The effect size, partial eta2, is interpreted in the same manner as an R2 statistic
Statistically significant differences compared to the “unstable” group
Statistically significant differences compared to the “control” group
Knee Kinematics and Angular Velocities
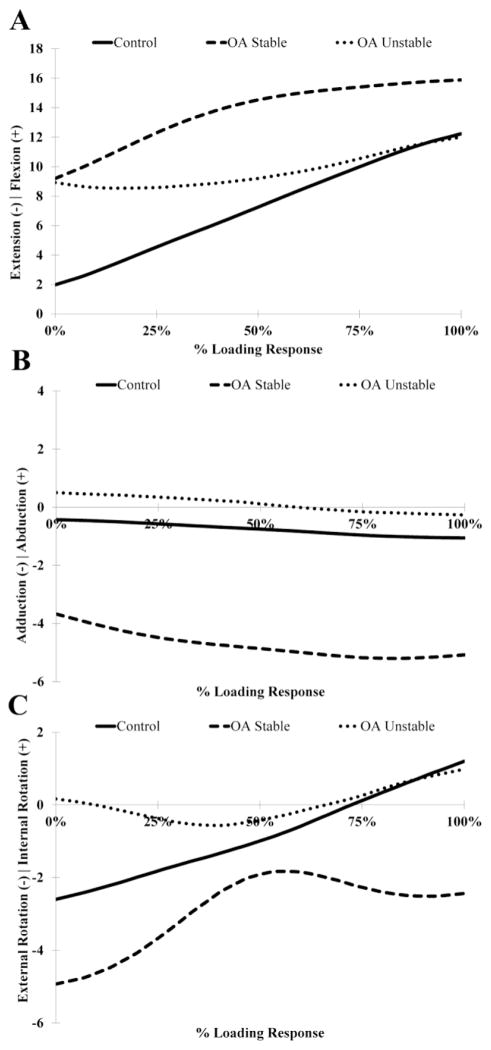
No differences in knee flexion angle were observed between groups (Table 1; Figure 2A). However, sagittal-plane knee angular velocity immediately after heel contact was negative for the unstable group who moved towards extension and positive for the stable (p=0.001) and the control groups (p=0.002; Table 2) who moved towards flexion. Additionally, the frontal plane knee adduction excursion range of motion was increased for the stable (p=0.002) and the unstable groups (p=0.02) compared to the control group (Figure 2B). The stable group also demonstrated significantly greater knee adduction angular velocities after heel contact compared to the control (p=0.0004) and the unstable groups (p=0.002) as well as greater peak knee adduction angular velocities compared to the control group (P=0.003; Table 2). No differences in internal/external rotation motions or angular velocities were detected between groups (Tables 1 & 2; Figure 2C).
Figure 2.
Ensemble average tibiofemoral rotations in the sagittal (A), coronal (B) and transverse (C) planes during the loading response phase of gait.
Angle-angle Plots
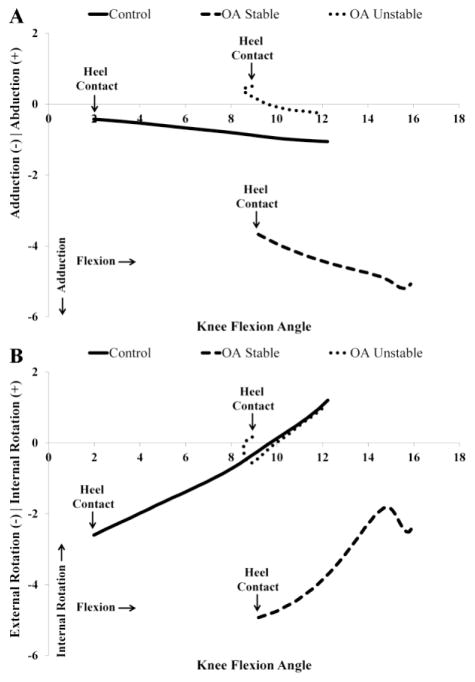
Angle-angle plots of the knee joint for the loading response phase of downhill gait provided insights about group variations in coupling of knee joint motion in different planes (i.e. intra-joint coordination). For the control group, the knee flexion versus adduction plots showed a progressive increase in knee adduction with increasing knee flexion angles (Figure 3A). A similar trend of increasing knee adduction angle as a function of increasing knee flexion angle was observed for the stable group, albeit at a steeper rate. Unlike the control and the stable groups, however, knee adduction was initially coupled with knee extension for the unstable group which then became coupled with knee flexion later in the loading response phase (Figure 3A).
Figure 3.
Angle-angle plots of knee flexion versus knee adduction/abduction (A) and knee flexion versus knee internal/external rotation (B).
Consistent with the normal screw-home motion of the knee joint during flexion, a progressive increase in internal rotation of the tibia with increasing knee flexion angles were observed for the control and stable groups (Figure 3B). In late loading response phase, however, a rounded trajectory signifying a change in rotation from internal to external rotation is observed with increasing knee flexion angle in the stable group. Unlike the control and the stable groups, the unstable group demonstrated a coupled movement pattern of knee extension and external rotation during the initial phase of loading response. This movement pattern was then reversed towards coupled motion of internal rotation with increasing knee flexion angle in the later phases of the loading response.
DISCUSSION
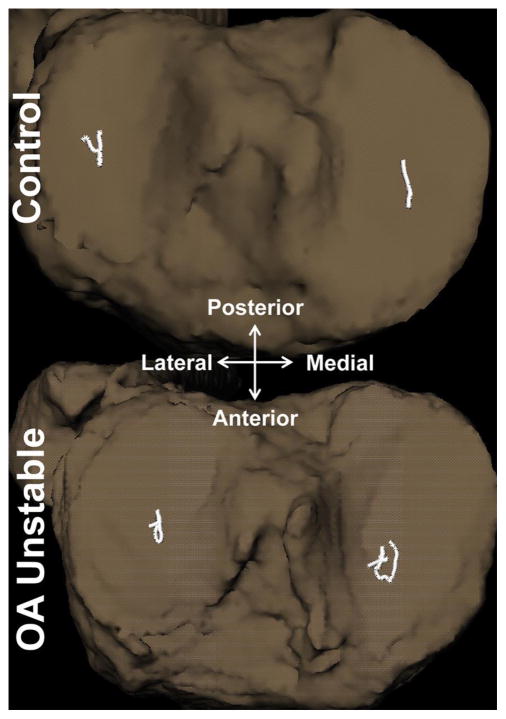
The findings from the current investigation support the hypothesis that knee OA patients with self-reported instability exhibit longer medial tibiofemoral joint contact point excursions during the loading response phase of downhill gait compared to their stable and control counterparts. More specifically, the unstable group demonstrated significantly longer medial compartment contact point excursions that were 83% longer compared to the stable group and 64% longer compared to the control group (Figure 4). Additionally, no differences in joint contact point excursions were noted between the stable and the control groups, suggesting that the excessive medial compartment contact point excursions observed in the unstable group are unique to this patient population and different than the common degenerative joint changes that occur solely due to the OA process alone.
Figure 4.
Representative tibiofemoral joint contact path profiles of a control and an unstable knee.
The unstable group also demonstrated increased average medial compartment contact point excursion velocities compared to the stable and the control groups (Table 2). This finding may have important clinical implications in terms of disease progression for unstable OA knees. In a longitudinal study of unstable canine knees, Anderst and colleagues (Anderst and Tashman 2009) reported that increased medial compartment contact velocity during the early stance phase of running was associated with greater medial compartment cartilage damage. Accelerated rates of disease progression in unstable knees are most likely due to increases in coefficient of friction (Nickel et al. 2004; Nickel et al. 2006), frictional forces (Drummond et al. 2003), as well as elevated contact and shear stresses (Drummond et al. 2003; Waldman and Bryant 1997) due to increasing velocity of the sliding joint surfaces. Increased tangential shear forces and stresses may in turn lead to greater breakdown of collagen and increased fibrillation of the articular surfaces in unstable knees (Andriacchi et al. 2004). Therefore, it stands to reason that finding ways to limit instability by restoring normal knee joint contact mechanics could have important clinical utility as decreased knee joint instability has shown to reduce medial compartment joint space loss in knee OA patients (Dayal et al. 2005).
As knee stability is determined by the interaction of the passive soft-tissue restraint and the active neuromuscular system, the longer and higher velocity medial compartment contact point excursions observed in the unstable group in our study are most likely affected by joint laxity and lack of adequate neuromuscular control. However, previous reports suggest little to no relationship between passive knee joint laxity and self-reported instability in patients with knee OA (Creaby et al. 2013; Knoop et al. 2012; Schmitt et al. 2008; Schmitt and Rudolph 2008). Additionally, neuromuscular adaptations such as higher muscle co-contractions used by knee OA patients with self-reported instability have been deemed ineffective in providing adequate knee joint stability (Schmitt and Rudolph 2008). However, no previous studies have investigated the relationship between either joint laxity or neuromuscular adaptations in knee OA patients with reports of instability with altered joint contact mechanics which warrants further investigation. As impairments in passive laxity and neuromuscular control could be addressed through interventions such as bracing (Ramsey et al. 2007) and neuromuscular training (Ageberg et al. 2010), identifying the underlying causes of functional instability could have important clinical implications for designing more effective interventions for knee OA patients with self-reported instability.
Our group has previously published detailed kinematic measurements obtained during a downhill gait condition from a subsample of subjects in the current study (Farrokhi et al. 2012). Traditional kinematic measurements (joint rotations) revealed that knee OA patients with reports of instability demonstrate reduced knee flexion and internal rotation motion excursions, as well as increased adduction range of motion when compared to a control group without knee OA or reports of instability (Farrokhi et al. 2012). The current study provides further kinematic evidence that the knee OA patients with reports of instability also demonstrate a distinctive pattern of coupled sagittal and transverse plane knee joint motion that is different than stable and control subjects without reports of instability (Figure 3B). To initiate knee flexion during the loading response phase of gait, the knee must first be unlocked through internal rotation of the tibia relative to the femur, which frees the tibial tubercles from the intercondylar notch and releases the tension in the joint capsule and the ligaments (Levangie and Norkin 2011). This so called “reverse screw-home” motion observed in both the control and the stable groups in our study allows the condyles of the tibia and femur to move freely on each other. Conversely, the unstable group in our study initiated the loading response phase of gait with coupled motions of knee extension and tibial external rotation which is consistent with the pattern of knee motion previously reported for unstable knees of patients after anterior curciate ligament ruptures and post total knee arthroplasty (Dennis et al. 2004; Dennis et al. 2005; Karrholm et al. 1994). It stands to reason that the combined motions of knee extension and external rotation may be an attempt at proving better knee joint stability by placing the knee joint closer to its close-packed (locked) position where the increased tension in the surrounding joint capsule and ligaments can provide better joint stability (Levangie and Norkin 2011). However, an undesirable consequence of this strategy may be decreased shock absorption (Perry and Burnfield 2010) and a further increase in medial compartment joint loading which can lead to increased rates of disease progression.
Knee OA patients with and without complaints of instability in the current study also demonstrated increased frontal-plane knee joint excursions towards knee adduction compared to the control group. Increased knee adduction motion theoretically shifts the line of ground reaction force medially and away from the knee joint center, thus intensifying the medial compartment compressive loads (i.e. increased knee adduction moment) (Andrews et al. 1996; Hurwitz et al. 2002). It could be argued that the potential additive effects of higher compressive loads along with increased joint surface translations at higher velocities could make the articular cartilage of the medial compartment in the unstable group more vulnerable to degeneration and disease progression (Miyazaki et al. 2002).
An additional unexpected finding of our current study was the increased knee adduction angular velocity after heel contact in the stable group who didn’t report episodic instability. A potential explanation for this observation could be that the stable group contacted the ground with a more adducted knee alignment compared to the stable and control groups who contacted the ground with near neutral alignments, although these differences did not reach statistical significance. Regardless of the cause, the observed increased knee adduction angular velocity after heel contact in our study provides in-vivo evidence for presence of frontal-plane knee joint instability in patients with knee OA. However, given that our stable patients did not report episodes of buckling, shifting, or giving way of their arthritic knee joints, the increased knee adduction joint velocities detected after heel contact may be a subclinical sign of joint instability and most likely different than the mechanism responsible for functional instability observed in the unstable patients. Given that increased knee adduction joint velocity can lead to greater medial compartment joint loading and increased rates of disease progression, further investigation of this finding in knee OA patients without reports of instability is warranted.
The findings of this study should be interpreted in light of its limitations. As no episodes of instability were reported during data collection, whether joint contact excursions or knee joint kinematics may differ during an actual episode of instability cannot be determined. Also, the potential influence of the menisci and the articular cartilage degeneration were not considered in our calculations of the tibiofemoral joint contact point location. However, considering the lack of differences in the medial compartment contact point excursions between the control and the stable groups who had distinctly different radiographic disease severity, it is unlikely that the excessive contact point excursions observed for the unstable group were affected by this limitation.
Conclusions
There is currently no adequate objective measure of knee joint stability in patients with knee OA. Findings from the current study suggest that knee OA patients with self-reported instability exhibit longer and higher velocity medial tibiofemoral joint contact point excursions compared to knee OA patients without instability or older adults without knee OA. To the best of our knowledge, this is the first objective evidence of functional instability during a common daily activity in this patient population. Given the localization of joint instability in the medial compartment in our study, the medial tibiofemoral joint in this patient population may be at high risk for OA progression.
Acknowledgments
This work was supported by the Pittsburgh Claude D. Pepper Older Americans Independence Center (Grant P30 AG024827), the National Center for Research Resources, a component of the National Institutes of Health (NIH) and Roadmap for Medical Research (Grant 1 UL1 RR024153) and a National Center Medical Rehabilitation Research, National Institute of Child Health and Human Development / National Institute Neurological Disorders and Stroke, National Institutes of Health career development award (K12 HD055931). The authors would like to thank Megan Robinson and Paige Kendal for their assistance with data analysis and management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ageberg E, Link A, Roos EM. Feasibility of neuromuscular training in patients with severe hip or knee OA: the individualized goal-based NEMEX-TJR training program. BMC Musculoskelet Disord. 2010;11:126. doi: 10.1186/1471-2474-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Anderst W, Zauel R, Bishop J, Demps E, Tashman S. Validation of three-dimensional model-based tibio-femoral tracking during running. Medical engineering & physics. 2009;31(1):10–16. doi: 10.1016/j.medengphy.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderst WJ, Les C, Tashman S. In vivo serial joint space measurements during dynamic loading in a canine model of osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2005;13(9):808–816. doi: 10.1016/j.joca.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Anderst WJ, Tashman S. The association between velocity of the center of closest proximity on subchondral bones and osteoarthritis progression. J Orthop Res. 2009;27(1):71–77. doi: 10.1002/jor.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M, Noyes FR, Hewett TE, Andriacchi TP. Lower limb alignment and foot angle are related to stance phase knee adduction in normal subjects: a critical analysis of the reliability of gait analysis data. J Orthop Res. 1996;14(2):289–295. doi: 10.1002/jor.1100140218. [DOI] [PubMed] [Google Scholar]
- Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- Astephen JL, Deluzio KJ. Changes in frontal plane dynamics and the loading response phase of the gait cycle are characteristic of severe knee osteoarthritis application of a multidimensional analysis technique. Clin Biomech (Bristol, Avon) 2005;20(2):209–217. doi: 10.1016/j.clinbiomech.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Benoit DL, Ramsey DK, Lamontagne M, Xu L, Wretenberg P, Renstrom P. Effect of skin movement artifact on knee kinematics during gait and cutting motions measured in vivo. Gait Posture. 2006;24(2):152–164. doi: 10.1016/j.gaitpost.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Briem K, Snyder-Mackler L. Proximal gait adaptations in medial knee OA. J Orthop Res. 2009;27(1):78–83. doi: 10.1002/jor.20718. [DOI] [PubMed] [Google Scholar]
- Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech (Bristol, Avon) 2004;19(1):44–49. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Creaby MW, Wrigley TV, Lim BW, Hinman RS, Bryant AL, Bennell KL. Self-reported knee joint instability is related to passive mechanical stiffness in medial knee osteoarthritis. BMC Musculoskelet Disord. 2013;14:326. doi: 10.1186/1471-2474-14-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal N, Chang A, Dunlop D, Hayes K, Chang R, Cahue S, Song J, Torres L, Sharma L. The natural history of anteroposterior laxity and its role in knee osteoarthritis progression. Arthritis Rheum. 2005;52(8):2343–2349. doi: 10.1002/art.21277. [DOI] [PubMed] [Google Scholar]
- Dennis DA, Komistek RD, Mahfouz MR, Walker SA, Tucker A. A multicenter analysis of axial femorotibial rotation after total knee arthroplasty. Clinical orthopaedics and related research. 2004;(428):180–189. doi: 10.1097/01.blo.0000148777.98244.84. [DOI] [PubMed] [Google Scholar]
- Dennis DA, Mahfouz MR, Komistek RD, Hoff W. In vivo determination of normal and anterior cruciate ligament-deficient knee kinematics. Journal of biomechanics. 2005;38(2):241–253. doi: 10.1016/j.jbiomech.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Drummond C, Israelachvili J, Richetti P. Friction between two weakly adhering boundary lubricated surfaces in water. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;67(6 Pt 2):066110. doi: 10.1103/PhysRevE.67.066110. [DOI] [PubMed] [Google Scholar]
- Farrokhi S, Tashman S, Gil AB, Klatt BA, Fitzgerald GK. Are the kinematics of the knee joint altered during the loading response phase of gait in individuals with concurrent knee osteoarthritis and complaints of joint instability? A dynamic stereo X-ray study. Clin Biomech (Bristol, Avon) 2012;27(4):384–389. doi: 10.1016/j.clinbiomech.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Niu J, McClennan C, Sack B, Aliabadi P, Hunter DJ, Guermazi A, Englund M. Knee buckling: prevalence, risk factors, and associated limitations in function. Ann Intern Med. 2007;147(8):534–540. doi: 10.7326/0003-4819-147-8-200710160-00005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GK, Piva SR, Irrgang JJ. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51(6):941–946. doi: 10.1002/art.20825. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP. The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res. 2002;20(1):101–107. doi: 10.1016/S0736-0266(01)00081-X. [DOI] [PubMed] [Google Scholar]
- Karrholm J, Jonsson H, Nilsson KG, Soderqvist I. Kinematics of successful knee prostheses during weight-bearing: three-dimensional movements and positions of screw axes in the Tricon-M and Miller-Galante designs. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 1994;2(1):50–59. doi: 10.1007/BF01552655. [DOI] [PubMed] [Google Scholar]
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the rheumatic diseases. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop J, van der Leeden M, van der Esch M, Thorstensson CA, Gerritsen M, Voorneman RE, Lems WF, Roorda LD, Dekker J, Steultjens MP. Association of lower muscle strength with self-reported knee instability in osteoarthritis of the knee: results from the Amsterdam Osteoarthritis cohort. Arthritis Care Res (Hoboken) 2012;64(1):38–45. doi: 10.1002/acr.20597. [DOI] [PubMed] [Google Scholar]
- Kuster M, Sakurai S, Wood GA. Kinematic and kinetic comparison of downhill and level walking. Clin Biomech (Bristol, Avon) 1995;10(2):79–84. doi: 10.1016/0268-0033(95)92043-l. [DOI] [PubMed] [Google Scholar]
- Lay AN, Hass CJ, Gregor RJ. The effects of sloped surfaces on locomotion: a kinematic and kinetic analysis. Journal of biomechanics. 2006;39(9):1621–1628. doi: 10.1016/j.jbiomech.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Leardini A, Chiari L, Della Croce U, Cappozzo A. Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait Posture. 2005;21(2):212–225. doi: 10.1016/j.gaitpost.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Levangie PK, Norkin CC. Joint structure and function : a comprehensive analysis. Philadelphia: F.A. Davis Co; 2011. p. xxii.p. 588. [Google Scholar]
- McIntosh AS, Beatty KT, Dwan LN, Vickers DR. Gait dynamics on an inclined walkway. Journal of biomechanics. 2006;39(13):2491–2502. doi: 10.1016/j.jbiomech.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61(7):617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Beatty MW, Marx DB. Laboratory stresses and tractional forces on the TMJ disc surface. J Dent Res. 2004;83(8):650–654. doi: 10.1177/154405910408300813. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Beatty MW, Moss MA, Marx DB. Static and dynamic loading effects on temporomandibular joint disc tractional forces. J Dent Res. 2006;85(9):809–813. doi: 10.1177/154405910608500906. [DOI] [PubMed] [Google Scholar]
- Perry J, Burnfield JM. Gait analysis : normal and pathological function. Thorofare, NJ: SLACK; 2010. p. xvi.p. 551. [Google Scholar]
- Ramsey DK, Briem K, Axe MJ, Snyder-Mackler L. A mechanical theory for the effectiveness of bracing for medial compartment osteoarthritis of the knee. The Journal of bone and joint surgery. 2007;89(11):2398–2407. doi: 10.2106/JBJS.F.01136. American volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern MS. Biomechanics of descending ramps. Gait Posture. 1997;6:119–125. [Google Scholar]
- Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9(1):113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- Schmitt LC, Fitzgerald GK, Reisman AS, Rudolph KS. Instability, laxity, and physical function in patients with medial knee osteoarthritis. Phys Ther. 2008;88(12):1506–1516. doi: 10.2522/ptj.20060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis Rheum. 2007;57(6):1018–1026. doi: 10.1002/art.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt LC, Rudolph KS. Muscle stabilization strategies in people with medial knee osteoarthritis: the effect of instability. J Orthop Res. 2008;26(9):1180–1185. doi: 10.1002/jor.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma L. Proprioceptive impairment in knee osteoarthritis. Rheum Dis Clin North Am. 1999;25(2):299–314. vi. doi: 10.1016/s0889-857x(05)70069-7. [DOI] [PubMed] [Google Scholar]
- Tashman S, Anderst W. In-vivo measurement of dynamic joint motion using high speed biplane radiography and CT: application to canine ACL deficiency. Journal of biomechanical engineering. 2003;125(2):238–245. doi: 10.1115/1.1559896. [DOI] [PubMed] [Google Scholar]
- van der Esch M, Knoop J, van der Leeden M, Voorneman R, Gerritsen M, Reiding D, Romviel S, Knol DL, Lems WF, Dekker J, et al. Self-reported knee instability and activity limitations in patients with knee osteoarthritis: results of the Amsterdam osteoarthritis cohort. Clin Rheumatol. 2012 doi: 10.1007/s10067-012-2025-1. [DOI] [PubMed] [Google Scholar]
- van der Esch M, Steultjens M, Harlaar J, Wolterbeek N, Knol DL, Dekker J. Knee varus-valgus motion during gait--a measure of joint stability in patients with osteoarthritis? Osteoarthritis Cartilage. 2008;16(4):522–525. doi: 10.1016/j.joca.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Waldman SD, Bryant JT. Dynamic contact stress and rolling resistance model for total knee arthroplasties. J Biomech Eng. 1997;119(3):254–260. doi: 10.1115/1.2796089. [DOI] [PubMed] [Google Scholar]