Abstract
Oxidative stress resulting from accumulation of reactive oxygen species (ROS) is involved in cell death associated with neurological disorders such as stroke, Alzheimer’s disease and traumatic brain injury. Antioxidant compounds that improve endogenous antioxidant defenses have been proposed for neural protection. The purpose of this study was to investigate the potential protective effects of total saponin in leaves of Panax notoginseng (LPNS) on oxidative stress and cell death in brain cells in vitro. Lactate dehydrogenase (LDH) assay indicated that LPNS (5μg/ml) reduced H2O2-induced cell death in primary rat cortical astrocytes (23±8% reduction in LDH release vs. control). Similar protection was found in oxygen and glucose deprivation/reoxygenation induced SH-SY5Y (a human neuroblastoma cell line) cell damage (78±7% reduction vs.control). The protective effects of LPNS in astrocytes were associated with attenuation of reactive oxygen species (ROS) accumulation. These effects involved activation of Nrf2 (nuclear translocation) and upregulation of downstream antioxidant systems including heme oxygenase-1 (HO-1) and glutathione S-transferase pi 1 (GSTP1). These results demonstrate for the first time that LPNS has antioxidative effects which may be neuroprotectionin neurological disorders.
Keywords: Oxidative stress, total saponin of Panaxnotoginseng leaves, astrocytes, SH-SY5Y cells
Introduction
Oxidative stress is an imbalance between formation of reactive oxygen and nitrogen species and endogenous antioxidant defenses and repair capacity (Crack and Taylor, 2005). It results from accumulation of reactive oxygen species (ROS) and is involved in cell death observed in multiple pathological processes including stroke, brain tumor and neurodegenerative diseases (Lotharius et al., 2005; Resende et al., 2008). Reactive oxygen and nitrogen species change cellular responses through diverse mechanisms. At low levels, they are signaling molecules, while at high levels they damage organelles, particularly the mitochondria. Oxidative damage and associated mitochondrial dysfunction may result in energy depletion, accumulation of cytotoxic mediators and cell death.
Various tissues differ in susceptibility to oxidative stress. Thus, the brain is very susceptible to energy deprivation and damage induced by oxidative stress, due to a high rate of oxidative metabolic activity and inadequate neuronal cell repair activity (Braughler and Hall, 1989). Astrocytes maintain high intracellular concentrations of certain antioxidants, such as vitamins E and C and antioxidant enzymes, which play a major neuroprotective role in brain resistance to the deleterious effects of superoxides (Lotharius et al., 2005; Papadopoulos et al., 1998; Wilson, 1997). In reactive gliosis, the neuroprotective role of astrocytes may be accentuated because of increases in a number of activities: expression of antioxidant enzymes; transport and metabolism of glucose that yields reducing equivalents for antioxidant regeneration and lactate for neuronal metabolism; synthesis of glutathione; and recycling of vitamin C. Although astrocytes are generally less susceptible to oxidative injury than neurons, there is strong evidence that oxidative stress also alters astrocyte function (Peuchen et al., 1997). Astrocytic apoptosis is observed in brain injuries caused by trauma and ischemia. In particular, they are extremely vulnerable to hydrogen peroxide (H2O2) induced damage (Giffard and Swanson, 2005; Takuma et al., 2004). Therefore, protection of astrocytes from oxidative stress appears essential to maintain cerebral antioxidant competence and to prevent neuronal damage as well as to facilitate neuronal recovery (Hamdi et al., 2011). Compounds which act to improve endogenous antioxidant defenses, have been proposed to serve as an avenue for therapeutic attempts aimed at limiting neural degeneration and improving neurological recovery.
In recent years, researchers have found that many herbal drugs and their extracts have antioxidative effects on brain injury. Panax notoginseng (Burk.) F.H. Chen (Sanqi), a traditional Chinese materia medica, is widely used in Chinese medicine to arrest internal and external hemorrhages, eliminate blood stasis, improve blood circulation, disperse bruises, reduce swelling and pain (Lei and Chiou, 1986). Ginsenosides or dammarane-type triterpenoidal saponins, the main bioactive constituents of P. notoginseng, are effective in preventing and treating cerebro- and cardiovascular diseases. Clinically, P. notoginseng Saponin (PNS) is used in the treatment of acute ischemic stroke (Chen et al., 2008; He et al., 2011) and cardiovascular disease (Chan et al., 2002; Chen et al., 2011). These compounds are also immunoregulatory, possessing properties such as hepatoprotection and anti-carcinogenesis (Ng, 2006). In experimental studies, a wide range of pharmacological activities of PNS had been reported include anti-inflammatory, hemostatic, hypolipidemic, hepatoprotective, renoprotective, and estrogen-like activities (Lei and Chiou, 1986; Ng, 2006). Recent research found that panaxatriol saponins protect against focal cerebral ischemia in rat by reducing cerebral edema, up-regulating heat shock protein HSP70 expression and down-regulating transferrin (Yao and Li, 2002). Antioxidative effects of the water extract of P. notoginseng have also been reported in on PC12 cells (Choi et al., 2010).
Although P.notoginseng has been shown to protect against neurological diseases, the underlying mechanisms remain poorly understood, especially with regards to any antioxidant effect in astrocytes. Furthermore, while the therapeutic effects of Panax notoginseng has been focused on its saponin of root, the pharmacological effects of other parts such as saponin in leaves of P. notoginseng (LPNS), rich in protopanoxadiol saponins, are largely unknown. It is of importance to explore the effect and mechanism of LPNS as a novel therapeutics for neurological disorders related to oxidative stress. This present study examines the antioxidative effects of LPNS in primary astrocytes and a neuroblastoma cell line, SH-SY5Y, and examines the effect of LPNS on the transcription factor Nuclear factor (erythroid-derived 2)-like 2(nrf2), a master regulator of antioxidative defense mechanisms, and downstream enzyme.
Materials and Methods
Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). Dulbecco’s modified Eagle’s medium (DMEM), L-glutamine, Antibiotic-Antimycotic (100X), trypsin-EDTA, H2O2 and collagen were obtained from Invitrogen (Carlsbad, CA).
The total saponin from the leaves of Panax notoginseng was a gift from Yunnan Hongyun Bioengineering Technology Co., Ltd. (Kunming, China). The fingerprint analysis of the LPNS sample was performed as described by Wagner et al. (Wagner et al., 2011) with slight modification. Five standard substances (ginsenoside Rb1, Rb2, Rb3, Rc and Rd) were purchased from the National Institute for the Pharmaceutical and Biological Products (Beijing, PR China). The tested sample was dissolved in methanol and the solution was filtered using a 0.45 μm Millipore® filtration unit. The HPLC system (SHIMADZU LC-2010A, SHIMADZU, Japan) was equipped with a binary solvent delivery system and an auto-sampler. 20 μl of the sample were separated on an Agilent Extand-C18 column (4.6×250 mm, 5 μm; Agilent Technologies, China) maintained at 20 °C. The mobile phase consisted of H2O (A) and CH3CN (B) went through a gradient as 15–30% (v/v) B for 20 min, 30–35% B for 20 min, and 35–50% B for 15 min. The flow rate was fixed at 1.0 ml/min and the DAD detector was set at 203 nm.
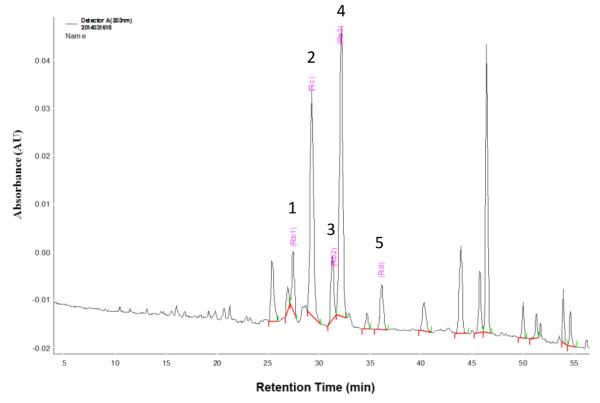
The LPNS-fingerprints (Fig. 1) were characterized by two dominating peaks of ginsenoside Rc (peak 2, Rt=29.18) and ginsenoside Rb3 (peak 4, Rt=32.06), with much lower contents of ginsenoside Rb1 (peak 1, Rt=27.33), ginsenoside Rb2 (peak 3, Rt=31.19), and ginsenoside Rd (peak 5, Rt=36.04) according to standard substance fingerprint. The other peak at Rt =47.0 may be assigned to panaxadiol or -triol. The content of these 5 compounds in LPNS was calculated as the peak area of the compound relative to the total peak area, respectively (Table 1).
Fig. 1.
HPLC-fingerprint analysis of LPNS. 1, ginsenoside Rb1; 2, ginsenoside Rc; 3, ginsenoside Rb2; 4, ginsenoside Rb3; 5, ginsenoside Rd
Table 1.
Contents of panaxadiols or –triols in LPNS
| Peak | Rt (min.) | Compound | Content |
|---|---|---|---|
| 1 | 27.33 | ginsenoside Rb1 | 2.80% |
| 2 | 29.18 | ginsenoside Rc | 11.72% |
| 3 | 31.19 | ginsenoside Rb2 | 2.61% |
| 4 | 32.06 | ginsenoside Rb3 | 17.58% |
| 5 | 36.04 | ginsenoside Rd | 3.87% |
The contents of different compounds in LPNS as determined by HPLC (see Fig. 1). Rt = retention time.
Cell cultures
Astrocytes were isolated and cultured from the cortex of 1-2 day old Sprague-Dawley rats using a modified version of the procedures previously described (McCarthy and de Vellis, 1980). This study followed the Animal Use and Care Guidelines issued by the National Institutes of Health using a protocol approved by the Animal Use and Care Committee at the University of Michigan. Cells were collected and seeded on collagen-coated 48-well-plate or 10cm dishes. The culture medium was changed every 48h in Dulbecco’s Modified Eagle’s Medium (DMEM) with 4.5 g/l glucose and L-glutamine containing 10% FBS, 2% glucose and 1% antibiotic-antimycotic agents. Cells were grown in a humidified incubator with 95% air-5% CO2 at 37°C.
SH-SY5Ycells(ATCC; Manassas, VA) were cultured as previously described (Sabirzhanova et al., 2009). Briefly, cells were grown in DMEM supplemented with 10% FBS in a humidified incubator with 95% air-5% CO2 at 37 °C. The cells were passaged (1:12–16) twice a week.
H2O2 treatment
A 30% solution of H2O2was diluted in FBS-free DMEM and added to the cultures to a final concentration of 0.5 mM. Prior to H2O2 insult, cell cultures were pretreated with vehicle (DMSO) or LPNS at 2.5, 5 or 10 μg/ml. The medium was then replaced and cells incubated with FBS-free DMEM with or without freshly made 0.5 mMH2O2 for 1 h or 4 h. In each experiment, cultures exposed to H2O2 were compared with normal controls (FBS-free DMEM not containing H2O2) and vehicle-treated cells exposed to H2O2.
Oxygen-glucose deprivation/reoxygenation
Oxygen-glucose deprivation (OGD) was performed as previously described (Goldberg and Choi, 1993; Shen et al., 2008). Briefly, SH-SY5Y cells were pretreated with vehicle or LPNS at 2.5, 5 or 10 μg/ml in culture medium for 24 h, then washed twice and incubated in glucose-free DMEM (Life Technologies, Grand Island, New York). The cultures were transferred into an anaerobic chamber filled with a gas mixture of 95% N2/5% CO2 at 37 °C for 5 h. At the end of the OGD, cells were incubated in regular culture medium and returned to normoxic conditions (37 °C in a humidified 95% air-5% CO2 atmosphere) for another 24 h. In each experiment, cultures exposed to OGD were compared with normoxic controls (supplied with DMEM containing glucose and maintained in standard incubation conditions) and vehicle-treated cells exposed to OGD.
Lactate dehydrogenase release
Cell injury was assessed by measuring extracellular lactate dehydrogenase (LDH) release. Primary astrocyte and SH-SY5Y cells were grown to confluence on polycarbonate 48-well plates. In the first set of experiments, cells were then pretreated with vehicle, 2.5, 5 or 10 μg/ml LPNS for 24 h in culture medium before exposure to 0.5 mMH2O2 (4 hours) or OGD. LDH release into the medium was measured using CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI), at 492 nm with a microplate reader (ELX, Bio-Tek, Winooski, VT). The amount of LDH released from each test sample was expressed as a percentage of the control and vehicle-treated cells exposed to H2O2. In a second set of experiments, astrocytes were treated with 5 μg/ml LPNS for 1 to 48 h before exposure to 0.5 mM H2O2 to examine the time course of protection.
Intracellular ROS production
Intracellular ROS production was detected using an OxiSelect™ Intracellular ROS Assay Kit (Cell Biolabs, San Diego, CA). The assay employs a cell-permeable fluorogenic probe 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) which is deacetylated by intracellular esterases and then oxidized by ROS to a fluorescent compound 2′,7′-dichlorofluorescein (DCF). Astrocytes were treated with LPNS at 2.5, 5, or 10 μg/ml for 24 h and then gently washed3x with Dulbecco’s PBS. Cells were then incubated at 37 °C for 30 min after adding 100 μl of DCFHDA/media solution followed by exposure to 0.5 mM H2O2 in FBS-free DMEM for 1 h. Cells were then washed 3x with Dulbecco’s PBS before adding 200 μl of cell lysis buffer. Five minutes later, 150 μl of the cell lysate was transferred to a 96-well plate and read with a fluorometric plate reader at 480 nm/530 nm.
Western blotting studies
Astrocytes pretreated with 5 μg/ml LPNS for 24 h were collected and lysed with RIPA buffer (Sigma, Saint Louis, Missouri) for heme oxygenase (HO-1) detection. Supernatants were collected and protein concentration determined by BCA assay kit (Thermo Fisher Scientific, Rockford, IL). Cells treated with 5 μg/ml LPNS and harvested at 1h were used to examine nuclear translocation of Nrf2. Nuclear fractions were isolated using a cell fractionation kit (Calbiochem, La Jolla, CA).
Fifty μg of each protein sample was run through a 10% SDS-PAGE gel and transferred onto a nitrocellulose membrane (Amershan, Piscataway, New Jersey). After being washed 3x with PBS buffer, membranes were blocked in 5% non-fat dry milk for 1h and incubated with rabbit anti-rat HO-1 (Assay Design, Ann Arbor, MI)or rabbit anti-rat Nrf2 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. Immunoreactive proteins were visualized by using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (GE Healthcare Biosciences, Pittsburgh, PA) and were exposed to Kodak X-OMAT film. The relative densities of Nrf2 and HO-1 bands were measured and quantified with NIH Image J. HO-1 was normalized against β-actin. The protein levels were expressed as percentage of control.
Quantitative real-time PCR
All of materials were from Life Techonologies unless otherwise stated. Total RNA was extracted from cultured rat primary astrocytes by Trizol reagent according to the manufacturer’s instructions. cDNA was synthesized from 1 μg of total RNA in a 20μl reaction mixture using a cDNA Reverse Transcription Kit with RNase Inhibitor. The mixture was incubated at 25 °C for 10 minutes, 37 °C for 120 minutes, and 85 °C for 5 minutes. The primers for GSTP1 were: forward, 5′-AGGCAAAGCTTTCATTGTGG-3′ and reverse, 5′-GTTGATGGGACGGTTCAAAT, and the primers for hypoxanthine phosphoribosyltransferase-1(an endogenous reference gene) were: forward, 5′-CTTTGCTGACCTGCTGGATT-3′ and reverse, 5′-GTTGAGAGATCATCTCCACC-3′. Real-time PCR was carried out with IQTM SYBR Supermix (Bio Rad, Hercules, CA) in an Eppendorf Thermal Cycler. The reaction mixture was incubated at 95 °C for 3 minutes and cycled 40 times from 95 °C for 15 seconds to 60 °C for 1 minute.
Statistical analysis
Data from at least three independent experiments are presented as means ± SEM and were analyzed statistically by t-test or one-way analysis of variance (ANOVA) followed by Dunnett post hoc test for comparisons to a single control or Tukey post hoc test for multiple comparisons between groups. A p value less than 0.05 was considered statistically significant (*p<0.05, **p<0.01 and ***p<0.001). All analysis was performed with Prism 5.0 (Graph Pad, San Diego, CA).
Results
Reduction in H2O2 and OGD/reoxygenation-induced cell death by LPNS
LDH release serves as an indicator of cell membrane damage and death. Pretreatment (24 h) with 5 and 10 μg/ml LPNS significantly reduced H2O2-induced LDH release in astrocytes compared to control by 23±8% (p<0.001) and 18±2% (p<0.01), respectively (Fig. 2A). 2.5 μg/ml LPNS did not show any protective effects. A time course indicated that pretreatment with 5μg/ml LPNS for 12 and 24 h reduced LDH by 71±14% (p<0.01) and 44±11% (p<0.05), respectively (Fig. 2B).
Fig. 2. Effect of LPNS on H2O2-induced release of lactate dehydrogenase (LDH) from cultured rat astrocytes.
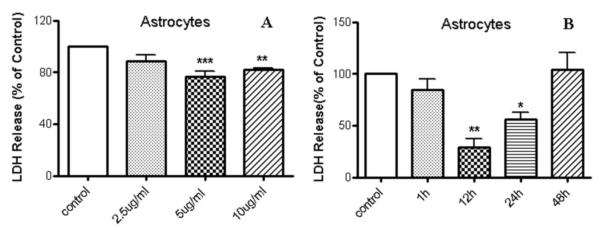
A: Cells were pre-incubated for 24 h in the presence of different LPNS concentrations (0 (control), 2.5, 5, 10 μg/ml) and then exposed for 4 h to 0.5 mM of H2O2 in FBS free medium. LDH release was determined and expressed as a % of control. B: LDH release from astrocyte cells pretreated with 5 μg/ml LPNS for different durations (up to 48 h) before exposure to 0.5 mM H2O2. The results are expressed as a % of LDH release from vehicle-treated cells exposed to H2O2 (control). Values are means ± SE, n =3-4 independent experiments. ANOVA followed by the Dunnett’s test. *, ** and *** indicate significant differences at the p < 0.05, p < 0.01 and p < 0.001 levels, respectively.
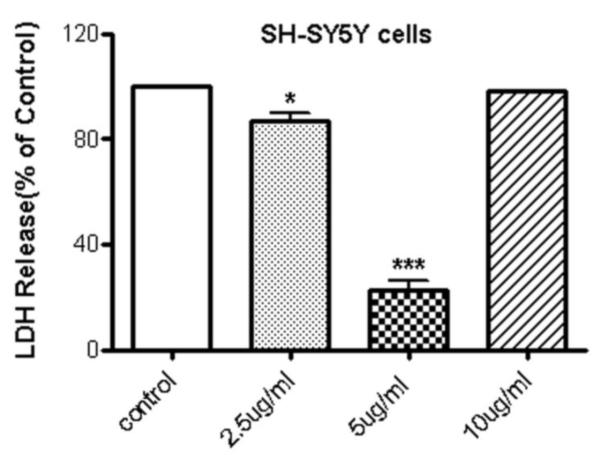
LPNS also protected SH-SY5Y cells from the damage induced by OGD/reoxygenation. Thus, SH-SY5Y cells pretreated with 2.5 and 5 μg/ml LPNS for 24 reduced the LDH release by 13±6 (p < 0.05) and 78±7% (p < 0.001), respectively (Fig. 3). There was no protection with10μg/ml pretreatment.
Fig. 3. Effect of LPNS on OGD-induced release of lactate dehydrogenase (LDH) from SHSY5Y cells.
Cells were pre-incubated for 24h in media containing 0 (control), 2.5, 5 or 10 μg/ml LPNS and then underwent 6h OGD and 24h reoxygenation with glucose as described in the Methods. LDH release to media was then determined and expressed as a % of vehicle-treated cells exposed to OGD/reoxygenation. Values are means ± SE, n =3 independent experiments. ANOVA followed by the Dunnett’s test. * and ***indicate significant differences at the p < 0.05, p < 0.001 levels, respectively.
Inhibition of intracellular ROS production induced by H2O2
In order to investigate the mechanisms underlying LPNS-induced protection, the effects of LPNS on H2O2-induced intracellular ROS accumulation were examined. Astrocytes were labeled with DCFH-DA which forms fluorescent DCF upon oxidation with ROS. There was an increase in ROS production in astrocyte cells after exposure to 0.5 mM H2O2for 1h (2951±92 vs. 2607±10 in controls, p < 0.05; Fig. 4). That increase was significantly reduced in cells pretreated with 5 and 10 μg/ml LPNS for 24 h (2691±149, p<0.05, and 2561±84, p<0.001, respectively). The levels of ROS in cells pretreated with LPNS before H2O2 exposure were not significantly different from those found in control cells.
Fig. 4. Effect of LPNS on H2O2-induced intracellular accumulation of reactive oxygen species (ROS) in cultured rat astrocytes.
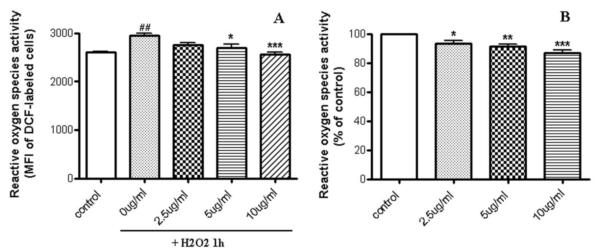
Cells were pre-treated with different concentration of LPNS (0, 2.5, 5, 10 μg/ml) and then incubated for 1 h with FBS-free medium alone (control) or with 0.5 mM of H2O2 and cellular ROS determined fluorometrically by DCF-labeling. Mean fluorescent intensity value (MFI) is proportional to intracellular levels of ROS. Each value is the mean (± SEM) calculated from at least three different wells from four independent cultures. B: The results are expressed as a percentage of fluorescence intensity in vehicle-treated control cells. ANOVA followed by the Tukey’s test. ##Significant differences from vehicle-treated cells exposed to H2O2 compare to normal cells (control) at the p < 0.01 levels; *, **and ***significant differences from vehicle-treated cells exposed to H2O2 (0.5 mM) at the p < 0.05, p < 0.01 and p < 0.001 levels, respectively.
Upregulation of Nrf2 and its downstream antioxidant systems
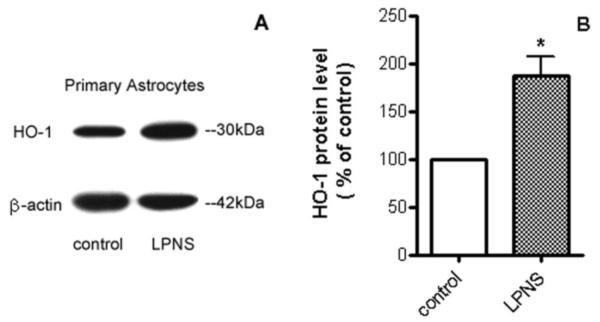
Nrf2 plays a pivotal role in cellular defense against oxidative stress. Under normal conditions, Nrf2 is bound to a specific inhibitory protein, KEAP1, and is targeted for proteasomal degradation. However, under conditions of oxidative stress, Nrf2 is released from KEAP1 and enters the nucleus where it upregulates antioxidant genes (Thimmulappa et al., 2002). Possible activation of Nrf2 by LPNS was investigated by Western blot. Results showed that nuclear Nrf2 levels in astrocytes after 1 h treatment with 5 μg/ml LPNS were 1.45±0.14 fold higher than controls (p < 0.05, Fig. 5). HO-1, an enzyme that catalyzes the degradation of heme, is regulated by Nrf2. Pretreatment (24 h) with 5 μg/ml LPNS upregulated cellular HO-1by 1.87±0.34 fold in cultured primary astroctyes (p < 0.05, Fig. 6). GSTP1 is another downstream protein of Nrf2 and plays an important role in detoxification of various environmental toxins and oxidative stress. Real-time PCR was used to examine mRNA express of GSTP1 in astrocytes after pretreated with 5 μg/ml LPNS and the results showed it was increased 231±47% compared to control (p < 0.05, Fig. 7).
Fig. 5. Effect of LPNS on nuclear Nrf2 levels in cultured astrocytes.
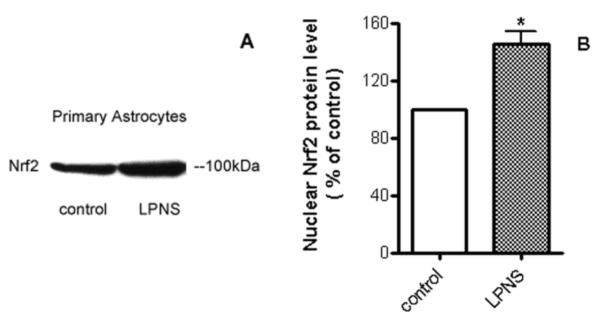
A: Representative Western blot of nuclear Nrf2 in primary astrocytes after 1 h treatment with vehicle or 5 μg/ml LPNS. B: Quantification (fold changes) of the Western blots. Values are means ± SE, n= 3. *Significant difference from vehicle-treated control at the p <0.05 level.
Fig. 6. Effect of LPNS on HO-1 protein levels in cultured astrocytes.
A: Representative HO-1 Western blot in primary astrocytes after 24 h treatment with vehicle or 5 μg/ml LPNS. B: Quantification (fold changes) of the Western blots. Values are means ± SE, n= 3. *Significant difference from vehicle-treated control at the p < 0.05 level.
Fig. 7. Effect of LPNS on GSTP1 mRNA levels in cultured astrocytes.
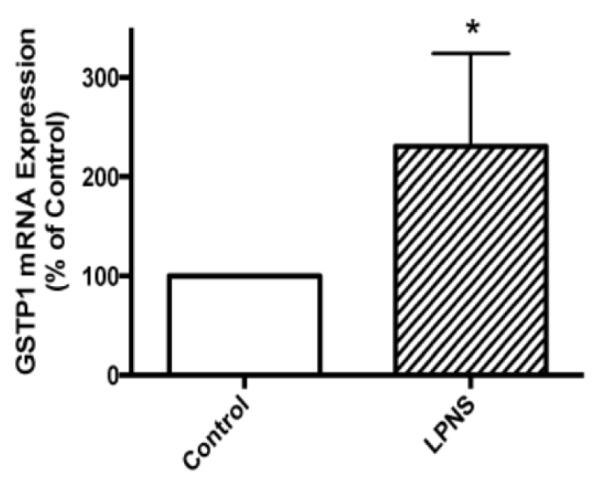
Real-time PCR of GSTP1 mRNA expression in astrocytes after 24 h pretreatment with 5 μg/ml LPNS. Values are means +/- S.E., n = 4. * indicates a significant difference from vehicle-treated control at the p < 0.05 level.
Discussion
This study examined the effects of total saponin in leaves of Panax notoginseng(LPNS)on oxidative stress-induced brain cell damage in vitro. In primary cortical astrocyte cells, LPNS reduced H2O2-induced cell damage. A similar protection was found in a neuronal cell line, SH SY5Y, with damage induced by OGD/reoxygenation. In primary astrocytes, the protective effects of LPNS were associated with reduced reactive oxygen species (ROS) levels, activation of Nrf2 and upregulation of downstream antioxidant mediators such as HO-1 and GSTP1. The present study demonstrates, for the first time, that LPNS exerts a protective effect on H2O2-induced astrocyte damage and ODG/reoxygenation-induced SH-SY5Y cells injury by activating antioxidant systems. These effects may also protect the brain from damage induced by oxidative stress in neurological disorders.
Oxidative stress culminates from an imbalance between pro- and anti-oxidants and consequent excessive production of ROS. ROS have particularly deleterious effects on the nervous system because the brain is relatively deficient in antioxidant systems (Kedar, 2003). Hydrogen peroxide (H2O2), which is a byproduct of both normal and aberrant metabolism, has been shown to elicit cellular injury by initiating lipid peroxidation, protein oxidation and DNA damage(Cochrane, 1991). At higher concentrations (200-800μM), H2O2 alters astrocyte membranes and the cytoskeleton (Zhu et al., 2005), which enable it to cross cell membranes and reach different subcellular compartments (de Almeida et al., 2008; Pedroso et al., 2009). Inside the cell and in the presence of transition metal ions such as Fe2+ or Cu+, H2O2 can be converted into the highly reactive hydroxyl radical. H2O2 can induce mitochondrial calcium overload, mitochondrial membrane potential depolarization, and opening of the mitochondrial permeability transition pore, resulting in an increasing of the mitochondrial ROS formation (Copin et al., 2001; Jou et al., 2004). Thus, an excess of H2O2 induces imbalance in ROS generation and impairs cellular antioxidant defenses, leading finally to cell death, as shown in various cell types, including astrocytes (Bienert et al., 2006; Gyulkhandanyan et al., 2003; Kaczara et al., 2010; Winterbourn, 1981). In the present study, LDH assay indicates pretreatment with LPNS significantly protected primary rat astrocytes from H2O2-induced cell damage and SH-SY5Y cells from OGD/reoxygenation-induced damage. It has been reported that the water extract of the biennial flower of P. notoginseng showed effects in protecting neuronal PC12 cells against H2O2-induced cytotoxicity by reducing the ROS formation (Choi et al., 2010). In this study, we also found that H2O2 increased the production of ROS in cultured rat astrocytes and LPNS significantly inhibited ROS formation in a dose-dependent manner. This is likely responsible for the reduction in cell death induced by H2O2 in astrocytes and OGD, which is a model of ischemia and associated with free radical production in SH-SY5Y cells (Guo et al., 2009). These results indicate that LPNS protected cells against the toxicity created by oxidative stress. It has been reported that LPNS (70-100mg/kg) can reduce ischemic injury and have anti-depressive effects in animals, those of diseases undergo the same basic pathology of oxidative damage (Fu et al., 2006; Xiang et al., 2011; Zhu et al., 2010).
Cells have evolved antioxidant defense mechanisms to neutralize ROS and maintain cellular redox homeostasis during oxidative stress. One of the most important cellular defense mechanisms against ROS and electrophilic intermediates is mediated through the antioxidant responsive element(ARE).ARE-dependent cellular defense systems are regulated by a transcription factor, Nrf2 (Jaiswal, 2004; Mathers et al., 2004), which is held in the cytoplasm by a cytoskeletal-associated specific inhibitory protein KEAP1 under conditions of normal cellular redox state and is continuously targeted to proteasomal degradation. Under conditions of oxidative stress, Nrf2 dissociates from KEAP1 and then translocates to the nucleus, binds to ARE to induce antioxidant and phase II detoxification enzymes such as HO-1 and GSTP1 (Kobayashi and Yamamoto, 2005; Park et al., 2011). There is increasing evidence that the Nrf2-ARE pathway is involved in neurodegenerative disease and the expression of ARE driven genes such as NQO1 and HO-1 is increased in post mortem brain tissue from PD patients (Park et al., 2011; Schipper et al., 1998; Yoo et al., 2003). These changes could be a neuroprotective response mediated by Nrf2 activation. Induction of Nrf2-mediated transcription, particularly in astrocytes, has been shown to protect against neurotoxicity from a variety of insults (Calkins et al., 2005). Consistent with those reports, the present study revealedNrf2 accumulation in the nucleus and upregulation of downstream antioxidative enzymes (HO-1, GSTP1) after treatment with LPNS in astrocytes. As ROS levels are tightly controlled by an inducible antioxidant program that responds to cellular stressors and is predominantly regulated by Nrf2 (DeNicola et al., 2011), LPNS may increase the transcription of Nrf2 to stably elevate the basalNrf2 antioxidant program and thereby reducing intracellular ROS.
Oxidative stress is an important molecular mediator of astrocyte injury indifferent neurological conditions and may be an important therapeutic target. One strategy for detoxifying different reactive oxygen and nitrogen species that are produced in such conditions is induction of the Phase II gene response by the use of chemicals or conditions that promote the translocation of Nrf2 from the cytosol to the nucleus, where it binds to genomic antioxidant response elements. The present study examined the hypothesis that pretreatment with LPNS would stimulate antioxidative responses in astrocytes and neurons, and would mitigate the effects of ROS-mediated neurotoxicity via the Nrf2 pathway. We demonstrate that LPNS, a potent stimulator of antioxidant responses, was effective in reducing neurotoxicity associated with decreasing H2O2-induced LDH release, decreasing ROS production and upregulating HO-1 and GSTP1 expression through the activation of Nrf2 in astrocytes. Data support a key role for astrocyte-produced antioxidants in modulating oxidative stress and neuronal damage in response to H2O2and other forms of oxidative damage (Dringen and Hirrlinger, 2003). LPNS may, therefore, promote neuronal survival through activating the astrocyte-mediated antioxidative pathway by decreasing the amount of ROS and increasing the activities of antioxidative enzymes.
In summary, LPNS has significant protective effects against cell damage induced by H2O2 and OGD/reoxygenation. It inhibits ROS by activating Nrf2 and downstream antioxidant systems. Moreover, this study supports the hypothesis that LPNS stimulates antioxidative responses in astrocytes, as well as neurons, thereby attenuating the effects of ROS-mediated neurotoxicity. This is the first report on the therapeutic effects of LPNS on oxidative damage after H2O2 or OGD/reoxygenation insult and the role of LPNS in regulating ROS and oxidative stress response in brain cells. All of these indicate that LPNS could be a potential therapeutic agent to ameliorate oxidative damage and neurodegenerative processes.
Acknowledgements
This study was supported by a grant, NS-034709 (RFK), from the National Institutes of Health (NIH) and 81260649, from the National Natural Science Foundation of China. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and National Natural Science Foundation of China.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Braughler JM, Hall ED. Central nervous system trauma and stroke. I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic Biol Med. 1989;6:289–301. doi: 10.1016/0891-5849(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102:244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P, Thomas GN, Tomlinson B. Protective effects of trilinolein extracted from panax notoginseng against cardiovascular disease. Acta Pharmacol Sin. 2002;23:1157–1162. [PubMed] [Google Scholar]
- Chen S, Liu J, Liu X, Fu Y, Zhang M, Lin Q, Zhu J, Mai L, Shan Z, Yu X, Yang M, Lin S. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. J Ethnopharmacol. 2011;137:263–270. doi: 10.1016/j.jep.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhou M, Li Q, Yang J, Zhang Y, Zhang D, Kong S, Zhou D, He L. Sanchi for acute ischaemic stroke. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006305.pub2. CD006305. [DOI] [PubMed] [Google Scholar]
- Choi RC, Jiang Z, Xie HQ, Cheung AW, Lau DT, Fu Q, Dong TT, Chen J, Wang Z, Tsim KW. Anti-oxidative effects of the biennial flower of Panax notoginseng against H2O2-induced cytotoxicity in cultured PC12 cells. Chin Med. 2010;5:38. doi: 10.1186/1749-8546-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane CG. Cellular injury by oxidants. Am J Med. 1991;91:23S–30S. doi: 10.1016/0002-9343(91)90280-b. [DOI] [PubMed] [Google Scholar]
- Copin JC, Gasche Y, Li Y, Chan PH. Prolonged hypoxia during cell development protects mature manganese superoxide dismutase-deficient astrocytes from damage by oxidative stress. FASEB J. 2001;15:525–534. doi: 10.1096/fj.00-0330com. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Taylor JM. Reactive oxygen species and the modulation of stroke. Free Radic Biol Med. 2005;38:1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- de Almeida LM, Leite MC, Thomazi AP, Battu C, Nardin P, Tortorelli LS, Zanotto C, Posser T, Wofchuk ST, Leal RB, Goncalves CA, Gottfried C. Resveratrol protects against oxidative injury induced by H2O2 in acute hippocampal slice preparations from Wistar rats. Arch Biochem Biophys. 2008;480:27–32. doi: 10.1016/j.abb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Hirrlinger J. Glutathione pathways in the brain. Biol Chem. 2003;384:505–516. doi: 10.1515/BC.2003.059. [DOI] [PubMed] [Google Scholar]
- Fu JH, Li XZ, Shang XH, Liu JX. Protective effects of saponines of stem and leaf of Panax notoginseng on acute myocardial ischemia in anaesthetic dogs] Zhongguo Zhong Yao Za Zhi. 2006;31:62–65. [PubMed] [Google Scholar]
- Giffard RG, Swanson RA. Ischemia-induced programmed cell death in astrocytes. Glia. 2005;50:299–306. doi: 10.1002/glia.20167. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Miyake M, Liu KJ, Shi H. Specific inhibition of hypoxia inducible factor 1 exaggerates cell injury induced by in vitro ischemia through deteriorating cellular redox environment. J Neurochem. 2009;108:1309–1321. doi: 10.1111/j.1471-4159.2009.05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyulkhandanyan AV, Feeney CJ, Pennefather PS. Modulation of mitochondrial membrane potential and reactive oxygen species production by copper in astrocytes. J Neurochem. 2003;87:448–460. doi: 10.1046/j.1471-4159.2003.02029.x. [DOI] [PubMed] [Google Scholar]
- Hamdi Y, Masmoudi-Kouki O, Kaddour H, Belhadj F, Gandolfo P, Vaudry D, Mokni M, Leprince J, Hachem R, Vaudry H, Tonon MC, Amri M. Protective effect of the octadecaneuropeptide on hydrogen peroxide-induced oxidative stress and cell death in cultured rat astrocytes. J Neurochem. 2011;118:416–428. doi: 10.1111/j.1471-4159.2011.07315.x. [DOI] [PubMed] [Google Scholar]
- He L, Chen X, Zhou M, Zhang D, Yang J, Yang M, Zhou D. Radix/rhizoma notoginseng extract (sanchitongtshu) for ischemic stroke: a randomized controlled study. Phytomedicine. 2011;18:437–442. doi: 10.1016/j.phymed.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Jou MJ, Peng TI, Reiter RJ, Jou SB, Wu HY, Wen ST. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J Pineal Res. 2004;37:55–70. doi: 10.1111/j.1600-079X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- Kaczara P, Sarna T, Burke JM. Dynamics of H2O2 availability to ARPE-19 cultures in models of oxidative stress. Free Radic Biol Med. 2010;48:1064–1070. doi: 10.1016/j.freeradbiomed.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedar NP. Can we prevent Parkinson’s and Alzheimer’s disease? J Postgrad Med. 2003;49:236–245. [PubMed] [Google Scholar]
- Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- Lei XL, Chiou GC. Cardiovascular pharmacology of Panax notoginseng (Burk) F.H. Chen and Salvia miltiorrhiza. Am J Chin Med. 1986;14:145–152. doi: 10.1142/S0192415X86000235. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Falsig J, van Beek J, Payne S, Dringen R, Brundin P, Leist M. Progressive degeneration of human mesencephalic neuron-derived cells triggered by dopamine-dependent oxidative stress is dependent on the mixed-lineage kinase pathway. J Neurosci. 2005;25:6329–6342. doi: 10.1523/JNEUROSCI.1746-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers J, Fraser JA, McMahon M, Saunders RD, Hayes JD, McLellan LI. Antioxidant and cytoprotective responses to redox stress. Biochem Soc Symp. 2004;157:176. doi: 10.1042/bss0710157. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Koumenis IL, Yuan TY, Giffard RG. Increasing vulnerability of astrocytes to oxidative injury with age despite constant antioxidant defenses. Neuroscience. 1998;82:915–925. doi: 10.1016/s0306-4522(97)00320-5. [DOI] [PubMed] [Google Scholar]
- Park JS, Jung JS, Jeong YH, Hyun JW, Le TK, Kim DH, Choi EC, Kim HS. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: critical role of hemeoxygenase-1 and NQO1 expression. J Neurochem. 2011;119:909–919. doi: 10.1111/j.1471-4159.2011.07395.x. [DOI] [PubMed] [Google Scholar]
- Pedroso N, Matias AC, Cyrne L, Antunes F, Borges C, Malho R, de Almeida RF, Herrero E, Marinho HS. Modulation of plasma membrane lipid profile and microdomains by H2O2 in Saccharomyces cerevisiae. Free Radic Biol Med. 2009;46:289–298. doi: 10.1016/j.freeradbiomed.2008.10.039. [DOI] [PubMed] [Google Scholar]
- Peuchen S, Bolanos JP, Heales SJ, Almeida A, Duchen MR, Clark JB. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–281. doi: 10.1016/s0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Resende R, Moreira PI, Proenca T, Deshpande A, Busciglio J, Pereira C, Oliveira CR. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med. 2008;44:2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Sabirzhanova IB, Sabirzhanov BE, Keifer J, Clark TG. Activation of mammalian Tolloid-like 1 expression by hypoxia in human neuroblastoma SH-SY5Y cells. Biochem Biophys Res Commun. 2009;389:338–342. doi: 10.1016/j.bbrc.2009.08.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp Neurol. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56:1747–1754. doi: 10.1002/glia.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Baba A, Matsuda T. Astrocyte apoptosis: implications for neuroprotection. Prog Neurobiol. 2004;72:111–127. doi: 10.1016/j.pneurobio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- Wagner H, Bauer R, Melchart D, Pei-Gen X, Staudinger A. The Analytical Monograph (TLC and HPLC) of Radix and Rhizoma Notoginseng (Sanqi) In: Wagner H, Bauer R, Melchart D, Pei-Gen X, Staudinger A, editors. Chromatographic Fingerprint Analysis of Herbal Medicines. Springer; Wien-New York: 2011. pp. 843–855. [Google Scholar]
- Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75:1149–1163. [PubMed] [Google Scholar]
- Winterbourn CC. Hydroxyl radical production in body fluids. Roles of metal ions, ascorbate and superoxide. Biochem J. 1981;198:125–131. doi: 10.1042/bj1980125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H, Liu Y, Zhang B, Huang J, Li Y, Yang B, Huang Z, Xiang F, Zhang H. The antidepressant effects and mechanism of action of total saponins from the caudexes and leaves of Panax notoginseng in animal models of depression. Phytomedicine. 2011;18:731–738. doi: 10.1016/j.phymed.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Yao XH, Li XJ. Protective effects and its mechanism of panaxatriol saponins isolated from Panax notoginseng on cerebral ischemia] Zhongguo Zhong Yao Za Zhi. 2002;27:371–373. [PubMed] [Google Scholar]
- Yoo MS, Chun HS, Son JJ, DeGiorgio LA, Kim DJ, Peng C, Son JH. Oxidative stress regulated genes in nigral dopaminergic neuronal cells: correlation with the known pathology in Parkinson’s disease. Brain Res Mol Brain Res. 2003;110:76–84. doi: 10.1016/s0169-328x(02)00586-7. [DOI] [PubMed] [Google Scholar]
- Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]
- Zhu JR, Tao YF, Lou S, Wu ZM. Protective effects of ginsenoside Rb(3) on oxygen and glucose deprivation-induced ischemic injury in PC12 cells. Acta Pharmacol Sin. 2010;31:273–280. doi: 10.1038/aps.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]